Abstract
PKCδ and p38δ are key proteins in a cascade that stimulates keratinocyte differentiation. This cascade activates transcription of involucrin (hINV) and other genes associated with differentiation. Protein arginine methyltransferase 5 (PRMT5) is an arginine methyltransferase that symmetrically dimethylates arginine residues. This protein interacts with a cofactor, MEP50, and symmetrically dimethylates arginine eight of histone 3 (H3R8me2s) and arginine three of histone 4 (H4R3me2s) to silence gene expression. We use the involucrin gene as a tool to understand the relationship between PKCδ/p38δ and PRMT5/MEP50 signaling. MEP50 suppresses hINV mRNA level and promoter activity. This is associated with increased arginine dimethylation of hINV gene-associated H3/H4. We further show that the PKCδ/p38δ keratinocyte differentiation cascade reduces PRMT5 and MEP50 expression, association with the hINV gene promoter, and H3R8me2s and H4R2me2s formation. We propose that PRMT5/MEP50-dependent methylation is an epigenetic mechanism that assists in silencing of hINV expression, and that PKCδ signaling activates gene expression by directly activating transcription and by suppressing PRMT5/MEP50 dependent arginine dimethylation of promoter associated histones. This is an example of crosstalk between PKCδ/p38δ signaling and PRMT5/MEP50 epigenetic silencing.
Keywords: PRMT5, p44, MEP50 arginine dimethylation, epigenetic regulation, keratinocyte differentiation, involucrin
Introduction
PKCδ and p38δ are key proteins that control keratinocyte differentiation and proliferation (Adhikary et al., 2010, Chew et al., 2011, Chew et al., 2013, Efimova et al., 1998, Efimova et al., 2002, Efimova et al., 2003, Efimova et al., 2004). PKCδ activates a MEK3/p38δ cascade which triggers events that enhance keratinocyte differentiation (Eckert et al., 2003, Eckert et al., 2004, Efimova et al., 1998, Efimova et al., 2003). This increases AP1, Sp1 and Kruppel-like transcription factor activity leading to activation of differentiation associated gene expression (Crish et al., 1998, Crish et al., 2002, Crish et al., 2006). In particular, involucrin gene expression is increased (Welter and Eckert, 1995, Welter et al., 1995, Han et al., 2012, Banks et al., 1999, Banks et al., 1998, Crish et al., 1998, Crish et al., 2002, Crish et al., 2006, Crish and Eckert, 2008, Adhikary et al., 2010, Chew et al., 2013). This regulation is mediated via specific elements in the involucrin promoter distal regulatory region (DRR) that function both in cultured cells and in vivo (Crish et al., 1998, Crish et al., 2002, Crish et al., 2006, Rorke et al., 2010).
We have recently been interested in epigenetic mechanisms that antagonize this pro-differentiation signaling mechanism. Modification of arginine side chain guanidine groups is quantitatively one of the most frequent modifications in cells (Bedford and Clarke, 2009). Three distinct types of methylated arginine residues occur in cells. The most prevalent is omega-NG,NG-dimethylarginine (Paik et al., 2007) where two methyl groups are placed on one of the terminal nitrogen atoms of the guanidine group to form asymmetric dimethylarginine. Other forms include symmetric dimethylated arginine (SDMA), where one methyl group is placed on each of the terminal guanidino nitrogens (omega-NG,NG-dimethylarginine) and the monomethylated derivative with a single methyl group on the terminal nitrogen atom (omega-NG-monomethylarginine) (Paik et al., 2007).
Protein arginine methyltransferase 5 (PRMT5) is an arginine methyltransferase that symmetrically dimethylates arginine residues on target proteins in both the cytoplasm and nucleus (Bedford and Clarke, 2009). Histones H3 and H4 are important targets. PRMT5 symmetrically dimethylates H3 and H4 as part of an epigenetic mechanism (Molina-Serrano et al., 2013). Histone H4 is symmetrically dimethylated on arginine 3 (H4R3me2s), and histone H3 is symmetrically dimethylated on arginine 8 (H3R8me2s), and this is associated with silencing of gene expression (Fabbrizio et al., 2002, Tae et al., 2011, Bedford and Clarke, 2009).
Our recent study shows that PRMT5 antagonizes differentiation-associated signaling and reduces involucrin gene expression (Kanade and Eckert, 2012). PRMT5 acts with MEP50 which forms a complex with and activates PRMT5 catalytic activity (Hosohata et al., 2003, Ho et al., 2013). In the present study, we examine the role of MEP50 in mediating this response. We show that MEP50 suppresses involucrin (hINV) gene expression as reflected by reduced hINV mRNA level and promoter activity. This is associated with increased arginine dimethylation of the hINV gene promoter. We further show that the PKCδ/p38δ keratinocyte differentiation cascade reduces PRMT5 and MEP50 expression and PRMT5/MEP50 association with the hINV gene promoter leading to increased promoter activity. We propose that PRMT5/MEP50 silencing of involucrin expression is an important epigenetic mechanism that suppresses expression of differentiation-associated genes in the undifferentiated epidermal basal layers. We further propose that loss of PRMT5/MEP50 activity during differentiation is permissive for expression of differentiation-associated genes.
Results
MEP50 and PRMT5 form a complex
Our previous study showed that PRMT5 has an important regulatory role in keratinocytes (Kanade and Eckert, 2012). In the present study, we examine the role of MEP50, a putative PRMT5 cofactor (Karkhanis et al., 2011, Bedford and Clarke, 2009, Yang and Bedford, 2013), as a regulator of keratinocyte differentiation. Recent studies show that MEP50 interacts with and is necessary for PRMT5 activity (Antonysamy et al., 2012). We first examined whether a PRMT5/MEP50 complex exists in keratinocytes. Keratinocytes extracts were prepared and immunoprecipitated with anti-MEP50. Fig. 1A shows that PRMT5 co-precipitates with MEP50. Moreover, as shown in Fig. 1B/C, treatment with MEP50-siRNA partially reduces MEP50 mRNA level and this is associated with reduced PRMT5 mRNA expression, and also reduced MEP50 and PRMT5 protein levels. Conversely, treatment with PRMT5-siRNA reduces PRMT5 and MEP50 mRNA level and this is associated with reduced levels of MEP50 and PRMT5 protein. To further study this regulation, we expressed PRMT5 or MEP50 and monitored the impact on level of the corresponding partner. Fig. 1D shows that overexpression of PRMT5 or MEP50 increases the level, respectively, of MEP50 and PRMT5. These findings suggest that MEP50 and PRMT5 are co-regulated.
Fig. 1.
