Abstract
Objective:
Bruguiera gymnorrhiza (BRG) (L.) Lamk (Rhizophoraceae), a mangrove species, is widely distributed in the Pacific region, eastern Africa, Indian subcontinent, and subtropical Australia. The leaves of this plant are traditionally used for treating burns and inflammatory lesions. This study isolates the bioactive compound from the methanol extract of BRG leaves and evaluates the possible mechanisms of anti-inflammatory activity involved.
Materials and Methods:
Bioassay-guided fractionation of BRG was performed to identify the bioactive fraction (displaying inhibition of cyclooxygenase 2 [COX2] - 5-lipoxygenase (5-LOX) activities and tumor necrosis factor-alpha (TNF-α) production at the tested concentrations of 100 and 10 μg/ml). The fractionation was performed by solvent extraction and preparative high-performance liquid chromatography. The bioactive compound was characterized by ultraviolet–visible, liquid chromatography–mass spectrometry and nuclear magnetic resonance spectroscopy. The antioxidant potential was evaluated by electron spin resonance spectrum of 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical at 250 μM. The effect of the compound was also studied on TNF-α converting enzyme and nuclear factor kappa B (NF-κB) activities at the concentrations 100, 10 and 1 μg/ml.
Results:
Bioassay-guided purification of BRG revealed the presence of a flavone (5,7-dihydroxy-2- [3-hydroxy-4,5-dimethoxy-phenyl]-chromen-4-one) of molecular weight 330Da. It demonstrated more than 80% inhibition against COX2, 5-LOX activities and TNF-α production at 100 μg/ml. It also displayed 40% inhibition against DPPH radical at the tested concentration along with 23.1% inhibition of NF-κB activity at 100 μg/ml.
Conclusions:
The isolated methoxy-flavone may play a predominant role in the anti-inflammatory properties displayed by BRG leaves. Such activity may involve multiple mechanisms, namely (a) modulation of oxidative stress (b) inhibition of arachidonic acid metabolism and (c) downregulation of pro-inflammatory cytokines probably through NF-κB inhibition.
KEY WORDS: 5-lipoxygenase, cyclooxygenase, cytokine, flavone, nuclear factor kappa B, transactivation assay
Introduction
In our continuing efforts to discover bioactive anti-inflammatory compounds from medicinal plants, we have screened different traditional medicinal plants from the Sundarban (West Bengal, India) mangroves. Our laboratory has reported the pharmacological activities of Pluchea indica,[1] Bryophyllum pinnatum[2] and Acanthus ilicifolius[3] collected from the mangrove regions of West Bengal. In a similar attempt, we have also reported that the methanolic extract of the Bruguiera gymnorrhiza (BRG) (Family: Rhizophoraceae) leaves displayed promising anti-inflammatory activity in different in vitro and in vivo models.[4]
Inflammation is the normal response to tissue injury or infection. A number of inflammatory mediators are known to be involved in the genesis, persistence and severity of inflammatory conditions.[2,3] Various cyclooxygenase (COX) and lipoxygenase (LOX) products, generated from arachidonic acid metabolism, are known to be upregulated during inflammation. Inhibition of prostaglandin biosynthesis has been proposed as the mechanism of action of aspirin-like drugs. However, these drugs have serious limitations owing to their various adverse effects.[3,4] Conventional nonsteroidal anti-inflammatory drugs (NSAIDs), which are potentially unsafe for the gastrointestinal (GI) tract and the liver, mediate their anti-inflammatory effects by blocking the COX enzymes, thereby causing a shift of the arachidonic acid metabolism to the 5-LOX pathway, whereas the dual inhibitors (owing to the blockade of both the enzymes) display reduced toxic manifestation.[5]
Based on the observed dual inhibitory activity of BRG,[4] the present investigation was carried out for isolation and characterization of the bioactive component. Efforts were also made to elucidate the possible mechanism of action of the purified component.
Materials and Methods
Plant Material
The leaves of BRG were collected from Jharkhali (Sundarban) region of West Bengal, India during the month of December 2008. The plant material was taxonomically identified and authenticated by Botanical Survey of India, Howrah, India (Authentication number CNH/I-I/[286/2008/Tech. II/328 dt. 12/12/2008]).
Extraction and Bioassay-guided Isolation
The air dried leaves (~2 kg) of BRG were defatted with petroleum ether followed by extraction with chloroform. Thereafter, the residue was exhaustively extracted with methanol. Upon evaporation of methanol in vacuum, a brown concentrated residue was obtained. The yield of the methanolic extract of BRG was 2.5%, w/w with respect to the dried starting material. The final product was then stored at 4°C until further use.
The bioactivity guided isolation and purification of the BRG was carried out using combination of different solvent extraction and chromatographic techniques. The choice of techniques largely depended on the nature of the bioactive substances present and their nature of separation in the particular adsorbent material.
Two hundred grams of BRG was dissolved in water (1 L) and extracted with equal volume of water saturated ethyl acetate. The remaining aqueous fraction was then extracted with water saturated n-Butanol. Solvents were removed with the help of rotary evaporator at 45°C and different fractions were collected. Three fractions of BRG (ethyl acetate fraction, n-butanol fraction and aqueous fraction) were then evaluated for their biological activity and from the results, the n-butanol fraction was found to possess maximum biological activity and therefore this particular fraction was considered for further fractionation with the help of Diaion Hp-20 resin.
