Abstract
Growth factors, cell-surface receptors, adhesion molecules, and extracellular matrix proteins play critical roles in vascular pathophysiology by affecting growth, migration, differentiation, and survival of vascular cells. In a search for secreted and cell-surface molecules expressed in the cardiovascular system, by using a retrovirus-mediated signal sequence trap method, we isolated a cell-surface protein named vasorin. Vasorin is a typical type I membrane protein, containing tandem arrays of a characteristic leucine-rich repeat motif, an epidermal growth factor-like motif, and a fibronectin type III-like motif at the extracellular domain. Expression analyses demonstrated that vasorin is predominantly expressed in vascular smooth muscle cells, and that its expression is developmentally regulated. To clarify biological functions of vasorin, we searched for its binding partners and found that vasorin directly binds to transforming growth factor (TGF)-β and attenuates TGF-β signaling in vitro. Vasorin expression was down-regulated during vessel repair after arterial injury, and reversal of vasorin down-regulation, by using adenovirus-mediated in vivo gene transfer, significantly diminished injury-induced vascular lesion formation, at least in part, by inhibiting TGF-β signaling in vivo. These results suggest that down-regulation of vasorin expression contributes to neointimal formation after vascular injury and that vasorin modulates cellular responses to pathological stimuli in the vessel wall. Thus, vasorin is a potential therapeutic target for vascular fibroproliferative disorders.
Vascular smooth muscle cells (VSMCs), the major cell type in the vessel wall, show a spectrum of phenotypes, depending on environmental cues. Various injurious stimuli provoke the proliferation of differentiated medial VSMCs, which migrate to the intima and produce extracellular matrix proteins, resulting in the narrowing of the vascular lumen. These processes, called VSMC phenotypic modulation, play a key role in development of atherosclerotic diseases, such as postangioplasty restenosis, vein graft disease, and transplant vasculopathy. Whereas tremendous progress has been made in identifying growth factors and transcription factors that regulate the vascular response to injury, much information is lacking regarding cell-surface molecules that are involved in the pathogenesis of vascular fibroproliferative disorders. The signal sequence trap (SST) is a strategy to identify cDNAs containing signal sequence that encode secreted and type I membrane proteins (1, 2). We recently developed a refined SST system based on retrovirus-mediated expression screening (SST-REX) (3). In a search for secreted and cell-surface molecules expressed in the cardiovascular system, by using SST-REX, we identified a TGF-β binding protein, vasorin. Vasorin is predominantly expressed in VSMCs and modulates the vascular response to injury, at least in part, by attenuating TGF-β signaling in vivo. Here, we describe the molecular and functional characteristics of vasorin.
Methods
Cells and Reagents. A murine pro-B cell line Ba/F3 was maintained in RPMI medium 1640 containing 10% FCS and 2 ng/ml murine IL-3 (R & D Systems). Chinese hamster ovary (CHO) cells were grown in DMEM supplemented with 5% FCS and 1% nonessential amino acids (Invitrogen). Stable transfectants were established by the retrovirus expression system, by using a bicistronic retroviral vector pMX-IRES-EGFP as reported (4). Rat aortic VSMCs, prepared from 8-week-old Wistar rats by using the explant method (5), were grown in DMEM supplemented with 10% FCS. Primary antibodies used in this study were anti-Flag monoclonal antibody (M2, Sigma), anti-Smad2/3 monoclonal antibody (BD Transduction Laboratories), anti-phospho-Smad2 polyclonal antibody (Upstate, Charlottesville, VA), and anti-rat CD45 monoclonal antibody (BD Pharmingen).
Screening of a Human Heart cDNA Library by SST-REX and Cloning of the Full-Length cDNA Encoding Vasorin. A human heart cDNA library was screened by SST-REX as described (3). Briefly, cDNA was synthesized from poly(A)+ RNA of human hearts (Clontech), by using the SuperScript Choice system (Invitrogen). The synthesized cDNA was separated based on size, and fractions >600 bp were inserted into BstXI sites of the pMX-SST vector, by using BstXI adapters (Invitrogen). Ba/F3 cells were infected with high-titer retroviruses expressing the human heart cDNA library, and the integrated cDNA fragments were isolated from factor-independent Ba/F3 clones by genomic PCR. All cDNA fragments were sequenced and analyzed. Subsequently, a human heart cDNA library in the pME18S vector was screened by using the 32P-labeled cDNA fragment of a clone so as to isolate the entire coding region.