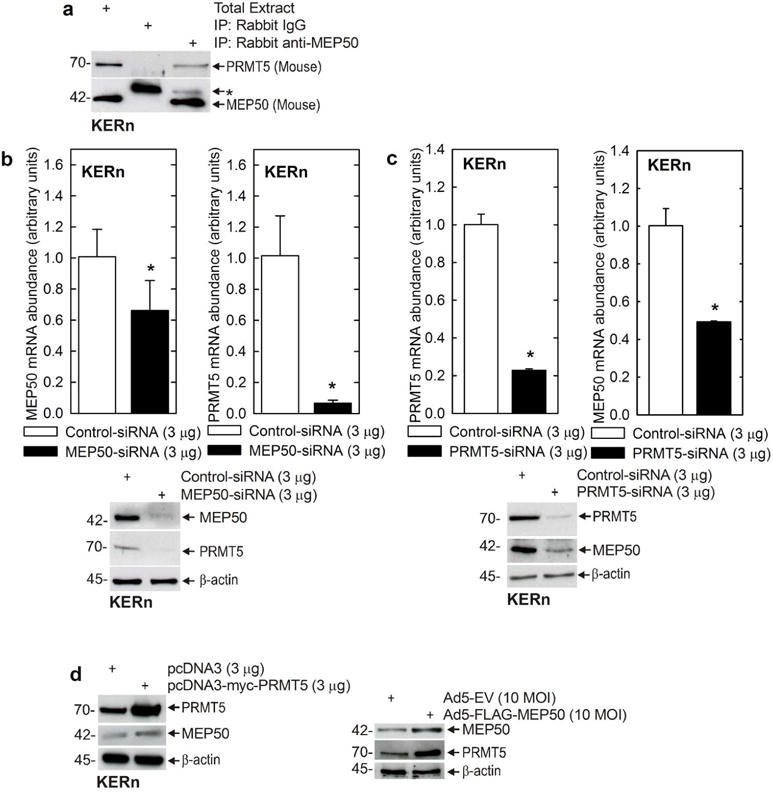
MEP50 and PRMT5 form a complex in keratinocytes. A Freshly isolated foreskin keratinocyte lysates (300 μg) were used for immunoprecipitation with Rabbit IgG or rabbit anti-MEP50, and 10 μg of total extract was electrophoresed. The antibodies for immunoblot are mouse anti-MEP50 and goat anti-PRMT5. Similar results were obtained in three separate experiments. The upper band (*) in the blot probed with MEP50 is non-specific. B/C Keratinocytes were electroporated with 3 μg of control-, MEP50- or PRMT5-siRNA. After 48h, RNA was isolated and MEP50 and PRMT5 mRNA levels were assessed by qRT-PCR. The values are mean ± SEM, n = 3. The asterisks indicate significant differences as determined by the students t-test (*, p<0.005). Extracts were also prepared to assess PRMT5 and MEP50 protein level. D MEP50 or PRMT5 overexpression. KERn were electroporated with 3 μg of control plasmid or plasmids encoding MEP50 or PRMT5, or MEP50-encoding adenovirus (10 MOI). After 48 h protein lysates were prepared for immunoblot with anti-MEP50 and anti-PRMT5. β-actin was used as the loading control. Similar results were observed in each of three experiments.
MEP50 suppresses hINV expression
To assess MEP50 function, we examined the impact of manipulating MEP50 expression on hINV mRNA level and promoter activity. Keratinocytes were transfected with empty vector or MEP50 expression vector and after 24 h the level of mRNA encoding involucrin (hINV) and filaggrin was monitored. These genes were selected because both are markers of terminal keratinocyte differentiation (Eckert et al., 1997, Presland et al., 1997). We show that expression of MEP50 reduces hINV and filaggrin mRNA level (Fig. 2A/B), suggesting that MEP50 acts to suppress differentiation-associated gene expression. We have previously identified a -2471/-1 segment of the hINV promoter that mediates differentiation-appropriate hINV expression in cell culture and in vivo (Welter and Eckert, 1995, Welter et al., 1995, Han et al., 2012, Banks et al., 1999, Banks et al., 1998, Crish et al., 1998, Crish et al., 2002, Crish et al., 2006, Crish and Eckert, 2008, Adhikary et al., 2010, Chew et al., 2013), and also a region of the promoter, called the distal regulatory region (DRR, nucleotides -2473/-2088), which encodes transcription factor binding elements that is essential for appropriate differentiation-associated gene expression (Banks et al., 1998, Crish et al., 1998, Crish et al., 2006). Transfection of hINV-2473 (full-length promoter) in the presence of MEP50 suppresses hINV promoter activity (Fig. 2C). Moreover, MEP50 expression suppresses and MEP50-siRNA increases activity of a reporter construct (-2473/-2088) encoding the hINV DRR region (Fig. 2D/E). Maps of the promoter constructs are shown in Fig. 3A/B. The hINV promoter encodes a key activator protein 1 (AP1) transcription factor binding site, AP1-5, located within the hINV gene DRR, that is absolutely required for correct differentiation-associated expression in cultured keratinocytes and in vivo (Crish et al., 1993, Crish et al., 1998, Crish et al., 2002, Crish et al., 2006). Fig. 3A shows the hINV(2473/-2088 and hINV(-2473/-2088)AP1-5m constructs, where the DRR region is fused to the minimal involucrin promoter at nucleotide -41. The AP1-5 site is mutated in hINV(-2473/-2088)AP1-5m. Fig. 3B shows promoter maps indicating hINV-2473, the full-length hINV gene promoter, and the hINV-241 and hINV-41 truncation constructs (Efimova et al., 1998, Welter and Eckert, 1995, Crish et al., 1993, Crish et al., 1998, Crish et al., 2002, Crish et al., 2006). We used these to examine the effect of mutating the hINV promoter AP1-5 site on MEP50 regulation of hINV promoter activity. Fig. 3C shows that the AP1-5 site mutant, in the context of the DRR, results in a loss of promoter activity and loss of MEP50-associated suppression of promoter activity, suggesting that AP1 transcription factor signaling and the MEP50 epigenetic silencing produces opposing responses on the hINV promoter.
Fig. 2.
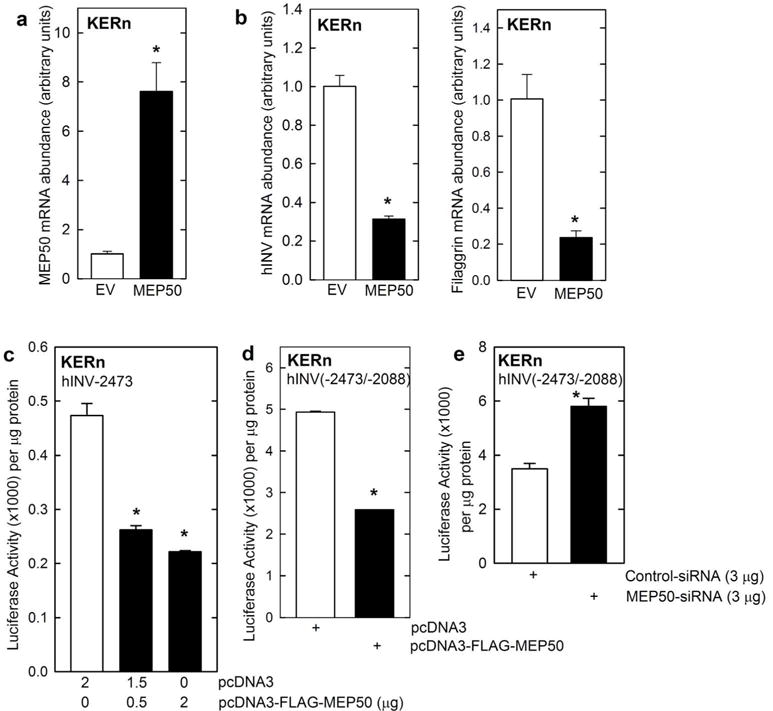
MEP50 suppresses involucrin expression. A/B KERn were electroporated with the indicated plasmids. After 24 h RNA was isolated and MEP50, involucrin and filaggrin mRNA levels were assessed by qRT-PCR. The values are mean ± SEM (n = 3). The asterisks indicate a significant difference (p < 0.005). C/D KERn were transfected with 0.5 μg of the indicated involucrin promoter plasmids in the presence of 1 μg of pcDNA3 or pcDNA3-FLAG-MEP50. At 24 h post-transfection, extracts were prepared and assayed for promoter activity. E KERn were electroporated with 3 μg of control-siRNA or MEP50-siRNA. After 48 h, the cells were re-electroporated with 3 μg of endo-free involucrin promoter. After an additional 24 h, extracts were prepared and promoter activity (luciferase) assay.
