Abstract
Diabetic embryopathy is a theoretical enigma and a clinical challenge. Both type 1 and type 2 diabetic pregnancy carry a significant risk for fetal maldevelopment, and the precise reasons for the diabetes-induced teratogenicity are not clearly identified. The experimental work in this field has revealed a partial, however complex, answer to the teratological question, and we will review some of the latest suggestions.
Keywords: Anomalies, development, diabetes in pregnancy, epigenetics, gene expression, malformations
Introduction
Claes Hellerström (1), the senior and mentor of the authors of this text, strongly inspired the early work in the field of embryonic development (2–4) and maldevelopment (5), particularly in a diabetic environment (6–9).
Despite increased clinical efforts to improve glycemic control during diabetic pregnancy, the rate of congenital malformations remains increased in studies of diabetic gestation of type 1 (10–21) and type 2 diabetes (16–25). In two recent large meta-studies it was found that the malformation rate in type 1 diabetic pregnancy did not differ from that of type 2 diabetic pregnancy (26,27), and both rates were estimated to be around 5%–6%. The similar rates of malformation may relate to the higher age and concomitant adiposity in type 2 diabetic women, both of which may increase the malformation incidence in this group (28–30).
The cell biological reason for the teratogenic effect of the diabetic state is not known. However, both environmental factors (maternal diabetic state and intrauterine conditions) and genetic predisposition seem to be of importance in diabetic embryopathy, i.e. this is a case of environment–gene interaction. The congenital malformations are likely to be induced in early gestation (31–33), and the risk for giving birth to a child with a malformation is enhanced by increased maternal metabolic dysregulation (34–37).
Alterations of maternal metabolism
Several teratological factors in maternal serum have been suggested, often from clinical experience, and subsequently characterized in various experimental systems. The maternal teratogenic factors most often indicated are hyperglycemia and hyperketonemia.
Glucose
Increased glucose levels are the hallmark of the diabetic state, and there is ample clinical evidence that increased glucose/HbA1c levels correlate with increased risk for congenital malformation in the offspring (32–35,37–40). In experimental studies the analogous correlation between increased serum glucose levels and increased risk for fetal malformations has been demonstrated in diabetic rodents (8,41–76). In addition, injections of glucose to pregnant non-diabetic animals have also yielded teratological effects (77,78). Furthermore, in vitro culture of rodent embryos in increased glucose concentrations (48,57,64,79–95) as well as in diabetic serum (83,96–105) yields disturbed development (Figure 1).
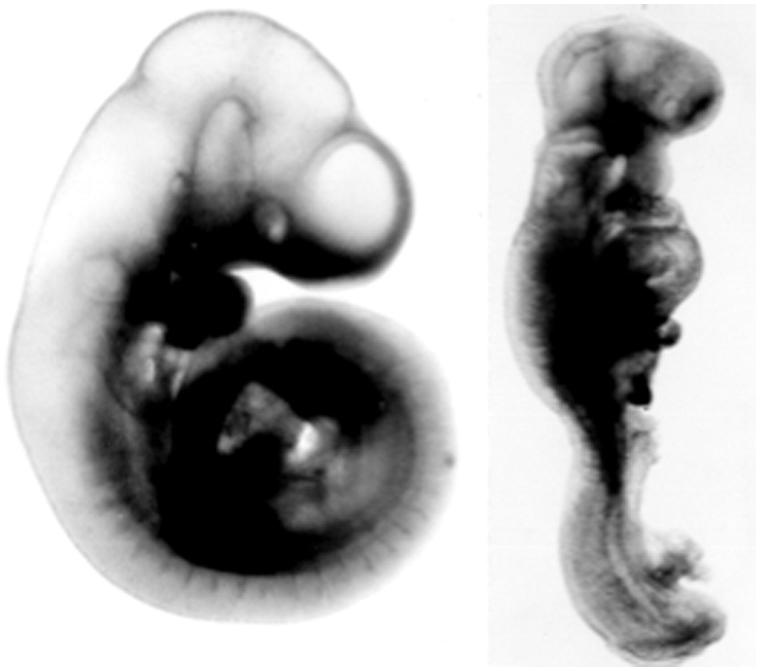
Figure 1.
Two day-9 rat embryos from a normal (left embryo) and a diabetic (right embryo) pregnancy. The latter embryo is growth-retarded (reduced crown-rump length and somite number) and malformed (rotational defect, open neural tube).
The alleged teratological effect of hyperglycemia is likely to be correlated to the metabolism of glucose, since exposure to L-glucose in vitro has no negative developmental effect (79). Increased embryonic uptake of glucose by existing (106), often up-regulated (107,108), glucose transporters also seems to be necessary for the teratogenic effect. The increased influx of glucose would yield increased glycolytic flux, as well as increased mitochondrial activity of the citric acid cycle and enhanced oxidative phosphorylation. Several consequences of increased glucose metabolism have been suggested (109) where increased mitochondrial production of superoxide may possess the most pronounced teratogenic potential. However, also increased hexose monophosphate shunt activity, reactive oxygen species (ROS)-mediated inhibition of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and increased formation of reactive alpha-oxoaldehydes may play roles in the diabetes-induced embryonic dysmorphogenesis. In addition, increased formation of advanced glycosylation end-products (AGEs) and decreased intracellular availability of arachidonic acid (and its products, the prostaglandins) are suspected players in the teratogenic drama of diabetic pregnancy (Figure 2).
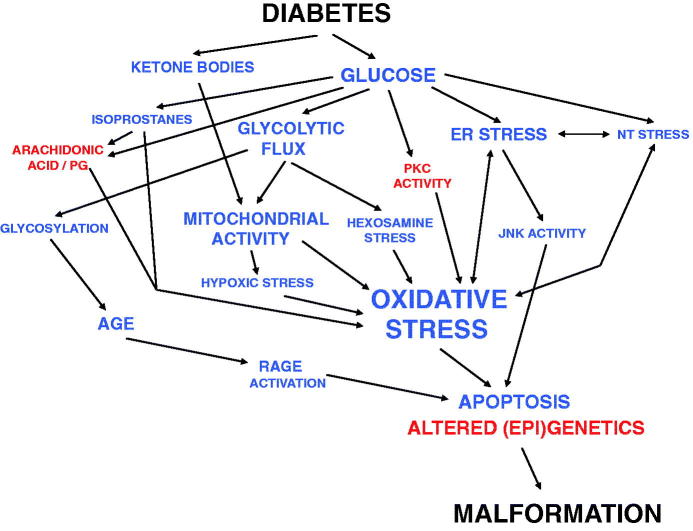
Figure 2.
Schematic outline of the development of diabetic embryopathy. Blue color marks increased activity/amount, and red color decreased or disturbed activity/amount of compounds or processes. Note that more interactions between the items are likely to be present than those denoted here, and that the putative importance of genetic predisposition is not included.
Of note, the pre-implantation embryo follows another route toward maldevelopment, since a diabetes-induced decrease of the inner cell mass (55,110) is likely to be induced by hypoglycemia, due to down-regulation of glucose transporters in the blastocyst, leading to decreased intraembryonic glucose levels (111–113) and enhanced apoptosis (110,114).
Ketone bodies
The ketone bodies are synthesized in increased amounts in the (maternal) liver in response to the diabetic state. The two major compounds, beta-hydroxybutyrate and acetoacetate, traverse the placenta (115) and are utilized as substrate (acetoacetyl-CoA) in the embryonic mitochondria. There is experimental evidence for a teratogenic role for diabetes-associated metabolites other than glucose. Thus, serum from diabetic rats with normalized glucose levels is still teratogenic in in vitro cultures (103,104), and the direct exposure to increased ketone body levels in vitro yields disturbed development in cultured rodent embryos (116–123). The mechanism for ketone body teratogenesis should also include metabolism of the compounds (124).
Combination effect
Culture of rat embryos in a combination of sub-teratogenic levels of glucose and beta-hydroxybutyrate yields disturbed in vitro embryonic development, thereby supporting the notion of a synergistic relationship between diabetes-associated metabolites (90). Both of these putative teratogens serve as metabolic substrates in the embryo (and mother), and the most prominent teratogen, glucose, has the most complex metabolism involving glycolysis. Furthermore, the metabolic pathways of the teratogens converge at the citric acid cycle and oxidative phosphorylation in the mitochondrion, which has led to the speculation that an overactivity of the oxidative phosphorylation pathway may be at the core of the diabetic teratogenicity.
Alterations of fetal metabolism
Major teratogenic processes in embryonic tissues so far identified include alterations of signaling systems such as metabolism of arachidonic acid/prostaglandins (73,86,94,125–129).
Arachidonic acid/prostaglandins
Arachidonic acid is a polyunsaturated fatty acid present in the phospholipids of membranes of the cells. The metabolism of arachidonic acid and its products, the prostaglandins, is crucial for cellular life. In particular, arachidonic acid is involved in cellular signaling as a lipid second messenger. Disturbed metabolism of arachidonic acid and prostaglandins has been found in previous studies of experimental diabetic pregnancy. Intraperitoneal injections of arachidonic acid to pregnant diabetic rats diminished the rate of neural tube damage (NTD) (48) as did enriching the diet of pregnant diabetic rats with arachidonic acid (130–132). Addition of arachidonic acid to the culture medium was shown to block the embryonic dysmorphogenesis elicited by high glucose concentration (48,82,94). Addition of PGE2 to the culture medium also blocked glucose-induced teratogenicity in vitro (86,94) as well as maldevelopment of embryos cultured in diabetic serum (101). Measurements of PGE2 have indicated that this prostaglandin is decreased in embryos of diabetic rodents during neural tube closure (128,133) in high-glucose-cultured embryos (128) as well as in the yolk sac of embryos of diabetic women (134).
Previous studies have shown that the uptake of arachidonic acid by embryonic yolk sacs is increased in a hyperglycemic environment (126). This finding would preclude an uptake deficiency of arachidonic acid in the conceptus of diabetic pregnancy, a result supported by the demonstration of unchanged concentration of arachidonic acid in membranes of high-glucose-cultured embryos in vitro (135). Measurements in day-12 embryos, however, indicated a decreased arachidonic acid concentration in the offspring of diabetic rats (136). Disturbances in the availability or metabolism of arachidonic acid affect the synthesis of prostaglandins. Alterations in the activity of the rate-limiting enzyme cyclo-oxygenase (COX), which converts arachidonic acid to prostaglandin H2, may be of major importance. There are two isoforms of cyclo-oxygenase, COX-1 (constitutive) and COX-2 (inducible). A glucose-induced down-regulation of the gene expression of COX-2, as well as a GSH-dependent decrease of the conversion of the precursor PGH2 to PGE2 (PGE synthase), has been demonstrated (128). Thus, the PGE2 concentration of day-10 embryos and membranes was decreased after exposure to high glucose in vitro or diabetes in vivo. In vitro addition of N-acetylcysteine (NAC) to high-glucose cultures restored the PGE2 concentration (128). Hyperglycemia/diabetes-induced down-regulation of embryonic COX-2 gene expression may be an early event in diabetic embryopathy, leading to lowered PGE2 levels and dysmorphogenesis, presumably because this pathway plays an important role in neural tube development. Antioxidant treatment does not prevent the decrease in COX-2 mRNA levels but restores PGE2 concentrations, suggesting that diabetes-induced oxidative stress aggravates the loss of COX-2 activity. From these data, it may be concluded that decreased availability of arachidonic acid and the resulting decrease in several prostaglandins, in particular PGE2, is likely to be involved in the teratogenicity of diabetic pregnancy (cf. Figure 2) (132).
Other studies have shown that a diabetes-like environment decreases embryonic PGE2 concentration (127,128,133) in embryonic tissues. Thus, affected arachidonic acid metabolism disturbs prostaglandins and embryogenesis (137) in several ways, which emphasizes the teratological importance of prostaglandin and prostaglandin-associated pathways.
Diabetes-induced teratological processes
Several studies have suggested that diabetic embryopathy is associated with alterations of various signaling systems, which results in disturbed intracellular conditions, such as oxidative stress (62,88,90,138), nitrosative stress (139,140), endoplasmic reticulum (ER) stress (140–142), and hexosamine stress (143,144). In addition, enhanced embryonic apoptosis (77,78,93,114,145–149) has been regarded as a component of diabetic embryopathy (Figure 2).
Oxidative stress
Oxidative stress reflects an imbalance between production of reactive oxygen species (ROS) and an ability to detoxify the reactive intermediates or to repair the resulting damage. ROS can damage all components of the cell, including proteins, lipids, and DNA. Some ROS act as cellular messengers in redox signaling, and a state of oxidative stress can therefore disturb normal cellular signaling. ROS are produced through multiple mechanisms, e.g. by NADPH oxidase (NOX) enzymes, and in mitochondria, where about 1%–2% of electrons passing through the electron transport chain are incompletely reduced and give rise to the superoxide radical (•O2-). The ROS with the highest capacity to cause cellular damage is the hydroxyl radical, which, once formed, will alter DNA, RNA, proteins, lipids, or carbohydrates without being removed by any scavenging enzyme system.
The notion that diabetes is associated with oxidative stress has been suggested by several authors (150–154). For instance, increased lipid peroxidation and ROS generation were found in diabetic rats, measured as increased serum 8-epi-PGF2a levels (155) and increased electron spin clearance rate (156). Cyclic voltammetric studies have also indicated increased levels of lipid peroxidation in diabetic rats (157), and the isoprostane 8-epi-PGF2a is increased in embryos exposed to high glucose levels in vitro (128) and diabetes in vivo (158). Examination of litters of diabetic rats demonstrated lowered a-tocopherol (vitamin E) concentration in day-11 embryos and in the liver of day-20 fetuses (69).