The resin bound material (4 g of n-butanol fraction) was eluted with gradient solvent systems of water-methanol, increasing the concentration of methanol. The different fractions (four) were then evaluated for their biological activity by measuring their inhibitory potential against COX-2 and 5-LOX enzymes and tumor necrosis factor-alpha (TNF-α) production in human peripheral blood mononuclear cells (PBMCs). The 3:1 (methanol: water) sub-fraction was found to possess highest activity and was therefore used for separation of the bio-active compound (s) with the help of preparative high-performance liquid chromatography (HPLC).
Preparative HPLC of 300 mg of the 3:1 sub-fraction was carried out using a Shimadzu LC-6AD instrument with an SPD-20A detector and a Xbridge C18 column (250 mm × 19 mm, 5 μm) with a flow rate of 16 ml/min where 90% of mobile phase A (0.01% formic acid in water) and 10% of mobile phase B (Acetonitrile) was continued for initial 3 min followed by linear gradient to reach to 70% of mobile phase A in next 15 min. Further gradient was applied to reach to 10% of mobile phase A in next 2 min followed by reverse gradient to reach to 90% of mobile phase A. Total run time was 25 min.
HPLC (detected at 210 nm) analysis revealed seven peaks; compound A (14.6 mg), compound B (14.8 mg), compound C (19.7 mg), compound D (9.1 mg), compound E (27.8 mg), compound F (12 mg) and compound G (14.8 mg). Compounds A-G were evaluated for their biological activity by measuring their inhibitory potential against COX-2, 5-LOX enzyme and TNF-α production by human PBMCs.
Spectroscopic Analysis
The isolated bioactive compound (Compound G) was analyzed to identify its chemical nature following the methods.[6] ultraviolet (UV) spectra (200–500 nm) was performed in a Spectramax M5 (Molecular Devices, California, USA) reader using appropriate blank. Nuclear magnetic resonance (NMR) experiments were performed on a Bruker DRX-400 spectrometer at 300 K. All the NMR spectra were acquired in DMSO-d6. The LC system comprises of LC-20AD pumps, CBM-20A controller (Shimadzu, Japan) and HTS-PAL autosampler (CTC Analytics, Switzerland). A Luna C18 (30 mm × 2.0 mm and 5 µm particle size, Phenomenex, USA) column was used in liquid chromatography–mass spectrometry (LC/MS and LC/MS/MSs) analysis. The mobile phase A was made up of 0.1% formic acid in water. A mixture of acetonitrile and water (80:20, v/v) containing 0.1% formic acid was as mobile phase B. Mass spectrometric analyses were performed on an API 4000 QTRAP system (AB Sciex, USA) equipped with Turbo ion spray ionization source operated at 5 kV and 400°C and the system was operated in both positive and negative ionization modes. Analysis was performed with Analyst software.
Inhibition of Cyclooxygenase 2 Mediated Prostaglandin E2 Production
Freshly collected blood (heparinised) from healthy human volunteers was incubated with acetylsalicylic acid (12 μg/ml) for 6 h at 37°C in a 96-well sterile tissue culture plate. Different concentrations of test materials or the standard drug (Rofecoxib) was added to the respective wells. Thereafter, lipopolysachharide (LPS: Escherichia coli B4) was added to the wells at a final concentration of 10 μg/ml. The plate was once again incubated at 37°C for 18 h and then centrifuged at 2000 rpm for 10 min and plasma was collected from individual wells for analyzing the levels of prostaglandin E2 (PGE2) production.[7,8] Levels of PGE2 in plasma samples were estimated using enzyme-linked immunosorbent assay (ELISA) kits as per the instructions provided by the manufacturer (R and D Systems, Minneapolis, USA).
5-Lipoxygenase Inhibition Assay
The potential of BRG and its different fractions to inhibit the process of leukotrienes production by 5-LOX enzyme was estimated by this assay. In this assay, freshly collected blood (heparinised) from healthy human volunteers was incubated with different concentrations of test materials or standard inhibitor (BWB70C) for 1 h at 37°C in a 96-well sterile tissue culture plate. Thereafter, calcium ionophore (A23187) was added to respective wells at a concentration of 5 μM and the plate was incubated for another 30 min at 37°C before collecting the plasma. Levels of LTB4(Leukotriene B4) in plasma samples were estimated using ELISA kits as per the instructions provided by the manufacturer (R and D Systems, Minneapolis, USA). Any reduction in the level of LTB4 by BRG or standard inhibitor indicates the inhibition of 5-LOX by the test sample.[9]
Inhibition of Tumor Necrosis Factor-alpha Production
BRG and its different fractions were tested for their ability to inhibit the production of pro-inflammatory cytokine TNF-α by human PBMC.[10] PBMCs were isolated from blood (from healthy volunteers) using BD Vacutainer CPT™ (Cell preparation tube, BD Bio Science, USA) and suspended in RPMI medium. Test compounds in different concentrations or dexamethasone were preincubated with PBMC (0.5 million/incubation well) for 30 min at 37°C and then stimulated with lipopolysaccharide (E. coli: B4; 1 μg/ml) for 18 h at 37°C under 5% CO2. The cell culture supernatants were collected and stored at −80°C until analysis. Levels of TNF-α in cell culture medium were estimated using ELISA kits as per the instructions provided by the manufacturer (R and D Systems, Minneapolis, USA).