Fig. 3.
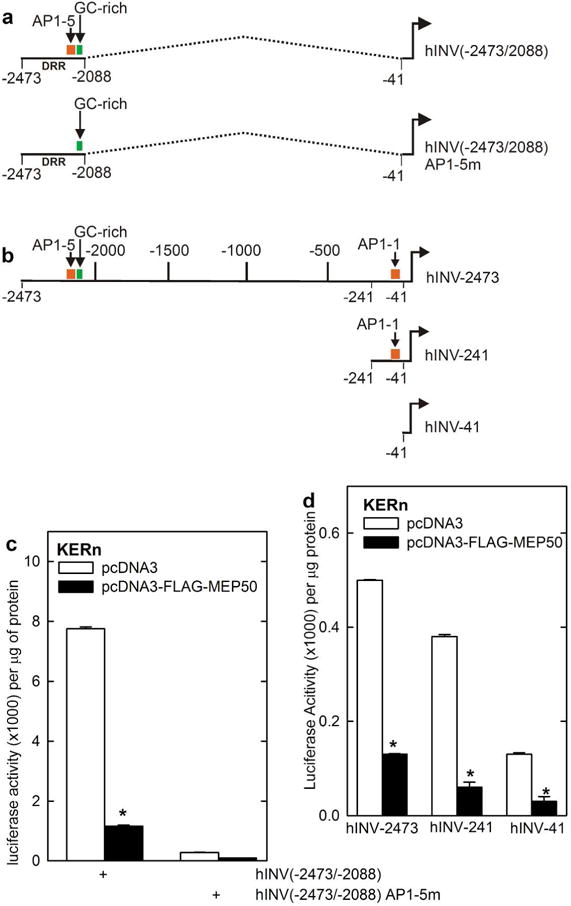
MEP50 suppression of hINV promoter activity. A/B Schematic showing key regulatory elements in the hINV promoter. hINV(-2473/-2088) is a construct in which the DRR (nucleotides -2473/-2088) is linked to the hINV minimal promoter. The dashed line indicates the fusion. hINV(-2473/2088)AP1-5M is identical, except that the AP1-5 site is mutated. hINV-2473 (full-length promoter), hINV-241 and hINV-41 (minimal promoter) comprise a truncation series. The functionally important AP1 (AP1-1 and AP1-5) sites and GC-rich (Sp1) element are indicated. The distances are in nucleotides relative to the transcription start site. C/D KERn were transfected with 0.5 μg of hINV-2473, which encodes the full-length wild-type human involucrin promoter, or the promoter harboring a mutant AP1-5 site (AP1-5m), or truncated promoters (hINV-241, hINV-41) and 1 μg of pcDNA3 or pcDNA3-FLAG-MEP50. At 24 h post-transfection cell extracts were prepared and assayed for promoter activity. The values are mean ± SEM, n = 3. The asterisks indicate a significant change, p < 0.005.
MEP50 controls histone arginine methylation at the hINV promoter We next examined the impact of MEP50 on activity of the constructs shown in Fig. 3B to identify promoter regions that are responsive to MEP50. As expected of a regulatory protein involved in arginine methylation of histones, MEP50 acts at various sites along the hINV promoter, as it suppresses activity of hINV-2473, hINV-241 and hINV-41 (Fig. 3D). However, because it is a key regulatory region (Crish et al., 1998, Crish et al., 2006), we studied effect of MEP50 on arginine methylation of histones surrounding the hINV promoter DRR region. Keratinocytes were electroporated with 3 μg of control- or MEP50-siRNA and after 48 h extracts were prepared for ChIP with anti-IgG, anti-PRMT5 or anti-MEP50. Fig. 4A shows that reducing PRMT5 or MEP50 level is associated with reduced binding of PRMT5 and MEP50 to the hINV promoter DRR region that encodes the AP1-5 and GC-rich (Sp1) binding sites (Fig. 4A). Moreover, the reduction in MEP50 level is associated with reduced H3R8me2s and H4R3me2s formation (Fig. 4B). These findings are consistent with MEP50 control of arginine methylation status at the hINV promoter.
Fig. 4.
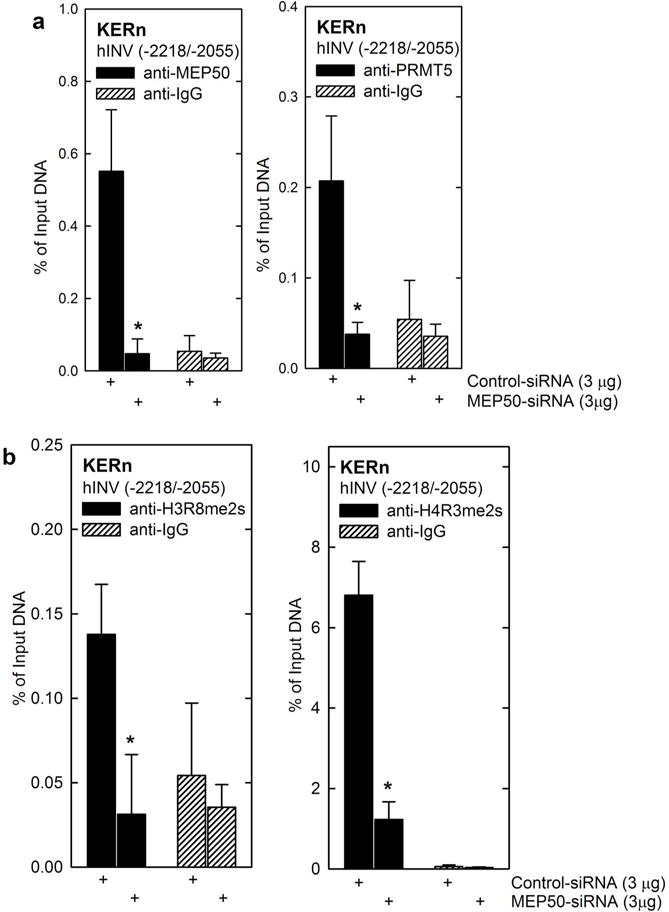
MEP50 controls histone arginine methylation at the hINV promoter. A/B KERn were electroporated with 3 μg of control-siRNA or MEP50-siRNA. After 48 h, extracts were prepared for ChIP analysis. DNA from 1 million cells was sheared, collected and 100,000 cell equivalents of DNA was immunoprecipitated. The primers include nucleotides -2218/-2055 of the DRR region of the hINV promoter region that includes the AP1-5 site. The values are mean ± SEM, n = 3 and the asterisks indicate significant difference, p < 0.005.
Impact of PKCδ/p38δ signaling on MEP50 and PRMT5 interaction and activity at the hINV promoter
We previously described a PKCδ/p38δ MAPK signaling pathway which increases hINV expression and promoter activity, and expression of other differentiation-associated genes in keratinocytes (Efimova and Eckert, 2000, Efimova et al., 1998, Efimova et al., 2003, Efimova et al., 2004). We asked whether activation of this cascade suppresses MEP50 level. Keratinocytes were infected with empty, PKCδ-encoding or p38δ-encoding adenovirus and after 48 h extracts were prepared to monitor MEP50, PRMT5, H3R8me2s and H4R3me2s. Fig. 5A shows that PKCδ and p38δ reduce intracellular MEP50 and PRMT5 level and PRMT5/MEP50-dependent histone modification. Fig. 5B/C show that this is associated with loss of MEP50 and PRMT5 interaction and reduced MEP50/PRMT5-dependent histone modification at the hINV promoter DRR.
