Abstract
Objective:
The study was conducted to develop the glucocorticoid-induced osteoporosis (GIO) model in Sprague-Dawley weanling rats using different doses of methylprednisolone (MP) and evaluate the antiosteoporotic effect of a classical ayurvedic formulation, Panchatikta Ghrita (PG), in this model.
Materials and Methods:
Institutional Animal Ethics Committee approval was obtained. Development of model was done by subcutaneous injection of 2 doses of MP (14 and 28 mg/kg/week) for 4 weeks in 21-day old weanlings. Following confirmation of the dose of MP that induced osteoporosis, the antiosteoporotic effect of PG was tested in this model in comparison to a known antiosteoporotic agent, alendronate. Both alendronate (2.9 mg/kg/day) and PG (1.35 g/kg/day) were administered orally 2 weeks after MP - 14 mg/kg/week injection and continued for 4 weeks. Serum and urine calcium and inorganic phosphate were analyzed at weekly intervals. Animals were sacrificed after 6 weeks, and femur bones were processed to measure bone hardness and elasticity and for histological studies.
Results:
Rats treated with MP - 14 mg/kg/week showed optimum osteoporotic effect with no mortality as compared to MP - 28 mg/kg/week; hence, this dose of MP was used further for the efficacy study. Osteoporotic rats treated with PG 1.35 g/kg showed increase in serum calcium and inorganic phosphate levels, whereas urine calcium and phosphate levels were significantly reduced. A significant decrease in a number of osteoclasts, whereas an increase in bone hardness and elasticity was observed as compared to diseased group demonstrating antiosteoporotic effect of PG.
Conclusion:
PG has an antiosteoporotic effect in GIO rat model.
KEY WORDS: Bone elasticity, bone hardness, methylprednisolone, nanoindentation technique, Panchatikta Ghrita, serum calcium, tartrate resistant acid phosphatase
Introduction
Osteoporosis is a rare, inherited disorder with abnormally dense bone throughout the skeleton. A disturbance in bone remodeling is the underlying mechanism for decrease in normal bone density. According to the World Health Organization, osteoporosis is second only to cardiovascular disease as a global healthcare problem and medical studies show a 50-year-old woman has a similar lifetime risk of dying from hip fracture as from breast cancer. Long-term exogenous glucocorticoid (GC) therapy causes profound reduction of bone mineral density (BMD), bone quality, bone formation, and bone mechanical properties, which leads to fracture. GCs inhibit osteoblastogenesis and reduce the lifespan of osteoblasts and osteocytes.[1] Alendronate, risedronate, and zoledronic acid were shown to prevent and reverse the loss of BMD in GC-induced osteoporosis (GIO) with greater effects. The main culprit in iatrogenic osteoporosis is GC therapy, which is used for many medical conditions including lung inflammation, inflammatory bowel disease, rheumatoid arthritis, systemic lupus erythematosus, renal disease, and diseases affecting nerve and muscle.[2] GCs impair the replication, differentiation, and function of osteoblasts and induce the apoptosis of mature osteoblasts and osteocytes.[3] The exact prevalence of GIO remains unclear. It is estimated, however, that in 30–50% of patients under chronic, long-term GC therapy, relevant bone loss occurs, and 1 out of 4 also develop some degree of osteonecrosis, demonstrating that current osteoporotic treatment is insufficient.[4] Hence, there is a need for the production of better quality drugs and the development of efficient methods of osteoporosis treatment.
Due to long-term adverse effects or lack of efficacy of synthetic drugs, the potential efficacy of traditional medicines has aroused the interest of scientists and doctors to seek the clues from traditional medicines for the treatment of certain chronic and difficult diseases including Osteoporosis. Although there is no direct mention of the condition “osteoporosis” in the ancient texts of Ayurveda; there are references stating to decrease in “asthi” or bone with advancing age. This condition leads to an imbalance of the vata dosha which further deteriorates the bone. This condition can be treated using medicinal plants with katu (bitter) and tikta (astringent) properties.[5] Panchatikta Ghrita (PG) is one such formulation having a combination of plants with katu and tikta properties. This formulation is said to help in relieving pain associated with bone degeneration along with regeneration of tissue function.[6] In this formulation, Azadirachta indica (Nimba), Trichosanthes dioica (Patola), Solanum surattense (Kantakari), Tinospora cordifolia (Guduchi), and Adhatoda vasica (Vasa) are the plants having primarily katu (bitter) properties along with tikta (astringent) properties which are helpful in slowing down the degeneration processes and thus useful in osteoarthritis. Based on the effect of these five plants, the name Panchatikta is given to the formulation. The ghrita present in the formulation acts as a carrier for these herbs to reach the level of the bones to exert a maximum effect.