The first evidence of an involvement of oxidative stress in the pathogenesis of diabetic embryopathy was the demonstration that treatment of rodent embryos with antioxidative agents largely normalizes increased glucose-induced malformation rates in vitro (88, 90), observations that were repeated (91,123,159,160) and extended to in vivo studies (62,65,66,69,70,94,105,131,161–163). Furthermore, antioxidative treatment was found to normalize several markers of oxidative stress, such as serum 8-epi-PGF2a levels (155), electron spin clearance rate (156), and the concentration of embryonic isoprostanes in vitro (128) and in vivo (158). Evidence for diabetes-induced oxidative stress has subsequently been found in several rat models of diabetic pregnancy (164).
Several different compounds with antioxidative properties have been shown to diminish embryonic maldevelopment resulting from exposure to high glucose levels in vitro or to a diabetic intrauterine environment in vivo. Thus, adding scavenging enzymes, e.g. superoxide dismutase (SOD) (88), catalase (88), or glutathione peroxidase (88), to the culture medium protects rat embryos from dysmorphogenesis induced by high glucose concentration in vitro. The antioxidant NAC blocks dysmorphogenesis in high-glucose-cultured rodent embryos (94,105,128,165–167) and neural crest cells (168,169), and addition of glutathione ester to high-glucose medium diminishes embryonic maldevelopment (91). Actually, teratogenic concentrations of beta-hydroxybutyrate or the branched chain amino-acid analog alpha-ketoisocaproic acid (KIC) can also be blocked by addition of SOD to the culture medium (90).
Analogously, dietary supplementations with antioxidative compounds have been shown to diminish diabetic embryopathy in vivo. Thus, administration of butylated hydroxytoulene (BHT) (62), vitamin E (66,69,163), vitamin C (70,163), and folic acid (170) decreases the malformation rate in the offspring of diabetic rats and largely improves embryonic and fetal growth in vivo. Alpha-lipoid acid supplementation has been found to reduce the diabetes-induced high incidence of resorptions and malformations in offspring of diabetic rodents (171–173). Embryos exposed to a diabetic intrauterine milieu have demonstrated diminished malformation rates after maternal supplementation of NAC (174). Combined supplementation of antioxidative compounds, e.g. vitamin E and C (162), or folic acid and vitamin E (147), to pregnant diabetic rats also diminished diabetes-induced dysmorphogenesis. In a study of glucose-induced cardiac malformations in a mouse model, the administered antioxidants decreased all negative effects of hyperglycemia/oxidative stress, such as hampered migration and increased apoptosis of neural crest cells, and prevented outflow tract defects (175). In addition, pregnant diabetic mice, transgenic for the CuZnSOD gene (SOD1) have fewer malformed offspring than the diabetic wild-type mice (138,139,142,176), illustrating the anti-teratogenic capacity of increased ROS scavenging activity.
Embryonic neural tissue subjected to high glucose concentrations shows increased superoxide production, as measured in a Cartesian diver system (177). One effect of increased intracellular ROS production would be inhibition of the rate-limiting enzyme of glycolysis, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), since this enzyme has displayed sensitivity to ROS in several different conditions of oxidative stress (178). This sensitivity resides in the thiol group of cysteine residue 149 in the active site of the enzyme (179,180). Oxidation of the thiol group by NO or ROS leads to decreased enzyme activity (181), and blocking of this process by antioxidants protects the activity of the enzyme (182). Another mechanism for GAPDH inhibition also results from mitochondrial production of ROS, activating poly-(ADP-ribose) polymerase 1 (PARP 1) by damaging DNA. PARP 1, in turn, induces ADP-ribosylation of GAPDH, leading to its inactivation and an accumulation of metabolites earlier in the metabolism pathway. In line with these considerations, decreased GAPDH activity was found in rat embryos subjected to a diabetic environment both in vivo and in vitro (183). Furthermore, addition of the antioxidant NAC prevented the decrease in activity (183). In addition, fetuses and embryos of diabetic rodents display increased rates of DNA damage (60,75,184), another indication of enhanced ROS activity and damage in embryonic tissues. High-amplitude mitochondrial swelling was demonstrated in embryonic neuroectoderm of embryos exposed to a diabetic environment (185,186). This swelling decreased after antioxidative treatment of the mother (74), implicating an embryonic ROS imbalance, with conceivable consequences for the rate of apoptosis in susceptible cell lineages in the embryo (167,187). The mitochondrion plays an important role in the apoptotic machinery, and previous studies have suggested that an altered apoptotic rate may affect the maldevelopment of embryos subjected to a diabetic milieu (93,145).
Furthermore, diabetic transgenic mice, overexpressing thioredoxin-1, have a lower incidence of malformations and decreased oxidative stress markers than diabetic wild-type mice (188), demonstrating a parallel relationship between dysmorphogenesis and degree of oxidative stress. Also, it has been suggested that diabetes-induced oxidative stress in embryos may cause maldevelopment via altered TNF-alpha levels (189).
In a recent study of the activity of AMP-activated kinase (AMPK) in embryos of hyperglycemic mice, it was demonstrated that maternal hyperglycemia stimulated AMPK activity and that stimulation of AMPK with 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside (AICAR) increased the rate of NTD in the embryos (190). In addition, stimulation of AMPK by hyperglycemia, hypoxia, or antimycin A could be inhibited by antioxidants. The AMPK inhibitor compound C blocked the effects of hyperglycemia or antimycin A on NTD occurrence, suggesting that diabetes/glucose-induced stimulation of embryonic AMPK activity is a teratogenic consequence of oxidative stress in diabetic embryopathy (190). In support of that notion, it was reported in a subsequent study that sole stimulation of AMPK disrupts embryonic gene expression and causes neural tube defects (191)
The bulk of data implicates oxidative stress and ROS excess as an important component in the etiology of diabetic embryopathy. The data also suggest that long-term exposure to high glucose creates embryonic ROS excess either from increased ROS production (177) or from diminished antioxidant defense capacity (91,159,192). The ROS excess may be small, restricted to particular cell populations (193,194), and likely to vary with gestational time and nutritional status, making direct ROS determinations difficult.
Increasing ROS levels in embryos lead to malformations (195,196), suggesting that ROS excess may also play a role in the teratogenic process(es) of phenytoin medication (197,198), ethanol abuse (193,194,199), and, possibly, thalidomide administration (200). Therefore, ROS excess may constitute a common element in a number of teratogenic situations, including diabetic pregnancy (cf. Figure 2) (201).
Hypoxic stress
In early organogenesis, oxygen levels are likely to be very low in the embryonic environment, and excess glucose metabolism could accelerate the rate of O2 consumption, thereby exacerbating the hypoxic state. Since hypoxia can increase mitochondrial superoxide production, excessive hypoxia may contribute to oxidative stress. In a study of O2 availability in embryos of glucose-injected hyperglycemic mice, it was found that O2 availability was reduced by 30% in embryos of hyperglycemic mice (202). When pregnant hyperglycemic mice were housed in 12% O2, the NTD rate increased 8-fold in the offspring. Conversely, housing pregnant hyperglycemic mice in 30% O2 significantly suppressed the effect of maternal diabetes to increase NTD (202). These observations suggest that maternal hyperglycemia depletes O2 in the embryo and that this contributes to oxidative stress and the adverse effects of maternal hyperglycemia on embryo development (Figure 2).
Isoprostane formation
Isoprostanes, e.g. 8-epi-PGF2a, are prostaglandin-like compounds formed in situ from peroxidation of arachidonic acid by non-enzymatic, free radical-catalyzed reactions, and they therefore serve as indicators of lipid peroxidation (203–205). These non-classical eicosanoids possess potent biological activity as inflammatory mediators. Also, the formation of isoprostanes may, in itself, consume arachidonic acid, and therefore diminish the available pool of arachidonic acid in the embryo (see above). It has been shown that a diabetes-like environment increases the isoprostane levels in embryonic tissues (128). In addition, supplementation of 8-epi-PGF2a to the culture medium caused malformations in whole-embryo culture, thereby illustrating the independent teratogenic activity of isoprostanes (206). Furthermore, adding SOD or NAC to the culture medium with isoprostane excess normalized almost all morphological and biochemical parameters, including the elevated tissue concentration of 8-epi-PGF2a, thereby illustrating the teratogenic potential of diabetes/glucose-induced oxidative stress (cf. Figure 2) (206).
AGE formation and RAGE activation
The biochemical process of advanced glycosylation end-product (AGE) formation, which is accelerated in diabetes as a result of chronic hyperglycemia and increased oxidative stress, has been postulated to play a central role in the development of diabetic complications (207). AGEs are known to accelerate oxidative damage to cells in a diabetic environment. Examples of AGE-modified sites are carboxymethyllysine (CML), carboxyethyllysine (CEL), and argpyrimidine. Under oxidative stress due to hyperglycemia in patients with diabetes, AGE formation is increased beyond normal levels.
RAGE, the receptor for AGE, is a transmembrane pattern recognition receptor. Except for AGEs, RAGE has also other agonistic ligands: high mobility group protein B1 (HMGB1), S100/calgranulins, amyloid-beta, and MAC-1. The interaction between RAGE and its ligands is thought to result in pro-inflammatory gene activation. Ligand stimulation of RAGE initiates a signaling cascade resulting in activation of NFκB and activation of NADPH-oxidase, thereby yielding increased intracellular oxidative stress. An increased accumulation of AGE has been found in the pathogenesis of several diabetic complications, such as cataract, retinopathy, atherosclerosis, neuropathy, and nephropathy. Inhibition or knockout of RAGE attenuates the detrimental effects of hyperglycemia in neuropathy and nephropathy (208).
It has also been suggested that AGE–RAGE activation is involved in diabetic embryopathy. Thus, the embryonic formation of glycated proteins (85,209,210) has been suggested to influence the teratological events in diabetic pregnancy. It has been shown in rodent embryos cultured in high glucose that the levels of the AGE precursor 3-DG increase in embryonic tissues, and the addition of 3-DG to the culture medium with physiologic concentrations of glucose induces malformations, an effect that is reversible with the addition of SOD (85).
In a recent study of diabetic embryopathy with RAGE knockout mice, it was found that maternal diabetes induced more fetal resorptions, malformations (facial skeleton, neural tube), and weight retardation in the wild-type fetuses than in the RAGE−/− fetuses, despite similar maternal hyperglycemia. In wild-type offspring, maternal diabetes increased fetal hepatic levels of 8-iso-PGF2a and activated NFκB in the embryos, in contrast to unchanged 8-iso-PGF2a levels and NFκB activity in diabetes-exposed RAGE−/− offspring. These findings suggest that RAGE activation and oxidative stress are associated phenomena in diabetic embryopathy (Figure 2).
Nitrosative stress
Nitrosamine overproduction, or ‘stress’, has been implicated in diabetic embryopathy, as a concomitant—and, in some studies, a ‘downstream’—event to oxidative stress. Thus, in a study of yolk sacs of CuZnSOD-(SOD1)-overexpressing embryos from normal and diabetic mice, it was found that the SOD1-transgenic embryos were largely protected from several of the negative effects of diabetes (139). Thus, diabetes-induced elevated markers of oxidative stress (4-hydroxynonenal and malondialdehyde reductions) were diminished in the SOD1-transgenic embryos compared to the wild-type embryos. Furthermore, hyperglycemia-increased iNOS expression and nitrosylated protein were also diminished, and caspase-3 and caspase-8 cleavages were blocked, in the SOD1-transgenic embryos. This finding suggests that oxidative stress induces iNOS expression, nitrosative stress, and apoptosis in diabetic embryopathy (139). In another study, diabetic pregnant mice were fed via gavage an inhibitor of nitric oxide (NO) synthase (NOS) 2,L-N6-(1-iminoethyl)-lysine (L-NIL; 80 mg/kg), once a day from embryonic (E) day 7.5 to 9.5 during early stages of neurulation. The treatment significantly reduced the NTD rate in the embryos, compared with that in vehicle (normal saline)-treated diabetic animals (140). In addition to alleviation of nitrosative stress, ER stress was also ameliorated, as assessed by quantification of associated factors. Apoptosis was reduced, indicated by caspase-8 activation. These results show that nitrosative stress is important in diabetes-induced NTDs via exacerbating ER stress, leading to increased apoptosis (140). The combined observations support a role for nitrosative stress in diabetic embryopathy, and suggest a ‘cross-talk’ between oxidative stress, nitrosamine stress, and ER stress (see below and Figure 2).
ER stress
ER stress, also named unfolded protein response (UPR), is a cellular stress response related to the ER, which is induced by an accumulation of misfolded proteins in the ER lumen. The ER stress/UPR diminishes protein translation, degrades misfolded proteins, and produces chaperones involved in protein folding in order to restore normal ER function. If this is not achieved, or the disruption is prolonged, the ER stress/UPR shifts towards promoting apoptosis.
In a study of the effects of maternal diabetes on the development of oocytes and early embryos by using time-lapse live cell imaging confocal microscopy, the ER displayed an increased percentage of homogeneous distribution patterns throughout the entire ooplasm during oocyte maturation and early embryo development. In addition, a higher frequency of large ER aggregations was detected in oocytes and two-cell embryos from diabetic mice. These results suggest that the diabetic condition adversely affects the ER distribution pattern during mouse oocyte maturation and early embryo development (211).