Fig. 5.
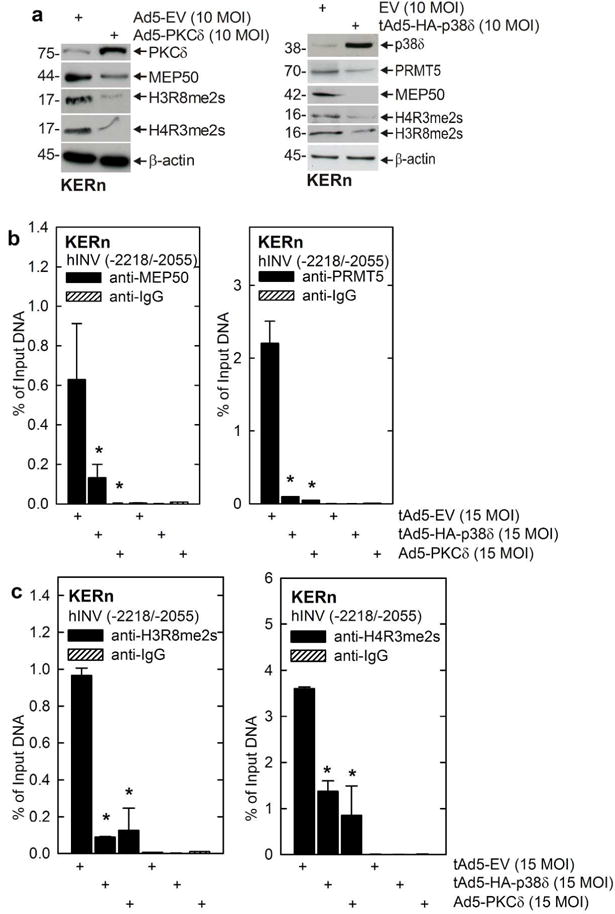
PKCδ/p38δ signaling reduces MEP50 and PRMT5 level and hINV promoter activity. A KERn were infected with 10 MOI of tAd5-EV or Ad5-PKCδ and at 48 h extracts were prepared for detection of PKCδ, MEP50, H3R8me2S and H4R3me2s. β-actin was used as a loading control. Similar results were obtained in three different experiments. B KERn were infected as above and after 48 h ChIP was performed using the Diagenode Low Cell ChIP Kit and primers spanning nucleotides -2218/-2055 if the hINV promoter region which includes the AP1-5 site. The values are mean ± SEM, n = 3. The asterisks indicate significant difference (p < 0.005).
Impact of phorbol ester on MEP50 and PRMT5 level and interaction and activity at the hINV promoter
12-O-tetradecanoylphorbol-13-acetate (TPA) is a known inducer of keratinocyte differentiation and involucrin gene expression. We wanted to assess whether an inducer of differentiation would replicate the regulation of MEP50 and PRMT5 observed following expression of PKCδ and p38δ. Treatment with 50 ng TPA/ml for 48 h suppresses MEP50 and PRMT5 mRNA level (Fig. 6A), and this is associated with a reduced PRMT5 and MEP50 level (Fig. 6B) and increased hINV mRNA level (Fig. 6C). To examine the impact of TPA treatment on PRMT5 and MEP50 interaction with the hINV promoter DRR region, we prepared extracts for ChIP analysis. Fig. 6D shows a reduction in PRMT5 and MEP50 association with the hINV promoter that is associated with reduced promoter-associated arginine-dimethylated histone (Fig. 6E).
Fig. 6.
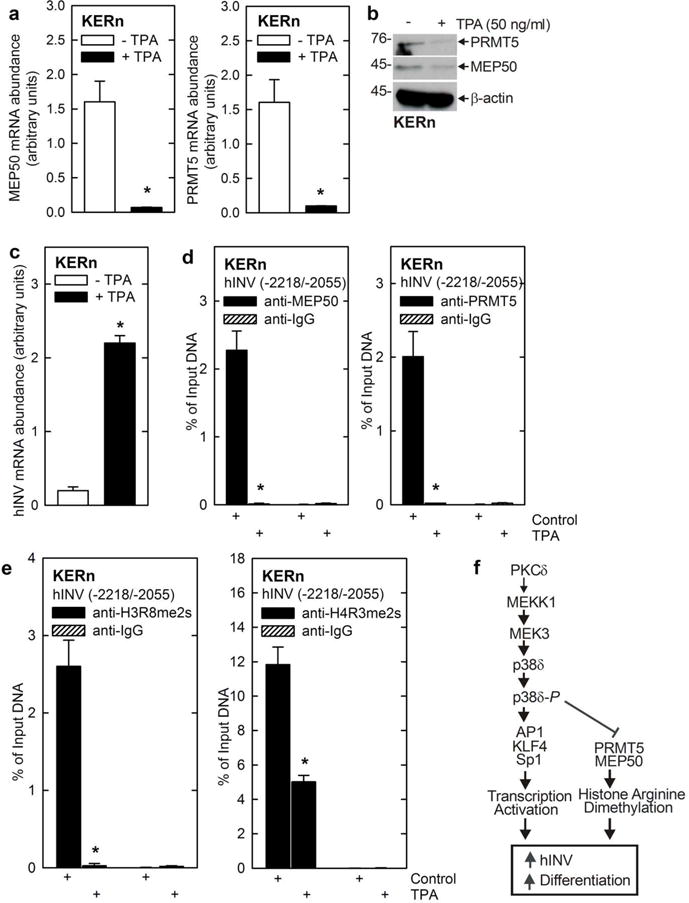
TPA suppresses MEP50 and PRMT5 level and activity. A/B KERn were treated with 50 ng/ml TPA for 48 h, RNA was isolated and MEP50 and PRMT5 mRNA levels were assessed by qRT-PCR. The values are mean ± SEM, n = 3, asterisk indicate a significant difference, p < 0.005. Simultaneously, protein extracts were prepared from identically treated cultures to detect MEP50, PRMT5, H3R8me2s and H4R3me2s. Similar results were obtained in three different experiments. C/D/E KERn were treated with 50 ng TPA/ml and after 48 h mRNA extracts were isolated for ChIP analysis and detection of MEP50 and PRMT5 interaction and H3R8-me2s and H4R3me2s formation at the hINV promoter. Similar results were obtained in three different experiments. The values are mean ± SEM, n = 3. The asterisks indicate significant difference (p < 0.005). F Proposed regulatory model – transcriptional activation and epigenetic de-repression. PKCδ activates the indicated p38δ MAPK cascade that triggers AP1, KLF4 and Sp1 transcription factor movement to the nucleus which activates hINV gene expression and other differentiation-related events. The PKCδ/p38δ cascade also suppresses PRMT5 and MEP50 level, leading to reduced histone arginine dimethylation of histone associated with the hINV promoter leading to de-repression of expression. This cascade can be triggered by expression of PKCδ, p38δ or by treatment with pro-differentiation agents (e.g., TPA).
