Abstract
Objective:
The underlying mechanisms for the analgesic action of paracetamol (PCT) are still under considerable debate. It has been recently proposed that PCT may act by modulating the Serotonin system. This study was conducted to verify the influence of Serotonin modulating drugs (buspirone, ondansetron, and fluoxetine) on the analgesic effect of PCT.
Materials and Methods:
Thirty adult albino mice were assigned to five groups: Normal saline, PCT, fluoxetine selective serotonin reuptake inhibitor (SSRI) + PCT, buspirone (5-HT1A Agonist) + PCT, and ondansetron (5HT3 antagonist) + PCT. Hot-plate and formalin test were used to determine pain threshold, tests being conducted 60 min after the last treatment. Statistical analysis was done using analysis of variance followed by Dunnet's test.
Results:
Coadministration of buspirone with PCT attenuated the antinociceptive activity of PCT (P < 0.001), whereas fluoxetine + PCT increased pain threshold in the hot-plate and formalin test (P = 0.0046). Analgesic effect of PCT was not affected by ondansetron in formalin models. It attenuated analgesic action of PCT in hot-plate test (P = 0.0137).
Conclusion:
The results suggest that 5-HT1 receptors could also be responsible for the analgesic effect of PCT. Also, higher analgesia is produced by co-administration of SSRI (fluoxetine) + PCT.
KEY WORDS: Acetaminophen, antinociceptive acton, buspirone, fluoxetine, ondansetron, selective serotonin reuptake inhibitor, serotonin
Introduction
Paracetamol (PCT) (acetaminophen) is used widely for its analgesic and antipyretic actions, despite being a weaker analgesic it is often preferred over other nonsteroidal anti-inflammatory drugs (NSAIDs) because of its better tolerance. Though it is a common belief that PCT inhibits cyclo-oxygenase pathway mainly in brain, the analgesic effects of PCT are attinuated by drugs that act via inhibition of serotonergic, opioid and cannabinoid systems.[1] Hence, its sites and mechanisms of action are not yet completely understood.
Extensive studies have shown that the analgesic action of PCT is significantly reduced when lesions are produced in the serotonergic pathway or by inhibiting synthesis of serotonin in animal models.[2,3] Conversely, PCT treatment increased the central levels of serotonin and reduced the density of cortical 5-HT receptors. Another hypothesis that has surfaced is that the analgesic action of systemically administered PCT can be attributed to both spinal adenosine A (1) receptors and serotonin (5-HT3 and 5-HT7) receptors.[4] It is becoming clearer that the analgesic action of PCT can be ascribed, to some extent, to the enhanced neurotransmitter release in the Descending Serotonergic pathway, which is responsible for modulation of pain at the spinal level, so it does not reach the higher centers.[2]
In clinical settings most commonly used drugs acting on serotonergic systems are 5-HT1 agonist (buspirone, sumatriptan) as anti-anxiety drugs, 5-HT3 antagonists (granisetron, ondansetron) as antiemetics, 5-HT reuptake inhibitor (fluoxetine, citalopram) as antidepressants. The diversity of the classes of these drugs illustrates the diversity of actions that can be credited to this one neurotransmitter – Serotonin. Serotonin is involved in a myriad of functions in the body – learning and memory, mood regulation, pain processing and modulation, cardiovascular functioning, gastrointestinal motility and many others.[1,2,3]
Consequently, it is critical to verify the effect of drugs modulating the serotonergic receptors on the analgesic effect of PCT, if it too acts via the same system. With this goal in mind, the current study was undertaken using rodent models of central and peripheral analgesia-Eddy's hot-plate method and formalin test.
Hot-plate model is one of the most commonly used tests of analgesic measure of analgesic drugs that act at the level of spine and higher centres.[5] hot-plate test involves higher brain functions and is considered to be a supraspinally organized response. The involvement of endogenous substances such as prostaglandins (PGs) is minimized in this model. Hence, we chose this model to assess central mechanism of the PCT as an analgesic.