In a study of oxidative and ER stress in SOD1-overexpressing mice, it was found that maternal diabetes causes increased levels of ER stress markers, e.g. C/EBP-homologous protein (CHOP), calnexin, phosphorylated (p)-eIF2alpha, p-PERK, and p-IRE1alpha; triggered XBP1 mRNA splicing; and enhanced ER chaperone gene expression in wild-type embryos, whereas all these changes were blocked in the embryos of diabetic transgenic mice. This supports the notion that diminishing diabetes-induced oxidative stress, e.g. by SOD1 overexpression, blocks ER stress in embryos (142)
A downstream effect of the ER stress would be an activation of C-Jun N-terminal kinase (JNK). The possible relationship between JNK1/2 activation and ER stress in diabetic embryopathy was investigated in mice. Maternal diabetes increased ER stress markers and induced swollen/enlarged ER lumens in embryonic neuroepithelial cells during neurulation. Deletion of both jnk1 or jnk2 genes diminished hyperglycemia-increased ER stress markers and ER chaperone gene expression. In high-glucose cultured embryos, the addition of the ER chaperone 4-phenylbutyric acid (4-PBA) diminished ER stress markers and abolished the activation of JNK1/2 and its downstream transcription factors, caspase-3 and caspase-8, as well as Sox1 neural progenitor apoptosis. Consequently, 4-PBA blocked high-glucose-induced NTD in vivo. It was concluded, therefore, that hyperglycemia induces ER stress, which yields activation of the proapoptotic JNK1/2 pathway, which yields induced neural tube apoptosis, and thereby NTD (212).
Autophagy is an intracellular process to degrade dysfunctional proteins and damaged cellular organelles, which, in addition, regulates embryonic cell proliferation, differentiation, and apoptosis. Furthermore, blockage of this process in embryos causes NTDs reminiscent of those observed in diabetic pregnancies. In a study of NTD induction in diabetic mice with or without 2%–5% trehalose water, the role of autophagy was investigated. Maternal diabetes suppressed autophagy in neuroepithelial cells and altered autophagy-related gene expression. Trehalose treatment reversed autophagy impairment and prevented NTDs in the embryos of diabetic pregnancies. The study demonstrates that maternal diabetes suppresses autophagy in neuroepithelial cells of the developing neural tube, leading to NTD formation (213).
In a recent study of diabetes-induced cardiac malformations, it was found that the rate of atrio-ventricular septal defects (AVSDs) was increased concomitant with enhanced ER stress in embryonic hearts. Blocking of glucose-induced ER stress with 4-PBA in an endocardial cushion explants culture restored endocardial cell migration. The findings suggest that development of the endocardial cushions is susceptible to the insult of maternal hyperglycemia, and that diabetes-induced ER stress in the developing heart mediates the negative effect on endocardial cell migration (214). The studies suggest that ER stress is involved in the teratogenesis of neural and cardiac malformations in embryos exposed to diabetes or hyperglycemia, and that ER stress, nitrosative stress, and oxidative stress enhance each other (Figure 2).
Hexosamine stress
Increased ambient glucose concentrations yield increased uptake, phosphorylation, and metabolism of glucose, primarily by enhanced flux in the glycolytic pathway and, also, in the hexosamine biosynthetic pathway (109). This is a pathway that converts glucose to uridine diphosphate N-acetylglucosamine (UDP-GlcNAc).
Under normoglycemic conditions, approximately 1%–3% of total glucose consumed by somatic cells are directed down the hexosamine pathway (215). UDP-GlcNAc is the substrate for the majority of glycosylation in the cell, producing mucopolysaccharides (216). In this process UDP-GlcNAc is attached to serine or threonine residues of proteins, thus becoming a post-translational modification (beta-O-linked glycosylation), which regulates protein function in an analogous manner to phosphorylation (217). Altered beta-O-linked glycosylation has been associated with a number of disease states, including cancer, inflammatory conditions, and neurodegenerative diseases (218). Notably, it is also implicated as a primary mechanism behind the development of insulin resistance and pancreatic beta-cell destruction in type-2 diabetes (215,218)
It has been suggested that hexosamine stress may play a role in diabetic teratogenesis (143,144,219). Indeed, defect development has been demonstrated in pre-implantation mouse embryos, treated with glucose (27 mM) or glucosamine (0.2 mM), which was added to embryo culture media. Both treatments disturbed embryo development, increased apoptosis, and decreased cell number in the resulting blastocysts (219). Addition of benzyl-2-acetamido-2-deoxy-a-D-galactopyranoside (BADGP), an inhibitor of O-linked beta-N-acetylglucosaminyltransferase (OGT), the enzyme which adds O-GlcNAc to proteins, rescued all these phenotypes in the hyperglycemia treatment group, although only mild improvement was seen in the glucosamine group (219). This may reflect the relative potencies of each hexose in their capacity to stimulate the UDP-GlcNAc production (215). In another study, pregnant mice were injected with glucose to induce hyperglycemia, or glucosamine, to activate the hexosamine pathway directly. Both treatments increased the NTD rate in the embryos, decreased GSH levels, and increased oxidative stress, as indicated by increased 2,7-dichloro-dihydrofluorescein fluorescence. Glucose and glucosamine also inhibited expression of Pax-3; however, all these effects were prevented by GSH ethyl ester administration (143). These findings suggest a role for hexosamine stress in diabetic embryopathy (Figure 2).
Apoptosis
The notion that apoptosis may be a component of the teratogenic process of diabetic pregnancy has been studied thoroughly. Thus, there are several reports of increased rates of apoptosis in embryos exposed to a diabetic environment (77,78,146–149,167,187,220–222). In particular, there are findings indicating increased apoptotic rates already in pre-implantation embryos (93,114,145).
In a study of early post-implantation embryos, the expression of Bcl-2 mRNA was decreased and the number of deoxynucleotidyl transferase-mediated nick end labeling (TUNEL)-positive cells increased in embryos of diabetic rats compared to control embryos. These results suggest that a Bax-regulated mitochondrial cytochrome c-mediated caspase-3 activation pathway might be involved in the diabetic embryopathy (146). In another early study, it was reported that combined supplementation of folic acid and vitamin E to pregnant diabetic rats diminished diabetes-induced dysmorphogenesis and normalized apoptotic-associated protein levels (147). In another study of rodent embryos subjected to high glucose in vitro or diabetes in vivo, disturbed development was found, concomitant with increased activation of caspase-3 and other markers of apoptosis. Supplementation of NAC or an apoptosis inhibitor diminished both the dysmorphogenesis and apoptosis (167). Exposure to a diabetic milieu during organogenesis thus increases dysmorphogenesis and apoptosis in embryos.
In mice transgenic for the thioredoxin-1 gene with overproduction of the antioxidant thioredoxin-1, the incidence of diabetes-induced malformation is markedly lower in diabetic transgenic mice compared with diabetic wild-type mice. Furthermore, the diabetes-induced increased markers for oxidative stress, apoptosis-promoting proteins, and cleaved caspase-3 production are all diminished in the offspring of diabetic transgenic mice compared with the offspring of diabetic wild-type mice (188).
A new apoptotic pathway was recently suggested to be activated by a diabetic/hyperglycemic environment, involving the gene products of the ASK1-FoxO3a-TRADD-caspase-8 gene (149). Blocking components of this pathway diminished the NTD rate in diabetic mice. Thus, hyperglycemia-induced apoptosis and the development of NTD was reduced with genetic blockage of either FoxO3a or Casp8, or by inhibition of ASK1 by thioredoxin. In addition, examination of human neural tissues affected by neural tube defects revealed increased activation or abundance of the genes in the cascade. The conclusion was that activation of the ASK1-FoxO3a-TRADD-caspase-8 pathway participates in the development of NTDs, which could be prevented by inhibiting intermediates in this cascade (149). There is, clearly, strong experimental evidence for a role of apoptosis in diabetic embryopathy (Figure 2).
Genetics and epigenetics of diabetic dysmorphogenesis
Several lines of evidence support the notion that the metabolic alterations in the embryonic tissues are followed by changes in genetics (gene expression) and epigenetics (regulation of gene expression). Also there should exist permissive conditions in the mother and offspring that pave the way for the diabetes-induced genetic/epigenetic changes that ultimately lead to embryonic maldevelopment, i.e. genetic predisposition in mother and child.
Genetic predisposition
Despite similar teratological exposure, the effect of any teratogen, including maternal diabetes/hyperglycemia, varies between individuals. In addition to stochastic conditions, genetic predisposition determines the effect of each teratogen on a particular individual (223,224). Although predisposing genetic conditions for diabetes are clearly present in offspring of diabetic parents (225,226), as the offspring of a diabetic father has higher risk of developing the disease than the offspring of a diabetic mother (227–231), it has been established that diabetic men do not have an increased risk of fathering malformed offspring (232,233). This indicates that the genes predisposing for diabetes do not induce congenital malformations. In contrast, maternal diabetes has been suggested to be associated with Down’s syndrome (234–236) and has also been suggested to predispose for optic nerve hypoplasia in female offspring (237). A genetic element may be present in the etiology of diabetic embryopathy (238), a notion supported by experimental data (58,84,138,239–242).
The contribution of the fetal genome and maternal (diabetic) environment was evaluated in a rat model where the outcome of diabetic pregnancy in two outbred substrains of Sprague-Dawley rats (with low incidence, H, and high incidence, U, of skeletal malformations in the offspring), and F1 hybrids between them was studied (84). The fetuses of diabetic H mothers had no skeletal malformations, regardless of embryo type (H/H or H/U). When the diabetic mother was U or from the hybrid strain (H/U) and the offspring of the mixed H/U type, increased skeletal malformation (3%–5%) rates resulted. When the embryos contained a major U genome, either U/U or U/(H/U), further increased skeletal malformations (17%–19%) were found. These findings indicate that both the maternal and fetal genomes are involved in the etiology of diabetes-induced (skeletal) malformations in rodent diabetic pregnancy (84). When pre-implantation embryos were transferred from diabetic NOD mice to non-diabetic ICR mice and allowed to develop until gestation day 13, as were embryos from the reverse transfers, as well as from ICR-to-ICR transfers, we found 8/58 (14%), 18/45 (40%), and 0/73 (0%) malformed embryos in the NOD–ICR, ICR–NOD, and ICR–ICR transfers, respectively. The result thus suggests that both the embryo genotype and the maternal environment are of importance for diabetic embryopathy (58). Moreover, in a recent study one-cell mouse zygotes and blastocysts were transferred from diabetic or control mice into non-diabetic pseudopregnant female recipients, and were evaluated at embryonic day 14 (243). The offspring from the diabetic rats had higher rates of malformations than the controls, and the conclusion was that exposure to maternal diabetes during oogenesis, fertilization, and the first 24 hours is enough to program permanently the fetus to develop significant morphological changes (243). In order to characterize the relative importance of the maternal and paternal genome in relation to the teratogenicity of the maternal (intrauterine) milieu, rats from two different strains were cross-mated. Thus, male rats from a malformation-prone (L) and a malformation-resistant (W) strain were mated with diabetic females from the other strain to produce F1 offspring: LW (L male × W female) and WL (W male × L female), which would be genetically identical with exception of imprinted genes and mitochondrial types. However, the malformation rate was 0% in the LW and 9% in the WL offspring (244), demonstrating both a teratologic ‘dilution’ effect, as well as the importance of the maternal environment. Based on metabolic data from the two types of pregnancy (indicating a more disturbed metabolic state in the diabetic L rats), the study suggested that the fetal genome controls the embryonic dysmorphogenesis in diabetic pregnancy by instigating a threshold level for the teratological insult and that the maternal genome controls the teratogenic insult by (dys)regulating the maternal metabolism (244). Furthermore, when the F1 crosses were mated in a subsequent study, the malformation rate of the F2 combinations WL × WLdiabetic and LW × LWdiabetic was around 5% (245), a further dilution of the teratogenic induction; however, the malformed WL × WL offspring had only agnathia/micrognathia, whereas the malformed LW × LW offspring had 60% agnathia/micrognathia and 40% cleft lip and palate. Thus, despite identical autosomal genotypes, the diabetic WL and LW female rats gave birth to offspring with markedly different malformation patterns. This study suggested a teratological mechanism in diabetic pregnancy influenced by maternal metabolism and parental strain epigenetics (245).
In a previous study, it could be demonstrated that a specific variant of the catalase enzyme is present in rats that are malformation-prone (Cs-1a), whereas another variant of the catalase protein was present in rats that do not develop malformations in response to maternal diabetes (Cs-1b) (241). Thus, embryonic catalase activity was lower in embryos from normal U rats than in embryos from normal H rats, and maternal diabetes augments this difference (242). The catalase cDNA and the promotor region of the catalase gene in the U and H rat were sequenced (246) and yielded one nucleotide mutation in the 5’-UTR region of the U rat cDNA and a heterozygocity in the U rat gene promoter. Therefore, the decreased catalase mRNA levels may result from different regulation of transcription (promotor), and the difference in the electrophoretic mobility in zymograms (241) may be a result of post-translational modifications of the catalase protein.
Using an inbred Sprague-Dawley strain (L) with about 20% skeletal malformations when the mother is diabetic, and inbred Wistar Furth rats (no diabetes-inducible skeletal malformations), a global gene linkage analysis of the skeletal malformations was performed with micro-satellites, a study which yielded strong coupling of the malformations to seven regions on chromosomes 4, 10, 14, 18, and 19, and a weaker coupling to 14 other loci in the genome. Altogether we found loci on 16 chromosomes. Searching for candidate genes within a distance of 10 cM from each micro-satellite yielded 18 genes that had been implicated in previous studies of diabetic embryopathy. These genes were involved in embryonic development/morphogenesis (Map1b, Shh, Tgfb3, Vegfa, Dvl2, Nf1, Gsk3b, Gap43, Tgfbr3, Gdf1, Csf1r) (247–253), regulation of DNA/RNA metabolism (En2, Brcc3, Tp53) (166,247,254–256), regulation of apoptosis (Nol3, Bak1) (247), and cellular metabolism (Folr1, Akr1b1) (170,254,257).