Discussion
Involucrin has been extensively studied as a model to understand the mechanisms that drive gene expression during keratinocyte differentiation (Eckert et al., 2004, Bikle et al., 2001, Bikle et al., 2003, Denning et al., 2000, Denning et al., 1995, Dlugosz et al., 1994). PKCδ/p38δ MAPK signaling is a key pro-differentiation/anti-proliferation pathway in keratinocytes (Eckert et al., 2002, Eckert et al., 2003). Genetic and inhibitor studies indicate that PKCδ and p38δ activity stimulate hINV gene transcription and increase hINV mRNA and protein level (Dashti et al., 2001, Efimova and Eckert, 2000, Efimova et al., 1998, Efimova et al., 2002, Efimova et al., 2003, Efimova et al., 2004, Kraft et al., 2007). Moreover, treating keratinocytes with agents that activate this cascade, including TPA and calcium, increase hINV gene expression (Adhikary et al., 2005, Chew et al., 2013, Deucher et al., 2002, Eckert et al., 2004). This pathway includes PKCδ, Ras, MEKK1, and MEK3 and activation results in increased p38δ and reduced ERK1/2 activity (Fig. 6F) (Efimova and Eckert, 2000, Efimova et al., 1998, Efimova et al., 2003, Efimova et al., 2004, Kraft et al., 2007). p38δ activation drives nuclear accumulation of AP1 transcription factors which interact with the AP1-5 transcription factor binding site in the hINV promoter DRR to increase hINV gene transcription (Welter and Eckert, 1995, Welter et al., 1995, Efimova et al., 1998). The DRR also includes a Sp1 (GC-rich) transcription factor binding site that is essential for optimal activity (Banks et al., 1999, Banks et al., 1998). Studies in transgenic mice, wherein the AP1-5 site is inactivated by mutation, reveal that this site is essential for differentiation-associated involucrin expression in vivo (Crish et al., 1993, Crish et al., 1998, Crish et al., 2000, Crish et al., 2002, Crish et al., 2006).
Epigenetic silencing of involucrin gene expression – MEP50
Understanding molecular events that occur at the AP1-5 element is important for understanding control of differentiation. Although substantial knowledge is available regarding pathways that positively regulate differentiation-associated gene expression (i.e., differentiation), less is known about pathways that prevent or suppress expression. This is important, as suppression of gene expression, in epidermis, prevents premature activation of differentiation. Epigenetic mechanisms often inhibit differentiation-associated gene expression (Saha et al., 2013, Gilbert et al., 2004) and we have shown that these mechanisms are important in controlling keratinocyte differentiation (Choudhury et al., 2011, Eckert et al., 2011, Kanade and Eckert, 2012, Kanade and Eckert, 2012). PRMT5 is the major type II arginine methyltransferase (Branscombe et al., 2001), and its first identified biological role was that of a transcriptional repressor (Fabbrizio et al., 2002). We recently reported that PRMT5 interacts with and antagonizes PKCδ/p38δ MAPK activation of hINV gene expression (Kanade and Eckert, 2012). We showed that PRMT5 interacts with a multiprotein regulatory complex that includes p38δ to antagonize PKCδ/p38δ signaling and suppress differentiation (Kanade and Eckert, 2012). However, PRMT5 also modifies histones and chromatin to arginine dimethylate histones to silences gene expression (Karkhanis et al., 2011). PRMT5 acts to symmetrical dimethylate arginine eight of H3 and arginine 3 of histone 4 to form, respectively, H3R8me2s and H4R3me2s, to silence gene expression (Yang and Bedford, 2013, Pal et al., 2004, Pal et al., 2007). In this context, PRMT5 interacts with MEP50, which has recently been shown to form an octamer with PRMT5 including four PRMT5 and four MEP50 subunits (Kanade and Eckert, 2012, Hosohata et al., 2003, Antonysamy et al., 2012, Ho et al., 2013). MEP50 is required for optimal PRMT5 activity.
Regulation of MEP50 and PRMT5 level
We show that MEP50 and PRMT5 exist as a complex in keratinocytes, and that MEP50 and PRMT5 are co-regulated such that a forced change in MEP50 level results in a parallel change in PRMT5 level and vice versa. These findings are consistent with recent information showing that PRMT5 and MEP50 form an octamer which requires that the cell maintain approximately equal amount of each (Antonysamy et al., 2012, Ratovitski et al., 2006, Rocco et al., 2006). Translational regulation, dependent upon specific miRNAs, has been described as a mechanism to control PRMT5 level (Karkhanis et al., 2011, Yang and Bedford, 2013). However our studies suggest that regulation of gene transcription or mRNA stability may also control PRMT5 and MEP50 levels, since knockdown of MEP50 causes a marked reduction in PRMT5 mRNA level, and PRMT5 knockdown reduces MEP50 mRNA level (Fig. 1), suggesting that MEP50 and PRMT5 mutually control the level of mRNA encoding the other factor. Additional future studies will be required to understand this mechanism.
MEP50 suppresses hINV expression
Functional studies indicate an inverse relationship between MEP50 level and hINV gene promoter activity and mRNA level. MEP50 suppresses transcription of hINV promoter luciferase reporter plasmids encoding nucleotides -2218/-2055, -2473/-1, -241/-1 or -41/-1 of the hINV promoter. This suggests that arginine dimethylation of chromatin, occurring at various regions in the promoter, can reduce gene expression. This is anticipated, as PRMT5 activity need not only impact a single target element in a promoter, but can impact large tracks of chromatin. To study the details of this regulation, we focused on the DRR region, located within nucleotides -2218/-2055, which encodes key transcription factor binding sites that are required for hINV gene expression (Banks et al., 1998, Welter et al., 1995, Crish et al., 1993, Crish et al., 1998). We observed a reduction in MEP50 interaction with DRR chromatin following MEP50 knockdown. Consistent with an obligatory interaction between PRMT5 and MEP50, MEP50 knockdown also reduced PRMT5 levels in chromatin, and these reductions were associated with reduced H3R8me2s and H4R3me2s formation. Thus, histone arginine dimethylation is associated with reduced hINV gene expression and demethylation is required for increased expression. The idea that PRMT5 and MEP50 act as anti-differentiation/pro-survival regulators is consistent with current reports showing that PRMT5 negatively controls transcription of growth inhibitory genes (Chang et al., 2010, Gu et al., 2012, Wang et al., 2008).
PKCδ/p38δ versus PRMT5/MEP50 signaling – a productive balance
We also show that activation of a classic PKCδ/p38δ cascade, which increases differentiation (Eckert et al., 2004, Efimova et al., 2002, Efimova et al., 2003, Efimova et al., 2004), reduces MEP50 and PRMT5 level and PRMT5-dependent chromatin modification. Reduced PRMT5 and MEP50 level and activity was observed when PKCδ or p38δ were overexpressed, or when activity of these kinases was stimulated by phorbol ester, a known inducer of keratinocyte differentiation (Eckert et al., 2004, Efimova et al., 2002, Efimova et al., 2003, Efimova et al., 2004). This observation suggests that pro-differentiation signaling inhibits PRMT5/MEP50 epigenetic silencing as a component of the process that drives differentiation. We also observed that PRMT5/MEP50 feedback regulates PKCδ and p38δ activity, suggesting that a balance between these regulatory mechanisms controls cell status. For example, MEP50 overexpression reduces PKCδ-Y311 phosphorylation, an indicator of reduced PKCδ activity (not shown). This finding is consistent with our recent report showing that PRMT5 arginine dimethylates proteins in the p38δ MAPK complex, that this part of the PKCδ/p38δ signaling cascade, to reduce p38δ phosphorylation and activity (Kanade and Eckert, 2012). This suggests that proper control of keratinocyte fate requires a balance between PKCδ/p38δ pro-differentiation signaling and PRMT5/MEP50 anti-differentiation/pro-survival signaling. It is interesting that a conventional differentiation stimulus, treatment with elevated (1.5 mM) calcium chloride, did not suppress PRMT5 or MEP50 level, suggesting that different differentiation stimuli may have differing impacts.