The formalin test is a simple and reliable model of chronic pain that involves 2 distinct phases, an early neurogenic phase, followed by a late phase of inflammation that is characterized by the release of inflammatory mediators and pain.[5] As both a central as well as peripheral mechanism of PCT has been proposed, we employed both Hot-plate and formalin test to assess effect of drugs modulating serotonergic system on the analgesic action of PCT in mice. We conducted this study with the objective to compare the analgesic effect of PCT alone and PCT administered with drugs acting on serotonergic system in mice.
Materials and Methods
The study was initiated after Institutional Animal Ethics Committee approval (Approval no. BVDUMC/185/2014–2015).
Animals
Thirty adult albino mice weighing 25–30 g of either sex were used in our study. Housing was done in standard cages (6 animals per cage) with food (standard chow) and water ad libitum and maintaining a 12-h light-dark cycle. Animal coding was done according to standard protocol and animals were randomly allocated to different experimental groups. All tests were performed between 09:00 a.m. and 04:00 p.m. to minimize the confounding effects of circadian rhythms. The mice were divided into five groups of six animals each as follows:
Group 1: Control - PCT alone
Group 2: PCT + buspirone
Group 3: PCT + ondansetron
Group 4: PCT + fluoxetine
Group 5: Negative control: Distilled water.
The drugs were administered to the various groups as follows
PCT: 200 mg/kg intraperitoneally
Buspirone: 10 mg/kg orally
Ondansetron: 4 mg/kg orally
Fluoxetine: 10 mg/kg orally.
Animals in Group 2 and 3 were administered respective drugs 30 min before PCT. Group 4 animals were pretreated with fluoxetine for 7 days and 30 min before PCT on the day of the experiment. Exactly 45 min after PCT injection the animals were evaluated for analgesic effect.
Following tests were used to assess analgesic activity of PCT.
Hot-plate method
The hot plate test was used as previously described.[5] The mice were randomly assigned to the groups mentioned above. The procedure was started 45 min after administration of PCT in treatment groups. The temperature of the hot-plate was maintained at 55°C. The time taken by the animal from placement on hot-plate to either licking of paws or jumping off the plate was recorded as the reaction time. A cut-off time of 30 s was instituted to avoid thermal injury to the paws of the animals.
Formalin test
Formalin test was carried out as described previously.[5] 0.02 ml of 5% formalin was injected subcutaneously into the plantar portion of the left hind paw of all animals using a Tuberculin syringe to produce chemically induced pain.[5,6] Each animal was kept in a transparent cage for observation. Readings were taken at both, early (0–5 min) and late (20–30 min) phases and scored according to a pain scale.[5,6]
Pain responses were observed by excessive licking and biting or elevation of the paw. Analgesic response or protection was indicated if both paws were seen stationary with no special attention towards the injected paw.[5]
Data Analysis
All values in the study were specified as mean ± standard error of mean. One-way analysis of variance followed by Dunnet’s
test was used for statistical analysis using Graph Pad Prism version 5. (GraphPad Software, Inc. La Jolla, CA 92037, USA). P < 0.05 was considered statistically significant.
Results
Analgesic Activity of Paracetamol when Co-administered with Drugs Acting on Serotonergic System in Eddy's Hot-plate Model in Mice
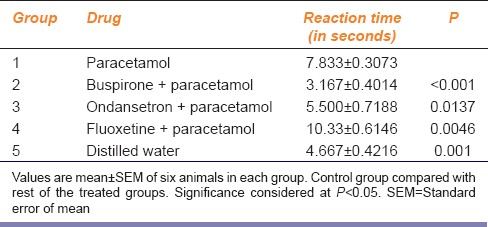
Reaction time in PCT treated group was significantly increased (P < 0.0001) as compared to the Control group. The most effective group found was fluoxetine + PCT group, with reaction time of 10.33 ± 0.614 s, whereas the buspirone + PCT showed a decrease in reaction time than PCT group (P < 0.0001). Ondansetron + PCT group also showed decrease in reaction time in comparison to PCT group (P = 0.013) [Table 1].
Table 1.