Thus, in the present study, we initially standardized the GIO model in Sprague-Dawley (SD) weanling rats using different doses of methylprednisolone (MP) (synthetic GC) and further evaluated the potential antiosteoporotic effect of the formulation Panchatikta in this model. The formulation was administered to the animals along with milk as per ayurvedic principles for maximum benefit.
Materials and Methods
Chemicals
Methyl prednisolone (MP) and Alendronate were purchased from M/S Sigma-Aldrich, USA. All other chemicals and reagents were purchased from SD Fine Chemicals Ltd., Mumbai, India.
Plant Material
Panchtikta ghrita (PG) was procured from Nagarjun Pharmaceuticals (P) Ltd., Naroda, Ahmedabad. It was mixed in warm milk before administration to the animals. The daily human dose of PG is 15 g once a day, which when extrapolated came to 1.35 g/kg/day in rats.
Animals
The 21-day-old SD weanlings weighing between 30 and 45 g of either sex were used for the experiment. The animals were housed in polycarbonate cages at room temperature (20 ± 3°C) and humidity (60 ± 10%) with 12:12 h light–dark cycle. The present study was approved by the Institutional Animal Ethics Committee for Animal Experimentation.
Phase I: Standardization of Glucocorticoid-Induced Osteoporosis Model
Osteoporosis was induced by subcutaneous injection of 2 doses of MP - 14 and 28 mg/kg/week[2,7] for 4 weeks in 21 days SD rats in two different groups. The effect of MP on serum and urine calcium and inorganic phosphate, bone morphology, osteoclasts, and bone hardness was studied in the two diseased groups, and the effect was evaluated in comparison with the normal control (NC) group. Biochemical parameters were assessed on 0, 7, 14, 21, and 28th day, whereas the histomorphometric parameters and bone strength were analyzed on day 28. Mortality was also recorded throughout the study period.
Biochemical parameters
Serum and urine were processed for calcium estimation using Colorimetric Arsenazo III Method and inorganic phosphate was assessed using phosphomolybdate U.V. Endpoint Method.[8,9]
Histomorphometrical analysis of femur
Following sacrifice of the animals on day 28, the femur bones were excised using bone cutter and cleaned for any remains of muscle fibers. The bones were deposited in 10% formalin solution for histomorphometric parameters. These bones were placed in metal cassettes and processed in an autoprocessor overnight. The processed bone samples were then placed in molds and embedded in paraffin to form paraffin blocks. On rotatory microtome, the lower end of femur was sectioned (5-μm thickness) longitudinally and processed for hematoxylin and eosin (H and E) staining and tartrate-resistant acid phosphatase (TRAP) staining which were assessed for bone morphology.[10]
Hardness of bones by nanoindentation method
Bone hardness was studied using nanoindentation test.[11] The indentation was carried out using CSM-Instrument make Ultra-Nano Hardness tester. Spherical diamond indenter with 5 µm radius was used for indentation. Indentation was carried out by following Oliver and Pharr method. Spherical indenter was used to avoid crack formation. Predefined load of 5 mN was applied on the bone samples. Loading-unloading rate was kept 10 mN/min with 30 s hold time at maximum load to avoid creeping of the sample.