Oxygen Radical Absorption Capacity Assay
Oxygen radical absorption capacity (ORAC) assay was performed in a black clear bottom 96-well microtitre plate. In brief, 20 µl of buffer, different concentrations of compound G (1.6–0.1 µM) or Trolox (6.3–0.4 µM) were incubated with 160 μL of a 78.75 nM fluorescein solution (prepared in 75 mM phosphate buffer; pH 7.4) at 37°C for 15 min. Thereafter, 20 μL of 178 mM AAPH solution was added to all the wells and fluorescence intensities (485Ex/520Em nm) were measured every minute up to a period of 90 min. The area under the curve (for fluorescein decay) corresponding to each concentrations of compound G and Trolox were prepared. Linear regression curves for compound G and Trolox were plotted and the ORAC value of compound G was calculated by comparing the slope of the regression curve of the compound to that of Trolox.[11]
Ferric Reducing Antioxidant Power
Total antioxidant activity of compound G was measured by Ferric Reducing Antioxidant Power (FRAP) assay.[12] In this assay, reduction of ferric tripyridyl triazine (Fe III TPTZ) complex to ferrous form (which has an intense blue color) was monitored by measuring the change in absorption at 593 nm and the reducing ability of test compound was compared to that of a known standard to obtain the FRAP value. Briefly, acetate buffer (300 mM; pH 3.6), TPTZ (10 mM in 40 mM HCl) and FeCl3 (20 mM) solutions were mixed in the ratio of 10:1:1 to prepare the FRAP working reagent at the time of use. 5 μl of different concentrations of compound G (3.16–0.3 g/l) or Vitamin C (0.18–0.002 g/l) was mixed with 150 μl of FRAP working reagent in a 96-well plate and the absorbance was measured at 593 nm. Thereafter, the plate was incubated at 37°C for 4 min and absorption was measured once again. The increase in absorbance corresponding to each concentration of compound G and Vitamin C were calculated. Linear regression curves for compound G and Vitamin C were plotted. The FRAP value of compound G was calculated by multiplying the ratio of the slopes of the regression curves with a factor of 2 (FRAP value of Vitamin C).
1,1-diphenyl-2-picrylhydrazyl Radical Scavenging Activity
DPPH radical scavenging activity of compound G was measured using the electron spin resonance (ESR) spectroscopy.[13] For the ESR spectroscopy, 50 μL of DPPH (3 mM in ethanol) solution was vigorously mixed with 50 μL of each dilution (in ethanol) of the test sample. After 1 min, the ESR spectrum was recorded in a JEOL JES-FA 200 ESR spectrophotometer (Measurement conditions: X band; modulation frequency, 100 kHz; magnetic field, 3360 ± 100 G; modulation, 2 G; microwave frequency, 9.44 GHz; microwave power, 5 mW; response, 0.3 s; temperature, 298 K.). For control experiment, 50 μL of DPPH solution was mixed with 50 μL of ethanol. Decrease in the relative intensity of the ESR signal indicated the DPPH radical scavenging activity of the test sample.
Metal Chelating Activity
The chelation of ferrous ions by compound G was estimated by the method of Dinis et al., 1994.[14] Briefly, 10 μl of 2 mM FeCl2 was added to 200 µl of different concentrations of the compound (100, 10 and 1 µg/ml) or quercetin. The reaction was initiated by the addition of 40 µl of 5 mM ferrozine solution. The mixture was vigorously shaken and incubated at room temperature for 10 min. Thereafter, the absorbance of the solution was measured at 562 nm. The percentage inhibition of Fe2+–ferrozine complex formation was calculated as ([A0-As]/As) ×100, where A0 was the absorbance of the control, and As was the absorbance of the sample.
Inhibition of Tumor Necrosis Factor-alpha Converting Enzyme
TNF-α converting enzyme (TACE) is responsible for releasing the 17 kDa extracellular form of TNF-α from the 26 kDa membrane-anchored TNF-α. Compound G was tested in the TACE assay to identify whether the effect was due to its inhibitory effect on TACE. The assay was performed in a black, 96-well plate (clear bottom). In brief, vehicle (positive control), different concentrations of compound G (100, 10 and 1 µg/ml) or standard (TAPI-0; 1, 0.1 and 0.01 µM) were preincubated with 10 ng/well of enzyme (hTACE: R and D Systems), in the presence of assay buffer (Tris 25 mm, ZnCl2 2.5 μM and Brij35 0.005%; pH 9) at 37°C for 30 min. 10 μM fluorogenic peptide substrate III (R and D Systems) was then added to the wells and the plate was incubated at 37°C for 2 h. Wells without any enzyme were considered as normal (negative control). The fluorescence values were measured at EX340/EM405 nm and compared to calculate any inhibition shown by compound G.[15]
Inhibition of Nuclear Factor Kappa B
Inhibitory potential of compound G on LPS induced nuclear factor kappa B (NF-ĸB) transcriptional activity was measured using mouse macrophage cell line RAW 264.7. The macrophage cell line was cotransfected with pGAL4/NF-ĸB in pCDNA4 (contains the yeast GAL4 DNA binding domain fused in frame with NF-ĸB binding ĸB sequence), pFR-Luc-Pur (containing the luciferase reporter vector with a fixed number of GAL4 DNA binding elements upstream of a minimal promoter followed by the luciferase gene) and pLacZ in pCDNA4 (containing a transfection-normalizing vector with the β-galactosidase gene). The transfected cells were incubated overnight in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum in a CO2 incubator at 37°C. On the following day, the cells were carefully removed from culture flask and 2.5 × 105 cells were plated in each well of a 96-well tissue culture plate. Different concentrations of the compound present in compound G (100, 10 and 1 µg/ml) were added to the respective wells followed by the addition of 1 µg/ml of LPS. The plate was then incubated for 16 h in a CO2 incubator at 37°C. Thereafter, lysis buffer (15 mM KPO4 buffer containing 1 mM DTT, 20 µM CoA, 2 mM ATP and 100 µM luciferin; pH 7.8) was added to each well and luciferase activity was measured using a luminescence reader. β-Galactosidase activity was monitored with 2-Nitrophenyl β-D-galactopyranoside at 410 nm using a spectrophotometer. The normalized activity in each well was calculated by dividing the relative luminescence unit (RLU) by the OD at 410 nm. Decrease in the RLU/optical density value in the sample well with respect to that of control indicated the inhibition of NF-ĸB activation by the test sample.[16]
Statistical Analysis
All the reported values represent the average of three independent experiments. Statistical analysis was performed with one-way analysis of variance followed by post hoc Dunnett's test. P <0.05 were considered to be statistically significant.