Analgesic activity of paracetamol when coadministered with drugs acting on serotonergic system in Eddy's hot-plate model in mice
Anti-nociceptive Activity of Paracetamol with Drugs Acting on Serotonergic System in Albino Mice in Formalin-Induced Paw-licking Test-early Phase
The results of orally administered PCT on the formalin-induced hind paw licking are presented in Figure 1. In this model, PCT was found to cause a significant reduction in the licking time compared to control group (P = 0.009) suggesting analgesic activity in the early phase (0–5 min). Co-administration of fluoxetine + PCT showed more analgesic effect than PCT alone, however this increase was not statistically significant (P > 0.05). The paw licking and shaking time following the administration of buspirone + PCT was significantly increased as compared to PCT group (P = 0.0122). Analgesic effect in the ondansetron + PCT group was less than PCT alone, however this decrease was not statistically significant (P > 0.05) [Figure 1].
Figure 1.
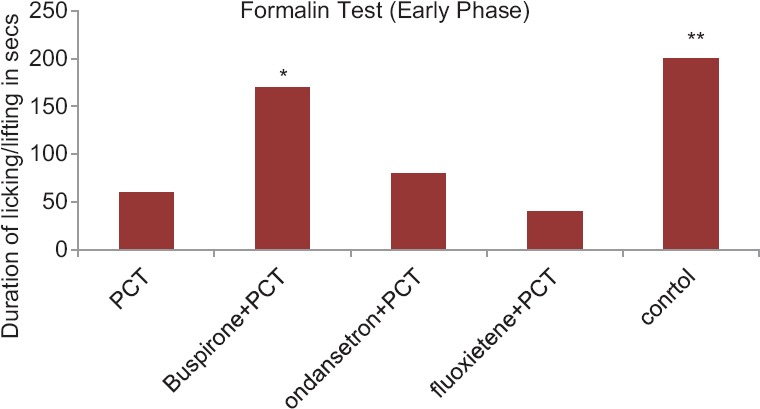
Anti-nociceptive activity of paracetamol with drugs acting on serotonergic system in albino mice in formalin-induced paw-licking test-early phase. Control group compared with rest of the treated groups. Significance at *P < 0.05; **P < 0.01; ***P < 0.001
Anti-Nociceptive Activity of Paracetamol with Drugs Acting on Serotonergic System in Albino Mice in Formalin-Induced Paw-licking Test-late Phase
During the late phase, the injection of PCT showed significant reduction in the duration of paw licking as compared to Control group. PCT + buspirone and PCT + ondansetron group also showed analgesic action comparable to PCT (P > 0.05). On the other hand, the PCT + fluoxetine group exhibited total nonexistence of the late phase as shown by lack of paw licking post the formalin injection (P < 0.001) [Figure 2].
Figure 2.
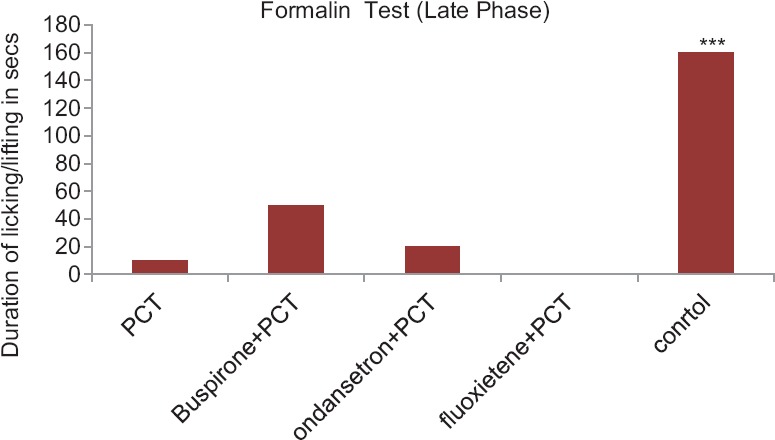
Anti-nociceptive activity of Paracetamol with drugs acting on serotonergic system in albino mice in formalin-induced paw-licking test-late phase. Control group compared with rest of the treated groups. Significance at *P < 0.05; **P < 0.01; ***P < 0.001
Discussion
PCT is one of the most widely used analgesic and antipyretic agent for several years, but its mechanism of action is yet to be fully understood. Although classified as NSAIDs-category drugs, it has a different profile, considering side effects and therapeutic activities. Many mechanisms have been postulated, the recent of them being, its ability to modulate the Serotonergic system to exert its analgesic action.
