Abstract
Objective:
Equisetum arvense has been used to treat bone diseases. The traditional supplementation of calcium and Vitamin D for osteoporosis patients is insufficient considering the rise in patients every year. We have observed that extending the calcium and Vitamin D supplement with L-lysine, L-proline, L-arginine, and L-ascorbic acid (N) positively affects bone mineralization in ovariectomized rat. Here, we report a further extension of the above supplement with E. arvense.
Materials and Methods:
The changes in serum biomarkers, bone mineral content, and femur bone histology were studied and compared to the standard drug for osteoporosis, namely raloxifene (RAL).
Results:
We report a significant change in formation and resorption markers of bone as well as in cortical bone thickness and trabecular width in N and N + EA groups. The treatment N + EA also restored lipid profile near to normal level compared to ovariectomized group.
Conclusions:
Treatment N + EA was found to be as effective as RAL in reversing the osteoporotic changes.
KEY WORDS: Bone mineralizing nutrients, Equisetum arvense, osteoporosis prevention
Introduction
Postmenopausal osteoporosis is a heterogeneous disorder characterized by the increased risk of fragility fractures due to deficiency of ovarian hormone, estrogen.[1] The available treatments such as antiresorptive (raloxifene [RAL]) and anabolic (parathyroid hormone) are reported to cause adverse effects and no substantial increase in bone mass. This has led to opting for other approaches such as medicinal herbs.[2] Equisetum arvense is reported to have highest silica content that helps in the absorption and utilization of calcium and synthesis and stabilization of collagen by prolyl hydroxylase enzyme.[3] Recently, E. arvense has been shown to enhance the proliferation of osteoblasts and inhibit osteoclasts in vitro.[4,5] Calcium, Vitamin D, and zinc are the accepted baseline supplements available for osteoporosis, but considering the load of the disease, it looks insufficient. There are few reports on the role of micronutrients in bone related disorders, but very few reports on combination therapy.
The nutrients selected for this study (L-lysine, L-proline, L-arginine, and L-ascorbic acid) other than calcium, Vitamin D, and zinc, have been reported to increase bone mass through collagen synthesis by activating prolyl hydroxylase and stimulating osteoblast development.[6] In addition, studies from our laboratory have shown that supplementation of basic bone mineralizing nutrients (L-lysine, L-proline, L-arginine, and L-ascorbic acid) along with calcium, Vitamin D, and zinc improved bone microarchitecture and serum biomarkers resulting in better bone health in ovariectomized female rats.[7] We, therefore, hypothesized that E. arvense containing silica may further increase the efficacy of the anabolic nutrient mixture for the treatment of osteoporosis.
Materials and Methods
Chemicals
All solvents and chemicals used in the study were of analytical grade and purchased from local suppliers.
Collection and Extraction of Plant Material
The whole plant of E. arvense was collected from Wainganga River at Bhandara District of Maharashtra, India and was identified and authenticated by University Department of Botany, RTM Nagpur University, Nagpur. Twenty grams of dried powder of aerial parts of E. arvense was extracted with 95% ethanol using a Soxhlet apparatus, was vacuum dried and weighed to determine its yield (14.17%).
Animals
After receiving approval from the Institutional Animal Ethics Committee (414/81/AB/CPCSEA), 3-month-old female wistar rats weighing (160–200 g) were purchased from the National Institute of Nutrition, India and housed in a temperature-controlled room under 12-h-light/dark period in polypropylene cages (4 rats/cage). After an adaptive period of 2 weeks, 32 rats were ovariectomized through dorsal bilateral approach, and 8 rats were SHAM operated.[8] The ovariectomized rats were randomly divided into four groups (8 rats/group):
Ovariectomy (OVX) and no supplementation
RAL (OVX and RAL (5.4 mg/kg body weight), a standard drug for osteoporosis)
N (OVX and nutrients such as calcium carbonate (104.16 mg/kg body weight), Vitamin D (100 IU), zinc sulfate (1.56 mg/kg body weight), L-lysine (93.75 mg/kg body weight), L-proline (46.85 mg/kg body weight), L-arginine (200 mg/kg body weight), and L-ascorbic acid (93.75 mg/kg body weight)
N + EA (N + E. arvenseEt extract [60 mg/kg body weight]).[9]
After 15 days of recovery period, SHAM and OVX group rats were fed with normal feed and other groups (RAL, N, and N + EA) with treatment mixed in feed for 3½ months.