RNA, Protein, and Histological Analyses. Northern blot, in situ hybridization, semiquantitative RT-PCR, immunoprecipitation, Western blot, and histological analyses were done as described in Supporting Text, which is published as supporting information on the PNAS web site.
Production of the Recombinant Vasorin-Fc Chimera Protein. The bicistronic retroviral vector containing the vasorin-Fc chimera, pMX-vasorin-Fc chimera-IRES-EGFP, was constructed by inserting the cDNA for the whole-extracellular domain of human vasorin and the cDNA for the human Ig Fc region. CHO cells stably expressing the vasorin-Fc chimera were expanded in the medium supplemented with 2.5% Ultralow IgG FCS (Invitrogen) and 1% nonessential amino acids (Invitrogen). Secreted recombinant vasorin-Fc chimera protein was purified from the media of infected CHO cells by using Hitrap protein A columns (Amersham Biosciences).
Surface Plasmon Resonance Analysis. The BIAcoreTM2000 system (BIAcore, Uppsala) was used to characterize the interaction and to determine binding characteristics between the recombinant vasorin-Fc and TGF-β1, according to the manufacturer's instructions. To immobilize TGF-β1 on CM5 sensor chips, recombinant human TGF-β1 (R & D Systems) solution in 10 mM acetic acid (pH 4.0) was injected until the desired amount of coupled TGF-β1 was achieved, by using the standard amine-coupling procedure. All experiments were performed at room temperature by using the KINJECT command at a flow rate of 20 μl/min. Responses obtained on control chips were subtracted from those obtained on chips coupled with TGF-β1. Sensorgrams were analyzed by using biaevaluation software (version 3.0).
Transient Transfection and Reporter Assay. CHO stable transfectants were transiently transfected with TGF-β1-responsive luciferase reporter plasmid (p3TP-lux) together with a β-galactosidase reporter plasmid driven by Rous sarcoma virus-LTR as an internal control, by using FuGENE6 (Roche Diagnostics, Roskilde, Denmark). After 24 h, the cells were stimulated for 24 h by adding TGF-β1 (R & D Systems) and were then assayed for luciferase and β-galactosidase activities. Experiments were performed several times and representative data are shown.
Rat Arterial Balloon-Injury Model. Adult rats (weighing 400–450 g) were anesthetized with chloral hydrate (370 mg/kg i.p.). Balloon denudation of the left common carotid artery was done by using a 2F Fogarty catheter (Baxter Edwards Healthcare, Irvine, CA), as described (6). The right common carotid artery served as a control. Rats were killed 3 or 14 days after injury, and the carotid arteries were perfused with 4% paraformaldehyde/PBS. Each injured left carotid artery was excised from the proximal edge of the omohyoid muscle to the carotid bifurcation. The middle third of the segment was isolated for subsequent analyses.
Generation of Recombinant Adenovirus (Ad) Expressing Vasorin. Replication-incompetent Ads expressing vasorin-Flag (Ad-vasorin) were prepared by using the Adeno-X system (Clontech) according to the manufacturer's instructions. Viral titer was measured by end-point dilution assay by using 293 cells.
Statistical Analysis. Quantitative values are expressed as the mean ± SD. Comparisons of means were made by using Student's t test for unpaired values; when >2 means were compared, an ANOVA with repeated measurements was used. If a significant F value was found, the Scheffé post hoc test for multiple comparisons was used to identify any differences among groups.
Results
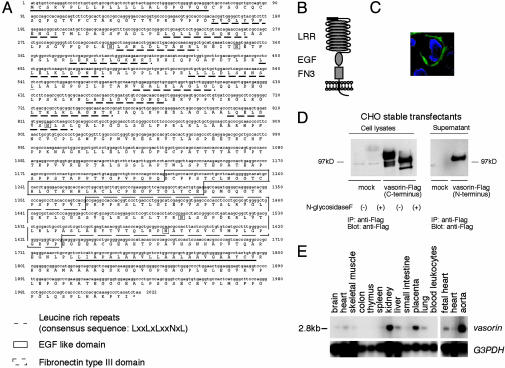
Cloning of Vasorin. We screened a human heart cDNA library, by using SST-REX (ref. 3 and Table 1, which is published as supporting information on the PNAS web site), and identified a type I membrane protein of 673 aa (Fig. 1A). The extracellular region was composed of a putative hydrophobic signal sequence, 10 tandem arrays of a leucine-rich repeat (LRR), an epidermal growth factor (EGF)-like domain, and a fibronectin type III-like domain, and the short intracellular region contained no obvious signaling domain (Fig. 1 A and B). We termed this protein vasorin, based on the expression pattern described below. By using human vasorin as a probe, we identified homologous mouse and rat protein sequences in the EMBL/GenBank/DDBJ database (accession nos. AK012169 and XM 220168, respectively).