Results
Effect on Prostaglandin E2 Production
Table 1 illustrates the effect of different fractions and sub-fractions of BRG on the COX-2 mediated PGE2 production in human whole blood stimulated with LPS. Compound G exhibited maximum inhibitory activity (83.3% and 36.3%; 100 µg/ml 10 µg/ml).
Table 1.
Effects of different fractions of Bruguiera gymnorrhiza on cyclooxygenase-2 mediated prostaglandin E2 production, 5-lipoxygenase mediated leukotriene B4 production using human whole blood assay. Tumor necrosis factor-α was estimated from lipopolysachharide (LPS) stimulated human peripheral blood mononuclear cellsa
Effect on 5-Lipoxygenase
The effect of different fractions and sub-fractions of BRG (obtained during the bioactivity guided fractionation) were studied for the inhibition of 5-LOX enzyme activity [Table 1]. Compound G exhibited maximum 5-LOX inhibitory activity. The enzyme activity nearly abolished in the presence of 100 µg/ml of compound G and 39.4% inhibition was observed at 10 µg/ml concentration of this compound.
Effect on Tumor Necrosis Factor-alpha Production
Compound E and compound G significantly inhibited the secretion of pro-inflammatory cytokine (TNF-α) in LPS-stimulated human PBMC [Table 1]. The cytokine levels in the cell culture supernatant of uninduced cells were negligible whereas LPS treated cells produced significantly higher level of cytokines. A dose dependent reduction of cytokine production was observed when the cells were preincubated with compound E or G. Compound G at a concentration of 100 µg/ml produced 86.1% inhibition of TNF-α production whereas 71.8% inhibition was observed at 10 µg/ml. However the inhibitory effect produced by Compound E was lesser (68.6% and 39.5%; 100 µg/ml 10 µg/ml).
From the evaluation of biological activities of different isolated compounds it was evident that compound G displayed maximum biological activity (COX-2, 5-LOX and TNF-α inhibition) towards different inflammatory mediators.
Chemical Characterization of the Bioactive Compound
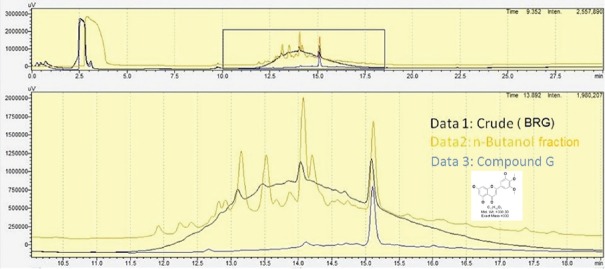
HPLC analysis [Figure 1] presented good separation and resolution of compound G at retention time of 15.12 min. The chemical characterization of the most potent bioactive compound (compound G) revealed it to be of flavonoid nature. The compound produced yellow coloration upon addition of ammonia solution. The yellow colour disappeared after the addition of concentrated H2 SO4. The UV-visible spectra of compound G indicated the presence of two absorbance peaks displaying λmax of at 340 nm and 263 nm for peak A and peak B respectively. According to reports, flavones are known to display maximum absorption at 340 nm.[17] The compound was also characterized by different NMR and LC/MS analysis. The molecular weight of compound G was found to be 330 Da, as confirmed by LC/ESI-MS analysis. The data obtained from1 H NMR (DMSO d6, 400 MHz),13 C APT NMR (DMSO d6, 100 MHz), LC/MS and LC/MS/MS-based analysis[17,18,19,20] indicated the presence of 5,7-Dihydroxy-2- (3-hydroxy-4,5-dimethoxy-phenyl)-chromen-4-one, reported earlier in Poa huecu.[21]
Figure 1.
HPLC overlay chromatogram of Bruguiera gymnorrhiza (BRG), n-butanol fraction of BRG and compound G. The HPLC fingerprint indicated the presence of compound G at retention time of 15.12 min. The analysis was carried out using Shimadzu high-performance liquid chromatography system attached with an ultraviolet detector and prepacked C18 column (4.6 mm × 250 mm, 5 μm; Agilent Technologies). The separation was carried out using a gradient conc. of Solvent A (Water with 0.1% TFA) and Solvent B (Acetonitrile with 0.1% TFA). The total run time for the separation was 30 min (flow rate of 1 ml/min) at λ = 220 nm
Oxygen Radical Absorption Capacity
The compound obtained from Compound G exhibited high ORAC when tested with the fluorescence-based assay. The calculated ORAC value of the compound was found to be 4.