Phase II: Evaluation of Efficacy of Panchatikta Ghrita on Glucocorticoid-Induced Osteoporosis Model
Based on the results of Phase I, the dose of 14 mg/kg/week MP for 4 weeks was used to induce osteoporosis and to evaluate the efficacy of PG. The 21-day-old SD weanling rats were divided into five groups, consisting of six animals per group, wherein the control group received only the vehicle, i.e. milk (vehicle control [VC] group), whereas the diseased group, i.e. MP - 14 mg/kg/week treated group (disease control [DC] group) and the other study groups were injected with the inducing agent, MP - 14 mg/kg/day administered subcutaneously for a period of 21 days to induce osteoporosis. The study drug, Panchatikta ghrita (1.35 g/kg/day) and standard drug, alendronate (2.9 mg/kg/day) were administered to the test control (TC) group and standard control (SC) group orally once in a day from day 1 and continued for a period of 42 days. Blood and urine samples were collected from all the groups on days 0, 14, 21, 28, and 42 to assess the biochemical parameters. At the end of the experimental period, the animals were sacrificed by cervical dislocation and the femur bones were dissected for further analysis; left femora for histomorphometrical studies and right femora to assess bone hardness by the nanoindentation test.
Statistical Analyses
Results were expressed as mean ± standard deviation. All experimental data were analyzed using one-way analysis of variance with Dunnett's post hoc test. Values of P < 0.05 were considered statistically significant.
Results
Phase I: Development of the Glucocorticoid-Induced Osteoporosis Model
Weanling rats were injected with either one of the 2 doses of MP (14 or 28 mg/kg/week for 4 weeks) and observed for a period of 28 days for the development of osteoporosis. Rats injected with 28 mg/kg/week of MP had a mortality rate of 66.66%. There was no mortality observed with the 14 mg/kg/week dose of MP and the NC group.
Effect on serum and urine calcium and inorganic phosphate levels
A decrease in serum and increase in urine calcium were observed in rats injected with both doses of MP as compared to the normal rats on the 28th day. However, a significant decrease was seen in animals treated with 14 mg/kg/week of MP. MP also significantly decreased the serum inorganic phosphate levels and increased urine inorganic phosphate levels in rats of both the groups on the 28th day as compared to NC group; however, a significant effect was seen in the MP - 14 mg/kg/week group.
Histomorphometrical analysis
As observed in the H and E staining, the thickness of the trabecular bone was decreased in the femur bones of rats in both the MP-treated groups as compared to NC group. However, the thickness loss of the trabecular bone in MP - 28 mg/kg/week group was more distinct as compared to MP - 14 mg/kg/week. The results are represented in Figure 1.
Figure 1.
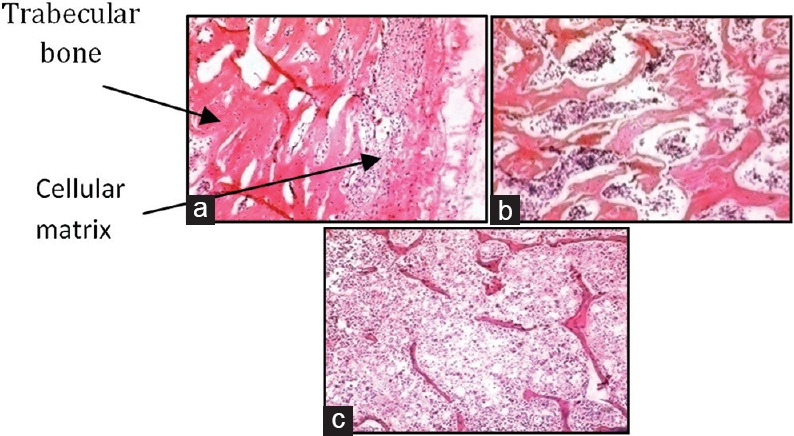
H and E staining of femur bones. (a) Normal control. (b) Methylprednisolone - 14 mg/kg/week. (c) Methylprednisolone - 28 mg/kg/week
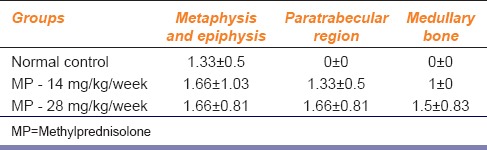
TRAP staining showed an increase in the number of osteoclasts in the femur bones in rats of both the MP-treated groups as compared to NC group rats. However, the 28 mg/kg/week MP group showed a maximum increase in the number of osteoclasts as compared to MP - 14 mg/kg/week group. The results are represented in Table 1.