Fig. 1.
Vasorin, an identified cell-surface protein. (A) Deduced amino acid sequence of human vasorin. The putative signal peptide (underlined), the LRRs (dotted underlines), the EGF motif (boxed), the fibronectin type III motif (dotted boxes), the transmembrane sequence (underlined), and five putative N-glycosylation sites (boxed) are indicated. (B) Structural model of vasorin. (C) Immunofluorescence analysis of subcellular localization of vasorin. Vasorin was expressed on the cell surface. (D) Cell lysates and supernatants of CHO cells stably expressing vasorin-Flag were subjected to immunoprecipitation and Western blot analysis by using an anti-Flag antibody. An ≈110-kDa protein for membrane-bound vasorin with C-terminal tag and a ≈100-kDa protein for soluble vasorin with N-terminal tag were detected under reducing conditions. N-glycosidase F treatment revealed that vasorin is N-glycosylated. (E) Northern blot analysis of adult human tissues. A single intense 2.8-kb band was detected and the strongest expression was observed in the aorta.
To observe the subcellular localization of vasorin, we stained CHO cells expressing human vasorin-Flag with an anti-Flag antibody (Fig. 1C), and confirmed this molecule to be expressed on the cell-surface membrane. Western blotting of cell lysates from CHO stable transfectants revealed a ≈110-kDa protein (Fig. 1D), which was larger than the predicted molecular mass. To determine whether this increase in molecular mass was due in part to N-linked glycosylation, immunoprecipitates of vasorin were treated with N-glycosydase F. An apparent shift in molecular mass of vasorin was observed, suggesting that vasorin is a cell-surface glycoprotein (Fig. 1D). Next, we examined the supernatant from CHO cells expressing human vasorin-Flag, and soluble vasorin was also detected (Fig. 1D).
Vasorin Is Predominantly Expressed in VSMCs. Tissue distribution of vasorin was examined by using Northern blot analysis of adult human tissues. The highest expression was detected in the aorta, and moderate expression was detected in the kidney and placenta (Fig. 1E). We also performed Northern analysis of various human cell lines. Interestingly, vasorin was not expressed abundantly in any cell lines we examined (Fig. 6, which is published as supporting information on the PNAS web site).
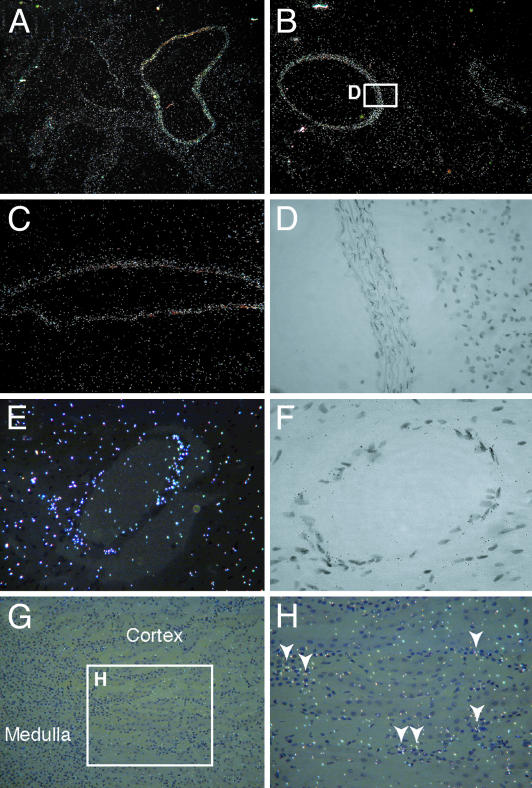
To determine the expression pattern of vasorin within the aorta, we performed in situ hybridization analyses. By using the antisense probe, strong expression of vasorin was detected in the tunica media of the proximal ascending aorta (Fig. 2A), the descending thoracic aorta (Fig. 2B), the abdominal aorta (Fig. 2C), and the coronary arteries (Fig. 2 E and F), suggesting that vasorin is expressed in VSMCs of different origins. We also performed in situ hybridization analyses in the kidney. Vasorin expression was detected in interstitial cells (Fig. 2 G and H).
Fig. 2.