The models we chose for assessing the pain in animals were intended to assess both central and peripheral pain stimulation.
In this study, PCT has shown significant analgesic effect in hot-plate as well as formalin (early and late phase) models. Our results support that PCT acts peripherally as well as centrally.
Such a hypothesis about the central mechanism of PCT is in concurrence with the studies about the ability of PCT to cross the blood brain barrier both in rodents and humans.[6] Several studies, using various pain models in animals, have concluded that the analgesic action of PCT could be attributed to the modulation of the serotonergic system, especially the descending serotonergic pathway. This pathway extends from the nucleus raphe magnus (NRM) to the substantia gelatinosa of the dorsal horn, and serotonin is its main neurotransmitter. Activation of this descending pathway inhibits transmission particularly in nociceptive pathways.[7] Thus, higher central 5-HT turnover due to PCT and its action on various serotonin receptor subtypes (5-HT1, 5-HT2 and 5-HT3 receptors) are now considered to have a role in pain control mechanisms.[8]
In line with the finding noted above, the current study suggests that the antinociceptive action of single systemic injection of PCT in hot-plate test and in early phase of formalin test get attenuated by buspirone which is 5-HT1A receptors selective agonist.
Buspirone is a 5-HT1A receptor agonist used as an anti-anxiety drug. The presynaptic 5HT1A receptor is an autoreceptor[9] and by stimulating these receptors, there is a decrease in the secretion of serotonin. Hence, when it is co-administered with PCT, which causes its analgesic action via serotonergic system, this effect of buspirone may result in decreased analgesic effect of PCT. This is reflected in our study. We observed attenuated analgesic action of PCT in hot-plate and formalin test model when co-administered with buspirone. Similar effects are demonstrated in previous animal studies where buspirone is co-administered with NSAIDs or other centrally acting analgesic like morphine.[9] In another study, subcutaneous injection of agonists selective for these receptors opposed the antinociceptive effect of acetaminophen in hot-plate test.[8] Conversely, analgesic effect of PCT was increased by the selective blockade of 5-HT1A receptors with WAY 100635 and the selective blockade of 5-HT1B receptors with SB 216641, both administered systemically.[10] Contradictory to our studies, some studies has also shown that buspirone itself has got good analgesic activity but the studies lack explanation for the same.[6] Buspirone may have some analgesic activity due to the stimulation of postsynaptic receptors which are not autoreceptors.
According to previous data by Millan,[11] the mechanism of antinociceptive action of 5HT1A agonists involves adrenergic receptor α2 activation. Also, buspirone has weak affinity to the α2 receptor but its main metabolite 1-pp is a potent antagonist.[9] Some authors state that antinociceptive effect of morphine may be weakened by spinal administration of α2 antagonist.[12] As buspirone is rapidly metabolized to 1-pp and in an hour after oral dose, its levels in the brain may be higher than the buspirone level itself.[13] This mechanism may be responsible for decreasing analgesic action of PCT when co-administered with buspirone.
Fluoxetine is a selective serotonin reuptake inhibitor (SSRI). As the name suggests, the antidepressant actions of SSRIs are due to an increase in the quantity and action of serotonin in the synaptic gap and to its inhibitory action on the presynaptic receptor.[14] Increased duration of analgesic effect of PCT can be attributed to the extension of the above mentioned effect to the descending serotonergic spinal pathways. When PCT is given along with fluoxetine, it showed an increased antinociceptive action in hot-plate model and early phase of formalin test. This coincides with the notion that PCT acts via serotonergic pathway and its analgesic activity is increased when the amount of serotonin in the synapse is increased.[6]
In the study conducted by Zhao et al.,[15] it was seen that systemic administration of fluoxetine led to a significant analgesic effect in wild-type mice that lasted over 3 h after the injection. In contrast, fluoxetine had negligible effect on thermal thresholds in Lmx1bf/f/p mice that is conditional knock-out mice which lack 5-HT neurons in the central nervous system (CNS). This gave conclusive proof that fluoxetine exerted its analgesic effect completely via the serotonergic system. Moreover, in wild-type mice, FLUOXETINE showed maximum analgesic effect only in the acute thermal pain model, whereas its was found to be less effective in the persistent pain models implying that in different settings of pain, the role of central 5-HT varies.[15]
Some studies also show that patients under chronic depression also suffer from various types of pain, also called as the pain syndrome which may be due to decreased levels of serotonin.[16]
Previous studies showed that fluoxetine itself has some analgesic effect[17] which has an additive effect on antinociceptive action of PCT. This may be again because it increases 5-HT in the synapse.