Table 1.
Effect of methylprednisolone on number and distribution of osteoclasts
Nanoindentation results
A significant dose-dependent decrease in bone hardness was observed in both the MP-treated groups as compared to the normal group. The results obtained are represented in Figure 2.
Figure 2.
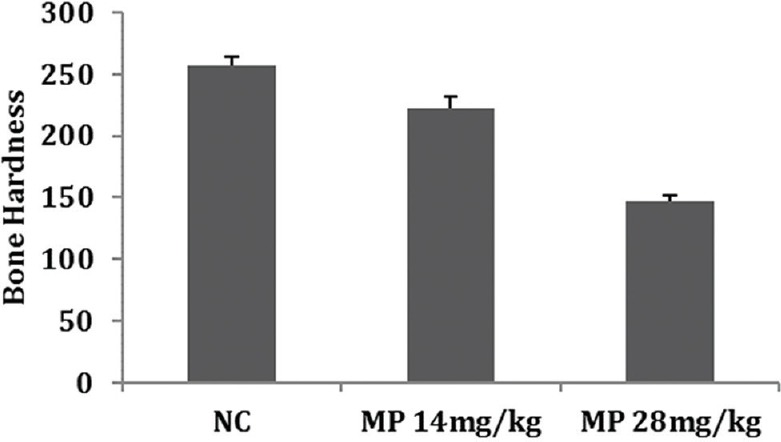
Effect of methylprednisolone on bone hardness
From the above results, it was seen that MP at a dose of 28 mg/kg/week had a more potent effect in inducing osteoporosis in weanling rats. However, a higher rate of mortality was also observed in this group (66.66%) as compared to no mortality in the MP - 14 mg/kg/week group and NC group. Hence, it was decided to use the dose of 14 mg/kg/week of MP for further studies.
Phase II: Evaluation of Efficacy of Panchatikta Ghrita on Glucocorticoid-Induced Osteoporosis Model
Effect on serum and urine calcium and inorganic phosphate levels
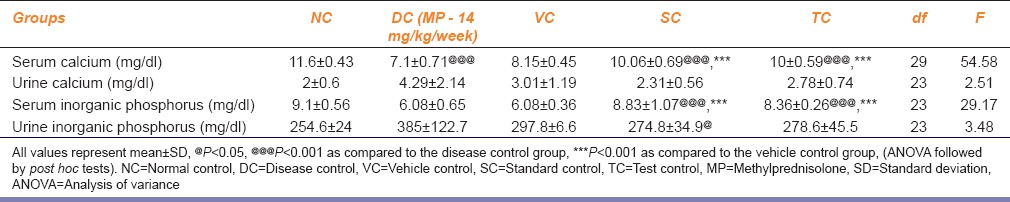
Rats treated with PG (TC) showed a significant increase in the serum calcium and serum inorganic phosphate levels at day 42 as compared to the VC and MP - 28 mg/kg/week treated group (DC). The effect was comparable to that exhibited by the SC group. Furthermore, a decrease in urine calcium and inorganic phosphate levels was seen in PG-treated rats when compared to the DC group. However, a significant decrease (P < 0.01) was observed only in the SC group. The results obtained are represented in Table 2.
Table 2.
Effect of Panchatikta Ghrita on serum and urine calcium and inorganic phosphate levels on 42 days of treatment
Histomorphometrical analysis
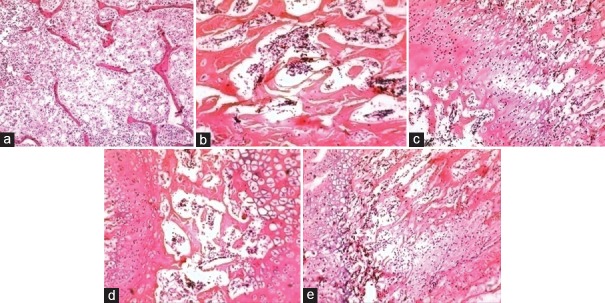
In H and E staining, as expected, the SC group showed increased trabecular bone thickness [Figure 3] as compared to DC group. Similarly, PG-treated group (TC) also showed increased trabecular bone thickness.