Bone Serum Biomarkers
At the end of the treatment period, final weights of the animals were recorded, and blood was withdrawn from orbital plexus into nonheparinized tubes. The animals were then sacrificed by CO2 asphyxiation. The serum parameters under study were: Calcium (OCPC kit), osteocalcin (Rat Gla-osteocalcin, Takara kit), alkaline phosphatase (ALP) (Innoline alkaline phosphatase kit), tartarate resistant acid phosphatase (TRAP),[10] high density lipoprotein (HDL) cholesterol (polyethylene glycol precipitation method), cholesterol (cholesterol oxidase/PAP method), and triglycerides (glycerol-3-phosphate oxidase/PAP method). Low density lipoprotein (LDL) cholesterol was calculated from the lipid profile. The internal organs were grossly examined for any lesion. Femur bones were removed to 10% neutral buffered formalin. Femur bone volume (cm3) was measured by the gravimetric method of water displacement.[11] To measure the bone mineral content, femur bones were dried in an oven (100°C) to constant bone weight and then ashed in a furnace (800°C). The percentage content (%) of calcium, phosphorus, and zinc was measured by inductively coupled plasma atomic emission spectroscopy.[12] The length of bone was measured by Vernier calipers. For histological analysis, the femur bone from each group was decalcified in a solution containing 8% HCl and 8% Formic acid. It was then sectioned (5 µm) and stained with Ehrlich's hematoxylin and eosin.
Statistical Analysis
Statistical analysis was done by AnalyseIt software (version 2.26), by Microsoft Partner, Silver Independent Software Vendor (ISV) for Windows Excel. The values given are mean ± standard error.
Results
Effect of treatments on serum biomarkers, bone mineral content, and femur bone histology were studied [Table 1]. OVX group recorded a significant increase in percentage weight gain, femur bone length, ALP, TRAP, cholesterol, triglycerides, and LDL and a significant decrease in serum calcium, osteocalcin, HDL, and trabecular width when compared to SHAM group.
Table 1.
Effect of various treatments on percentage weight gain, femur bone physical parameters, bone mineral content, serum parameters, lipid profile, and histological analyses
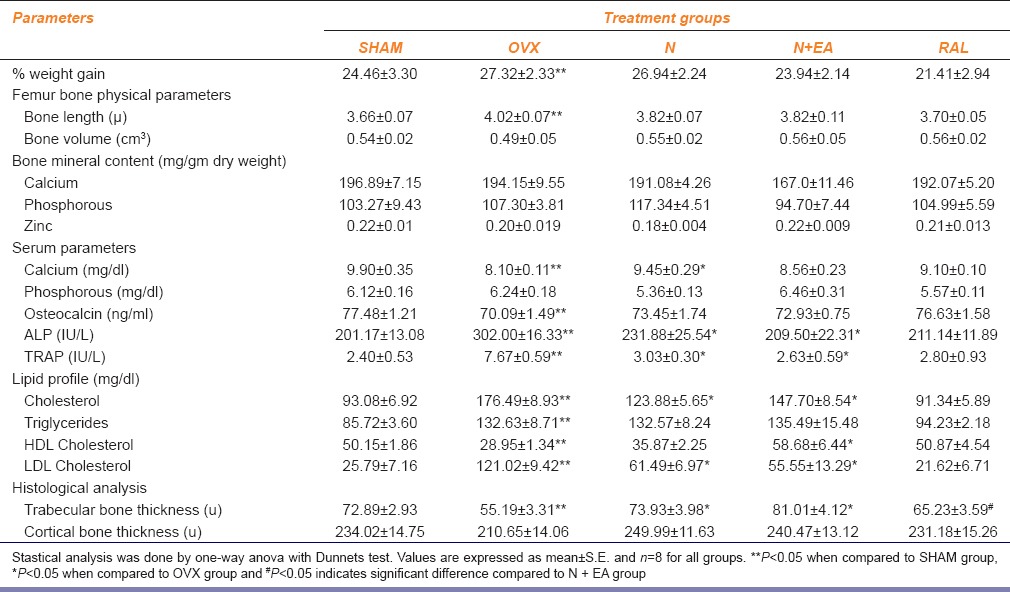
No significant change in percentage weight gain and femur bone volume was observed in all the groups. The weight of organs did not record significant differences in all treatment groups except N + EA group which recorded significantly low-liver weight compared to OVX group (data not given). Histological examination of the liver showed no abnormality.
