Abstract
Objectives:
Xanthones are flavonoids with numerous activities, including antioxidant, antidepressant., or anxiolytic-like. Therefore, the aim of our study was to determine antidepressant- and anxiolytic-like properties of four xanthone derivatives (3-chloro-5-[(4-methylpiperazin-1-yl)methyl]-9H-xanthen-9-one dihydrochloride [HBK-5], 6-methoxy-2-[(4-methylpiperazin-1-yl) methyl]-9H-xanthen-9-one dihydrochloride, 2-[(4-benzylpiperazin-1-yl) methyl]-6-methoxy-9H-xanthen-9-one dihydrochloride, 2-{[4-(2-methoxyphenyl) piperazin-1-yl] methyl}-9H-xanthen-9-one hydrochloride), as well as the influence on cognitive and motor function of active compounds, using animal models.
Materials and Methods:
To determine the antidepressant-like activity, we used forced swim test (FST) and tail suspension test (TST) in mice. We evaluated anxiolytic-like properties in the four-plate test in mice. We studied the influence on cognitive and motor function in passive avoidance step-through and chimney tests, respectively.
Results:
The antidepressant-like activity (in both FST and TST) showed only HBK-5. Moreover, the compound was also active in the four-plate test, which suggests that it possessed anxiolytic-like properties. HBK-5 did not cause any cognitive and motor deficits in mice at antidepressant- and anxiolytic-like doses.
Conclusions:
HBK-5 may have potential in the treatment of depression or anxiety disorders, but this issue needs further studies.
KEY WORDS: Antidepressant-like, anxiolytic-like, behavioral tests, buspirone, moclobemide, xanthone derivative
Introduction
Depression is a severe mental condition, and according to the World Health Organization, a leading cause of disability worldwide. The pathophysiology of the disease is very complex, but not fully understood.[1,2,3,4] Since the effectiveness of the drugs is limited, and over 30% of patients do not respond to current pharmacotherapy, scientists still search for new compounds with antidepressant properties.
Xanthones-flavonoids with numerous activities-are one of the group of compounds that attract scientists’ attention. Xanthones and their derivatives possess many pharmacological properties, including antiarrhythmic or hypotensive.[5,6,7,8,9] Moreover, they show activity within central nervous system (CNS). In addition to antioxidant[10] or neuroprotective properties,[11] xanthone derivatives showed antidepressant-[12,13,14] or anxiolytic-like effects[15] in behavioral studies.
Taking the above into account, we aimed to investigate antidepressant- and anxiolytic-like properties of four xanthone derivatives, as well as the influence on cognitive and motor function of active compounds.
Materials and Methods
All experimental procedures were approved by the I Local Ethics Committee for Experiments on Animals of the Jagiellonian University in Krakow, Poland, and performed in accordance with the guidelines provided by the committee for the purpose of control and supervision of experiments on animals (CPCSEA).
Animals and Housing
In all experiments, adult male Albino-Swiss mice (CD-1) weighing 18–21 g were used. The animals were kept in groups of 15 mice in cages at room temperature of 22 ± 2°C under light/dark (12:12) cycle and they had free access to food (standard laboratory pellets) and water before experiments. Humidity and ambient temperature of the room were kept constant throughout all tests, which were conducted between 9 a.m. and 4 p.m. The animals were used only once in each test. All injections were given in a volume of 10 ml/kg. A trained observer blind to the treatments scored behavioral experiments.
Drugs
Four tested compounds, xanthone derivatives with piperazine moiety: 3-chloro-5-[(4-methylpiperazin-1-yl)methyl]-9H-xanthen-9-one dihydrochloride (HBK-5, Figure 1a), 6-methoxy-2-[(4-methylpiperazin-1-yl)methyl]-9H-xanthen-9-one dihydrochloride (HBK-8, Figure 1b), 2-[(4-benzylpiperazin-1-yl)methyl]-6-methoxy-9H-xanthen-9-one dihydrochloride (HBK-9, Figure 1c), 2-{[4-(2-methoxyphenyl)piperazin-1-yl] methyl}-9H-xanthen-9-one hydrochloride (HBK-12, Figure 1d) were synthesized in the Department of Bioorganic Chemistry, Chair of Organic Chemistry, Faculty of Pharmacy, Jagiellonian University [Figure 1].[16] The studied compounds: Moclobemide (Sigma-Aldrich, Germany) and buspirone (Sigma-Aldrich, Germany) were dissolved in saline and administered intraperitoneally 60 or 30 min before the test, respectively.
Figure 1.