Figure 3.
H and E staining of femur bones. (a) Normal control (untreated). (b) Disease control (methylprednisolone - 14 mg/kg/week) treated group. (c) Vehicle control (milk treated group). (d) Standard control (alendronate treated group). (e) Test control (Panchatikta Ghrita treated group)
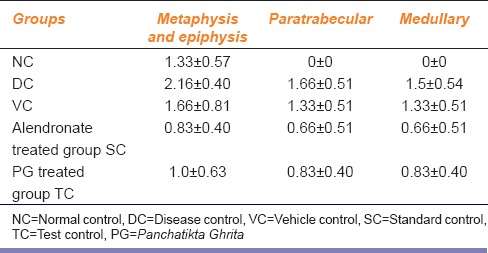
In TRAP staining, the DC group exhibited an increase in the number of osteoclasts in the femur bones. PG-treated rats showed a decreased in the number of osteoclasts as compared to the DC group. The SC group also exhibited a decrease in the number of osteoclasts [Table 3].
Table 3.
Effect of Panchatikta Ghrita on number and distribution of osteoclasts
Nanoindentation results
Bone hardness was found to be the highest in the NC group as compared to the DC group. PG-treated rats showed an increase in bone hardness as compared to the DC group and the effect was compared to that exhibited by alendronate. Bone hardness in the VC group was low indicating the per se effect of the study drug. Similar results were also observed with regard to bone elasticity in all the study groups except for the NC group. Hardness is dependent on inorganic mineral components, and elasticity depends on interconnectivity of organic and inorganic matrices. The results obtained on hardness and elasticity of bones are summarized in Figures 4 and 5.
Figure 4.
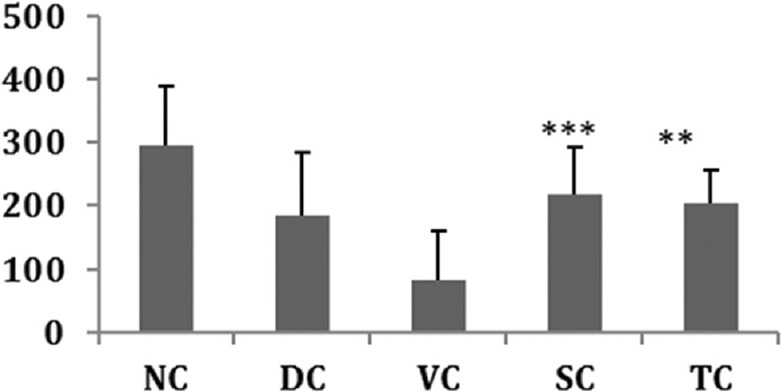
Effect on bone hardness. All values represent mean ± standard deviation; **P<0.01; **P<0.001 as compared to Vehicle control group (analysis of variance followed by post hoc tests)
Figure 5.
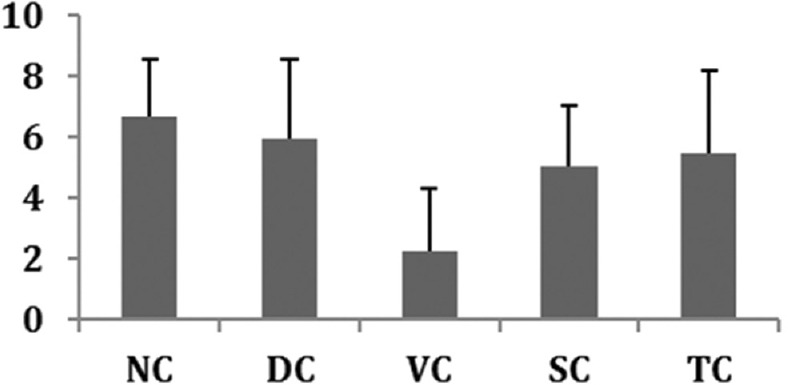
Effect on Bone elasticity. All values represent mean ± standard deviation
Discussion
We selected MP to induce osteoporosis in rats due to the widespread use of GC therapy which causes profound reduction of BMD, bone quality, bone formation, and bone mechanical properties which lead to fracture. Initially, the model was standardized by subcutaneously injecting 2 doses of MP (14 and 28 mg/kg/week) in two different groups of animals. The extent of osteoporosis induced by MP was estimated every 7 days, in terms of serum and urine calcium and inorganic phosphate and on day 28, in terms of bone morphology, number and distribution of osteoclasts, and bone hardness.