However, as is evident from the existing data and the current study, this combination may be possible only with drugs that have at least some serotonergic properties. This kind of combination has almost exclusively and extensively been studies with antidepressants.[18] Many studies have been conducted to study the effect of CNS modulators like SSRIs, clomipramine, morphine, tramadol, etc., to assess the effect these drugs have on Serotonin and how it translates to the various functions in the body. Much of this research has been conducted on the concentration of serotonin around the raphe nucleus and while many drugs seem to potentiate this neurotransmitter, others had limited acute effects. A possible explanation for this phenomenon could be the inadvertent activation of the somatodendritic 5-HT1A autoreceptors in this region, either by the drug itself or by the excessive Serotonin produced due to the drug. Thus, the analgesic potential of these drugs could be stunted due to this negative feedback mechanism. Considering this phenomenon, it could be postulated that the combination of fluoxetine and PCT may have inhibited these 5-HT1A autoreceptors, thus inhibiting the negative feedback and increasing the Serotonin levels, which resulted in the potentiation of the overall analgesia.
Related to this hypothesis is the fact that drugs that increase 5-HT and norepinephrine (NE) neurotransmission, such as tricyclic antidepressants like amitriptyline and desipramine or SSRIs which inhibit 5-HT and/or NE reuptake, are mostly used in the clinical management of chronic pain.[14,16]
Ondansetron is a 5HT3 antagonist. It did not interfere with the analgesic effect of PCT in formalin models. It attenuated analgesic action of PCT in hot-plate test to some extent (P = 0.0137) as shown in Table 1.
Studies evaluating the effect of ondansetron on the analgesic activity of PCT has already been demonstrated using a rat model of pain (paw pressure test [Randall and Selitto] and hot-plate test) have shown that this 5-HT3 antagonist has negligible to no effect on PCT.[3] Girard et al.[19] found that 5-HT1B and 5-HT2C, but not 5-HT3 receptor subtypes are involved in the antinociceptive effect of Nefopam, centrally-acting nonopioid analgesic. These results indicate that the analgesia exerted by PCT may be due to involvement of only specific serotonin receptor subtypes (5-HT1 and 5-HT2) but not all (e.g., 5-HT3).
Libert et al.[20] demonstrated that the 5-HT3 receptor antagonists, ondansetron and granisetron given intrathecally, did not decrease the analgesic effect of PCT. Ondansetron is a substrate of the phosphoglycoprotein (P-gp) transport pump encoded by the MDR1a gene. Ondansetron is actively pumped out of the CNS across blood-brain barrier against the concentration gradient and so it is unable to cross the blood-brain barrier. We postulate that the no change in the analgesic effect of PCT may result from the failure of ondansetron accumulation in the CNS to sufficient concentration due to the extrusion by P-gp transport pump.[21] The findings of this study support the use ondansetron as an antiemetic with PCT during the perioperative period for analgesia.
Our results showed that drugs modulating serotonergic system interfered/changed analgesic action of PCT only in hot-plate model but not much difference was seen in formalin test except decreased pain threshold by co-administration of buspirone in early phase.
This failure in formalin test is related to the mechanism of action of these drugs. As mentioned above, the mechanism of PCT involved both central and peripheral pathways. Peripheral pathway consisting of inhibition of cyclooxygenase and PGs may not be affected by drugs acting on serotonergic system. This is clearly seen in formalin test. Only buspirone attenuated analgesic action of PCT, suggesting dominant role of 5HT1 receptor in central action of PCT.
Secondly, nature of stimulus is different in both tests. In tests of nociception, stimuli are usually applied to cutaneous and also to visceral structures to some extent. The application of a gradually increasing thermal stimulus will lead to systematic and unalterable sequence of activation, namely thermoreceptors, then thermoreceptors plus nociceptors, then nociceptors alone, and finally (possibly) nociceptors plus “paradoxical cold” receptors.[22] Therefore, the response of the animal to the stimuli may be due to thermoreceptors with only partial stimulation of nociceptors and may not purely be a nociceptive reaction.