In situ hybridization analysis of vasorin. Sections of adult mouse aorta at different levels (A–D), the coronary artery (E and F), and the kidney (G and H) are shown. Vasorin is expressed in VSMCs of different origins. White spots represent hybridization signals. (A) The proximal ascending aorta. (B) The descending thoracic aorta. (C) The abdominal aorta. (D) Partial magnification of bright-field image of B. Black spots within the elastic fibers represent hybridization signals. (E) The coronary artery. (F) A bright-field image of the coronary artery. Black spots represent hybridization signals. (G) The kidney. (H) Partial magnification of G. Vasorin is expressed in interstitial cells.
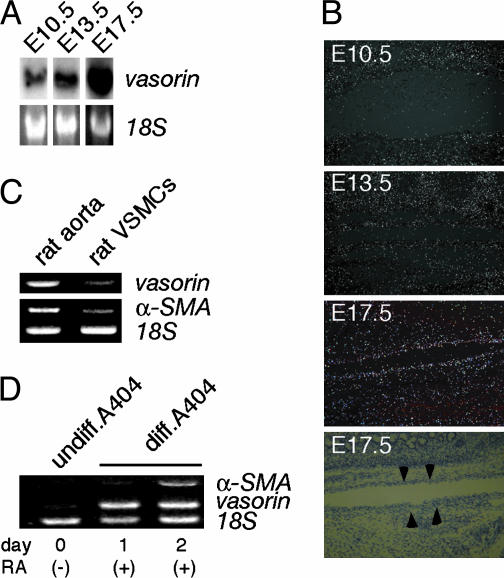
Developmental Regulation of Vasorin Expression. The developmental regulation of vasorin was investigated by using Northern blot and in situ hybridization analyses of staged mouse embryos. As shown in Fig. 3A, vasorin mRNA expression was detected in embryonic day (E)10.5 embryos, with increasing levels of expression observed at subsequent stages (E13.5 and E17.5). With in situ hybridization analyses, we examined the expression pattern of vasorin during aortic development (Fig. 3B). The expression of vasorin increased gradually in parallel with the differentiation of VSMCs in aortas at different stages of development (E11.5, E13.5, and E17.5).
Fig. 3.
Developmental regulation of vasorin. (A) Northern blot analysis of staged mouse embryos (E10.5, E13.5, and E17.5). (B) Expression pattern of vasorin during aortic development examined by in situ hybridization analyses. The fourth image is the corresponding bright-field image of the third representation. Arrowheads indicate the aorta in the mouse embryo (E17.5). (C) Semiquantitative RT-PCR comparing the expression of vasorin in the adult rat aorta with that in cultured rat aortic VSMCs. Rat α-smooth muscle actin (α-SMA) and 18S rRNA were used as a positive and an internal control, respectively. (D) Semiquantitative RT-PCR showing the induction of the vasorin gene in RA-treated A404 cells. Rat α-SMA and 18S rRNA were used as a positive and an internal control, respectively.
When VSMCs are established in culture, a rapid transition from a contractile differentiated phenotype to a synthetic dedifferentiated phenotype occurs (5). To investigate the influence of this phenotypic modulation on the expression of vasorin, semiquantitative RT-PCR was performed to compare the expression of vasorin in the adult rat aorta with that in cultured rat aortic VSMCs. The expression of vasorin was significantly down-regulated in the cultured VSMCs (Fig. 3C).
P19 embryonal carcinoma cells differentiate into SMCs when given retinoic acid (RA) treatment. Recently, this in vitro differentiation system was improved by generating stable cell lines of P19 carrying a smooth muscle α-actin promoter/puromycin resistance gene cassette to enrich SMC lineage cells, by using RA plus puromycin selection. One such stable line, designated as A404, shows a high propensity for SMC differentiation even before puromycin selection (7). As expected, vasorin gene expression was induced by RA-treatment in A404 cells (Fig. 3D).