In both the groups, although MP decreased serum calcium and inorganic phosphate levels on day 28, only MP 14 mg/kg/week dose showed a significant decrease in the serum calcium and inorganic phosphate levels as compared to serum calcium and inorganic phosphate levels of the NC group. Mahgoub et al. demonstrated a similar pattern of fall in serum calcium in his study on the calcium-lowering action of GCs in adrenalectomized rats,[12] whereas Ferraro et al. have demonstrated depletion in phosphate levels in his study on intestinal absorption of phosphate in rats.[13]
Urine calcium and inorganic phosphate levels were found to increase on day 28 in both the MP-treated groups. However, MP at 14mg/kg/week showed a significant increase in the urine calcium and inorganic phosphate level as compared to urine calcium and inorganic phosphate levels of the NC group. Thus, optimum induction of osteoporosis was observed with MP at a dose of 14 mg/kg/week when given for 28 days. These results are in agreement with the study conducted by Rubin and Bilezikian;[14] wherein, it was demonstrated that GCs increase the urine calcium level and decrease serum calcium levels in GIO.
GCs usually target trabecular bone loss in lumbar spine or femur[15] leading to osteoporosis, which was successfully demonstrated by H and E staining. MP reduced the trabecular thickness in both the groups as compared to the NC group. Furthermore, there was a distinct difference in the trabecular thickness of both the MP-treated groups. Rubin and Bilezikian in a similar study of GCs demonstrated a decrease in trabecular wall thickness and a typical disruption of trabecular microarchitecture with a reduction in connectivity.[14]
GCs are known to favor osteoclastogenic bone resorption,[16,17] i.e. GCs promote osteoclast formation from precursor cells in bone marrow.[18] In addition, osteoclasts are found in the region where continuous formation and resorption of bone takes place. In our study, TRAP staining successfully demonstrated these changes. There was an increase in the number of osteoclasts in the metaphysis and epiphysis, paratrabecular, and medullary regions in the bones of animals treated with MP as compared to the NC group. Since the metaphysis and the epiphyseal region are the growing ends of the bone, the presence of osteoclasts was also seen in the bone regions of NC group animals.
Although MP at the dose of 28 mg/kg/week group showed a significant decrease in bone hardness as compared to the 14 mg/kg/week and NC groups by the nanoindentation test, this dose was found to be lethal to the animals with a mortality of 66.66% as compared to MP - 14 mg/kg/week and NC groups. Thus, based on these results, the 14 mg/kg/week MP dose was used to induce osteoporosis in the efficacy study.
As expected MP - 14 mg/kg/week exhibited decrease in serum calcium and inorganic phosphate levels in the DC group, drug control, and SC group up to day 14 as compared to NC group. However, at the end of treatment, i.e. on day 42, a significant increase in the serum calcium and inorganic phosphate levels was seen in the drug and SC groups as compared to disease and VC group. Similarly, MP - 14 mg/kg/week also exhibited increase in urine calcium and inorganic phosphate levels in the DC group, drug control, and SC group up to day 14 as compared to NC group. On day 42, the drug and SC groups demonstrated a significant decrease in the urine calcium and inorganic phosphate levels as compared to disease and VC group.
Nanoindentation and microtomography are reliable techniques in assessing the quality of bone at the micron- and nano-meter scale. In addition, the findings of more obvious changes in morphology at the micron level might help in more accurate or earlier diagnosis of osteoporosis. These techniques can also be used to study the efficacy of various treatment methods.