Ferric Reducing Antioxidant Power
The antioxidant potential of compound G was ascertained from FRAP assay (the ability of an antioxidant to reduce TPTZ-Fe [III] complex to TPTZ-Fe [II]). The FRAP value of the compound was found to be 0.13.
1,1-diphenyl-2-picrylhydrazyl Radical Scavenging Activity
Addition of the isolated compound G significantly decreased the ESR signals as evident from Figure 2. The extent of inhibition was found to be around 40% at a concentration of 0.25 mM (82.5 µg/ml) of the compound, whereas, 68% and 59% inhibitions were observed with BRG at 2 and 1 mg/ml concentrations respectively.
Figure 2.
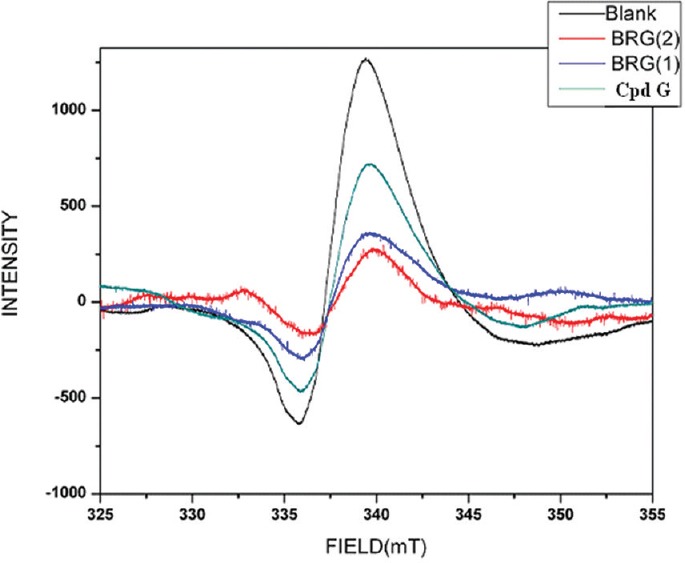
Electron spin resonance (ESR) spectrum o f 1,1- diphenyl-2-picrylhydrazyl radical in presence of Bruguiera gymnorrhiza (2 and 1 mg/ml) and compound G (0.25 mM)
Metal Chelating Activity
The isolated compound (Compound G) exhibited significant (25.82%) metal chelating activity only at the highest tested concentration (100 µg/ml). However, the standard drug quercetin exhibited significant inhibition (64.69% at 12.5 µg/ml; 33.35% at 6.25 µg/ml) in our assay [Figure 3].
Figure 3.
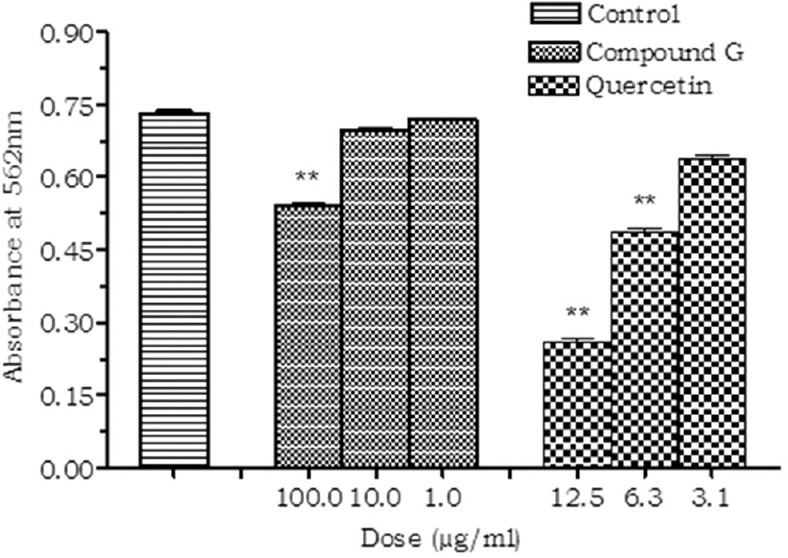
Effect of compound G on metal chelating activity. Values are expressed as mean ± standard deviation.; (n = 3), **P < 0.01 (vs. control)
Inhibition of Tumor Necrosis Factor-alpha Converting Enzyme
The isolated compound (Compound G) did not produce any significant inhibition of TACE enzyme when studied in vitro. However, the standard inhibitor TAPI significantly inhibited the TACE activity in a dose-dependent manner [Figure 4].
Figure 4.