Gene expression
Altered gene expression in the offspring has been demonstrated in several studies (247,250,252–254,258–262) and appears to be an integral component of the diabetic embryopathy (263). Pax3 gene expression was found to be reduced in embryos of diabetic mice (77,78), and this transcription factor may regulate the gene expression of the licensing factor cdc-46 (264) and a gene, Dep-1 (265), as well as p53 (255), all of which may be of importance for a correct neural tube closure. Null mutation of the Pax3 gene yields the Splotch mouse displaying neural tube defects (77,266). It has also been shown that the decreased Pax3 expression in embryos of diabetic mice could be normalized by treatment of the mother with antioxidants (202), thereby demonstrating a coupling between ROS excess and a teratologically important change in gene expression. In a later study, neural crest cells (NCCs) of Pax3-deficient embryos displayed impaired migration and increased apoptosis. Suppression of p53, either by null mutation of the p53 gene, or administration of a p53 inhibitor, pifithrin-alpha, prevented the defective NCC migration and apoptosis in Pax3-deficient embryos, and also restored proper development of cardiac outflow tracts. Pax3 thus appears required for cardiac outflow tract septation because it blocks p53 expression during NCC migration (256).
The embryonic expression of genes controlling the defense against oxidative stress is sensitive to maternal diabetes. Thus, in a study of pregnant diabetic rats, the embryos demonstrated decreased expression of CuZnSOD and MnSOD (267). In addition, the expression of Gpx-1 and Gpx-2 was decreased compared with embryos from normal rats, enzymes that function in the detoxification of hydrogen peroxide, an important antioxidant system. The decrease in gene expression of Gpx-1 was further enhanced in the malformed embryos. The immunostaining of Gpx-1 displayed a general accumulation of positive cells in the cardiac tissue of all embryos. However, the non-malformed embryos had less staining than embryos from normal rats, and malformed embryos from diabetic rats had almost no staining at all (267).
In a study of cardiac malformations in diabetic mouse pregnancy demonstrating dilated heart tube, smaller ventricles, conotruncal stenosis, and abnormal heart looping, ventricular septal defects were observed and actors in the TGF-β signaling that regulate heart development were down-regulated by maternal diabetes. It was concluded that the TGF-β signaling is involved in cardiac malformations in diabetic embryopathy (250). Also, in a study of global gene expression in a transgenic mouse model of caudal dysgenesis, and in a pharmacological model using in situ hybridization and quantitative real-time PCR, altered expression of several molecules that control developmental processes and embryonic growth was observed. The most pronounced finding was that of altered Wnt signaling, which suggests that impaired signaling in this pathway may be involved in diabetic embryopathy (252). A genome-wide investigation of gene expression in embryos from normal and diabetic mice followed by quantitative RT-PCR yielded several genes with altered expression. Sequence motifs in the promoters of diabetes-affected genes suggest potential binding of transcription factors that are involved in responses to oxidative stress and/or to hypoxia, and, furthermore, around 30% of the diabetes-affected genes encoded transcription factors and chromatin-modifying proteins or components of signaling pathways that affect transcription (258). In a genome-wide expression profiling in the developing heart of embryos from diabetic and control mice it was found that a total of 878 genes exhibited more than 1.5-fold changes in expression level in the hearts of experimental embryos compared with their respective controls. Several genes involved in a number of molecular signaling pathways such as apoptosis, proliferation, migration, and differentiation in the developing heart were differentially expressed in embryos of diabetic pregnancy (262).
Several different genes and pathways have been demonstrated to be affected in diabetic pregnancy; however, there is not, as yet, any universal gene identified to be responsible for enhanced (or decreased) susceptibility to diabetic embryopathy (Figure 2).
Epigenetics
There are considerable indications that epigenetic processes play a role in diabetic embryopathy (259,268–274). In a seminal study, it was found that transient hyperglycemia induced long-lasting activating epigenetic changes in the promoter of the NFκB subunit p65 in aortic endothelial cells both in vitro and in non-diabetic mice, resulting in increased p65 gene expression. Both the epigenetic changes and the gene expression changes persisted for at least 6 days of subsequent normoglycemia. Furthermore, the hyperglycemia-induced epigenetic changes and increased p65 expression were prevented by reducing mitochondrial superoxide production or superoxide-induced alpha-oxaldehydes (268).
Analysis of gene expression data from two sets of embryos of diabetic mice suggested that maternal diabetes may increase the overall variability of gene expression levels in embryos. The suggestion was that altered gene expression and increased variability of gene expression together constitute the molecular correlates for the incomplete phenotype penetrance in diabetic pregnancy. Based on this model, it was suggested that maternal diabetes reduces the precision of gene regulation in exposed individuals. Loss of precision in embryonic gene regulation may include changes to the epigenome via deregulated expression of chromatin-modifying factors (259,269).
In a study of maternal diabetes effect on DNA methylation of imprinted genes in oocytes, it was found in SZ-diabetic and NOD mice that the methylation pattern of Peg3 differential methylation regions (DMR) was altered, and in the SZ mice demethylation was observed on day 35 after SZ injection. The expression level of DNA methyltransferases (DNMTs) was also decreased in diabetic oocytes. These results indicate that maternal diabetes has adverse effects on DNA methylation of the maternally imprinted gene Peg3 in oocytes, but also that methylation in the oocytes of the offspring is normal (271). Also, the expression was increased and the methylation level of H19 was decreased, whereas the expression and methylation levels of Peg3 were completely opposite in placentas of diabetic mice. When embryos of normal females were transferred to normal/diabetic pseudopregnant females, the methylation and expression of Peg3 in placentas were also clearly altered in the normal-to-diabetic group compared to the normal-to-normal group. However, when the embryos of diabetic females were transferred to normal pesudopregnant female mice, the methylation and expression of Peg3 and H19 in placentas were similar in the two groups. The data suggest that the effects of maternal diabetes on imprinted genes may primarily be caused by the adverse uterus environment (272).
In a study neural stem cells (NSCs) were exposed to high glucose/hyperglycemia, and alterations were found in chromatin reorganization, global histone H3 status, and global DNA methylation, as well as increased expression of Dcx and Pafah1b1 and decreased expression of four microRNAs targeting these genes. This study suggested that hyperglycemia alters the epigenetic mechanisms in NSCs, resulting in altered expression of developmental genes (273). In a genome-wide survey of histone acetylation in neurulation stage embryos from diabetic mouse pregnancies, it was found that exposure to maternal diabetes and, independently, exposure to high-fat diet were associated with increases and decreases of H3 and H4 histone acetylation, respectively, in the embryo. These data suggest that epigenetic changes in response to diet and metabolic conditions may contribute to increased risk for NTD in diabetic and obese pregnancies (270).
In a study of embryos of pregnant hyperglycemic mice and mouse embryonic stem cells (ESC), the methylation of a Pax3 CpG island was decreased in embryos and ESC. Use of shRNA in ESC demonstrated that DNA methyltransferase 3b (Dnmt3b) was responsible for methylation and silencing of Pax3 prior to differentiation and by oxidative stress. These results indicate that hyperglycemia-induced oxidative stress stimulates Dnmt3b activity, thereby inhibiting chromatin modifications necessary for induction of Pax3 expression, and, thus, providing a molecular mechanism for defects caused by Pax3 insufficiency in diabetic pregnancy (274).
Epigenetic changes in the embryo caused by maternal diabetes are likely to be transferring the teratogenic input of the diabetic environment. Identifying these changes is important both for the increased knowledge generated, and also for the possible anti-teratological treatment that may emerge from the identified mechanisms (Figure 2).
Conclusions and future directions
Diabetic embryopathy has a complex etiology and pathogenesis. The studies of etiologic factors in the pathogenesis of congenital malformations have revealed a scenario in which the diabetic state simultaneously induces alterations in a series of teratogenically capable pathways. These pathways are intertwined, and several of them result in an imbalance of the ROS metabolism, yielding ROS excess in teratogenically sensitive cell populations, an imbalance ultimately causing the congenital malformations. Blocking the ROS excess may therefore be one valid way to diminish the disturbed development caused by the diabetic environment.
Upstream and downstream of the oxidative stress, however, several conditions of cellular stress are present, such as nitrosative, ER, hypoxic, and hexosamine stress, as well as a possible teratogenic involvement of AGEs. In this area of metabolites and pathways, there may be possibilities of finding molecules to use for intervention therapy.
There is also a growing understanding of the diabetes-induced alterations in genetic and epigenetic systems, which will, again, increase our knowledge, and inspire to develop new ways to block diabetic embryopathy in the future.
Disclosure statement
The authors report no conflicts of interest.
Funding information
The authors gratefully acknowledge the support from The Swedish Research Council, The Family Ernfors Fund, The Novo Nordisk Foundation, and The Swedish Diabetes Association.
References
- 1.Andersson A, Eriksson UJ, Jansson L, Sandler S, Welsh M, Welsh N.. Claes Hellerstrom: a friendly islet explorer. Diabetologia. 2007;50:4 p following 496. [PubMed] [Google Scholar]
- 2.Asplund K, Westman S, Hellerstrom C.. Glucose stimulation of insulin secretion from the isolated pancreas of foetal and newborn rats. Diabetologia. 1969;5:260–2. [DOI] [PubMed] [Google Scholar]
- 3.Aoyagi K, Bergsten P, Eriksson UJ, Ebendal T, Hellerström C.. In vitro regulation of insulin release and biosynthesis of fetal rat pancreatic cells explanted on pregnancy day16. Biol Neonate. 1997;71:60–8. [DOI] [PubMed] [Google Scholar]
- 4.Bergsten P, Aoyagi K, Persson E, Eriksson UJ, Hellerström C.. Appearance of glucose-induced insulin release in fetal rat b-cells. J Endocrinol. 1998;158:115–20. [DOI] [PubMed] [Google Scholar]
- 5.Hellerstrom C, Swenne I, Eriksson UJ.. Is there an animal model for gestational diabetes? Diabetes. 1985;34:28–31. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson UJ, Andersson A, Efendic S, Elde R, Hellerström C.. Diabetes in pregnancy: effects on the fetal and newborn rat with particular regard to body weight, serum insulin concentration and pancreatic contents of insulin, glucagon and somatostatin. Acta Endocrinol (Copenh). 1980;94:354–64. [DOI] [PubMed] [Google Scholar]
- 7.Eriksson UJ, Hellerström C, Andersson A.. Diabetes in pregnancy: effects on the maturation of (pro)insulin biosynthesis in fetal and neonatal rat islets. Diabete Metab. 1981;7:243–9. [PubMed] [Google Scholar]
- 8.Eriksson UJ, Dahlstrom E, Larsson KS, Hellerstrom C.. Increased incidence of congenital malformations in the offspring of diabetic rats and their prevention by maternal insulin therapy. Diabetes. 1982;31:1–6. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson UJ, Dahlström E, Hellerström C.. Diabetes in pregnancy: skeletal malformations in the offspring of diabetic rats after intermittent withdrawal of insulin in early gestation. Diabetes. 1983;32:1141–5. [DOI] [PubMed] [Google Scholar]