Bones of rats treated with PG showed increased trabecular bone thickness and hardness and decreased a number of osteoclasts in all the three regions, namely metaphysis and epiphysis, paratrabecular and medullary as compared to the bones of disease and VC group. However, the effect exhibited by the SC group was much more than exhibited by the drug control group. The bones of VC group showed the presence of osteoclasts at the growing ends, i.e., metaphysis and epiphysis. These results thus demonstrate the bone forming ability of PG against the bone resorption ability of GCs. The VC, i.e., milk used in the study had no active role in protecting against the osteoporotic effect of MP. Uchida et al. in his study on effects of alendronate on bone metabolism in GIO measured by 18F-fluoride positron emission tomography[19] have demonstrated that alendronate is effective in the prevention and treatment of GIO. He reported that alendronate prevented bone loss and improved the BMD of lumbar vertebrae by reducing both bone formation and resorption further suppressing bone metabolism. These results are in line with the results exhibited by the SC group.
Akhtar et al.,[20] in his clinical study “Sandhigata Vata” with respect to osteoarthritis and its management by PG Guggulu has reported that PG along with local Abhyanga and Nadi Swedana provides better relief in osteoarthritis. Most of the plants used in the PG are said to possess Tikta Rasa, Ushna Virya, Madhura, and Katu Vipaka properties. Tikta Rasa increases the Dhatvagni (metabolic stage) which in turn improves the nutrition of all the Dhatus. As a result of which, the Asthi Dhatu and Majja Dhatu become stable, and the Asthi Dhatu and Majja Dhatu kshaya will decrease the degeneration seen in the Asthi Dhatu. PG is said to slow down the degeneration processes and has been shown to be useful in osteoarthritis. All the plants included in Panchatikta are known to have osteoprotective or antiarthritic property; hence, further studies are required to investigate the possible mechanism of action by which the individual plant and its chemical constituents show its action.
Conclusion
The data obtained from the present study demonstrated the antiosteoporotic potential of PG and thus provides evidence to confirm its traditional use in osteoarthritis and bone disorders.
Financial Support and Sponsorship
Research Society, TNMC & BYL Nair Ch. Hospital.
Conflicts of Interest
There are no conflicts of interest.
Acknowledgment
We thank Mr. Alok Sabnis and Ms. Bhagyashree Rane for their technical assistance. We also thank Dr. Supriya Bhalerao for selection of the formulation for the study. We also thank Dr. Vikas Kavishwar, Department of Pathology, TNMC and BYL Nair Hospital for helping us in histomorphometric analysis.
References
- 1.Raisz LG. Pathogenesis of osteoporosis: Concepts, conflicts, and prospects. J Clin Invest. 2005;115:3318–25. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nansie A, McHugh NA, Vercesi HM, Egan RW, Hey JA. In vivo rat assay: Bone remodeling and steroid effects on juvenile bone by pQCT quantification in 7 days. Am J Physiol Endocrinol Metab. 2003;284:E70–5. doi: 10.1152/ajpendo.00102.2002. [DOI] [PubMed] [Google Scholar]
- 3.Bitto A, Polito F, Burnett B, Levy R, Di Stefano V, Armbruster MA, et al. Protective effect of genistein aglycone on the development of osteonecrosis of the femoral head and secondary osteoporosis induced by methylprednisolone in rats. J Endocrinol. 2009;201:321–8. doi: 10.1677/JOE-08-0552. [DOI] [PubMed] [Google Scholar]
- 4.Hanada K, Furuya K, Yamamoto N, Nejishima H, Ichikawa K, Nakamura T, et al. Bone anabolic effects of S-40503, a novel nonsteroidal selective androgen receptor modulator (SARM), in rat models of osteoporosis. Biol Pharm Bull. 2003;26:1563–9. doi: 10.1248/bpb.26.1563. [DOI] [PubMed] [Google Scholar]