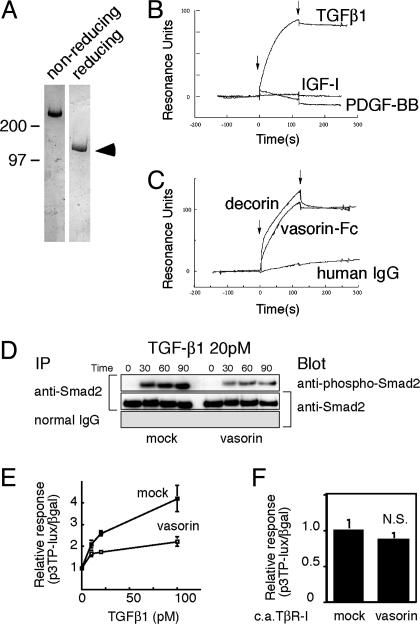
Vasorin Directly Binds to TGF-β and Modulates TGF-β Signaling in Vitro. An LRR, an EGF-like domain, and a fibronectin type III-like domain are characteristic motifs involved in protein–protein interactions (8). To clarify biological functions of vasorin, we generated recombinant vasorin-Fc fusion protein (Fig. 4A), and searched for binding partners of vasorin by using vasorin-Fc as a probe. When comparing the extracellular domain of vasorin with the EMBL/GenBank/DDBJ database, several other LRR protein family members, including decorin, were found to share a significant homology with vasorin. Decorin is a small leucine-rich proteoglycan that interacts directly with TGF-β (9, 10). Considering that vasorin has the same number of LRRs as decorin, and that TGF-β plays an important role in vascular pathophysiology, we tested whether TGF-β binds directly to vasorin. By using a surface plasmon resonance biosensor, we found that the extracellular domain of vasorin directly binds to TGF-β1 in a specific and significant manner (Fig. 4 B and C). The equilibrium dissociation constant (Kd) was calculated to be 0.86 nM. We also tested the binding of TGF-β2 and TGF-β3 to vasorin. TGF-β2 and TGF-β3 showed a specific binding to vasorin with a similar binding affinity to that of TGF-β1 (data not shown).
Fig. 4.
Vasorin directly binds to TGF-β and modulates TGF-β signaling in vitro. (A) Purified recombinant vasorin-Fc fusion protein was free of protein contamination, as estimated by SDS/PAGE, followed by Coomassie blue staining. (B) Sensorgrams obtained from injection of vasorin-Fc on immobilized TGF-β1, PDGF-BB, and insulin-like growth factor I (IGF-I) are shown. (C) Sensorgrams obtained from injection of vasorin-Fc, decorin, and human IgG on immobilized TGF-β1 are shown. Arrowheads indicate initiation and termination of injections. (D) TGF-β-induced Smad2 phosphorylation was significantly inhibited in vasorin-expressing cells. Stable transfectants were treated with TGF-β1 (20 pM), and then immunoprecipitated with an anti-Smad2/3 antibody, followed by blotting with an anti-phospho-Smad2 antibody. (E) A reporter assay was performed by using the TGF-β-responsive reporter p3TP-lux. Stable transfectants were stimulated with TGF-β1 at various concentrations, and vasorin inhibited TGF-β-induced reporter gene activation. (F) Vasorin inhibited TGF-β signaling at the extracellular and/or cell-surface level. The p3TP-lux reporter and the constitutively active TβR-I were cotransfected into stable transfectants. Transfection of the constitutively active TβR-I activated the p3TP-lux reporter, but vasorin did not significantly inhibit this activation. N.S., not significant.
Next, we examined the functional role of vasorin in TGF-β signaling. First, stable transfectants expressing vasorin were stimulated by TGF-β1 (20 pM). Cells expressing vasorin showed a significant reduction in Smad2 phosphorylation (Fig. 4D). Second, we did a reporter assay, by using the TGF-β-responsive reporter p3TP-lux. TGF-β1 activated this reporter in a dose-dependent manner, and vasorin significantly inhibited this effect (Fig. 4E). This inhibitory effect of vasorin was specific to TGF-β signaling because vasorin did not affect the cellular responses to irrelevant cytokine stimulation (data not shown). Stable transfectants were also stimulated by using the constitutively active TGF-β type I receptor (constitutively active TβR-I) instead of TGF-β1 stimulation. Transfection of constitutively active TβR-I activated the p3TP-lux reporter, but vasorin did not significantly inhibit this activation (Fig. 4F). These findings indicate that vasorin inhibits TGF-β signaling at the extracellular and/or cell-surface level.
Vasorin Expression Was Down-Regulated During Vessel Repair After Arterial Injury, and Reversal of Vasorin Down-Regulation, by Using Ad-Mediated in Vivo Gene Transfer, Significantly Reduced Neointimal Formation at Least in Part by Modulating TGF-β Signaling in the Vessel Wall. To investigate in vivo functions of vasorin, we used a rat arterial balloon-injury model, because it is a well characterized atherosclerosis model of VSMC-derived lesions, and it is well established that TGF-β contributes to neointimal formation by promoting fibrosis.