- 10.Mølsted-Pedersen L, Tygstrups I, Pedersen J.. Congenital malformations in newborn infants of diabetic women: correlation with maternal diabetic vascular complications. Lancet. 1964;1:1124–6. [DOI] [PubMed] [Google Scholar]
- 11.Kucera J. Rate and type of congenital anomalies among offspring of diabetic women. J Reprod Med. 1971;7:61–70. [PubMed] [Google Scholar]
- 12.Mills JL. Malformations in infants of diabetic mothers. Teratology. 1982;25:385–94. [DOI] [PubMed] [Google Scholar]
- 13.Ray JG, O'Brien TE, Chan WS.. Preconception care and the risk of congenital anomalies in the offspring of women with diabetes mellitus: a meta-analysis. Q J Med. 2001;94:435–44. [DOI] [PubMed] [Google Scholar]
- 14.Platt MJ, Stanisstreet M, Casson IF, Howard CV, Walkinshaw S, Pennycook S, et al. . St Vincent's Declaration 10 years on: outcomes of diabetic pregnancies. Diabet Med. 2002;19:216–20. [DOI] [PubMed] [Google Scholar]
- 15.Evers IM, de Valk HW, Visser GH.. Risk of complications of pregnancy in women with type 1 diabetes: nationwide prospective study in the Netherlands. BMJ. 2004;328:915–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verheijen EC, Critchley JA, Whitelaw DC, Tuffnell DJ.. Outcomes of pregnancies in women with pre-existing type 1 or type 2 diabetes, in an ethnically mixed population. Br J Obstet Gynaecol. 2005;112:1500–3. [DOI] [PubMed] [Google Scholar]
- 17.Macintosh MC, Fleming KM, Bailey JA, Doyle P, Modder J, Acolet D, et al. . Perinatal mortality and congenital anomalies in babies of women with type 1 or type 2 diabetes in England, Wales, and Northern Ireland: population based study. BMJ. 2006;333:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy HR, Steel SA, Roland JM, Morris D, Ball V, Campbell PJ, et al. . Obstetric and perinatal outcomes in pregnancies complicated by type 1 and type 2 diabetes: influences of glycaemic control, obesity and social disadvantage. Diabet Med. 2011;28:1060–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knight KM, Thornburg LL, Pressman EK.. Pregnancy outcomes in type 2 diabetic patients as compared with type 1 diabetic patients and nondiabetic controls. J Reprod Med. 2012;57:397–404. [PubMed] [Google Scholar]
- 20.Bell R, Glinianaia SV, Tennant PW, Bilous RW, Rankin J.. Peri-conception hyperglycaemia and nephropathy are associated with risk of congenital anomaly in women with pre-existing diabetes: a population-based cohort study. Diabetologia. 2012;55:936–47 [DOI] [PubMed] [Google Scholar]
- 21.Vinceti M, Malagoli C, Rothman KJ, Rodolfi R, Astolfi G, Calzolari E, et al. . Risk of birth defects associated with maternal pregestational diabetes. Eur J Epidemiol. 2014;29:411–18. [DOI] [PubMed] [Google Scholar]
- 22.Schaefer-Graf UM, Buchanan TA, Xiang A, Songster G, Montoro M, Kjos SL.. Patterns of congenital anomalies and relationship to initial maternal fasting glucose levels in pregnancies complicated by type 2 and gestational diabetes. Am J Obstet Gynecol. 2000;182:313–20. [DOI] [PubMed] [Google Scholar]
- 23.Brydon P, Smith T, Proffitt M, Gee H, Holder R, Dunne F.. Pregnancy outcome in women with type 2 diabetes mellitus needs to be addressed. Int J Clin Pract. 2000;54:418–19. [PubMed] [Google Scholar]
- 24.Dunne F, Brydon P, Smith K, Gee H.. Pregnancy in women with type 2 diabetes: 12 years outcome data 1990-2002. Diabet Med. 2003;20:734–8. [DOI] [PubMed] [Google Scholar]
- 25.Knight KM, Pressman EK, Hackney DN, Thornburg LL.. Perinatal outcomes in type 2 diabetic patients compared with non-diabetic patients matched by body mass index. J Matern Fetal Neonatal Med. 2012;25:611–15. [DOI] [PubMed] [Google Scholar]
- 26.Balsells M, Garcia-Patterson A, Gich I, Corcoy R.. Maternal and fetal outcome in women with type 2 versus type 1 diabetes mellitus: a systematic review and metaanalysis. J Clin Endocrinol Metab. 2009;94:4284–91. [DOI] [PubMed] [Google Scholar]
- 27.Gizzo S, Patrelli TS, Rossanese M, Noventa M, Berretta R, Di Gangi S, et al. . An update on diabetic women obstetrical outcomes linked to preconception and pregnancy glycemic profile: a systematic literature review. ScientificWorldJournal. 2013;2013:254901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blomberg MI, Kallen B.. Maternal obesity and morbid obesity: the risk for birth defects in the offspring. Birth Defects Res A Clin Mol Teratol. 2010;88:35–40. [DOI] [PubMed] [Google Scholar]
- 29.Rankin J, Tennant PW, Stothard KJ, Bythell M, Summerbell CD, Bell R.. Maternal body mass index and congenital anomaly risk: a cohort study. Int J Obes (Lond). 2010;34:1371–80. [DOI] [PubMed] [Google Scholar]
- 30.Brite J, Laughon SK, Troendle J, Mills J.. Maternal overweight and obesity and risk of congenital heart defects in offspring. Int J Obes (Lond). 2014;38:878–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mills JL, Baker L, Goldman AS.. Malformations in infants of diabetic mothers occur before the seventh gestational week. Implications for treatment. Diabetes. 1979;28:292–3. [DOI] [PubMed] [Google Scholar]
- 32.Ylinen K, Aula P, Stenman UH, Kesaniemi-Kuokkanen T, Teramo K.. Risk of minor and major fetal malformations in diabetics with high haemoglobin A1c values in early pregnancy. Br Med J (Clin Res Ed). 1984;289:345–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuhrmann K, Reiher H, Semmler K, Glöckner E.. The effect of intensified conventional insulin therapy before and during pregnancy on the malformation rate in offspring of diabetic mothers. Exp Clin Endocrinol Diabetes. 1984;83:173–7. [DOI] [PubMed] [Google Scholar]
- 34.Leslie RDG, Pyke DA, John PN, White JM.. Hemoglobin A1 in diabetic pregnancy. Lancet. 1978;2:958–9. [Google Scholar]
- 35.Miller E, Hare JW, Cloherty JP, Dunn PJ, Gleason RE, Soeldner JS, et al. . Elevated maternal hemoglobin A1c in early pregnancy and major congenital anomalies in infants of diabetic mothers. N Engl J Med. 1981;304:1331–4. [DOI] [PubMed] [Google Scholar]
- 36.Ylinen K, Raivio K, Teramo K.. Haemoglobin AIc predicts the perinatal outcome in insulin-dependent diabetic pregnancies. Br J Obstet Gynaecol. 1981;88:961–7. [DOI] [PubMed] [Google Scholar]
- 37.Suhonen L, Hiilesmaa V, Teramo K.. Glycaemic control during early pregnancy and fetal malformations in women with type I diabetes mellitus. Diabetologia. 2000;43:79–82. [DOI] [PubMed] [Google Scholar]
- 38.Rose BI, Graff S, Spencer R, Hensleigh P, Fainstat T.. Major congenital anomalies in infants and glycosylated hemoglobin levels in insulin-requiring diabetic mothers. J Perinatol. 1988;8:309–11. [PubMed] [Google Scholar]
- 39.Mills JL, Knopp RH, Simpson JL, Jovanovic-Peterson L, Metzger BE, Holmes LB, et al. . Lack of relation of increased malformation rates in infants of diabetic mothers to glycemic control during organogenesis. N Engl J Med. 1988;318:671–6. [DOI] [PubMed] [Google Scholar]
- 40.Hanson U, Persson B, Thunell S.. Relationship between haemoglobin A1c in early type 1 (insulin-dependent) diabetic pregnancy and the occurrence of spontaneous abortion and fetal malformation in Sweden. Diabetologia. 1990;33:100–4. [DOI] [PubMed] [Google Scholar]
- 41.Kim JN, Runge W, Wells LJ, Lazarow A.. Effects of experimental diabetes on the offspring of the rat. Fetal growth, birth, weight, gestation period and fetal mortality. Diabetes. 1960;9:396–404. [DOI] [PubMed] [Google Scholar]
- 42.Lazarow A, Kim JN, Wells LJ.. Birth weight and fetal mortality in pregnant subdiabetic rats. Diabetes. 1960;9:114–17. [DOI] [PubMed] [Google Scholar]
- 43.Endo A. Teratogenesis in diabetic mice treated with alloxan prior to conception. Arch Environ Health. 1966;12:492–500. [DOI] [PubMed] [Google Scholar]
- 44.Deuchar EM. Embryonic malformation in rats, resulting from maternal diabetes: preliminary observations. J Embryol Exp Morphol. 1977;41:93–9. [PubMed] [Google Scholar]
- 45.Ellington SKL. In vivo and in vitro studies on the effects of maternal fasting during embryonic organogenesis in the rat. J Reprod Fertil. 1980;60:383–8. [DOI] [PubMed] [Google Scholar]
- 46.Brownscheidle M, Wootten V, Mathieu MH, Davis DL, Hofman IA.. The effects of maternal diabetes on fetal maturation and neonatal health. Metabolism. 1983;32(Suppl 1):148–55. [DOI] [PubMed] [Google Scholar]
- 47.Funaki K, Mikamo K.. Developmental-stage-dependent teratogenic effects of maternal spontaneous diabetes in the Chinese hamster. Diabetes. 1983;32:637–43. [DOI] [PubMed] [Google Scholar]
- 48.Goldman AS, Baker L, Piddington R, Marx B, Herold R, Egler J.. Hyperglycemia-induced teratogenesis is mediated by a functional deficiency of arachidonic acid. Proc Natl Acad Sci USA. 1985;82:8227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eriksson UJ, Dahlstrom VE, Lithell HO.. Diabetes in pregnancy: influence of genetic background and maternal diabetic state on the incidence of skeletal malformations in the fetal rat. Acta Endocrinol Suppls (Copenh). 1986;277:66–73. [DOI] [PubMed] [Google Scholar]
- 50.Giavini E, Broccia ML, Prati M, Roversi GD, Vismara C.. Effects of streptozotocin-induced diabetes on fetal development of the rat. Teratology. 1986;34:81–8. [DOI] [PubMed] [Google Scholar]
- 51.Zusman I, Yaffe P, Ornoy A.. The effects of high-sucrose diets and of maternal diabetes on the ultrastructure of the visceral yolk sac endoderm in rat embryos developing in vivo and in vitro. Acta Anat. 1987;128:11–18. [DOI] [PubMed] [Google Scholar]
- 52.Diamond MP, Moley KH, Pellicer A, Vaughn WK, DeCherney AH.. Effects of streptozotocin- and alloxan-induced diabetes mellitus on mouse follicular and early embryo development. J Reprod Fertil. 1989;86:1–10. [DOI] [PubMed] [Google Scholar]
- 53.Eriksson UJ, Bone AJ, Turnbull DM, Baird JD.. Timed interruption of insulin therapy in diabetic BB/E rat pregnancy: effect on maternal metabolism and fetal outcome. Acta Endocrinol (Copenh). 1989;120:800–10. [DOI] [PubMed] [Google Scholar]
- 54.Chartrel NC, Clabaut MT, Boismare FA, Schrub JC.. Uteroplacental hemodynamic disturbances in establishment of fetal growth retardation in streptozocin-induced diabetic rats. Diabetes. 1990;39:743–6. [DOI] [PubMed] [Google Scholar]
- 55.Pampfer S, de Hertogh R, Vanderheyden I, Michiels B, Vercheval M.. Decreased inner cell mass proportion in blastocysts from diabetic rats. Diabetes. 1990;39:471–6. [DOI] [PubMed] [Google Scholar]
- 56.Akashi M, Akazawa S, Akazawa M, Trocino R, Hashimoto M, Maeda Y, et al. . Effects of insulin and myo-inositol on embryo growth and development during early organogenesis in streptozocin-induced diabetic rats. Diabetes. 1991;40:1574–9. [DOI] [PubMed] [Google Scholar]
- 57.Moley KH, Vaughn WK, DeCherney AH, Diamond MP.. Effect of diabetes mellitus on mouse pre-implantation embryo development. J Reprod Fertil. 1991;93:325–32. [DOI] [PubMed] [Google Scholar]
- 58.Otani H, Tanaka O, Tatewaki R, Naora H, Yoneyama T.. Diabetic environment and genetic predisposition as causes of congenital malformations in NOD mouse embryos. Diabetes. 1991;40:1245–50. [DOI] [PubMed] [Google Scholar]
- 59.De Hertogh R, Vanderheyden I, Pampfer S, Robin D, Delcourt J.. Maternal insulin treatment improves pre-implantation embryo development in diabetic rats. Diabetologia. 1992;35:406–8. [DOI] [PubMed] [Google Scholar]
- 60.Lee AT, Plump A, DeSimone C, Cerami A, Bucala R.. A role for DNA mutations in diabetes-associated teratogenesis in transgenic embryos. Diabetes. 1995;44:20–4. [DOI] [PubMed] [Google Scholar]
- 61.Styrud J, Thunberg L, Nybacka O, Eriksson UJ.. Correlations between maternal metabolism and deranged development in the offspring of normal and diabetic rats. Pediatr Res. 1995;37:343–53. [DOI] [PubMed] [Google Scholar]
- 62.Eriksson UJ, Simán CM.. Pregnant diabetic rats fed the antioxidant butylated hydroxytoluene show decreased occurrence of malformations in the offspring. Diabetes. 1996;45:1497–502. [DOI] [PubMed] [Google Scholar]
- 63.Moley K, Chi M, Manchester J, McDougal D, Lowry O.. Alterations of intraembryonic metabolites in preimplantation mouse embryos exposed to elevated concentrations of glucose: a metabolic explanation for the developmental retardation seen in preimplantation embryos from diabetic animals. Biol Reprod. 1996;54:1209–16. [DOI] [PubMed] [Google Scholar]