This situation is inevitable, as it is, in practice, not possible to separate thermoception from nociception, and thus tests like the hot-plate test may be confounded by the stimulation of the thermoregulatory mechanism, as a result of simultaneous stimulation of the paws and tail. In rodents, the tail is an important organ of thermoregulation and balance. Thus, its stimulation can introduce considerable bias in the results, which may be unavoidable.[23]
The study has some limitations. We used only the hot-plate test to evaluate central action of PCT. The study should be conducted in other central analgesic models of nociception to further confirm/strengthen the results.
Tropisetron had been used in most of previous studies as it crosses blood brain barrier and interfer with antinociceptive action of centrally acting drugs. We did not use tropisetron (5HT3 antagonist) as we could not procure it, instead we used ondansetron.
Conclusion
Pain threshold of mice who were administered PCT + buspirone was decreased suggesting involvement of 5HT1 receptors in mechanism of nociception. Whereas higher analgesia is produced by co-administration of SSRI (fluoxetine) + PCT. These findings support the hypothesis that there is an underlying role of central serotonergic system in the mechanism of analgesic action of PCT. It can further be explored, if this action of PCT is increased by 5-HT1A and 5HT1B antagonists or SSRI and SNRI. If so, this combination might lead to development of new strategy in therapeutics of pain.
Financial Support and Sponsorship
The study was approved and received grant under ICMR-STS programme (2014).
Conflicts of Interest
There are no conflicts of interest.
Acknowledgment
We are grateful to ICMR for funding the project under STS scheme.
References
- 1.Toussaint K, Yang XC, Zielinski MA, Reigle KL, Sacavage SD, Nagar S, et al. What do we (not) know about how paracetamol (acetaminophen) works? J Clin Pharm Ther. 2010;35:617–38. doi: 10.1111/j.1365-2710.2009.01143.x. [DOI] [PubMed] [Google Scholar]
- 2.Tiippana E, Hamunen K, Kontinen V, Kalso E. The effect of paracetamol and tropisetron on pain: Experimental studies and a review of published data. Basic Clin Pharmacol Toxicol. 2013;112:124–31. doi: 10.1111/j.1742-7843.2012.00935.x. [DOI] [PubMed] [Google Scholar]
- 3.Sandrini M, Pini LA, Vitale G. Differential involvement of central 5-HT1B and 5-HT3 receptor subtypes in the antinociceptive effect of paracetamol. Inflamm Res. 2003;52:347–52. doi: 10.1007/s00011-003-1185-5. [DOI] [PubMed] [Google Scholar]
- 4.Dogrul A, Seyrek M, Akgul EO, Cayci T, Kahraman S, Bolay H. Systemic paracetamol-induced analgesic and antihyperalgesic effects through activation of descending serotonergic pathways involving spinal 5-HT7 receptors. Eur J Pharmacol. 2012;677:93–101. doi: 10.1016/j.ejphar.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Vogel HG. Drug Discovery and Evaluation: Pharmacological Assays. 2nd ed. Vol. 2. Germany: Springer-Verlag Berlin Heidelberg; 2002. Analgesic, anti-inflammatory, and anti-pyretic activity; pp. 670–773. [Google Scholar]
- 6.Bardin L, Schmidt J, Alloui A, Eschalier A. Effect of intrathecal administration of serotonin in chronic pain models in rats. Eur J Pharmacol. 2000;409:37–43. doi: 10.1016/s0014-2999(00)00796-2. [DOI] [PubMed] [Google Scholar]
- 7.Raffa RB, Walker EA, Sterious SN. Opioid receptors and acetaminophen (paracetamol) Eur J Pharmacol. 2004;503:209–10. doi: 10.1016/j.ejphar.2004.08.055. [DOI] [PubMed] [Google Scholar]
- 8.Pini LA, Sandrini M, Vitale G. The antinociceptive action of paracetamol is associated with changes in the serotonergic system in the rat brain. Eur J Pharmacol. 1996;308:31–40. doi: 10.1016/0014-2999(96)00261-0. [DOI] [PubMed] [Google Scholar]