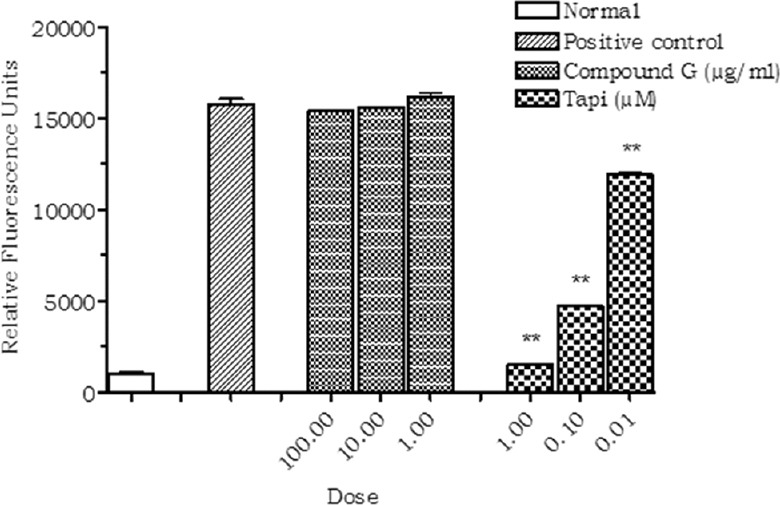
Effect of compound G on tumor necrosis factor-alpha converting enzyme (TACE). Values are expressed as mean ± standard deviation.; (n = 3), **P < 0.01 (vs. control)
Inhibition of Nuclear Factor Kappa B
The compound G exhibited significant inhibition (23.1%) of NF-ĸB activation at a concentration of 100 µg/ml. No significant inhibition was observed at the concentrations of 10 and 1 µg/ml [Figure 5].
Figure 5.
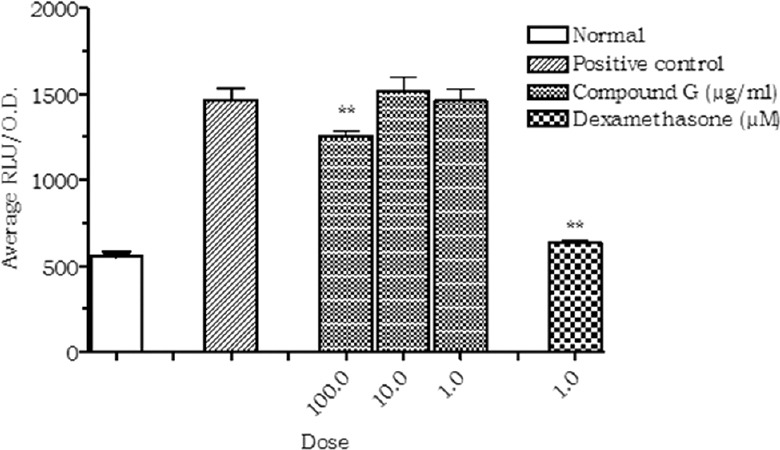
Effect of compound G on NF-κB activation. Values are expressed as mean ± standard deviation.; (n = 3), **P < 0.01 (vs. control)
Discussion
The cellular response to inflammation involves the migration of neutrophils and macrophages to the damaged sites. These cells combat the invading organism by phagocytosis and production of reactive oxygen species (ROS). The superoxide radicals are produced first which in turn lead to the generation of hydrogen peroxide, hypochlorite, and other radicals.[22] Hydrogen peroxide in the presence of suitable transitional elements may be transformed to highly reactive hydroxyl radical per se, which in turn is responsible for the activation of the NF-κB pathway,[23] a key transcription factor that regulates the expression of pro-inflammatory genes encoding cytokines such as TNF-α, interleukin-1 beta (IL-1β), and IL-6. In both acute and chronic inflammatory conditions (particularly associated arthritis, psoriasis), an elevated level of these pro-inflammatory cytokines has been observed. These pro-inflammatory cytokines are known to activate the signaling cascade of inflammation which eventually causes tissue injury. As evident from our earlier studies, the anti-inflammatory activity of BRG was found to be mediated through dual COX2 and 5-LOX enzyme inhibition along with inhibition of TNF-α production.[4] As an alternative to the existing NSAIDS, dual COX-LOX inhibitors have been reported to show better GI and cardiovascular tolerance. Thus, in the present investigation an attempt was made to isolate the bioactive component (s) from this mangrove medicinal plant.
In the present study, bioactivity guided purification was carried out and preparative HPLC was used in the final step of the purification procedure, for isolation of the active constituent, with a yield of 0.01%. Spectroscopic analysis, coupled with LC-MS/MS was utilized for the characterization and structure elucidation.[19] The isolated molecule was found to be a flavone (5,7-Dihydroxy-2- (3-hydroxy-4,5-dimethoxy-phenyl)-chromen-4-one) with a molecular weight of 330 Daltons. Although the presence of this flavone in another plant species (P. huecu) has been reported earlier,[21] its presence in Bruguiera species is being reported for the first time. Moreover, detailed report of the pharmacological and biochemical characteristics of the compound is yet to be ascertained.
The isolated flavone produced significant inhibition of COX-2 mediated PGE2 production and also reduced 5-LOX enzyme activity.
The primary pro-inflammatory cytokines like TNF-α, IL-1β and IL-6 are particularly important for initiating the inflammatory process and production of secondary cytokines and various chemokines.[24] These pro-inflammatory cytokines are known to activate the signaling cascade of inflammation which eventually causes tissue injury. In both acute and chronic inflammatory conditions (particularly associated arthritis, psoriasis), an elevated level of these pro-inflammatory cytokines has been observed. The isolated compound present inhibited the TNF-α production in the LPS stimulated human PBMC. Interestingly, the inhibition of TNF-α production was not mediated through the inhibition of TACE enzyme, which is known to convert membrane bound TNF-α to a more soluble form.