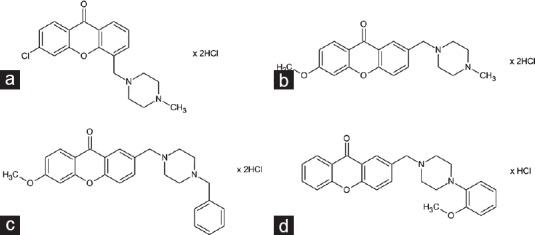
Chemical structures of xanthone derivatives HBK-5 (a), HBK-8 (b), HBK-9 (c), HBK-12 (d). HBK-5: 3-chloro-5-[(4-methylpiperazin-1-yl)methyl]-9H-xanthen-9-one; HBK-8: 6-methoxy-2-[(4-methylpiperazin-1-yl) methyl]-9H-xanthen-9-one dihydrochloride; HBK-9: 2-[(4-benzylpiperazin-1-yl) methyl]-6-methoxy-9H-xanthen-9-one dihydrochloride; HBK-12: 2-{[4-(2-methoxyphenyl) piperazin-1-yl] methyl}-9H-xanthen-9-one hydrochloride
Forced Swim Test in Mice (Porsolt's Test)
The experiments were conducted on mice according to the method previously described.[17,18] The animals were dropped separately into glass cylinders (height 25 cm, diameter 10 cm) containing 10 cm3 of water (maintained at 23–25°C) and they were left there for 6 min. After the initial 2 min, the total duration of immobility was measured during the final 4 min of the assay. The mice were considered to be motionless when they remained floating passively in the water.
Tail Suspension Test in Mice
The studies were performed on mice according to the method previously described by Steru et al. and Waszkielewicz et al.[19,20] The animals were securely suspended to a flat surface by medical adhesive tape placed approximately 1 cm from the tip of the tail about 50 cm below the surface. The entire immobility time was measured manually during the total 6 min of the test. Immobility was considered when the animals hung passively without limb movement, completely motionless.
Four-plate Test in Mice
The test was carried out on mice according to the method previously described by Gunia-Krzyz.ak et al. and Partyka et al.[21,22] Mice were placed individually in the four-plate apparatus connected to the power source. After a 15 s of habituation period, each mouse crossing from one plate to another (two limbs on one plate, two on another) was punished by an electric shock (0.6 mA, 0.5 s). There was 3 s protection period between two following punishments. The number of punished crossings was calculated during the 60 s of the test.
Spontaneous Locomotor Activity
Photoresistor actometers (Ugo Basile, Varese, Italy) connected to a counter for the recording of light-beam interruptions were used to investigate the effect of various doses of the compounds on locomotor activity in mice. Locomotor activity as the number of light-beam crossing was counted automatically during the session (either 1-, 4-, or 6-min). The tested compounds were administered at the dose, which was active in forced swim test (FST), tail suspension test (TST), or four-plate test to determine whether the observed effect was specific. Moreover, data obtained during 1, 4, and 6 min (i.e. time equal to the observation period in the four-plate test, FST, and TST, respectively) were used for the statistical analysis.
Passive Avoidance Step-through Test in Mice
The passive avoidance step-through test was performed according to the method described by Jarvik and Kopp.[23] The apparatus used for this test consisted of two compartments, one light (20 cm × 21 cm × 20 cm3, 1000 lux) and one dark (7.3 cm × 7.5 cm × 14 cm3, 10 lux), both separated by an automated sliding door (LE872, Bioseb, Vitrolles, France). The smaller dark compartment was equipped with an electric grid floor. First for the training session, mice were placed individually in an illuminated white compartment and after 30 s the door was opened. As far as the mice came into the dark compartment, the door closed automatically and the animal was punished by an electric foot shock through the grid floor (0.8 mA for 2 s). The mouse, which did not enter the dark compartment within 50 s, were excluded from the study. On the following day (24 h after training trial), the proper experiment was conducted. The pretrained animals were placed in the light compartment again and observed for 180 s (retention session). The mice, which avoided the dark compartment for 180 s, were considered to remember the task. The elapsed time before mice entered the dark compartment was recorded (in both sessions) and the median latencies (retention times) with 25th and 75th percentiles were calculated.