- 5.Harishahtri P, editor. 11th Adhyaya’ Sutra Sthana, Asthanghridaya. Varanasi: Chaukhamba Sanskrit Sansthan; 2000. p. 186. [Google Scholar]
- 6.Ratnavali B, Chikitsa K. In: Ratnavali B, Shastri A, Shastri R, editors. Varanasi: Chaukhambha Sanskrit Sansthan; 2001. p. 633. [Google Scholar]
- 7.Wang Y, Ohtsuka-Isoya M, Shao P, Sakamoto S, Shinoda H. Effects of methylprednisolone on bone formation and resorption in rats. Jpn J Pharmacol. 2002;90:236–46. doi: 10.1254/jjp.90.236. [DOI] [PubMed] [Google Scholar]
- 8.Fiske CH, Subbarow Y. The colorimetric determination of phosphorus. J Biol Chem. 1925;66:375–400. [Google Scholar]
- 9.Smith HG, Jr, Bauer PJ. Light-induced permeability changes in sonicated bovine disks: Arsenazo III and flow system measurements. Biochemistry. 1979;18:5067–73. doi: 10.1021/bi00590a007. [DOI] [PubMed] [Google Scholar]
- 10.Hayman AR, Bune AJ, Bradley JR, Rashbass J, Cox TM. Osteoclastic tartrate-resistant acid phosphatase (Acp 5): Its localization to dendritic cells and diverse murine tissues. J Histochem Cytochem. 2000;48:219–28. doi: 10.1177/002215540004800207. [DOI] [PubMed] [Google Scholar]
- 11.Hoffler CE, Guo XE, Zysset PK, Goldstein SA. An application of nanoindentation technique to measure bone tissue lamellae properties. J Biomech Eng. 2005;127:1046–53. doi: 10.1115/1.2073671. [DOI] [PubMed] [Google Scholar]
- 12.Mahgoub A, Hirsch PF, Munson PL. Calcium-lowering action of glucocorticoids in adrenalectomized-parathyroidectomized rats. Specificity and relative potency of natural and synthetic glucocorticoids. Endocrine. 1997;6:279–83. doi: 10.1007/BF02820504. [DOI] [PubMed] [Google Scholar]
- 13.Ferraro C, Ladizesky M, Cabrejas M, Montoreano R, Mautalen C. Intestinal absorption of phosphate: Action of protein synthesis inhibitors and glucocorticoids in the rat. J Nutr. 1976;106:1752–6. doi: 10.1093/jn/106.12.1752. [DOI] [PubMed] [Google Scholar]
- 14.Rubin MR, Bilezikian JP. Clinical review 151: The role of parathyroid hormone in the pathogenesis of glucocorticoid-induced osteoporosis: A re-examination of the evidence. J Clin Endocrinol Metab. 2002;87:4033–41. doi: 10.1210/jc.2002-012101. [DOI] [PubMed] [Google Scholar]
- 15.Reid IR. Glucocorticoid effects on bone. J Clin Endocrinol Metab. 1998;83:1860–2. doi: 10.1210/jcem.83.6.4911. [DOI] [PubMed] [Google Scholar]
- 16.Reid IR. Glucocorticoid osteoporosis – Mechanisms and management. Eur J Endocrinol. 1997;137:209–17. doi: 10.1530/eje.0.1370209. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki N, Kusano E, Ando Y, Yano K, Tsuda E, Asano Y. Glucocorticoid decreases circulating osteoprotegerin (OPG): Possible mechanism for glucocorticoid induced osteoporosis. Nephrol Dial Transplant. 2001;16:479–82. doi: 10.1093/ndt/16.3.479. [DOI] [PubMed] [Google Scholar]
- 18.Shuto T, Kukita T, Hirata M, Jimi E, Koga T. Dexamethasone stimulates osteoclast-like cell formation by inhibiting granulocyte-macrophage colony stimulating factor production in mouse bone marrow cultures. Endocrinology. 1994;134:1121–6. doi: 10.1210/endo.134.3.8119150. [DOI] [PubMed] [Google Scholar]
- 19.Uchida K, Nakajima H, Miyazaki T, Yayama T, Kawahara H, Kobayashi S, et al. Effects of alendronate on bone metabolism in glucocorticoid-induced osteoporosis measured by 18F-fluoride PET: A prospective study. J Nucl Med. 2009;50:1808–14. doi: 10.2967/jnumed.109.062570. [DOI] [PubMed] [Google Scholar]
- 20.Akhtar B, Mahto RR, Dave AR, Shukla VD. Clinical study on Sandhigata Vata w.s.r. to osteoarthritis and its management by Panchatikta Ghrita Guggulu. Ayu. 2010;31:53–7. doi: 10.4103/0974-8520.68210. [DOI] [PMC free article] [PubMed] [Google Scholar]