N group also showed a significant increase in serum calcium, but N + EA recorded low serum level of calcium when compared to OVX group. Osteocalcin level after treatment with N and N + EA was found to be nearly as equal to as SHAM. Treatment with N and N + EA restored serum ALP and TRAP level when compared to OVX group. Furthermore, addition of E. arvense to N resulted in significant difference in cholesterol, HDL, and LDL and marginal or no change in triglycerides. A significant increase in trabecular width (P < 0.05) was observed in N and N + EA groups as compared to OVX group. No significant change in cortical bone thickness was recorded in any groups. Sparse, disrupted, enlarged space, and diminished area of trabecular bone were found to be restored by treatments with N and N + EA as seen in histological examination [Figure 1].
Figure 1.
Effect of various treatments on trabecular region and cortical region in rats of different groups
Discussion
This study was conducted to study the effect of E. arvense addition to the formulated mixture (N) on ovariectomized rats. OVX in rats leads to weight gain[13] and increased bone length as a consequence of decreased chondroclast differentiation.[14] OVX elicits increased bone turnover with increase in ALP and TRAP activity.[15] Serum calcium level decreases due to increased calcium excretion and decreased absorption.[16] Absence of estrogen during the menopausal period results in variations in lipid profile and predominant abdominal fat accumulation. This marks the onset of cardiovascular disease.[17] Our results also are in agreement with the reported changes indicating successful induction of osteoporosis. Histological examination of femur bone showed sparse and thinned trabecular network and enlarged marrow cavity in the cortical region in OVX group as compared to normal architecture observed in SHAM group.
N showed anabolic effect on OVX induced changes in bone metabolism as reported by a previous study from our laboratory. Significantly increased serum calcium, trabecular bone thickness and significantly decreased ALP and TRAP activity, cholesterol, and LDL were recorded in N group. Treatment with N + EA inhibited the percentage weight gain as recorded in OVX group and brought down it to SHAM level. Silica is reported to enhance the bone mineralization by decreasing excretion of calcium, but addition of E. arvense could not bring about a significant change in calcium level. This might be due to the addition of arginine in the formulation of N as arginine and silica are reported to affect the mineral element composition of rat femur and vertebra,[18] and same was observed in N + EA group probably due to the masking effect of the arginine on the action of the herb. Normal levels of ALP and TRAP enzymes in N + EA group indicate that the herb accelerated the mineralization of the organic matrix, thus increasing bone formation. Increase in cortical bone thickness and trabecular width in N + EA group and as observed in histological examination of N + EA group, it clearly indicates the bone mineralizing activity of the herb.
In comparison to the other parameters, N + EA did not show increased efficacy in restoring lipid profile when compared to RAL. RAL, being the standard drug for the treatment of postmenopausal osteoporosis is reported to show beneficial cardiovascular effects by lowering cholesterol level.[19] Although no significant difference was observed in other parameters, result of histological analysis and significant increase in trabecular bone thickness shows that the treatment N + EA has better efficacy than RAL. When compared to RAL group, treatment with N + EA reversed the OVX induced changes and normalized the changes to SHAM level. In this study, the treatments were started immediately after OVX so, a significant change in the bone mineral content was not observed. Increase in the induction period might have resulted in significant change in the mineral content.
Conclusion
It is proved that calcium, Vitamin D, zinc sulfate L-lysine, L-proline, L-arginine, and L-ascorbic acid (N) accelerate the mineralization of bone matrix and bone formation. The addition of ethanolic extract of E. arvense to the mixture N was beneficial for bone formation. Thus, it can be concluded that formulation of E. arvense and bone mineralizing nutrients helps in the prevention of osteoporosis and that N + EA was more effective in preventing osteoporotic bone loss when compared to RAL, the standard drug available for the treatment of osteoporosis. Further studies will be needed to assess the role of all the nutrients except arginine and E. arvense extract to determine the bone protective effects of the formulation.
Financial Support and Sponsorship
This research was supported by The Council of Scientific and Industrial Research, Human Resource Development Group, New Delhi (Grant No. 20–12/2009 (ii) EU-IV).