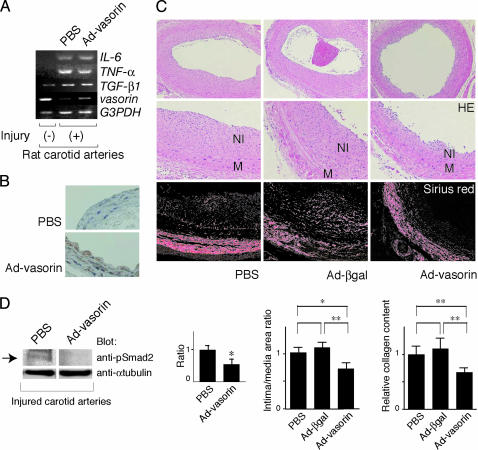
Adult rats were subjected to balloon injury with a catheter inserted through the external carotid artery. Vascular injury provokes fibroproliferative activity in quiescent VSMCs, and the phenotypic modulation of VSMCs is induced. Because the fibroproliferative activity of VSMCs peaks at 3 days after injury (6), balloon-injured carotid arteries were harvested at 3 days after insertion to examine the expression levels of vasorin by semiquantitative RT-PCR. Consistent with our findings described above (Fig. 3 A–D), down-regulation of vasorin expression was induced by mechanical vascular injury (Fig. 5A). In contrast, the expression of several cytokines, including TGF-β, was up-regulated by vascular injury, and the ratio of TGF-β to vasorin was increased (Fig. 5A).
Fig. 5.
Vasorin expression was down-regulated during vessel repair after arterial injury, and reversal of vasorin down-regulation significantly reduced neointimal formation, at least in part, by attenuating TGF-β signaling in vivo. (A) Rat carotid arteries were harvested at 3 days after injury to examine the expression levels of vasorin by semiquantitative RT-PCR analysis. Down-regulation of vasorin expression was induced by mechanical vascular injury, and Ad-vasorin treatment partially reversed this down-regulation. In contrast to vasorin, the expression of TGF-β1, TNF-α, and IL-6 was up-regulated by vascular injury and was not altered by vasorin administration. GAPDH was used as an internal control. (B) Vessels treated with Ad-vasorin were harvested 3 days afterward and were subjected to immunostaining to confirm protein expression by using the anti-Flag antibody. (C) Effects of Ad-vasorin on neointimal formation in rat carotid arteries at 14 days after injury (n = 5 arteries for each group). Representative hematoxylin/eosin-stained cross sections (Top and Middle) and Sirius red-stained cross sections (Bottom) of balloon-injured arteries treated with PBS (Left), Ad-β-galactosidase (Center), and Ad-vasorin (Right) are shown. (Middle) Partial magnifications of the respective Top images. Ad-vasorin administration significantly reduced the intima/media area ratio of injured arteries and collagen content in the lesions (P < 0.01), as compared with Ad-β-galactosidase administration. NI, neointima; M, media; HE, hematoxylin/eosin. *, P < 0.05; **, P < 0.01. (D) The inhibitory effects of vasorin administration on TGF-β signaling in vivo. Arteries were harvested 3 days after injury and were subjected to Western blot analysis by using the anti-phospho-Smad2 antibody. Representative data are shown. Smad2 phosphorylation was significantly reduced in all Ad-vasorin-treated arteries (P < 0.05). The blots were stripped and reprobed with the anti-α-tubulin antibody to ensure equal loading of proteins. The relative intensities of phospho-Smad2 bands were measured by densitometric scanning from three independent experiments. *, P < 0.05.
Next, we investigated the functional role of vasorin down-regulation in neointimal formation. To restore vasorin expression during vessel repair after injury, we did Ad-mediated vasorin gene transfer to balloon-injured rat carotid arteries. Replication-defective Ad-vasorin were constructed, and after denudation with a balloon catheter, the vessel wall was exposed to the adenoviral solution (1 × 109 pfu) for 20 min to deliver vasorin gene locally. First, arteries were harvested 3 days afterward to examine the expression of vasorin by RT-PCR analysis (Fig. 5A), immunostaining (Fig. 5B), and Western blot analysis (data not shown). Ad-mediated vasorin gene transfer was successful (Fig. 5 A and B), and the expression of TGF-β, TNF-α, and IL-6 was not altered by vasorin administration (Fig. 5A). Second, arteries were harvested to assess the effect of vasorin gene transfer on neointimal formation (n = 5 arteries for each group) at 14 days after injury. The administration of Ad-vasorin significantly reduced the intima/media area ratio of injured arteries by 35% (P < 0.01), as compared with the administration of Ad-β-galactosidase (Fig. 5C), suggesting that restoration of vasorin expression had a significant inhibitory effect on neointimal formation. Because TGF-β stimulates extracellular matrix protein synthesis, and also functions as an antiinflammatory cytokine, we examined collagen content and leukocyte infiltration in the lesions quantitatively, by using Sirius red staining and CD45 staining, respectively. As shown in Fig. 5C Lower, vasorin administration significantly reduced collagen content in the lesions (P < 0.01), whereas leukocyte infiltration was not altered (data not shown).