- 64.Ornoy A, Kimyagarov D, Yaffe P, Abir R, Raz I, Kohen R.. Role of reactive oxygen species in diabetes-induced embryotoxicity: studies on pre-implantation mouse embryos culture in serum from diabetic pregnant women. Isr J Med Sci. 1996;32:1066–73. [PubMed] [Google Scholar]
- 65.Sivan E, Reece EA, Wu YK, Homko CJ, Polansky M, Borenstein M.. Dietary vitamin E prophylaxis and diabetic embryopathy: morphologic and biochemical analysis. Am J Obstet Gynecol. 1996;175:793–9. [DOI] [PubMed] [Google Scholar]
- 66.Viana M, Herrera E, Bonet B.. Teratogenic effects of diabetes mellitus in the rat. Prevention with vitamin E. Diabetologia. 1996;39:1041–6. [DOI] [PubMed] [Google Scholar]
- 67.Hunter SK, Wang Y, Weiner CP, Niebyl J.. Encapsulated beta-islet cells as a bioartificial pancreas to treat insulin-dependent diabetes during pregnancy. Am J Obstet Gynecol. 1997;177:746–52. [DOI] [PubMed] [Google Scholar]
- 68.Malaisse-Lagae F, Vanhoutte C, Rypens F, Louryan S, Malaisse WJ.. Anomalies of fetal development in GK rats. Acta Diabetol. 1997;34:55–60. [DOI] [PubMed] [Google Scholar]
- 69.Simán CM, Eriksson UJ.. Vitamin E decreases the occurrence of malformations in the offspring of diabetic rats. Diabetes. 1997;46:1054–61. [DOI] [PubMed] [Google Scholar]
- 70.Simán CM, Eriksson UJ.. Vitamin C supplementation of the maternal diet reduces the rate of malformation in the offspring of diabetic rats. Diabetologia. 1997;40:1416–24. [DOI] [PubMed] [Google Scholar]
- 71.Sivan E, Lee Y, Wu Y, Reece E.. Free radical scavenging enzymes in fetal dysmorphogenesis among offspring of diabetic rats. Teratology. 1997;56:343–9. [DOI] [PubMed] [Google Scholar]
- 72.Torchinsky A, Toder V, Carp H, Orenstein H, Fein A.. In vivo evidence for the existence of a threshold for hyperglycemia-induced major fetal malformations: relevance to the etiology of diabetic teratogenesis. Early Pregnancy. 1997;3:27–33. [PubMed] [Google Scholar]
- 73.Jawerbaum A, Gonzalez ET, Novaro V, Faletti A, Sinner D, Gimeno MA.. Increased prostaglandin E generation and enhanced nitric oxide synthase activity in the non-insulin-dependent diabetic embryo during organogenesis. Reprod Fertil Dev. 1998;10:191–6. [DOI] [PubMed] [Google Scholar]
- 74.Yang X, Borg LAH, Simán CM, Eriksson UJ.. Maternal antioxidant treatments prevent diabetes-induced alterations of mitochondrial morphology in rat embryos. Anat Rec. 1998;251:303–15. [DOI] [PubMed] [Google Scholar]
- 75.Lee AT, Reis D, Eriksson UJ.. Hyperglycemia induced embryonic dysmorphogenesis correlates with genomic DNA mutation frequency in vitro and in vivo. Diabetes. 1999;48:371–6. [DOI] [PubMed] [Google Scholar]
- 76.Jawerbaum A, Gonzalez ET, Sinner D, Pustovrh C, White V, Gimeno MA.. Diminished PGE2 content, enhanced PGE2 release and defects in 3H-PGE2 transport in embryos from overtly diabetic rats. Reprod Fertil Dev. 2000;12:141–7. [DOI] [PubMed] [Google Scholar]
- 77.Phelan SA, Ito M, Loeken MR.. Neural tube defects in embryos of diabetic mice: role of the Pax-3 gene and apoptosis. Diabetes. 1997;46:1189–97. [DOI] [PubMed] [Google Scholar]
- 78.Fine EL, Horal M, Chang TI, Fortin G, Loeken MR.. Evidence that elevated glucose causes altered gene expression, apoptosis, and neural tube defects in a mouse model of diabetic pregnancy. Diabetes. 1999;48:2454–62. [DOI] [PubMed] [Google Scholar]
- 79.Cockroft DL, Coppola PT.. Teratogenic effect of excess glucose on head-fold rat embryos in culture. Teratology. 1977;16:141–6. [DOI] [PubMed] [Google Scholar]
- 80.Sadler TW. Effects of maternal diabetes on early embryogenesis. II. Hyperglycemia-induced exencephaly. Teratology. 1980;21:349–56. [DOI] [PubMed] [Google Scholar]
- 81.Hod M, Star S, Passonneau JV, Unterman TG, Freinkel N.. Effect of hyperglycemia on sorbitol and myo-inositol content of cultured rat conceptus: failure of aldose reductase inhibitors to modify myo-inositol depletion and dysmorphogenesis. Biochem Biophys Res Commun. 1986;140:974–80. [DOI] [PubMed] [Google Scholar]
- 82.Pinter E, Reece EA, Leranth CZ, Garcia-Segura M, Hobbins JC, Mahoney MJ.. Arachidonic acid prevents hyperglycemia-associated yolk sac damage and embryopathy. Am J Obstet Gynecol. 1986;155:691–702. [DOI] [PubMed] [Google Scholar]
- 83.Ornoy A, Zusman I, Cohen AM, Shafrir E.. Effects of sera from Cohen, genetically determined diabetic rats, streptozotocin diabetic rats and sucrose fed rats on in vitro development of early somite rat embryos. Diabetes Res. 1986;3:43–51. [PubMed] [Google Scholar]
- 84.Eriksson UJ. Importance of genetic predisposition and maternal environment for the occurrence of congenital malformations in offspring of diabetic rats. Teratology. 1988;37:365–74. [DOI] [PubMed] [Google Scholar]
- 85.Eriksson UJ, Wentzel P, Minhas HS, Thornalley PJ.. Teratogenicity of 3-deoxyglucosone and diabetic embryopathy. Diabetes. 1998;47:1960–6. [DOI] [PubMed] [Google Scholar]
- 86.Baker L, Piddington R, Goldman A, Egler J, Moehring J.. Myo-inositol and prostaglandins reverse the glucose inhibition of neural tube fusion in cultured mouse embryos. Diabetologia. 1990;33:593–6. [DOI] [PubMed] [Google Scholar]
- 87.Hashimoto M, Akazawa S, Akazawa M, Akashi M, Yamamoto H, Maeda Y, et al. . Effects of hyperglycaemia on sorbitol and myo-inositol contents of cultured embryos: treatment with aldose reductase inhibitor and myo-inositol supplementation. Diabetologia. 1990;33:597–602. [DOI] [PubMed] [Google Scholar]
- 88.Eriksson UJ, Borg LAH.. Protection by free oxygen radical scavenging enzymes against glucose-induced embryonic malformations in vitro. Diabetologia. 1991;34:325–31. [DOI] [PubMed] [Google Scholar]
- 89.Strieleman PJ, Connors MA, Metzger BE.. Phosphoinositide metabolism in the developing conceptus. Effects of hyperglycemia and scyllo-inositol in rat embryo culture. Diabetes. 1992;41:989–97. [DOI] [PubMed] [Google Scholar]
- 90.Eriksson UJ, Borg LA.. Diabetes and embryonic malformations. Role of substrate-induced free-oxygen radical production for dysmorphogenesis in cultured rat embryos. Diabetes. 1993;42:411–19. [DOI] [PubMed] [Google Scholar]
- 91.Trocino RA, Akazawa S, Ishibashi M, Matsumoto K, Matsuo H, Yamamoto H, et al. . Significance of glutathione depletion and oxidative stress in early embryogenesis in glucose-induced rat embryo culture. Diabetes. 1995;44:992–8. [DOI] [PubMed] [Google Scholar]
- 92.Ellington S. Effects of excess glucose on mammalian post-implantation embryos. Int J Dev Biol. 1997;41:299–306. [PubMed] [Google Scholar]
- 93.Moley K, Chi M, Knudson C, Korsmeyer S, Mueckler M.. Hyperglycemia induces apoptosis in pre-implantation embryos through cell death effector pathways. Nat Med. 1998;4:1421–4. [DOI] [PubMed] [Google Scholar]
- 94.Wentzel P, Eriksson UJ.. Antioxidants diminish developmental damage induced by high glucose and cyclooxygenase inhibitors in rat embryos in vitro. Diabetes. 1998;47:677–84. [DOI] [PubMed] [Google Scholar]
- 95.Moley KH, Chi MM, Mueckler MM.. Maternal hyperglycemia alters glucose transport and utilization in mouse preimplantation embryos. Am J Physiol. 1998;275:E38–47. [DOI] [PubMed] [Google Scholar]
- 96.Sadler TW. Effects of maternal diabetes on early embryogenesis. I. The teratogenic potential of diabetic serum. Teratology. 1980;21:339–47. [DOI] [PubMed] [Google Scholar]
- 97.Sadler TW, Phillips LS, Balkan W, Goldstein S.. Somatomedin inhibitors from diabetic rat serum alter growth and development of mouse embryos in culture. Diabetes. 1986;35:861–5. [DOI] [PubMed] [Google Scholar]
- 98.Rashbass P, Ellington SKL.. Development of rat embryos cultured in serum prepared from rats with streptozotocin-induced diabetes. Teratology. 1988;37:51–61. [DOI] [PubMed] [Google Scholar]
- 99.Mulder EJ, Brader LJ, Verhoef A, Piersma AH, Visser GH, Peters PW.. Whole rat embryo culture in serum from insulin-dependent (Type 1) diabetic women. Toxicol Vitro. 1989;3:221–6. [DOI] [PubMed] [Google Scholar]
- 100.Zusman I, Yaffe P, Raz I, Bar-On H, Ornoy A.. Effects of human diabetic serum on the in vitro development of early somite rat embryos. Teratology. 1989;39:85–92. [DOI] [PubMed] [Google Scholar]
- 101.Goto MP, Goldman AS, Uhing MR.. PGE2 prevents anomalies induced by hyperglycemia or diabetic serum in mouse embryos. Diabetes. 1992;41:1644–50. [DOI] [PubMed] [Google Scholar]
- 102.Styrud J, Eriksson UJ.. Development of rat embryos in culture media containing different concentrations of normal and diabetic rat serum. Teratology. 1992;46:473–83. [DOI] [PubMed] [Google Scholar]
- 103.Buchanan TA, Denno KM, Sipos GF, Sadler TW.. Diabetic teratogenesis. In vitro evidence for a multifactorial etiology with little contribution from glucose per se. Diabetes. 1994;43:656–60. [DOI] [PubMed] [Google Scholar]
- 104.Wentzel P, Eriksson UJ.. Insulin treatment fails to abolish the teratogenic potential of serum from diabetic rats. Eur J Endocrinol. 1996;134:459–66. [DOI] [PubMed] [Google Scholar]
- 105.Wentzel P, Thunberg L, Eriksson UJ.. Teratogenic effect of diabetic serum is prevented by supplementation of superoxide dismutase and N-acetylcysteine in rat embryo culture. Diabetologia. 1997;40:7–14. [DOI] [PubMed] [Google Scholar]
- 106.Takao Y, Akazawa S, Matsumoto K, Takino H, Akazawa M, Trocino RA, et al. . Glucose transporter gene expression in rat conceptus during high glucose culture. Diabetologia. 1993;36:696–706. [DOI] [PubMed] [Google Scholar]
- 107.Trocino RA, Akazawa S, Takino H, Takao Y, Matsumoto K, Maeda Y, et al. . Cellular-tissue localization and regulation of the Glut-1 protein in both the embryo and the visceral yolk sac from normal and experimental diabetic rats during the early postimplantation period. Endocrinology. 1994;134:869–79. [DOI] [PubMed] [Google Scholar]
- 108.Li R, Thorens B, Loeken MR.. Expression of the gene encoding the high-Km glucose transporter 2 by the early postimplantation mouse embryo is essential for neural tube defects associated with diabetic embryopathy. Diabetologia. 2007;50:682–9. [DOI] [PubMed] [Google Scholar]
- 109.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. [DOI] [PubMed] [Google Scholar]
- 110.Pampfer S, Vanderheyden I, McCracken JE, Vesela J, De Hertogh R.. Increased cell death in rat blastocysts exposed to maternal diabetes in utero and to high glucose or tumor necrosis factor-alpha in vitro. Development. 1997;124:4827–36. [DOI] [PubMed] [Google Scholar]
- 111.Moley KH. Diabetes and preimplantation events of embryogenesis. Semin Reprod Endocrinol. 1999;17:137–51. [DOI] [PubMed] [Google Scholar]
- 112.Chi MM, Pingsterhaus J, Carayannopoulos M, Moley KH.. Decreased glucose transporter expression triggers BAX-dependent apoptosis in the murine blastocyst. J Biol Chem. 2000;275:40252–7. [DOI] [PubMed] [Google Scholar]
- 113.Wang Q, Chi MM, Schedl T, Moley KH.. An intercellular pathway for glucose transport into mouse oocytes. Am J Physiol Endocrinol Metab. 2012;302:E1511–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pampfer S. Apoptosis in rodent peri-implantation embryos: differential susceptibility of inner cell mass and trophectoderm cell lineages–a review. Placenta. 2000;21:S3–S10. [DOI] [PubMed] [Google Scholar]
- 115.Herrera E. Lipid metabolism in pregnancy and its consequences in the fetus and newborn. Endocrine. 2002;19:43–55. [DOI] [PubMed] [Google Scholar]
- 116.Sadler TW, Horton WEJ.. Effects of maternal diabetes on early embryogenesis: the role of insulin and insulin therapy. Diabetes. 1983;32:1070–4. [DOI] [PubMed] [Google Scholar]
- 117.Horton WEJ, Sadler TW.. Effects of maternal diabetes on early embryogenesis. Alterations in morphogenesis produced by the ketone body B-hydroxybutyrate. Diabetes. 1983;32:610–16. [DOI] [PubMed] [Google Scholar]
- 118.Lewis NJ, Akazawa S, Freinkel N.. Teratogenesis from beta-hydroxybutyrate during organogenesis in rat embryo organ culture and enhancement by subteratogenic glucose. Diabetes. 1983;32(Suppl 1):11A. [Google Scholar]
- 119.Hunter ES, Sadler TW, Wynn RE.. A potential mechanism of DL-beta-hydroxybutyrate-induced malformations in mouse embryos. Am J Physiol. 1987;253:E72–80. [DOI] [PubMed] [Google Scholar]