Chimney Test
The chimney test was conducted according to the method described by Boissier et al.[24] First, the animals were trained and selected, then after the administration of the tested compounds they were placed individually in a 25 cm long and 2.5 cm in diameter tube, located horizontally. Afterward, the tube was reversed vertically and mice was expected to climb backward up to get out the pipe within 60 s. Motor impairment as the number of animals unable to performed the test within 60 s was signified and TD<Subscript>50</Subscript> values were calculated. This test was performed only for those compounds, which showed significant activity in the FST, TST, and four-plate test. To establish TD<Subscript>50</Subscript> values the compounds were administered at the dose active in the FST and/or TST and/or four-plate test and then the dose was gradually increased until the neurotoxic effects appeared.
Data Analysis
The obtained results were estimated using one-way analysis of variance, followed by Newman–Keuls multiple comparisons test, Student's t-test, or nonparametric Kruskal–Wallis (KW) test and presented as means ± standard error of mean or median retention times. Differences between groups were considered as significant if P < 0.05.
To establish TD50 value in chimney test for the active compound, the log-probit method described by Litchfield and Wilcoxon was used.[25] In this test, TD50 value was described as the dose of the investigated compound that impaired motor coordination in 50% of mice compared to vehicle-treated group.
Results
Antidepressant-like Activity in the Forced Swim Test in Mice
HBK-5 compared with vehicle treatment significantly decreased immobility time in mice at the doses 10 and 20 mg/kg by 24% and 28% (F (3, 36) = 8.392, P < 0.001), respectively. HBK-9 significantly decreased the immobility time in mice at the dose 10 mg/kg by 25% (F (3, 36) = 7.322, P < 0.001). HBK-8 (F (3, 36) = 1.411, NS) and HBK-12 (F (3, 36) = 2.110, NS) did not influence immobility time in the FST in mice at the doses 5–20 mg/kg. The reference compound: Moclobemide at the dose 20 mg/kg significantly decreased immobility time in mice by 28% (F (2, 26) = 8.490, P < 0.01). The results of the FST in mice are summarized in Figure 2.
Figure 2.
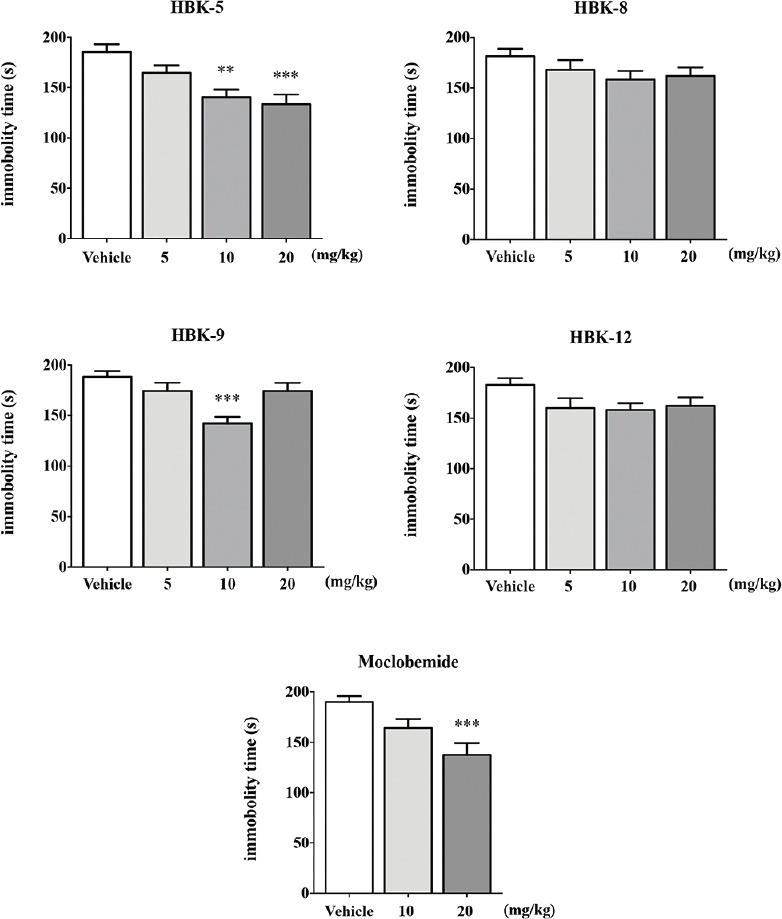
The effect of xanthone derivatives and moclobemide on the duration of immobility time in forced swim test in mice. Moclobemide and the tested compounds were administered (intraperitoneally) 60 or 30 min before the test, respectively. Vehicle-treated group received 0.9% NaCl. Statistical analysis: One-way analysis of variance (Newman–Keuls post hoc); **P <0.01, ***P <0.001 versus respective control group; n = 9.10 mice per group
Antidepressant-like Activity in the Tail Suspension Test in Mice
HBK-5 at the doses 5 and 10 mg/kg significantly decreased immobility time in mice by 41% and 45% (F (3, 36) = 3.462, P < 0.05), respectively. HBK-8 (F (3, 36) = 0.140, NS), HBK-9 (F (3, 36) = 1.528, NS), and HBK-12 (F (3, 36) = 0.749, NS) did not influence immobility time in the TST in mice at the doses 2.5–10 mg/kg. Moclobemide at the dose 20 mg/kg significantly decreased immobility time in mice by 56% (F (2, 27) = 13.320, P < 0.001). The results of the TST in mice are shown in Figure 3.