Regulation of PRMT5 level has been extensively studied at the translational and post-translation level. An important study showed that mutant JAK kinase, in myeloproliferative neoplasms, phosphorylates PRMT5 (Liu et al., 2011) to reduce PRMT5 association with MEP50 leading to reduced PRMT5 activity, but no change in PRMT5 level (Liu et al., 2011). Additional studies show that specific miRNAs control translation of PRMT5 and MEP50 protein (Yang and Bedford, 2013). Moreover, another study suggests that MEP50 is phosphorylated by CDK4 and that this leads to increased PRMT5/MEP50 activity (Aggarwal et al., 2010). Our present studies suggest a third option – that PRMT5 and MEP50 levels can be controlled by transcriptional mechanisms or by altered mRNA stability. We show that stimulation of PKCδ/p38δ signaling reduces PRMT5 and MEP50 mRNA level, leading to reduced production of these proteins, as a mechanism to reduce PRMT5 activity and arginine methylation of the hINV promoter (Fig. 6F). We have previously shown that a PKCδ, MEKK1, MEK3, p38δ cascade increases AP1/Sp1 and KLF4 transcription factor binding to the DRR element to activate transcription (Eckert et al., 2004, Efimova et al., 1998, Efimova et al., 2002, Efimova et al., 2003). We now propose that this same pathway acts to suppress PRMT5/MEP50 expression, leading to reduced histone arginine dimethylation of the involucrin DRR. It is an interesting feature that PKCδ/MAPK signaling converges via two mechanisms, transcriptional activation of hINV gene expression and inhibition of hINV promoter histone arginine dimethylation, to increase hINV promoter activity (Fig. 6F). We propose that this may be a general mechanism to regulate differentiation-associated gene expression in keratinocytes.
Materials and Methods
Cell culture, plasmids and viruses
The human epidermal keratinocytes used in our studies were obtained from foreskin epidermis. Foreskin epidermis, obtained from newborn infants, was separated from dermis by overnight dispase treatment. KERn were obtained by trypsin treatment and maintained in supplemented keratinocyte serum-free medium (KSFM) (Efimova et al., 2003, Efimova et al., 2004). The human involucrin (hINV) promoter constructs were previously described (Welter et al., 1995, Banks et al., 1998). The human MEP50-encoding plasmid was constructed by primer amplification using plasmid p-OTB7-FLAG-MEP50 (pOTB7-WDR77, MHS1011-202830316) from Open Biosystems (Huntsville, AL) as template. The primers used for amplification of FLAG-MEP50 (BamHI/NotI fragment) were 5′-GATC GGA TCC ATG GAC TAC AAG GAC GAC GAC GAC AAG ATG CGG AAG GAA ACC CCA and 5′-GATC GCG GCC GCC TAC TCA GTA ACA CTT GCA GG. The ATG start codon is bold and the FLAG epitope is underlined. The product was then cloned into pcDNA3 to get pcDNA3-FLAG-MEP50. Adenoviruses encoding HA-p38δ, PKCδ, and empty control virus (tAd5-HA-p38δ, Ad5-PKCδ, Ad5-FLAG-p38δ, and Ad5-EV) were prepared by propagation in HEK293 cells and cesium gradient purification (Chew et al., 2011). The MEP50-FLAG-His tag-encoding adenovirus was from Vigene Biosciences (#VH804847). Culture of human foreskin keratinocytes was approved as an exempt study by the University of Maryland Human Subjects Institutional Review Board.
Promoter luciferase assay
FUGENE 6 (4.5 μl) was diluted in 95.5 μl KSFM and the mixture was incubated at room temperature for 15 minutes. Next, the human involucrin promoter reporter plasmid (0.5 μg) and 1 μg of pcDNA3 or pcDNA3-FLAG-MEP50 were added to the mixture followed by a 20 min incubation. This mixture was added to 2 ml of KSFM in dishes containing fifty percent confluent KERn cultures. After 24 h, cell lysates were processed for luciferase activity assay (Adhikary et al., 2010).
Keratinocyte electroporation
Our studies used the AMAXA electroporator and VPD-1002 nucleofection kit (Cologne, Germany) for keratinocyte electroporation. KERn were harvested with trypsin and replated one day prior to electroporation. After an additional 24 h, the cells were harvested with trypsin and 1 million cells were used per electroporation. The cells were washed with 1 ml of PBS and suspended in 100 μl of keratinocyte nucleofection solution containing 3 μg of control-, MEP50- or PRMT5-siRNA. The mixture was mixed by gentle pipetting and transferred to the electroporation cuvette. The T-018 setting was used for electroporation. This was followed with addition of warm KSFM (500 μl) and the mixture was then transferred to a 55 cm2 dishes containing 10 ml of KSFM. The cells were maintained for various time points before the extracts were prepared for mRNA or protein analysis. This method achieves electroporation efficiencies of > 90% (Adhikary et al., 2010).
Chromatin immunoprecipitation assay (ChIP)
ChIP assays were performed using the Diagenode Low Cell ChIP assay kit (C01010073: kch-maglow-G48). Keratinocytes (0.5 × 106) cells were infected with 15 MOI of empty adenovirus or adenoviruses encoding HA-p38δ or PKCδ. After 48 h, the cells were harvested and 1 × 106 cells per group were used for sonication. Extract equivalent to 100,000 cells was used for immunoprecipitation per sample. MEP50, PRMT5, H4R3Me2s and H3R8me2s antibodies were used for ChIP analysis and binding to the distal regulatory region of the hINV promoter was detected by qRT-PCR using sequence-specific primers and the LightCycler 480 SYBR Green I master mix. The primers used to detect the distal regulatory region of the involucrin promoter (nucleotides -2218/-2055), encoding the AP1-5/Sp1 binding sites, were 5′-TCA GCT GTA TCC ACT GCC CTC TTT (forward) and 5′-TCA CAC CGG TCT TAT GGG TTA GCA (reverse).
Additional methods
Sections describing antibodies and reagents, immunoprecipitation and immunoblot, and quantitative RT-PCR are included in the appendix.
