Abstract
Finasteride and dutasteride are commonly used 5-alpha reductase inhibitors. While finasteride is a selective inhibitor of 5-alpha reductase Type II, dutasteride inhibits 5- alpha reductase Type I and II. The United States Food and Drug Administration approved the use of finasteride for benign prostatic hypertrophy (BPH) as well as androgenic alopecia (AGA) while dutasteride is approved only for BPH. Off-label use of dutasteride is not uncommon in AGA as well. Although the postfinasteride syndrome (PFS) is a well-established entity, its symptomatology is quite variable. Here, we describe a case of an atypical PFS in a patient treated with dutasteride and finasteride for AGA. The multisystem involvement and irreversible nature of this case warrant its reporting.
KEY WORDS: 5-alpha reductase inhibitors, dutasteride, finasteride, postfinasteride syndrome
Introduction
Postfinasteride syndrome (PFS) is a symptom complex which occurs in patients taking finasteride. This symptom complex can be physical, sexual, as well as neurological.[1] The United States Food and Drug Administration approved the use of finasteride for benign prostatic hypertrophy (BPH) as well as androgenic alopecia (AGA) while dutasteride is approved only for BPH.[2] The exact prevalence of PFS is not documented so far. We describe a case of PFS with an atypical presentation in a patient treated with finasteride for AGA. The present case illustrates the atypical as well as so far irreversible nature of PFS in the patient as well as challenges in its management.
Case Report
In January 2010, a 33-year-old adult male presented with a history of hair loss. He was diagnosed to be a case of AGA and was prescribed dutasteride 0.5 mg once a day for a month. During the course of treatment, he complained of generalized itching, burning micturition, abdominal discomfort, and seborrhea. The total serum testosterone level was around 600 ng/dL. He stopped the treatment but the symptoms persisted. These symptoms subsided after the patient resorted to regular exercise. The total serum testosterone level was 495.14 ng/dL in September 2012. In October 2014, the patient returned for the treatment of hair loss. He was prescribed finasteride 1 mg (instead of dutasteride 0.5 mg) once a day. Similar adverse effects such as itching, burning micturition, abdominal discomfort, skin rash, and seborrhea were observed again [Figure 1]. Only this time, the symptoms persisted and did not subside even with exercise. The symptoms are so far irreversible in nature.
Figure 1.
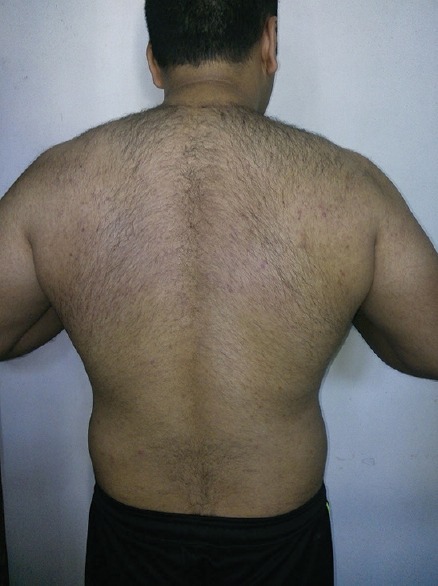
Keratotic follicular papules on shoulders and back along with few pustules
Since there was no resolution of symptoms, a series of tests were ordered. Of which hemogram (Hb 12.8 g/dL, packed cell volume 42.10%, red blood cell count 6.79 mill/mm3, mean corpuscular volume 62 fL, mean corpuscular hemoglobin 18.8 pg, mean corpuscular hemoglobin concentration 30.30, red cell distribution width 15.7%, total lymphocyte count 6800/mm3, and platelet count 261,000), renal function tests (serum urea 21.60 mg/dL; serum creatinine 1.00 mg/dL) and urine examination were found to be normal. However, the total serum testosterone level had dropped down to 227.06 ng/dL in February 2016. Moreover, semen analysis was found to have pus cells while semen culture showed the moderate growth of Enterococcus faecalis.
The patient was managed at three different centers. The first center prescribed desloratadine 5 mg twice a day, combination of clindamycin 1% and benzoyl peroxide 5%, and povidone iodine (antidandruff) shampoo with restricted use of hair oil. The second center prescribed ketoconazole shampoo and adapalene gel. The third center prescribed oxiconazole cream, loratadine, combination of benzoyl peroxide 2.5% and clindamycin 1%, alprazolam 0.5 mg, and dexchlorpheniramine along with antiseborrheic soap.
However, the symptoms persisted even after a complete course of the treatment.
Discussion
Clinical adverse experiences that are reported as possibly, probably, or definitely drug-related in ≥1% of patients treated with finasteride are decreased libido, erectile dysfunction, ejaculation disorder (decreased volume of ejaculate), etc. Postmarketing surveillance has reported finasteride to be associated with breast tenderness and enlargement and even male breast cancer; depression; hypersensitivity reactions including rash, pruritus, urticaria, and swelling of the lips; and testicular pain.[3] This symptom-complex together constitute PFS. Here, in the present case, there were some symptoms that did not fit into the aforementioned list. These symptoms include burning micturition, seborrhea, as well as abdominal discomfort. Moreover, these symptoms were so far irreversible. It prompted us to label this patient as a case of “Atypical PFS.”
We could establish a “certain” causal relationship between finasteride and the atypical symptom complex according to the WHO-UMC causality assessment system. Further assessment using the Naranjo's et al. adverse drug reaction probability scale showed a “probable relation” between finasteride and the atypical symptom complex.[4] Both of these inferences were carried out assuming that rechallenge test was positive as finasteride and dutasteride belong to the 5- alpha-reductase group. The severity of the reaction as determined according to Hartwig et al.[5] revealed the adverse drug reaction to be severe (Level 6) as so far the adverse reaction seems to have caused permanent harm to the patient.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Post-Finasteride Syndrome: Overview. Post-Finasteride Syndrome Foundation. Somerset, New Jersey: [Last cited on 2016 Jan 18]. Available from: http://www.pfsfoundation.org/post-finasteride-syndrome-overview/ [Google Scholar]
- 2.Synder PJ. Androgens. In: Brunton LL, Chabner BA, Knollmann BC, editors. Goodman and Gilman's the Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw-Hill Publication; 2011. pp. 1195–207. [Google Scholar]
- 3.McConnell JD, Bruskewitz R, Walsh P, Andriole G, Lieber M, Holtgrewe HL, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N Engl J Med. 1998;338:557–63. doi: 10.1056/NEJM199802263380901. [DOI] [PubMed] [Google Scholar]
- 4.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 5.Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49:2229–32. [PubMed] [Google Scholar]