Figure 3.
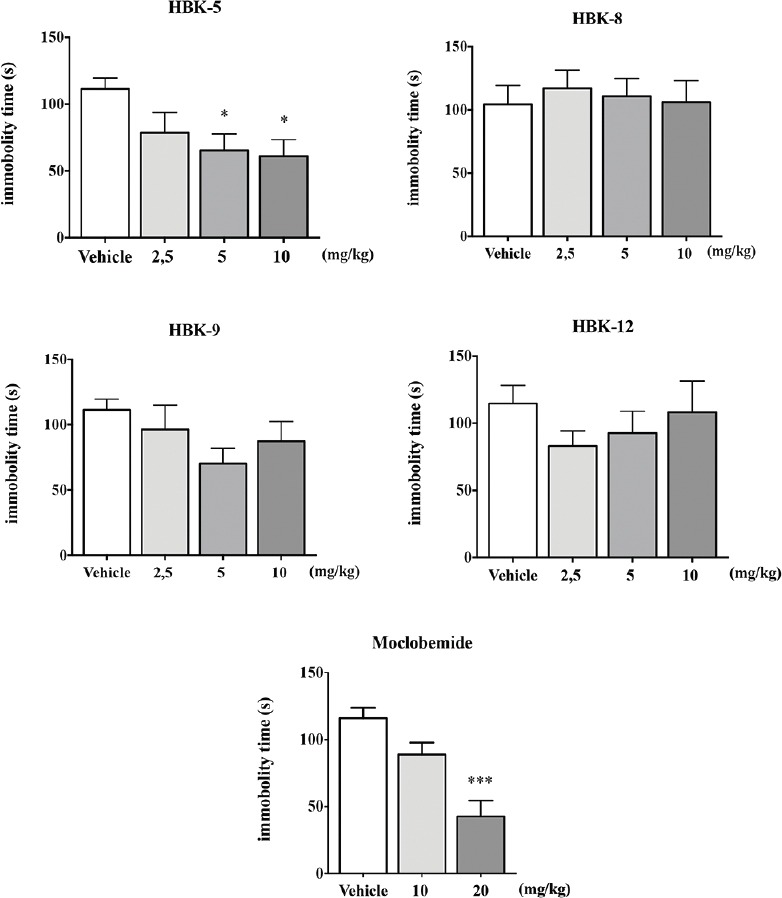
The effect of xanthone derivatives and moclobemide on the duration of immobility time in tail suspension test in mice. Moclobemide and the tested compounds were administered (intraperitoneally) 60 or 30 min before the test, respectively. Vehicle-treated group received 0.9% NaCl. Statistical analysis: One-way analysis of variance (Newman–Keuls post hoc); *P <0.05, ***P <0.001 versus respective control group; n = 10 mice per group
Anxiolytic-like Activity in Four-plate Test in Mice
HBK-5 at the doses 5 and 10 mg/kg significantly increased the number of punished crossings in mice by 100% and 96% (F (3, 36) = 5.116, P < 0.01), respectively. HBK-8 (F (3, 36) = 0.314, NS), HBK-9 (F (3, 36) = 0.685, NS), and HBK-12 (F (3, 36) = 0.333, NS) did not influence on the number of punished crossings in mice at the doses 2.5–10 mg/kg. The reference compound: Buspirone at the doses 2.5 and 5 mg/kg significantly increased the number of punished crossings in mice by 46% and 61% (F (3, 36) = 5.660, P < 0.01), respectively. The results of four-plate test in mice are presented in Figure 4.
Figure 4.