According to available reports, DPPH scavenging activity is a widely employed method to evaluate antioxidant properties of unknown test compounds. In the present study, the isolated compound significantly scavenged the DPPH free radical as evident from the ESR spectrum. The significant ORAC (ORAC; used for evaluating antioxidant capacity against peroxy radicals induced with AAPH) and FRAP (Ferric reducing antioxidant power; a measure of specific oxidant reducing capacity determined by quantifying the conversion of Fe3+ TPTZ to Fe2+ TPTZ) values[25] of the compound is also comparable to that of quercetin and ascorbic acid. Moreover, the isolated compound produced about 25% reduction of Fe2+–ferrozine complex formation. However, in this study, quercetin (1/8th of the flavone concentration) showed almost 3-fold superior inhibitory properties. All the above findings indicate a strong antioxidant property of the isolated flavone.
ROS can initiate and also perpetuate inflammatory cascades and cause subsequent tissue damage through the upregulation of a number of different genes involved in the inflammatory response. One potential mechanism is through activation of NF-κB, a ubiquitous transcription factor involved in regulating several genes involved in immune and inflammatory responses.[26] The cellular response to inflammation involves the migration of neutrophils and macrophages to the damaged sites. These cells combat the invading organism by phagocytosis and production of ROS. As evident from reports, inhibition of NF-κB signaling remains an attractive target for anti-inflammatory drug discovery. Apart from antioxidants like N-acetyl cysteine and CAPE,[27] a number of herbal extracts and dietary plants are also known to inhibit NF-κB activation. Moreover, a number of natural antioxidants displaying anti-cancer and anti-inflammatory activity have also been found to inhibit NF-κB.[28]
In our findings, the isolated compound inhibited the activation of NF-κB by about 23% when applied at a concentration of 100 µg/ml, in the transactivation assay. Considering the nature of this assay, this amount of inhibition can be considered to be significant in modulating the transcription of the inflammatory mediators.[29] Furthermore, it can be postulated that the inhibition of NF-κB, might play an important role towards inhibition of LPS induced TNF-α production in human PBMC, considering the fact that NF-κB is one of the key transcription factors responsible for regulating the expression of this pro-inflamatory cytokines.[26]
Thus, on the basis of the various pharmacological and biochemical experiments conducted with the methanolic fraction of BRG leaf and the isolated compound, it may be suggested that the anti-inflammatory activity may be attributed to the presence of the bioactive flavone. Our observation related to inhibition of both COX and 5-LOX, by the methanolic fraction of BRG leaf and by the bioactive flavone, is the first of its kind to be reported. However, as the extract also contains several other bioactive constituents, the synergistic effect of the other constituents might be possible, and therefore further studies are warranted to explore such possibilities.
Conclusions
The results described in this paper show that the compound 5,7-Dihydroxy-2 -(3-hydroxy-4,5- dimethoxy-phenyl) -chromen-4-one, a flavone, reported for the first time to be present in this plant, may primarily be responsible for the anti-inflammatory activities of BRG leaves. Moreover, our results also suggest that, the observed anti-inflammatory activity is not mediated through a single mechanism, rather it may be mediated through the following (i) modulation of oxidative stress, (ii) inhibition of arachidonic acid metabolism (Dual inhibition of COX-2 and 5-LOX), and (iii) downregulation of pro-inflammatory cytokines probably through NF-κB inhibition.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
Acknowledgments
We thankfully acknowledge the active support of Prof. Sugata Hazra, Director, School of Oceanographic Studies, Jadavpur University. We are also thankful to the University Grants Commission (DSA Phase-III and UPE-II program of UGC, New Delhi) and DST, New Delhi (PURSE program) for their support.
References
- 1.Pal S, Chaudhuri AK. Studies on the effects of Pluchea indica less root extract on gastroduodenal ulcer models in rats and guineapigs. Phytother Res. 1989;3:156–8. [Google Scholar]
- 2.Pal S, Nag Chaudhuri AK. Studies on the anti-ulcer activity of a Bryophyllum pinnatum leaf extract in experimental animals. J Ethnopharmacol. 1991;33:97–102. doi: 10.1016/0378-8741(91)90168-d. [DOI] [PubMed] [Google Scholar]
- 3.Mani Senthil Kumar KT, Gorain B, Roy DK, Zothanpuia, Samanta SK, Pal M, et al. Anti-inflammatory activity of Acanthus ilicifolius. J Ethnopharmacol. 2008;120:7–12. doi: 10.1016/j.jep.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Barik R, Sarkar R, Biswas P, Pattnaik A, Samanta SK, Manisenthilkumar KT, et al. Inhibition of arachidonic acid metabolism and pro-inflammatory cytokine production by Bruguiera gymnorrhiza leaf. Orient Pharm Exp Med. 2013;13:41–9. [Google Scholar]