- 120.Shum L, Sadler TW.. Embryonic catch-up growth after exposure to the ketone body D,L,-beta-hydroxybutyrate in vitro. Teratology. 1988;38:369–79. [DOI] [PubMed] [Google Scholar]
- 121.Moore DCP, Stanisstreet M, Clarke CA.. Morphological and physiological effects of beta-hydroxybutyrate on rat embryos grown in vitro at different stages. Teratology. 1989;40:237–51. [DOI] [PubMed] [Google Scholar]
- 122.Shum L, Sadler TW.. Biochemical basis for D,L,beta-hydroxybutyrate-induced teratogenesis. Teratology. 1990;42:553–6. [DOI] [PubMed] [Google Scholar]
- 123.Ornoy A, Zaken V, Kohen R.. Role of reactive oxygen species (ROS) in the diabetes-induced anomalies in rat embryos in vitro: reduction in antioxidant enzymes and low-molecular-weight antioxidants (LMWA) may be the causative factor for increased anomalies. Teratology. 1999;60:376–86. [DOI] [PubMed] [Google Scholar]
- 124.Yang X, Borg LAH, Eriksson UJ.. Metabolic alterations in neural tissue of rat embryos exposed to beta-hydroxybutyrate during organogenesis. Life Sci. 1998;62:293–300. [DOI] [PubMed] [Google Scholar]
- 125.Pinter E, Reece EA, Leranth CS, Sanyal MK, Hobbins JC, Mahoney MJ, et al. . Yolk sac failure in embryopathy due to hyperglycemia: ultrastructural analysis of yolk sac differentiation associated with embryopathy in rat conceptuses under hyperglycemic conditions. Teratology. 1986;33:73–84. [DOI] [PubMed] [Google Scholar]
- 126.Engström E, Haglund A, Eriksson UJ.. Effects of maternal diabetes or in vitro hyperglycemia on uptake of palmitic and arachidonic acid by rat embryos. Pediatr Res. 1991;30:150–3. [DOI] [PubMed] [Google Scholar]
- 127.Jawerbaum A, Gonzalez ET, Carolina P, Debora S, Christian P, Gimeno MA.. Diminished levels of prostaglandin E in type I diabetic oocyte-cumulus complexes. Influence of nitric oxide and superoxide dismutase. Reprod Fertil Dev. 1999;11:105–10. [DOI] [PubMed] [Google Scholar]
- 128.Wentzel P, Welsh N, Eriksson UJ.. Developmental damage, increased lipid peroxidation, diminished cyclooxygenase-2 gene expression, and lowered PGE2 levels in rat embryos exposed to a diabetic environment. Diabetes. 1999;48:813–20. [DOI] [PubMed] [Google Scholar]
- 129.White V, Jawerbaum A, Sinner D, Pustovrh C, Capobianco E, Gonzalez E.. Oxidative stress and altered prostanoid production in the placenta of streptozotocin-induced diabetic rats. Reprod Fertil Dev. 2002;14:117–23. [DOI] [PubMed] [Google Scholar]
- 130.Reece EA, Wu YK, Wiznitzer A, Homko C, Yao J, Borenstein M, et al. . Dietary polyunsaturated fatty acid prevents malformations in offspring of diabetic rats. Am J Obstet Gynecol. 1996;175:818–23. [DOI] [PubMed] [Google Scholar]
- 131.Reece AE, Wu YK.. Prevention of diabetic embryopathy in offspring of diabetic rats with use of a cocktail of deficient substrates and an antioxidant. Am J Obstet Gynecol. 1997;176:790–8. [DOI] [PubMed] [Google Scholar]
- 132.Wiznitzer A, Furman B, Mazor M, Reece EA.. The role of prostanoids in the development of diabetic embryopathy. Semin Reprod Endocrinol. 1999;17:175–81. [DOI] [PubMed] [Google Scholar]
- 133.Piddington R, Joyce J, Dhanasekaran P, Baker L.. Diabetes mellitus affects prostaglandin E2 levels in mouse embryos during neurulation. Diabetologia. 1996;39:915–20. [DOI] [PubMed] [Google Scholar]
- 134.Schoenfeld A, Erman A, Warchaizer S, Ovadia J, Bonner G, Hod M, et al. . Yolk sac concentration of prostaglandin E2 in diabetic pregnancy: further clues to the etiology of diabetic embryopathy. Prostaglandins. 1995;50:121–6. [DOI] [PubMed] [Google Scholar]
- 135.Pinter E, Reece EA, Ogburn PJ, Turner S, Hobbins JC, Mahoney MJ, et al. . Fatty acid content of yolk sac and embryo in hyperglycemia-induced embryopathy and effect of arachidonic acid supplementation. Am J Obstet Gynecol. 1988;159:1484–90. [DOI] [PubMed] [Google Scholar]
- 136.Khandelwal M, Reece EA, Wu YK, Borenstein M.. Dietary myo-inositol therapy in hyperglycemia-induced embryopathy. Teratology. 1998;57:79–84. [DOI] [PubMed] [Google Scholar]
- 137.Jawerbaum A, Gonzalez E.. The role of alterations in arachidonic acid metabolism and nitric oxide homeostasis in rat models of diabetes during early pregnancy. Curr Pharm Des. 2005;11:1327–42. [DOI] [PubMed] [Google Scholar]
- 138.Hagay ZJ, Weiss Y, Zusman I, Peled-Kamar M, Reece EA, Eriksson UJ, et al. . Prevention of diabetes-associated embryopathy by overexpression of the free radical scavenger copper zinc superoxide dismutase in transgenic mouse embryos. Am J Obstet Gynecol. 1995;173:1036–41. [DOI] [PubMed] [Google Scholar]
- 139.Weng H, Li X, Reece EA, Yang P.. SOD1 suppresses maternal hyperglycemia-increased iNOS expression and consequent nitrosative stress in diabetic embryopathy. Am J Obstet Gynecol. 2012;206:448e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhao Z, Eckert RL, Reece EA.. Reduction in embryonic malformations and alleviation of endoplasmic reticulum stress by nitric oxide synthase inhibition in diabetic embryopathy. Reprod Sci. 2012;19:823–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cao Y, Zhao Z, Eckert RL, Reece EA.. The essential role of protein kinase Cdelta in diabetes-induced neural tube defects. J Matern Fetal Neonatal Med. 2012;25:2020–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang F, Reece EA, Yang P.. Superoxide dismutase 1 overexpression in mice abolishes maternal diabetes-induced endoplasmic reticulum stress in diabetic embryopathy. Am J Obstet Gynecol. 2013;209:345e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Horal M, Zhang Z, Stanton R, Virkamaki A, Loeken MR.. Activation of the hexosamine pathway causes oxidative stress and abnormal embryo gene expression: involvement in diabetic teratogenesis. Birth Defects Res A Clin Mol Teratol. 2004;70:519–27. [DOI] [PubMed] [Google Scholar]
- 144.Frank LA, Sutton-McDowall ML, Gilchrist RB, Thompson JG.. The effect of peri-conception hyperglycaemia and the involvement of the hexosamine biosynthesis pathway in mediating oocyte and embryo developmental competence. Mol Reprod Dev. 2014;81:391–408. [DOI] [PubMed] [Google Scholar]
- 145.Moley KH. Hyperglycemia and apoptosis: mechanisms for congenital malformations and pregnancy loss in diabetic women. Trends Endocrinol Metab. 2001;12:78–82. [DOI] [PubMed] [Google Scholar]
- 146.Sun F, Kawasaki E, Akazawa S, Hishikawa Y, Sugahara K, Kamihira S, et al. . Apoptosis and its pathway in early post-implantation embryos of diabetic rats. Diabetes Res Clin Pract. 2005;67:110–18. [DOI] [PubMed] [Google Scholar]
- 147.Gareskog M, Eriksson UJ, Wentzel P.. Combined supplementation of folic acid and vitamin E diminishes diabetes-induced embryotoxicity in rats. Birth Defects Res A Clin Mol Teratol. 2006;76:483–90. [DOI] [PubMed] [Google Scholar]
- 148.Li X, Weng H, Xu C, Reece EA, Yang P.. Oxidative stress-induced JNK1/2 activation triggers proapoptotic signaling and apoptosis that leads to diabetic embryopathy. Diabetes. 2012;61:2084–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang P, Li X, Xu C, Eckert RL, Reece EA, Zielke HR, et al. . Maternal hyperglycemia activates an ASK1-FoxO3a-caspase 8 pathway that leads to embryonic neural tube defects. Sci Signal. 2013;6:ra74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Oberley LW. Free radicals and diabetes. Free Radic Biol Med. 1988;5:113–24. [DOI] [PubMed] [Google Scholar]
- 151.Gillery P, Monboisse JC, Maquart FX, Borel JP.. Does oxygen free radical increased formation explain long term complications of diabetes mellitus? Med Hypotheses. 1989;29:47–50. [DOI] [PubMed] [Google Scholar]
- 152.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–12. [DOI] [PubMed] [Google Scholar]
- 153.Schmidt AM, Weidman E, Lalla E, Yan SD, Hori O, Cao R, et al. . Advanced glycation endproducts (AGEs) induce oxidant stress in the gingiva: a potential mechanism underlying accelerated periodontal disease associated with diabetes. J Periodontal Res. 1996;31:508–15. [DOI] [PubMed] [Google Scholar]
- 154.West IC. Radicals and oxidative stress in diabetes. Diabet Med. 2000;17:171–80. [DOI] [PubMed] [Google Scholar]
- 155.Palmer AM, Thomas CR, Gopaul N, Dhir S, Änggård EE, Poston L, et al. . Dietary antioxidant supplementation reduces lipid peroxidation but impairs vascular function in small mesenteric arteries of the streptozotocin-diabetic rat. Diabetologia. 1998;41:148–56. [DOI] [PubMed] [Google Scholar]
- 156.Sano T, Umeda F, Hashimoto T, Nawata H, Utsumi H.. Oxidative stress measurements by in vivo electron spin resonance spectroscopy in rats with streptozotocin-induced diabetes. Diabetologia. 1998;41:1355–60. [DOI] [PubMed] [Google Scholar]
- 157.Elangovan V, Shohami E, Gati I, Kohen R.. Increased hepatic lipid soluble antioxidant capacity as compared to other organs of streptozotocin-induced diabetic rats: a cyclic voltammetry study. Free Radic Res. 2000;32:125–34. [DOI] [PubMed] [Google Scholar]
- 158.Wentzel P, Eriksson UJ.. A diabetes-like environment increases malformation rate and diminishes prostaglandin E(2) in rat embryos: reversal by administration of vitamin E and folic acid. Birth Defects Res A Clin Mol Teratol. 2005;73:506–11. [DOI] [PubMed] [Google Scholar]
- 159.Ishibashi M, Akazawa S, Sakamaki H, Matsumoto K, Yamasaki H, Yamaguchi Y, et al. . Oxygen-induced embryopathy and the significance of glutathione-dependent antioxidant system in the rat embryo during early organogenesis. Free Radic Biol Med. 1997;22:447–54. [DOI] [PubMed] [Google Scholar]
- 160.Zaken V, Kohen R, Ornoy A.. Vitamins C and E improve rat embryonic antioxidant defense mechanism in diabetic culture medium. Teratology. 2001;64:33–44. [DOI] [PubMed] [Google Scholar]
- 161.Sakamaki H, Akazawa S, Ishibashi M, Izumino K, Takino H, Yamasaki H, et al. . Significance of glutathione-dependent antioxidant system in diabetes-induced embryonic malformations. Diabetes. 1999;48:1138–44. [DOI] [PubMed] [Google Scholar]
- 162.Cederberg J, Siman CM, Eriksson UJ.. Combined treatment with vitamin E and vitamin C decreases oxidative stress and improves fetal outcome in experimental diabetic pregnancy. Pediatr Res. 2001;49:755–62. [DOI] [PubMed] [Google Scholar]
- 163.Cederberg J, Eriksson UJ.. Antioxidative treatment of pregnant diabetic rats diminishes embryonic dysmorphogenesis. Birth Defects Res A Clin Mol Teratol. 2005;73:498–505. [DOI] [PubMed] [Google Scholar]
- 164.Ornoy A. Embryonic oxidative stress as a mechanism of teratogenesis with special emphasis on diabetic embryopathy. Reprod Toxicol. 2007;24:31–41. [DOI] [PubMed] [Google Scholar]
- 165.Wentzel P, Wentzel CR, Gareskog MB, Eriksson UJ.. Induction of embryonic dysmorphogenesis by high glucose concentration, disturbed inositol metabolism, and inhibited protein kinase C activity. Teratology. 2001;63:193–201. [DOI] [PubMed] [Google Scholar]
- 166.Gareskog M, Wentzel P.. N-Acetylcysteine and {alpha}-cyano-4-hydroxycinnamic acid alter protein kinase C (PKC)-{delta} and PKC-{zeta} and diminish dysmorphogenesis in rat embryos cultured with high glucose in vitro. J Endocrinol. 2007;192:207–14. [DOI] [PubMed] [Google Scholar]
- 167.Gareskog M, Cederberg J, Eriksson UJ, Wentzel P.. Maternal diabetes in vivo and high glucose concentration in vitro increases apoptosis in rat embryos. Reprod Toxicol. 2007;23:63–74. [DOI] [PubMed] [Google Scholar]
- 168.Suzuki N, Svensson K, Eriksson UJ.. High glucose concentration inhibits migration of rat cranial neural crest cells in vitro. Diabetologia. 1996;39:401–11. [DOI] [PubMed] [Google Scholar]
- 169.Roest PA, van Iperen L, Vis S, Wisse LJ, Poelmann RE, Steegers-Theunissen RP, et al. . Exposure of neural crest cells to elevated glucose leads to congenital heart defects, an effect that can be prevented by N-acetylcysteine. Birth Defects Res A Clin Mol Teratol. 2007;79:231–5. [DOI] [PubMed] [Google Scholar]
- 170.Wentzel P, Gareskog M, Eriksson UJ.. Folic acid supplementation diminishes diabetes- and glucose-induced dysmorphogenesis in rat embryos in vivo and in vitro. Diabetes. 2005;54:546–53. [DOI] [PubMed] [Google Scholar]
- 171.Wiznitzer A, Ayalon N, Hershkovitz R, Khamaisi M, Reece EA, Trischler H, et al. . Lipoic acid prevention of neural tube defects in offspring of rats with streptozocin-induced diabetes. Am J Obstet Gynecol. 1999;180:188–93. [DOI] [PubMed] [Google Scholar]