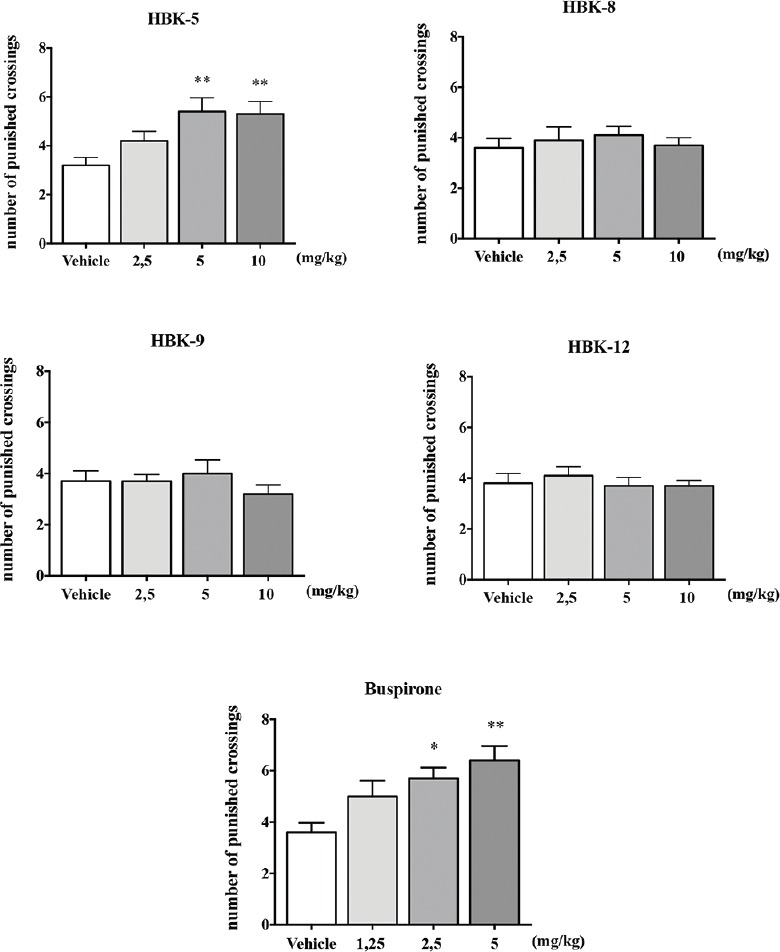
The effect of xanthone derivatives and buspirone on the number of punished crossings in the four-plate test in mice. The tested compounds and buspirone were administered (intraperitoneally) 30 min before the test. Vehicle-treated group received 0.9% NaCl. Statistical analysis: One-way analysis of variance (Newman–Keuls post hoc); *P < 0.05, **P < 0.01 versus respective control group; n = 10 mice per group
The Influence on Locomotor Activity
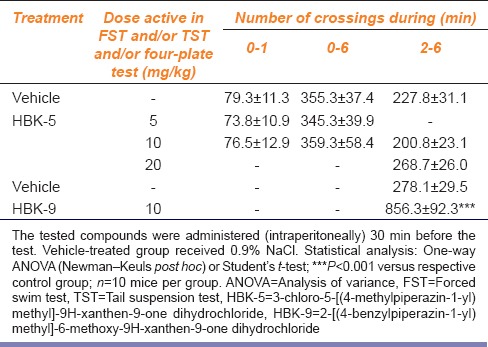
HBK-5 did not affect locomotor activity in mice at the doses active in the FST (F (2, 27) = 1.611, NS), TST (F (2, 27) = 0.024, NS), and four-plate test (F (2, 27) = 0.055, NS) in mice. HBK-9 at the dose 10 mg/kg significantly increased locomotor activity in mice by 208% (t (18) = 5.967, P < 0.001). The results are shown in Table 1.
Table 1.
The influence of 3-chloro-5-[(4-methylpiperazin-1-yl) methyl]-9H-xanthen-9-one dihydrochloride and 2-[(4-benzylpiperazin-1-yl) methyl]-6-methoxy-9H-xanthen-9-one dihydrochloride on locomotor activity in mice
The Influence on Cognitive Function in Passive Avoidance Step-through Test and Motor Coordination in Chimney Test
HBK-5 at the doses 5, 10, and 20 mg/kg did not influence memory in mice (KW = 2.138, NS). The results are presented in Table 2.
Table 2.