- 5.Martel-Pelletier J, Lajeunesse D, Reboul P, Pelletier JP. Therapeutic role of dual inhibitors of 5-LOX and COX, selective and non-selective non-steroidal anti-inflammatory drugs. Ann Rheum Dis. 2003;62:501–9. doi: 10.1136/ard.62.6.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harborne JB. Phytochemical Methods. London: Champman and Hall; 1973. A guide to modern techniques of plant analysis; pp. 49–188. [Google Scholar]
- 7.Warner TD, Giuliano F, Vojnovic I, Bukasa A, Mitchell JA, Vane JR. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: A full in vitro analysis. Proc Natl Acad Sci. 1999;96:7563–74. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patrignani P, Panara MR, Greco A, Fusco O, Natoli C, Iacobelli S, et al. Biochemical and pharmacological characterization of the cyclooxygenase activity of human blood prostaglandin endoperoxide synthases. J Pharmacol Exp Ther. 1994;271:1705–12. [PubMed] [Google Scholar]
- 9.Brideau C, Van Staden C, Styhler A, Rodger IW, Chan CC. The effects of phosphodiesterase type 4 inhibitors on tumour necrosis factor-alpha and leukotriene B4 in a novel human whole blood assay. Br J Pharmacol. 1999;126:979–88. doi: 10.1038/sj.bjp.0702387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janský L, Reymanová P, Kopecký J. Dynamics of cytokine production in human peripheral blood mononuclear cells stimulated by LPS or infected by Borrelia. Physiol Res. 2003;52:593–8. [PubMed] [Google Scholar]
- 11.Ou B, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem. 2001;49:4619–26. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- 12.Benzie IF, Strain JJ. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999;299:15–27. doi: 10.1016/s0076-6879(99)99005-5. [DOI] [PubMed] [Google Scholar]
- 13.Nanjo F, Goto K, Seto R, Suzuki M, Sakai M, Hara Y. Scavenging effects of tea catechins and their derivatives on 1,1-diphenyl-2-picrylhydrazyl radical. Free Radic Biol Med. 1996;21:895–902. doi: 10.1016/0891-5849(96)00237-7. [DOI] [PubMed] [Google Scholar]
- 14.Dinis TC, Maderia VM, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–9. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 15.Primakoff P, Myles DG. The ADAM gene family: Surface proteins with adhesion and protease activity. Trends Genet. 2000;16:83–7. doi: 10.1016/s0168-9525(99)01926-5. [DOI] [PubMed] [Google Scholar]
- 16.Berger J, Leibowitz MD, Doebber TW, Elbrecht A, Zhang B, Zhou G, et al. Novel peroxisome proliferator-activated receptor (PPAR) gamma and PPARdelta ligands produce distinct biological effects. J Biol Chem. 1999;274:6718–25. doi: 10.1074/jbc.274.10.6718. [DOI] [PubMed] [Google Scholar]
- 17.Tsimogiannis D, Samiotaki M, Panayotou G, Oreopoulou V. Characterization of flavonoid subgroups and hydroxy substitution by HPLC-MS/MS. Molecules. 2007;12:593–606. doi: 10.3390/12030593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Justesen U. Negative atmospheric pressure chemical ionisation low-energy collision activation mass spectrometry for the characterisation of flavonoids in extracts of fresh herbs. J Chromatogr A. 2000;902:369–79. doi: 10.1016/s0021-9673(00)00861-x. [DOI] [PubMed] [Google Scholar]
- 19.Cheng XL, Wan JY, Li P, Qi LW. Ultrasonic/microwave assisted extraction and diagnostic ion filtering strategy by liquid chromatography-quadrupole time-of-flight mass spectrometry for rapid characterization of flavonoids in Spatholobus suberectus. J Chromatogr A. 2011;1218:5774–86. doi: 10.1016/j.chroma.2011.06.091. [DOI] [PubMed] [Google Scholar]
- 20.Wu W, Liu Z, Song F, Liu S. Structural analysis of selected characteristic flavones by electrospray tandem mass spectrometry. Anal Sci. 2004;20:1103–5. doi: 10.2116/analsci.20.1103. [DOI] [PubMed] [Google Scholar]
- 21.Rofi RD. Pomilio AB.5,7,3′-trihydroxy-4′,5′-dimethoxyflavone and other phenolics from Poa huecu. Phytochemistry. 1985;24:2131–2. [Google Scholar]
- 22.Edwards SW. Biochemistry and Physiology of Neutrophil. Cambridge: Cambridge University Press; 1994. The respiratory burst: The generation of reactive oxygen metabolites and their role in microbial killing; pp. 149–79. [Google Scholar]
- 23.Ye J, Zhang X, Young HA, Mao Y, Shi X. Chromium(VI)-induced nuclear factor-kappa B activation in intact cells via free radical reactions. Carcinogenesis. 1995;16:2401–5. doi: 10.1093/carcin/16.10.2401. [DOI] [PubMed] [Google Scholar]
- 24.Lind L. Circulating markers of inflammation and atherosclerosis. Atherosclerosis. 2003;169:203–14. doi: 10.1016/s0021-9150(03)00012-1. [DOI] [PubMed] [Google Scholar]
- 25.Ou B, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem. 2001;49:4619–26. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- 26.Hayden MS, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132:344–62. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 27.Bowie A, O’Neill LA. Oxidative stress and nuclear factor-kappaB activation: A reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol. 2000;59:13–23. doi: 10.1016/s0006-2952(99)00296-8. [DOI] [PubMed] [Google Scholar]
- 28.Paur I, Balstad TR, Kolberg M, Pedersen MK, Austenaa LM, Jacobs DR, Jr, et al. Extract of oregano, coffee, thyme, clove, and walnuts inhibits NF-kappaB in monocytes and in transgenic reporter mice. Cancer Prev Res (Phila) 2010;3:653–63. doi: 10.1158/1940-6207.CAPR-09-0089. [DOI] [PubMed] [Google Scholar]
- 29.Janssen YM, Sen CK. Nuclear factor κB activity in response to oxidants and antioxidants. Methods Enzymol. 1999;300:363–70. doi: 10.1016/s0076-6879(99)00141-x. [DOI] [PubMed] [Google Scholar]