Supplementary Material
Acknowledgments
This work was supported by grants NIH R21AR065266 and NIH RO1CA131064 to RLE and a pilot award from the Greenebaum Cancer Center.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- Adhikary G, Chew YC, Reece EA, et al. PKC-delta and -eta, MEKK-1, MEK-6, MEK-3, and p38-delta Are Essential Mediators of the Response of Normal Human Epidermal Keratinocytes to Differentiating Agents. J Invest Dermatol. 2010 doi: 10.1038/jid.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Adhikary G, Crish JF, Bone F, et al. An involucrin promoter AP1 transcription factor binding site is required for expression of involucrin in the corneal epithelium in vivo. Invest Ophthalmol Vis Sci. 2005;46:1219–1227. doi: 10.1167/iovs.04-1285. [DOI] [PubMed] [Google Scholar]
- Aggarwal P, Vaites LP, Kim JK, et al. Nuclear cyclin D1/CDK4 kinase regulates CUL4 expression and triggers neoplastic growth via activation of the PRMT5 methyltransferase. Cancer Cell. 2010;18:329–340. doi: 10.1016/j.ccr.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonysamy S, Bonday Z, Campbell RM, et al. Crystal structure of the human PRMT5:MEP50 complex. Proc Natl Acad Sci U S A. 2012;109:17960–17965. doi: 10.1073/pnas.1209814109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks EB, Crish JF, Eckert RL. Transcription factor Sp1 activates involucrin promoter activity in non-epithelial cell types. Biochem J. 1999;337(Pt 3):507–512. [PMC free article] [PubMed] [Google Scholar]
- Banks EB, Crish JF, Welter JF, et al. Characterization of human involucrin promoter distal regulatory region transcriptional activator elements-a role for Sp1 and AP1 binding sites. Biochem J. 1998;331(Pt 1):61–68. doi: 10.1042/bj3310061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford MT, Clarke SG. Protein arginine methylation in mammals: who, what, and why. Mol Cell. 2009;33:1–13. doi: 10.1016/j.molcel.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD, Ng D, Tu CL, et al. Calclium- and vitamin D-regulated keratinocyte differentiation. Mol Cell Endocrinol. 2001;177:161–171. doi: 10.1016/s0303-7207(01)00452-x. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Tu CL, Xie Z, et al. Vitamin D regulated keratinocyte differentiation: role of coactivators. J Cell Biochem. 2003;88:290–295. doi: 10.1002/jcb.10339. [DOI] [PubMed] [Google Scholar]
- Branscombe TL, Frankel A, Lee JH, et al. PRMT5 (Janus kinase-binding protein 1) catalyzes the formation of symmetric dimethylarginine residues in proteins. J Biol Chem. 2001;276:32971–32976. doi: 10.1074/jbc.M105412200. [DOI] [PubMed] [Google Scholar]
- Chang YI, Hua WK, Yao CL, et al. Protein-arginine methyltransferase 1 suppresses megakaryocytic differentiation via modulation of the p38 MAPK pathway in K562 cells. J Biol Chem. 2010;285:20595–20606. doi: 10.1074/jbc.M109.092411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew YC, Adhikary G, Wilson GM, et al. PKCdelta suppresses keratinocyte proliferation by increasing p21CIP1 level by a KLF4-dependent mechanism. J Biol Chem. 2011;286:28771–28782. doi: 10.1074/jbc.M110.205245. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chew YC, Adhikary G, Xu W, et al. Protein Kinase C delta Increases Kruppel-like Factor 4 Protein, which Drives Involucrin Gene Transcription in Differentiating Keratinocytes. J Biol Chem. 2013;288:17759–17768. doi: 10.1074/jbc.M113.477133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury SR, Balasubramanian S, Chew YC, et al. (−)-Epigallocatechin-3-gallate and DZNep reduce polycomb protein level via a proteasome-dependent mechanism in skin cancer cells. Carcinogenesis. 2011;32:1525–1532. doi: 10.1093/carcin/bgr171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crish JF, Bone F, Balasubramanian S, et al. Suprabasal expression of the human papillomavirus type 16 oncoproteins in mouse epidermis alters expression of cell cycle regulatory proteins. Carcinogenesis. 2000;21:1031–1037. doi: 10.1093/carcin/21.5.1031. [DOI] [PubMed] [Google Scholar]
- Crish JF, Bone F, Banks EB, et al. The human involucrin gene contains spatially distinct regulatory elements that regulate expression during early versus late epidermal differentiation. Oncogene. 2002;21:738–747. doi: 10.1038/sj.onc.1205038. [DOI] [PubMed] [Google Scholar]
- Crish JF, Eckert RL. Synergistic activation of human involucrin gene expression by Fra-1 and p300–evidence for the presence of a multiprotein complex. J Invest Dermatol. 2008;128:530–541. doi: 10.1038/sj.jid.5701049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crish JF, Gopalakrishnan R, Bone F, et al. The distal and proximal regulatory regions of the involucrin gene promoter have distinct functions and are required for in vivo involucrin expression. J Invest Dermatol. 2006;126:305–314. doi: 10.1038/sj.jid.5700019. [DOI] [PubMed] [Google Scholar]
- Crish JF, Howard JM, Zaim TM, et al. Tissue-specific and differentiation-appropriate expression of the human involucrin gene in transgenic mice: an abnormal epidermal phenotype. Differentiation. 1993;53:191–200. doi: 10.1111/j.1432-0436.1993.tb00708.x. [DOI] [PubMed] [Google Scholar]
- Crish JF, Zaim TM, Eckert RL. The distal regulatory region of the human involucrin promoter is required for expression in epidermis. J Biol Chem. 1998;273:30460–30465. doi: 10.1074/jbc.273.46.30460. [DOI] [PubMed] [Google Scholar]
- Dashti SR, Efimova T, Eckert RL. MEK7-dependent activation of p38 MAP kinase in keratinocytes. J Biol Chem. 2001;276:8059–8063. doi: 10.1074/jbc.C000862200. [DOI] [PubMed] [Google Scholar]
- Denning MF, Dlugosz AA, Cheng C, et al. Cross-talk between epidermal growth factor receptor and protein kinase C during calcium-induced differentiation of keratinocytes. Exp Dermatol. 2000;9:192–199. doi: 10.1034/j.1600-0625.2000.009003192.x. [DOI] [PubMed] [Google Scholar]
- Denning MF, Dlugosz AA, Williams EK, et al. Specific protein kinase C isozymes mediate the induction of keratinocyte differentiation markers by calcium. Cell Growth Differ. 1995;6:149–157. [PubMed] [Google Scholar]
- Deucher A, Efimova T, Eckert RL. Calcium-dependent Involucrin Expression Is Inversely Regulated by Protein Kinase C (PKC)alpha and PKCdelta. J Biol Chem. 2002;277:17032–17040. doi: 10.1074/jbc.M109076200. [DOI] [PubMed] [Google Scholar]
- Dlugosz AA, Cheng C, Williams EK, et al. Alterations in murine keratinocyte differentiation induced by activated rasHa genes are mediated by protein kinase C-alpha. Cancer Res. 1994;54:6413–6420. [PubMed] [Google Scholar]
- Eckert RL, Adhikary G, Rorke EA, et al. Polycomb group proteins are key regulators of keratinocyte function. J Invest Dermatol. 2011;131:295–301. doi: 10.1038/jid.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert RL, Crish JF, Efimova T, et al. Regulation of involucrin gene expression. J Invest Dermatol. 2004;123:13–22. doi: 10.1111/j.0022-202X.2004.22723.x. [DOI] [PubMed] [Google Scholar]
- Eckert RL, Crish JF, Robinson NA. The epidermal keratinocyte as a model for the study of gene regulation and cell differentiation. Physiol Rev. 1997;77:397–424. doi: 10.1152/physrev.1997.77.2.397. [DOI] [PubMed] [Google Scholar]
- Eckert RL, Efimova T, Balasubramanian S, et al. p38 Mitogen-Activated Protein Kinases on the Body Surface – A Function for p38delta. J Invest Dermatol. 2003;120:823–828. doi: 10.1046/j.1523-1747.2003.12120.x. [DOI] [PubMed] [Google Scholar]