The influence of antidepressant- or anxiolytic-like doses of 3-chloro-5-[(4-methylpiperazin-1-yl) methyl]-9H-xanthen-9-one dihydrochloride on cognitive and motor function in passive avoidance step-through and chimney tests in mice, respectively
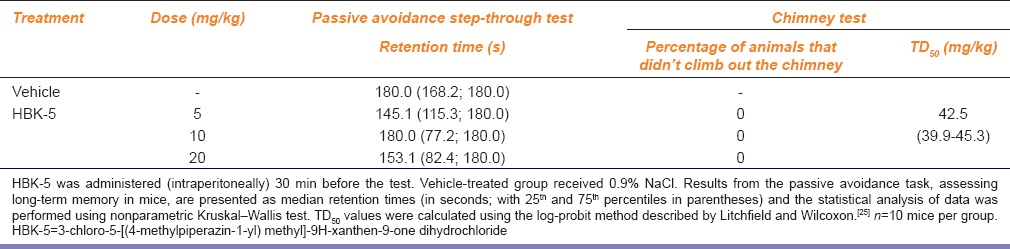
HBK-5 did not affect motor coordination in chimney test at the doses active in the FST, TST, and four-plate test in mice. In comparison with vehicle-treated group, HBK-5 at the doses 40, 45, 50 mg/kg, statistically significantly increased time spent in the chimney in mice by 134%, 382%, and 385% (F (3, 36) = 38.60, P < 0.0001), respectively. TD<Subscript>50</Subscript> value in the chimney test for HBK-5 is shown in Table 2.
Discussion
We found that HBK-5 showed both antidepressant- and anxiolytic-like properties in behavioral tests on mice. Moreover, the compound did not alter cognitive or motor function at active doses.
In our previous studies, the tested compounds showed moderate affinity for serotonergic 5-hydroxytryptamine (5-HT) receptors, and particularly 5-HT<Subscript>1A</Subscript> receptors, as well as for adrenergic <Symbol>α</Symbol><Subscript>1</Subscript> receptors.[16] Based on the scientific reports about xanthone derivatives and their potential role in the treatment of CNS disorders, such as: Depression or anxiety,[15,26] we decided to investigate antidepressant- and anxiolytic-like properties of four xanthone derivatives. To examine the antidepressant-like activity, we performed two tests: The FST and TST. Both are widely used to assess antidepressant-like properties of novel compounds. The advantage of the TST is a good predictive validity.[27] In the FST, only HBK-5 and HBK-9 significantly decreased immobility of mice, and their effect was stronger than that of moclobemide. In the TST, only HBK-5 showed significant activity, which was stronger than that of the reference drug.
Since psychostimulants may give false positive results in both FST and TST, to confirm our findings, we determined the influence of HBK-5 and HBK-9 on locomotor activity of mice. HBK-5 did not affect locomotor activity of animals at the doses active in the FST and TST, which implies that its antidepressant-like effect was specific. HBK-9 administered at the dose active in the FST significantly increased locomotor activity of mice, which suggests that the effect observed in this test was due to psychostimulant rather than antidepressant-like activity of the compound.
Our findings correlate with other studies on xanthone derivatives.[26,28] Zhao et al. demonstrated antidepressant-like activity of two xanthone derivatives in the TST and FST in mice and rats.[29] Moreover, the results of this study are in agreement with our previous reports on a xanthone derivatives.[13,14]
Since Tovilović et al. suggested that xanthone derivatives may have anxiolytic-like properties,[15] we decided to investigate anxiolytic-like activity of the tested compounds in the four-plate test in mice. The four-plate test is a model using conditioned fear to predict potential anxiolytic activity. In this experiment, only HBK-5 showed significant anxiolytic-like properties, which were not as strong as the effect elicited by buspirone. As the treatment with HBK-5 did not impair locomotor activity in mice, we can conclude that the observed effect was specific.
Among the studied xanthone derivatives, HBK-5 showed the best pharmacological profile. We think that this may be due to the insertion of chloride atom in the position 3 of 9H-xanthen-9-one moiety, which probably improved its lipophilic properties, and thereby increased its penetration to the CNS. Nevertheless, this issue requires further studies.
Drugs acting within CNS may influence brain function negatively. Therefore, we investigated the influence of HBK-5 on cognitive and motor function using passive avoidance step-through and chimney tests, respectively. Since the step-through passive avoidance task provides information about the ability to acquire the task (learning) and to recall the task (retrieval) by rodent, it may be regarded as a measure of learning and memory.[30] HBK-5 did not impair memory in mice challenged with this task at antidepressant- and anxiolytic-like doses. Moreover, the tested compound did not affect motor coordination in chimney test at the doses active in the FST, TST, and four-plate test. Motor impairment was observed at about 8-fold higher dose than the lowest active dose. Thus, we can assume that HBK-5 did not induce motor or cognitive deficits at active doses.