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Shirwaikar A, Khan S, Kamariya HY, Patel DB, Gajera PF. Medicinal plants for the management of post menopausal osteoporosis: A review. Open Bone J. 2010;2:1–13. [Google Scholar]
- 2.McBane S. Osteoporosis: A review of current recommendations and emerging treatment options. [Last accessed on 2014 Feb 20];Formulary. 2011 46:432–46. Available from: http//www.formularyjournal.modernmedicine.com . [Google Scholar]
- 3.Badole S, Kotwal S. Equisetum arvense: Ethnopharmacological and phytochemical review with reference to osteoporosis. Int J Pharm Sci Health Care. 2014;1:131–41. [Google Scholar]
- 4.Costa-Rodrigues J, Carmo SC, Silva JC, Fernandes MH. Inhibition of human in vitro osteoclastogenesis by Equisetum arvense. Cell Prolif. 2012;45:566–76. doi: 10.1111/j.1365-2184.2012.00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bessa Pereira C, Gomes PS, Costa-Rodrigues J, Almeida Palmas R, Vieira L, Ferraz MP, et al. Equisetum arvense hydromethanolic extracts in bone tissue regeneration:In vitro osteoblastic modulation and antibacterial activity. Cell Prolif. 2012;45:386–96. doi: 10.1111/j.1365-2184.2012.00826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponrasu T, Jamuna S, Mathew A, Madhukumar KN, Ganeshkumar M, Iyappan K, et al. Efficacy of L-proline administration on the early responses during cutaneous wound healing in rats. Amino Acids. 2013;45:179–89. doi: 10.1007/s00726-013-1486-0. [DOI] [PubMed] [Google Scholar]
- 7.Siriah S, Mishra S, Kotwal S. Interrelation of calcium with other bone mineralizing nutrients for preventing bone loss in ovariectomized wistar rats: A prospective study. Eur J Biomed Pharm Sci. 2015;2:469–80. [Google Scholar]
- 8.Park SB, Lee YJ, Chung CK. Bone mineral density changes after ovariectomy in rats as an osteopenic model: Stepwise description of double dorso-lateral approach. J Korean Neurosurg Soc. 2010;48:309–12. doi: 10.3340/jkns.2010.48.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badole S, Kotwal S. Biochemical, hematological and histological changes in response to graded dose of extract of Equisetum arvense in adult female wistar rats. Int J Pharm Sci Res. 2015;6:1000–6. [Google Scholar]
- 10.Lau KH, Onishi T, Wergedal JE, Singer FR, Baylink DJ. Characterization and assay of tartrate-resistant acid phosphatase activity in serum: Potential use to assess bone resorption. Clin Chem. 1987;33:458–62. [PubMed] [Google Scholar]
- 11.Zhang Y, Yu L, Ao M, Jin W. Effect of ethanol extract of Lepidium meyenii Walp. on osteoporosis in ovariectomized rat. J Ethnopharmacol. 2006;105:274–9. doi: 10.1016/j.jep.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Kern J, Mathiason L. The determination of copper, zinc and lead in human teeth using inductively coupled plasma atomic emission spectrometry (ICP-AES) Concordia Coll J Anal chem. 2012;3:33–9. [Google Scholar]
- 13.Kalu DN, Liu CC, Salerno E, Hollis B, Echon R, Ray M. Skeletal response of ovariectomized rats to low and high doses of 17 beta-estradiol. Bone Miner. 1991;14:175–87. doi: 10.1016/0169-6009(91)90021-q. [DOI] [PubMed] [Google Scholar]
- 14.Orwoll E. S, Bliziotes M. Osteoporosis: Pathophysiology and clinical management book, Chapter 15. The basic biology of estrogen and bone. Springer Science and Business Media Publishers. 2002. [Last accessed on 2012 Dec 15]. Available from https://www.books.google.co.in/books?isbn=1592592783 .
- 15.Rahnama M, Świątkowski W. Effect of ovariectomy on biochemical markers of bone turnover (ALP, ACP) and calcium content in rat mandible and teeth. Bull Vet Inst Pulawy. 2002;46:281–7. [Google Scholar]
- 16.Riggs B.L, Khosla S, Melton L.J., III . Osteoporosis book, Estrogen, Bone Homeostasis, and Osteoporosis. 3rd edi. Chapter 40. Burlington: Elsevier Academic Press Publishers; 2008. [Google Scholar]
- 17.Lizcano F, Guzmán G. Estrogen deficiency and the origin of obesity during menopause. Biomed Res Int 2014. 2014 doi: 10.1155/2014/757461. 757461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seaborn CD, Nielsen FH. Dietary silicon and arginine affect mineral element composition of rat femur and vertebra. Biol Trace Elem Res. 2002;89:239–50. doi: 10.1385/bter:89:3:239. [DOI] [PubMed] [Google Scholar]
- 19.Black LJ, Sato M, Rowley ER, Magee DE, Bekele A, Williams DC, et al. Raloxifene (LY139481 HCI) prevents bone loss and reduces serum cholesterol without causing uterine hypertrophy in ovariectomized rats. J Clin Invest. 1994;93:63–9. doi: 10.1172/JCI116985. [DOI] [PMC free article] [PubMed] [Google Scholar]