- Eckert RL, Efimova T, Dashti SR, et al. Keratinocyte survival, differentiation, and death: many roads lead to mitogen-activated protein kinase. J Invest Dermatol Symp Proc. 2002;7:36–40. doi: 10.1046/j.1523-1747.2002.19634.x. [DOI] [PubMed] [Google Scholar]
- Efimova T, Broome AM, Eckert RL. A regulatory role for p38 delta MAPK in keratinocyte differentiation. Evidence for p38 delta-ERK1/2 complex formation. J Biol Chem. 2003;278:34277–34285. doi: 10.1074/jbc.M302759200. [DOI] [PubMed] [Google Scholar]
- Efimova T, Broome AM, Eckert RL. Protein kinase Cdelta regulates keratinocyte death and survival by regulating activity and subcellular localization of a p38delta-extracellular signal-regulated kinase 1/2 complex. Mol Cell Biol. 2004;24:8167–8183. doi: 10.1128/MCB.24.18.8167-8183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimova T, Deucher A, Kuroki T, et al. Novel protein kinase C isoforms regulate human keratinocyte differentiation by activating a p38 delta mitogen-activated protein kinase cascade that targets CCAAT/enhancer-binding protein alpha. J Biol Chem. 2002;277:31753–31760. doi: 10.1074/jbc.M205098200. [DOI] [PubMed] [Google Scholar]
- Efimova T, Eckert RL. Regulation of human involucrin promoter activity by novel protein kinase C isoforms. J Biol Chem. 2000;275:1601–1607. doi: 10.1074/jbc.275.3.1601. [DOI] [PubMed] [Google Scholar]
- Efimova T, LaCelle P, Welter JF, et al. Regulation of human involucrin promoter activity by a protein kinase C, Ras, MEKK1, MEK3, p38/RK, AP1 signal transduction pathway. J Biol Chem. 1998;273:24387–24395. doi: 10.1074/jbc.273.38.24387. [DOI] [PubMed] [Google Scholar]
- Fabbrizio E, El MS, Polanowska J, et al. Negative regulation of transcription by the type II arginine methyltransferase PRMT5. EMBO Rep. 2002;3:641–645. doi: 10.1093/embo-reports/kvf136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert J, Gore SD, Herman JG, et al. The clinical application of targeting cancer through histone acetylation and hypomethylation. Clin Cancer Res. 2004;10:4589–4596. doi: 10.1158/1078-0432.CCR-03-0297. [DOI] [PubMed] [Google Scholar]
- Gu Z, Gao S, Zhang F, et al. Protein arginine methyltransferase 5 is essential for growth of lung cancer cells. Biochem J. 2012;446:235–241. doi: 10.1042/BJ20120768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Rorke EA, Adhikary G, et al. Suppression of AP1 transcription factor function in keratinocyte suppresses differentiation. PLoS One. 2012;7:e36941. doi: 10.1371/journal.pone.0036941. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ho MC, Wilczek C, Bonanno JB, et al. Structure of the arginine methyltransferase PRMT5-MEP50 reveals a mechanism for substrate specificity. PLoS One. 2013;8:e57008. doi: 10.1371/journal.pone.0057008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosohata K, Li P, Hosohata Y, et al. Purification and identification of a novel complex which is involved in androgen receptor-dependent transcription. Mol Cell Biol. 2003;23:7019–7029. doi: 10.1128/MCB.23.19.7019-7029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanade SR, Eckert RL. Protein Arginine Methyltransferase 5 (PRMT5) Signaling Suppresses Protein Kinase Cdelta- and p38delta-dependent Signaling and Keratinocyte Differentiation. J Biol Chem. 2012;287:7313–7323. doi: 10.1074/jbc.M111.331660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis V, Hu YJ, Baiocchi RA, et al. Versatility of PRMT5-induced methylation in growth control and development. Trends Biochem Sci. 2011;36:633–641. doi: 10.1016/j.tibs.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft CA, Efimova T, Eckert RL. Activation of PKCdelta and p38delta MAPK during okadaic acid dependent keratinocyte apoptosis. Arch Dermatol Res. 2007;299:71–83. doi: 10.1007/s00403-006-0727-4. [DOI] [PubMed] [Google Scholar]
- Liu F, Zhao X, Perna F, et al. JAK2V617F-mediated phosphorylation of PRMT5 downregulates its methyltransferase activity and promotes myeloproliferation. Cancer Cell. 2011;19:283–294. doi: 10.1016/j.ccr.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Serrano D, Schiza V, Kirmizis A. Cross-talk among epigenetic modifications: lessons from histone arginine methylation. Biochem Soc Trans. 2013;41:751–759. doi: 10.1042/BST20130003. [DOI] [PubMed] [Google Scholar]
- Paik WK, Paik DC, Kim S. Historical review: the field of protein methylation. Trends Biochem Sci. 2007;32:146–152. doi: 10.1016/j.tibs.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Pal S, Baiocchi RA, Byrd JC, et al. Low levels of miR-92b/96 induce PRMT5 translation and H3R8/H4R3 methylation in mantle cell lymphoma. EMBO J. 2007;26:3558–3569. doi: 10.1038/sj.emboj.7601794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Vishwanath SN, Erdjument-Bromage H, et al. Human SWI/SNF-associated PRMT5 methylates histone H3 arginine 8 and negatively regulates expression of ST7 and NM23 tumor suppressor genes. Mol Cell Biol. 2004;24:9630–9645. doi: 10.1128/MCB.24.21.9630-9645.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presland RB, Kimball JR, Kautsky MB, et al. Evidence for specific proteolytic cleavage of the N-terminal domain of human profilaggrin during epidermal differentiation. J Invest Dermatol. 1997;108:170–178. doi: 10.1111/1523-1747.ep12333356. [DOI] [PubMed] [Google Scholar]
- Ratovitski E, Trink B, Sidransky D. p63 and p73: teammates or adversaries? Cancer Cell. 2006;9:1–2. doi: 10.1016/j.ccr.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Rocco JW, Leong CO, Kuperwasser N, et al. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell. 2006;9:45–56. doi: 10.1016/j.ccr.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Rorke EA, Adhikary G, Jans R, et al. AP1 factor inactivation in the suprabasal epidermis causes increased epidermal hyperproliferation and hyperkeratosis but reduced carcinogen-dependent tumor formation. Oncogene. 2010;29:5873–5882. doi: 10.1038/onc.2010.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha K, Hornyak TJ, Eckert RL. Epigenetic cancer prevention mechanisms in skin cancer. AAPS J. 2013;15:1064–1071. doi: 10.1208/s12248-013-9513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tae S, Karkhanis V, Velasco K, et al. Bromodomain protein 7 interacts with PRMT5 and PRC2, and is involved in transcriptional repression of their target genes. Nucleic Acids Res. 2011;39:5424–5438. doi: 10.1093/nar/gkr170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Pal S, Sif S. Protein arginine methyltransferase 5 suppresses the transcription of the RB family of tumor suppressors in leukemia and lymphoma cells. Mol Cell Biol. 2008;28:6262–6277. doi: 10.1128/MCB.00923-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welter JF, Crish JF, Agarwal C, et al. Fos-related antigen (Fra-1), junB, and junD activate human involucrin promoter transcription by binding to proximal and distal AP1 sites to mediate phorbol ester effects on promoter activity. J Biol Chem. 1995;270:12614–12622. doi: 10.1074/jbc.270.21.12614. [DOI] [PubMed] [Google Scholar]
- Welter JF, Eckert RL. Differential expression of fos and jun family members c-fos, fosB, Fra-1, Fra-2, c-jun, junB and junD during human epidermal keratinocyte differentiation. Oncogene. 1995;11:2681–2687. [PubMed] [Google Scholar]
- Yang Y, Bedford MT. Protein arginine methyltransferases and cancer. Nat Rev Cancer. 2013;13:37–50. doi: 10.1038/nrc3409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