The limitation of our study was that the antidepressant-like effects were studied after acute administration and using tests, not models of depression. Current antidepressants act after 2–4 weeks of treatment. Therefore, it will be reasonable to evaluate antidepressant potential of HBK-5 after chronic administration in animal models of depression.
In conclusion, we demonstrated that HBK-5 – a new xanthone derivative with piperazine moiety – possessed antidepressant-like activity (stronger than moclobemide), and anxiolytic-like properties. The tested compound at active doses did not influence motor or cognitive function negatively. Given the promising results, we plan to perform further studies on HBK-5 to verify its therapeutic potential in the treatment of depression and/or anxiety.
Financial Support and Sponsorship
This study was supported by Jagiellonian University grant number K/DSC/000040 and K/DSC/001955.
Conflicts of Interest
There are no conflicts of interest.
Acknowledgments
We wish to thank Agnieszka Niedbał and Teresa Dobrut for their technical assistance.
References
- 1.Pytka K, Podkowa K, Rapacz A, Podkowa A, Zmudzka E, Olczyk A, et al. The role of serotonergic, adrenergic and dopaminergic receptors in antidepressant-like effect. Pharmacol Rep. 2016;68:263–74. doi: 10.1016/j.pharep.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Pytka K, Dziubina A, Mlyniec K, Dziedziczak A, Zmudzka E, Furgala A, et al. The role of glutamatergic, GABA-ergic, and cholinergic receptors in depression and antidepressant-like effect. Pharmacol Rep. 2016;68:443–50. doi: 10.1016/j.pharep.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Mlyniec K, Gawel M, Doboszewska U, Starowicz G, Pytka K, Davies CL, et al. Essential elements in depression and anxiety. Part II. Pharmacol Rep. 2015;67:187–94. doi: 10.1016/j.pharep.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Mlyniec K, Davies CL, de Agüero Sánchez IG, Pytka K, Budziszewska B, Nowak G. Essential elements in depression and anxiety. Part I. Pharmacol Rep. 2014;66:534–44. doi: 10.1016/j.pharep.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Rapacz A, Pytka K, Sapa J, Kubacka M, Filipek B, Szkaradek N, et al. Antiarrhythmic, hypotensive and a1-adrenolytic properties of new 2-methoxyphenylpiperazine derivatives of xanthone. Eur J Pharmacol. 2014;735:10–6. doi: 10.1016/j.ejphar.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Rapacz A, Sapa J, Pytka K, Dudek M, Filipek B, Szkaradek N, et al. Antiarrhythmic activity of new 2-methoxyphenylpiperazine xanthone derivatives after ischemia/reperfusion in rats. Pharmacol Rep. 2015;67:1163–7. doi: 10.1016/j.pharep.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Rapacz A, Sapa J, Nowinski L, Mogilski S, Pytka K, Filipek B, et al. Biofunctional studies of new 2-methoxyphenylpiperazine xanthone derivatives with a1-adrenolytic properties. Pharmacol Rep. 2015;67:267–74. doi: 10.1016/j.pharep.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Szkaradek N, Rapacz A, Pytka K, Filipek B, Zelaszczyk D, Szafranski P, et al. Cardiovascular activity of the chiral xanthone derivatives. Bioorg Med Chem. 2015;23:6714–24. doi: 10.1016/j.bmc.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Szkaradek N, Rapacz A, Pytka K, Filipek B, Siwek A, Cegla M, et al. Synthesis and preliminary evaluation of pharmacological properties of some piperazine derivatives of xanthone. Bioorg Med Chem. 2013;21:514–22. doi: 10.1016/j.bmc.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Panda SS, Chand M, Sakhuja R, Jain SC. Xanthones as potential antioxidants. Curr Med Chem. 2013;20:4481–507. doi: 10.2174/09298673113209990144. [DOI] [PubMed] [Google Scholar]
- 11.Wezeman T, Bräse S, Masters KS. Xanthone dimers: A compound family which is both common and privileged. Nat Prod Rep. 2015;32:6–28. doi: 10.1039/c4np00050a. [DOI] [PubMed] [Google Scholar]
- 12.Jastrzebska-Wiesek M, Librowski T, Czarnecki R, Marona H, Nowak G. Central activity of new xanthone derivatives with chiral center in some pharmacological tests in mice. Pol J Pharmacol. 2003;55:461–5. [PubMed] [Google Scholar]