- 9.Zuideveld KP, Rusiç-Pavletiç J, Maas HJ, Peletier LA, Van der Graaf PH, Danhof M. Pharmacokinetic-pharmacodynamic modeling of buspirone and its metabolite 1-(2-pyrimidinyl)-piperazine in rats. J Pharmacol Exp Ther. 2002;303:1130–7. doi: 10.1124/jpet.102.036798. [DOI] [PubMed] [Google Scholar]
- 10.Millan MJ. Colpaert FC 5-hydroxytryptamine (HT) 1A receptors and the tail-flick response. II. High efficacy -HTA agonists attenuate morphine-induced antinociception in mice in a competitive-like manner. J Pharmacol Exp Ther. 1991;256:983–92. [PubMed] [Google Scholar]
- 11.Millan MJ. Serotonin and pain: Evidence that activation of 5-HT1A receptors does not elicit antinociception against noxious thermal, mechanical and chemical stimuli in mice. Pain. 1994;58:45–61. doi: 10.1016/0304-3959(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 12.Aley KO, Levine JD. Multiple receptors involved in peripheral alpha 2, mu, and A1 antinociception, tolerance, and withdrawal. J Neurosci. 1997;17:735–44. doi: 10.1523/JNEUROSCI.17-02-00735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuller RW, Perry KW. Effects of buspirone and its metabolite, 1-(2-pyrimidinyl) piperazine, on brain monoamines and their metabolites in rats. J Pharmacol Exp Ther. 1989;248:50–6. [PubMed] [Google Scholar]
- 14.Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997;340:249–58. doi: 10.1016/s0014-2999(97)01393-9. [DOI] [PubMed] [Google Scholar]
- 15.Zhao ZQ, Chiechio S, Sun YG, Zhang KH, Zhao CS, Scott M, et al. Mice lacking central serotonergic neurons show enhanced inflammatory pain and an impaired analgesic response to antidepressant drugs. J Neurosci. 2007;27:6045–53. doi: 10.1523/JNEUROSCI.1623-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nekovarova T, Yamamotova A, Vales K, Stuchlik A, Fricova J, Rokyta R. Common mechanisms of pain and depression: Are antidepressants also analgesics? Front Behav Neurosci. 2014;8:99. doi: 10.3389/fnbeh.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raffa RB, Stone DJ, Jr, Tallarida RJ. Discovery of “self-synergistic” spinal/supraspinal antinociception produced by acetaminophen (paracetamol) J Pharmacol Exp Ther. 2000;295:291–4. [PubMed] [Google Scholar]
- 18.Millan MJ, Brocco M, Veiga S, Cistarelli L, Melon C, Gobert A. WAY 100,635 enhances both the ‘antidepressant’ actions of duloxetine and its influence on dialysate levels of serotonin in frontal cortex. Eur J Pharmacol. 1998;341:165–7. doi: 10.1016/s0014-2999(97)01445-3. [DOI] [PubMed] [Google Scholar]
- 19.Girard P, Coppé MC, Verniers D, Pansart Y, Gillardin JM. Role of catecholamines and serotonin receptor subtypes in nefopam-induced antinociception. Pharmacol Res. 2006;54:195–202. doi: 10.1016/j.phrs.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Libert F, Bonnefont J, Bourinet E, Doucet E, Alloui A, Hamon M, et al. Acetaminophen: A central analgesic drug that involves a spinal tropisetron-sensitive, non-5-HT(3) receptor-mediated effect. Mol Pharmacol. 2004;66:728–34. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 21.Scott JA, Wood M, Flood P. The pronociceptive effect of ondansetron in the setting of P-glycoprotein inhibition. Anesth Analg. 2006;103:742–6. doi: 10.1213/01.ane.0000228861.80314.22. [DOI] [PubMed] [Google Scholar]
- 22.Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- 23.Le Bars D, Willer JC, De Broucker T, Villanueva L. Neurophysiological mechanisms involved in the pain-relieving effects of counter-irritation and related techniques. In: Pomerantz B, Stux G, editors. Scientific Bases of Acupuncture. Berlin: Springer Verlag; 1989. pp. 79–112. [Google Scholar]