Finally, we investigated whether in vivo vasorin administration inhibits TGF-β signaling in the vessel wall. Balloon-injured rat carotid arteries treated with Ad-vasorin were subjected to Western blot analysis, by using the anti-phospho-Smad2 antibody, when the fibroproliferative activity of VSMCs peaked (3 days after injury). Smad2 phosphorylation was significantly reduced in Ad-vasorin-treated arteries (P < 0.05; Fig. 5D). These results suggest that enhanced TGF-β signaling after vascular injury (Fig. 5A) is significantly inhibited by in vivo vasorin administration, and that vasorin inhibits neointimal formation at least in part by modulating TGF-β signaling in the vessel wall.
Discussion
VSMCs respond to various growth factors, and the best investigated situation in which VSMCs proliferate and produce extracellular matrix proteins in vivo is after vascular injury. Whereas the rat carotid arterial balloon-injury model has been studied extensively, the underlying mechanisms that regulate vessel repair and neointimal formation appear to be the same in other arteries and in other species, including humans. The phenotype of VSMCs following arterial injury is similar to that observed during embryonic angiogenesis, and molecular mechanisms that regulate VSMC differentiation during embryonic development are thought to be recapitulated during vessel repair. Vasorin gene expression was developmentally regulated (Fig. 3 A–D), and, consistent with this finding, vasorin was down-regulated during vessel repair after arterial injury (Fig. 5A). This finding suggests its involvement in injury-induced vascular lesion formation. Therefore, we explored the in vivo functions of vasorin, by using a rat arterial balloon-injury model combined with Ad-mediated in vivo gene transfer. As shown in Fig. 5C, reversal of vasorin down-regulation had a significant inhibitory effect on neointimal formation. These results indicate that down-regulation of vasorin expression contributes to the fibroproliferative response to vascular injury.
Numerous factors that regulate VSMC activity have been studied in the arterial-injury model, and the results of these investigations suggest the importance of pathological extracellular stimuli, such as the renin-angiotensin system, catecholamines, EGF, platelet-derived growth factor (PDGF), insulin-like growth factor I, endothelin 1, TGF-β, and oxidative stress. TGF-β is a context-dependent pleiotropic cytokine, which plays a key role in the vascular response to injury. Several studies using gene transfer techniques have shown that locally enhanced TGF-β signaling enables matrix-rich neointima to develop in uninjured normal arteries of rats (11, 12). In contrast, localized blockade of TGF-β signaling results in the inhibition of neointimal formation, accompanied by reduced extracellular matrix synthesis in a rat balloon-injury model (13, 14). Of clinical relevance is the observation that the expression levels of TGF-β mRNA in restenotic lesions are higher than those in primary atherosclerotic lesions (15). These investigations indicate that TGF-β functions as a fibrogenic cytokine in a balloon-injury model, and apparently aggravates neointimal formation by promoting fibrosis. In this paper, we found that vasorin directly binds to TGF-β and negatively modulates TGF-β signaling in the vessel wall (Figs. 4 and 5D). Considering the functional role of TGF-β in this model, it is reasonably assumed that the in vivo phenotype induced by Ad-vasorin administration is mediated at least in part by the inhibitory effects of vasorin on TGF-β signaling in the vessel wall.
The extracellular region of vasorin is composed of ten tandem arrays of an LRR, an EGF-like domain, and a fibronectin type III-like domain, that are known to be involved in protein–protein interactions (Fig. 1B). Secreted and cell-surface molecules containing those domains, such as extracellular matrix proteins and adhesion molecules, sometimes have multiple binding partners. PDGF and TGF-β are prominent growth factors that have been suggested to play an important role in neointimal formation after arterial injury, and we demonstrated here that vasorin directly binds to TGF-β, but not to PDGF (Fig. 4B). However, it is possible that vasorin has other binding partners and that vasorin affects not only TGF-β signaling but also other signaling pathways, through another yet-to-be-identified mechanism. Further investigations will be needed to clarify these issues.