- 13.Pytka K, Rapacz A, Zygmunt M, Olczyk A, Waszkielewicz A, Sapa J, et al. Antidepressant-like activity of a new piperazine derivative of xanthone in the forced swim test in mice: The involvement of serotonergic system. Pharmacol Rep. 2015;67:160–5. doi: 10.1016/j.pharep.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Pytka K, Walczak M, Kij A, Rapacz A, Siwek A, Kazek G, et al. The antidepressant-like activity of 6-methoxy-2-[4-(2-methoxyphenyl) piperazin-1-yl]-9H-xanthen-9-one involves serotonergic 5-HT(1A) and 5-HT(2A/C) receptors activation. Eur J Pharmacol. 2015;764:537–46. doi: 10.1016/j.ejphar.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 15.Tovilović G, Krstić D, Ignjatović D, Janać B. Anxiolytic-like effects of xanthone-rich diethylether extract of Gentiana kochiana in rodents. Dig J Nanomater Biostruct. 2011;6:1385–92. [Google Scholar]
- 16.Waszkielewicz AM, Gunia A, Szkaradek N, Pytka K, Siwek A, Satala G, et al. Synthesis and evaluation of pharmacological properties of some new xanthone derivatives with piperazine moiety. Bioorg Med Chem Lett. 2013;23:4419–23. doi: 10.1016/j.bmcl.2013.05.062. [DOI] [PubMed] [Google Scholar]
- 17.Pytka K, Partyka A, Jastrzebska-Wiesek M, Siwek A, Gluch-Lutwin M, Mordyl B, et al. Antidepressant- and anxiolytic-like effects of new dual 5-HT1A and 5-HT7 antagonists in animal models. PLoS One. 2015;10:e0142499. doi: 10.1371/journal.pone.0142499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: A primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–36. [PubMed] [Google Scholar]
- 19.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–70. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 20.Waszkielewicz AM, Pytka K, Rapacz A, Welna E, Jarzyna M, Satala G, et al. Synthesis and evaluation of antidepressant-like activity of some 4-substituted 1-(2-methoxyphenyl) piperazine derivatives. Chem Biol Drug Des. 2015;85:326–35. doi: 10.1111/cbdd.12394. [DOI] [PubMed] [Google Scholar]
- 21.Gunia-Krzyzak A, Pytka K, Słoczyńska K, Waszkielewicz AM, Sałata G, Bojarski AJ, et al. Preliminary evaluation of central nervous system activity of (E)-N-2-methyl-3-phenylprop-2-enyl ((E)-N- <Symbol>α</Symbol>-methylcin-namyl) derivatives of selected aminoalkanols. Acta Pol Pharm. 2016;73:345–57. [PubMed] [Google Scholar]
- 22.Partyka A, Chlon-Rzepa G, Wasik A, Jastrzebska-Wiesek M, Bucki A, Kolaczkowski M, et al. Antidepressant- and anxiolytic-like activity of 7-phenylpiperazinylalkyl-1,3-dimethyl-purine-2,6-dione derivatives with diversified 5-HT1A receptor functional profile. Bioorg Med Chem. 2015;23:212–21. doi: 10.1016/j.bmc.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Jarvik ME, Kopp R. An improved one-trial passive avoidance learning situation. Psychol Rep. 1967;21:221–4. doi: 10.2466/pr0.1967.21.1.221. [DOI] [PubMed] [Google Scholar]
- 24.Boissier JR, Tardy J, Diverres JC. <AQ>A new simple method to explore the tranquilizing action: The chimney test</AQ>. Pharmacology. 1960;3:81–4. [Google Scholar]
- 25.Litchfield JT, Jr, Wilcoxon F. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther. 1949;96:99–113. [PubMed] [Google Scholar]
- 26.Sela VR, Hattanda I, Albrecht CM, De Almeida CB, Obici S, Cortez DA, et al. Effect of xanthone from Kielmeyera coriacea stems on serotonergic neurons of the median raphe nucleus. Phytomedicine. 2010;17:274–8. doi: 10.1016/j.phymed.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Martins J, Otobone FJ, Sela VR, Obici S. Behavioral effects of Kielmeyera coriacea extract in rats. Indian J Pharmacol. 2006;38:427. [Google Scholar]
- 29.Zhao X, Chen Q, Liu Y, Xia C, Shi J, Zheng M. Effect of xanthone derivatives on animal models of depression. Curr Ther Res Clin Exp. 2014;76:45–50. doi: 10.1016/j.curtheres.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venault P, Chapouthier G, de Carvalho LP, Simiand J, Morre M, Dodd RH, et al. Benzodiazepine impairs and beta-carboline enhances performance in learning and memory tasks. Nature. 1986;321:864–6. doi: 10.1038/321864a0. [DOI] [PubMed] [Google Scholar]