In the present study, we found that down-regulation of vasorin expression was induced by acute vascular injury, and that reversal of vasorin down-regulation during vessel repair inhibits neointimal formation, at least in part, by modulating cellular responses to TGF-β. These data raise a possibility that the gene expression profile of cell-surface molecules is changed by mechanical vascular injury, and that altered cellular responses to growth factors in dedifferentiated VSMCs are in part due to this change. Thus, identification and modification of the pivotal gene expression of cell-surface molecules in VSMCs may be a potential therapeutic approach to vascular fibroproliferative disorders.
Supplementary Material
Acknowledgments
We thank Dr. G. K. Owens for A404 cells, M. Ohara for language assistance, and Drs. H. Ono and H. Ogasawara for valuable advice. This work was also supported in part by grants from the Ministry of Education, Science, Technology, Sports, and Culture of Japan. The Division of Hematopoietic Factors was supported in part by the Chugai Pharmaceutical Company, Ltd.
Abbreviations: VSMC, vascular smooth muscle cell; TGF, transforming growth factor; EGF, epidermal growth factor; SST, signal sequence trap; SST-REX, retrovirus-mediated SST; CHO, Chinese hamster ovary; RA, retinoic acid; En, embryonic day n; LRR, leucine-rich repeat; Ad, adenovirus; Ad-vasorin, Ads expressing vasorin-Flag; PDGF, platelet-derived growth factor.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AY166584).
References
- 1.Tashiro, K., Tada, H., Heilker, R., Shirozu, M., Nakano, T. & Honjo, T. (1993) Science 261, 600-603. [DOI] [PubMed] [Google Scholar]
- 2.Klein, R. D., Gu, Q., Goddard, A. & Rosenthal, A. (1996) Proc. Natl. Acad. Sci. USA 93, 7108-7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kojima, T. & Kitamura, T. (1999) Nat. Biotechnol. 17, 487-490. [DOI] [PubMed] [Google Scholar]
- 4.Nosaka, T., Kawashima, T., Misawa, K., Ikuta, K., Mui, A. L. & Kitamura, T. (1999) EMBO J. 18, 4754-4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chamley-Campbell, J., Campbell, G. R. & Ross, R. (1979) Physiol. Rev. 59, 1-61. [DOI] [PubMed] [Google Scholar]
- 6.Clowes, A. W., Reidy, M. A. & Clowes, M. M. (1983) Lab. Invest. 49, 327-333. [PubMed] [Google Scholar]
- 7.Manabe, I. & Owens, G. (2001) Circ. Res. 88, 1127-1134. [DOI] [PubMed] [Google Scholar]
- 8.Kajava, A. V. (1998) J. Mol. Biol. 277, 519-527. [DOI] [PubMed] [Google Scholar]
- 9.Iozzo, R. V. (1999) J. Biol. Chem. 274, 18843-18846. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi, Y., Mann, D. M. & Ruoslahti, E. (1990) Nature 346, 281-284. [DOI] [PubMed] [Google Scholar]
- 11.Nabel, E. G., Shum, L., Pompili, V. J., Yang, Z. Y., San, H., Shu, H. B., Liptay, S., Gold, L., Gordon, D., Derynck, R., et al. (1993) Proc. Natl. Acad. Sci. USA 90, 10759-10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulick, A. H., Taylor, A. J., Zuo, W., Qiu, C. B., Dong, G., Woodward, R. N., Agah, R., Roberts, A. B., Virmani, R. & Dichek, D. A. (1998) Proc. Natl. Acad. Sci. USA 95, 6983-6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto, K., Morishita, R., Tomita, N., Shimozato, T., Nakagami, H., Kikuchi, A., Aoki, M., Higaki, J., Kaneda, Y. & Ogihara, T. (2000) Circulation 102, 1308-1314. [DOI] [PubMed] [Google Scholar]
- 14.Kingston, P. A., Sinha, S., David, A., Castro, M. G., Lowenstein, P. R. & Heagerty, A. M. (2001) Circulation 104, 2595-2601. [DOI] [PubMed] [Google Scholar]
- 15.Nikol, S., Isner, J. M., Pickering, J. G., Kearney, M., Leclerc, G. & Weir, L. (1992) J. Clin. Invest. 90, 1582-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.