- 172.Al Ghafli MH, Padmanabhan R, Kataya HH, Berg B.. Effects of alpha-lipoic acid supplementation on maternal diabetes-induced growth retardation and congenital anomalies in rat fetuses. Mol Cell Biochem. 2004;261:123–35. [DOI] [PubMed] [Google Scholar]
- 173.Sugimura Y, Murase T, Kobayashi K, Oyama K, Hayasaka S, Kanou Y, et al. . Alpha-lipoic acid reduces congenital malformations in the offspring of diabetic mice. Diabetes Metab Res Rev. 2009;25:287–94. [DOI] [PubMed] [Google Scholar]
- 174.Moazzen H, Lu X, Ma NL, Velenosi TJ, Urquhart BL, Wisse LJ, et al. . N-acetylcysteine prevents congenital heart defects induced by pregestational diabetes. Cardiovasc Diabetol. 2014;13:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Morgan SC, Relaix F, Sandell LL, Loeken MR.. Oxidative stress during diabetic pregnancy disrupts cardiac neural crest migration and causes outflow tract defects. Birth Defects Res A Clin Mol Teratol. 2008;82:453–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li X, Weng H, Reece EA, Yang P.. SOD1 overexpression in vivo blocks hyperglycemia-induced specific PKC isoforms: substrate activation and consequent lipid peroxidation in diabetic embryopathy. Am J Obstet Gynecol. 2011;205:84e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yang X, Borg LAH, Eriksson UJ.. Altered metabolism and superoxide generation in neural tissue of rat embryos exposed to high glucose. Am J Physiol. 1997;272:E173–80. [DOI] [PubMed] [Google Scholar]
- 178.Janero DR, Hreniuk D, Sharif HM.. Hydroperoxide-induced oxidative stress impairs heart muscle cell carbohydrate metabolism. Am J Physiol. 1994;266:C179–88. [DOI] [PubMed] [Google Scholar]
- 179.Rivera-Nieves J, Thompson WC, Levine RL, Moss J.. Thiols mediate superoxide-dependent NADH modification of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1999;274:19525–31. [DOI] [PubMed] [Google Scholar]
- 180.Ishii T, Sunami O, Nakajima H, Nishio H, Takeuchi T, Hata F.. Critical role of sulfenic acid formation of thiols in the inactivation of glyceraldehyde-3-phosphate dehydrogenase by nitric oxide. Biochem Pharmacol. 1999;58:133–43. [DOI] [PubMed] [Google Scholar]
- 181.Morgan PE, Dean RT, Davies MJ.. Inhibition of glyceraldehyde-3-phosphate dehydrogenase by peptide and protein peroxides generated by singlet oxygen attack. Eur J Biochem. 2002;269:1916–25. [DOI] [PubMed] [Google Scholar]
- 182.McKenzie SM, Doe WF, Buffinton GD.. 5-Aminosalicylic acid prevents oxidant mediated damage of glyceraldehyde-3-phosphate dehydrogenase in colon epithelial cells. Gut. 1999;44:180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wentzel P, Ejdesjo A, Eriksson UJ.. Maternal diabetes in vivo and high glucose in vitro diminish GAPDH activity in rat embryos. Diabetes. 2003;52:1222–8. [DOI] [PubMed] [Google Scholar]
- 184.Viana M, Aruoma OI, Herrera E, Bonet B.. Oxidative damage in pregnant diabetic rats and their embryos. Free Radic Biol Med. 2000;29:1115–21. [DOI] [PubMed] [Google Scholar]
- 185.Horton WE, Sadler TW.. Mitochondrial alterations in embryos exposed to beta-hydroxybutyrate in whole embryo culture. Anat Rec. 1985;213:94–101. [DOI] [PubMed] [Google Scholar]
- 186.Yang X, Borg LAH, Eriksson UJ.. Altered mitochondrial morphology of rat embryos in diabetic pregnancy. Anat Rec. 1995;241:255–67. [DOI] [PubMed] [Google Scholar]
- 187.Forsberg H, Eriksson UJ, Welsh N.. Apoptosis in embryos of diabetic rats. Pharmacol Toxicol. 1998;83:104–11. [DOI] [PubMed] [Google Scholar]
- 188.Kamimoto Y, Sugiyama T, Kihira T, Zhang L, Murabayashi N, Umekawa T, et al. . Transgenic mice overproducing human thioredoxin-1, an antioxidative and anti-apoptotic protein, prevents diabetic embryopathy. Diabetologia. 2010;53:2046–55. [DOI] [PubMed] [Google Scholar]
- 189.Torchinsky A, Toder V.. TNFalpha in the pathogenesis of diabetes-induced embryopathies: functions and targets. Rev Diabet Stud. 2007;4:200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wu Y, Viana M, Thirumangalathu S, Loeken MR.. AMP-activated protein kinase mediates effects of oxidative stress on embryo gene expression in a mouse model of diabetic embryopathy. Diabetologia. 2012;55:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lee HY, Wei D, Loeken MR.. Lack of metformin effect on mouse embryo AMPK activity: implications for metformin treatment during pregnancy. Diabetes Metab Res Rev. 2014;30:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Menegola E, Broccia ML, Prati M, Ricolfi R, Giavini E.. Glutathione status in diabetes-induced embryopathies. Biol Neonate. 1996;69:293–7. [DOI] [PubMed] [Google Scholar]
- 193.Davis WL, Crawford LA, Cooper OJ, Farmer GR, Thomas DL, Freeman BL.. Ethanol induces the generation of reactive free radicals by neural crest cells in vitro. J Craniofac Genet Dev Biol. 1990;10:277–93. [PubMed] [Google Scholar]
- 194.Chen SY, Sulik KK.. Free radicals and ethanol-induced cytotoxicity in neural crest cells. Alcohol Clin Exp Res. 1996;20:1071–6. [DOI] [PubMed] [Google Scholar]
- 195.Jenkinson PC, Anderson D, Gangolli SD.. Malformations induced in cultured rat embryos by enzymically generated active oxygen species. Teratog Carcinog Mutagen. 1986;6:547–54. [DOI] [PubMed] [Google Scholar]
- 196.Anderson D, Francis AJ.. The modulating effects of antioxidants in rat embryos and Sertoli cells in culture. Basic Life Sci. 1993;61:189–200. [DOI] [PubMed] [Google Scholar]
- 197.Winn LM, Wells PG.. Phenytoin-initiated DNA oxidation in murine embryo culture, and embryoprotection by the antioxidative enzymes superoxide dismutase and catalase: evidence for reactive oxygen species mediated DNA oxidation in the molecular mechanism of phenytoin teratogenecity. Mol Pharmacol. 1995;48:112–20. [PubMed] [Google Scholar]
- 198.Winn LM, Wells PG.. Maternal administration of superoxide dismutase and catalase in phenytoin teratogenicity. Free Radic Biol Med. 1999;26:266–74. [DOI] [PubMed] [Google Scholar]
- 199.Kotch LE, Chen S-E, Sulik KK.. Ethanol-induced teratogenesis: free radical damage as a possible mechanism. Teratology. 1995;52:128–36. [DOI] [PubMed] [Google Scholar]
- 200.Parman T, Wiley MJ, Wells PG.. Free radical-mediated oxidative DNA damage in the mechanism of thalidomide teratogenicity. Nat Med. 1999;5:582–5. [DOI] [PubMed] [Google Scholar]
- 201.Eriksson UJ. Oxidative DNA damage and embryo development. Nat Med. 1999;5:715. doi: 10.1038/10420. [DOI] [PubMed] [Google Scholar]
- 202.Li R, Chase M, Jung SK, Smith PJ, Loeken MR.. Hypoxic stress in diabetic pregnancy contributes to impaired embryo gene expression and defective development by inducing oxidative stress. Am J Physiol Endocrinol Metab. 2005;289:E591–9. [DOI] [PubMed] [Google Scholar]
- 203.Morrow JD, Harris TM, Roberts LJ 2nd. Noncyclooxygenase oxidative formation of a series of novel prostaglandins: analytical ramifications for measurement of eicosanoids. Anal Biochem. 1990;184:1–10. [DOI] [PubMed] [Google Scholar]
- 204.Morrow JD, Awad JA, Boss HJ, Blair IA, Roberts LJ. II. Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc Natl Acad Sci USA. 1992;89:10721–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Davi G, Ciabattoni G, Consoli A, Mezzetti A, Falco A, Santarone S, et al. . In vivo formation of 8-iso-prostaglandin f2alpha and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation. 1999;99:224–9. [DOI] [PubMed] [Google Scholar]
- 206.Wentzel P, Eriksson UJ.. 8-Iso-PGF(2alpha) administration generates dysmorphogenesis and increased lipid peroxidation in rat embryos in vitro. Teratology. 2002;66:164–8. [DOI] [PubMed] [Google Scholar]
- 207.Goh SY, Cooper ME.. Clinical review: The role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metab. 2008;93:1143–52. [DOI] [PubMed] [Google Scholar]
- 208.Coughlan MT, Thorburn DR, Penfold SA, Laskowski A, Harcourt BE, Sourris KC, et al. . RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol. 2009;20:742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wolff SP, Jiang ZY, Hunt JV.. Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic Biol Med. 1991;10:339–52. [DOI] [PubMed] [Google Scholar]
- 210.Thornalley PJ. Advanced glycation and the development of diabetic complications. Unifying the involvement of glucose, methylglyoxal and oxidative stress. Endocrinol Metab. 1996;3:149–66. [Google Scholar]
- 211.Zhang CH, Qian WP, Qi ST, Ge ZJ, Min LJ, Zhu XL, et al. . Maternal diabetes causes abnormal dynamic changes of endoplasmic reticulum during mouse oocyte maturation and early embryo development. Reprod Biol Endocrinol. 2013;11:31. doi: 10.1186/1477-7827-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Li X, Xu C, Yang P.. c-Jun NH2-terminal kinase 1/2 and endoplasmic reticulum stress as interdependent and reciprocal causation in diabetic embryopathy. Diabetes. 2013;62:599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Xu C, Li X, Wang F, Weng H, Yang P.. Trehalose prevents neural tube defects by correcting maternal diabetes-suppressed autophagy and neurogenesis. Am J Physiol Endocrinol Metab. 2013;305:E667–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhao Z. Endoplasmic reticulum stress in maternal diabetes-induced cardiac malformations during critical cardiogenesis period. Birth Defects Res B Dev Reprod Toxicol. 2012;95:1–6. [DOI] [PubMed] [Google Scholar]
- 215.Marshall S, Bacote V, Traxinger RR.. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem. 1991;266:4706–12. [PubMed] [Google Scholar]
- 216.Anderson JW, Nicolosi RJ, Borzelleca JF.. Glucosamine effects in humans: a review of effects on glucose metabolism, side effects, safety considerations and efficacy. Food Chem Toxicol. 2005;43:187–201. [DOI] [PubMed] [Google Scholar]
- 217.Whelan SA, Hart GW.. Proteomic approaches to analyze the dynamic relationships between nucleocytoplasmic protein glycosylation and phosphorylation. Circ Res. 2003;93:1047–58. [DOI] [PubMed] [Google Scholar]
- 218.Yang YR, Suh PG.. O-GlcNAcylation in cellular functions and human diseases. Adv Biol Regul. 2013;54:68–73. [DOI] [PubMed] [Google Scholar]
- 219.Pantaleon M, Tan HY, Kafer GR, Kaye PL.. Toxic effects of hyperglycemia are mediated by the hexosamine signaling pathway and o-linked glycosylation in early mouse embryos. Biol Reprod. 2010;82:751–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Reece EA, Ma XD, Zhao Z, Wu YK, Dhanasekaran D.. Aberrant patterns of cellular communication in diabetes-induced embryopathy in rats: II, apoptotic pathways. Am J Obstet Gynecol. 2005;192:967–72. [DOI] [PubMed] [Google Scholar]
- 221.Yang P, Zhao Z, Reece EA.. Activation of oxidative stress signaling that is implicated in apoptosis with a mouse model of diabetic embryopathy. Am J Obstet Gynecol. 2008;198:130e1–7. [DOI] [PubMed] [Google Scholar]
- 222.Zhao Z, Yang P, Eckert RL, Reece EA.. Caspase-8: a key role in the pathogenesis of diabetic embryopathy. Birth Defects Res B Dev Reprod Toxicol. 2009;86:72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kapron C, Trasler D.. Genetic determinants of teratogen-induced abnormal development in mouse and rat embryos in vitro. Int J Dev Biol. 1997;41:337–44. [PubMed] [Google Scholar]
- 224.Morrison K, Papapetrou C, Hol FA, Mariman EC, Lynch SA, Burn J, et al. . Susceptibility to spina bifida; an association study of five candidate genes. Ann Hum Genet. 1998;62:379–96. [DOI] [PubMed] [Google Scholar]
- 225.Degnbol B, Green A.. Diabetes mellitus among first and second-degree relatives of early onset diabetes. Ann Hum Genet. 1978;42:25–47. [DOI] [PubMed] [Google Scholar]
- 226.Tillil H, Köbberling J.. Age-corrected empirical genetic risk estimates for first degree relatives of IDDM patients. Diabetes. 1982;36:93–9. [DOI] [PubMed] [Google Scholar]
- 227.Warram JH, Krolewski AS, Gottlieb MS, Kahn CR.. Differences in risk of insulin-dependent diabetes in offspring of diabetic mothers and diabetic fathers. N Engl J Med. 1984;311:149–52. [DOI] [PubMed] [Google Scholar]
- 228.Dahlquist G, Blom L, Tuvemo T, Nyström L, Sandström A, Wall S.. The Swedish childhood diabetes study - results from nine year case register and a one year case-referrent study indicating that type 1 (insulin-dependent) diabetes mellitus is associated with both type 2 (non-insulin-dependent) diabetes mellitus and autoimmune disorders. Diabetologia. 1989;32:2–6. [DOI] [PubMed] [Google Scholar]
- 229.McCarthy BJ, Dorman JS, Aston CE.. Investigating genomic imprinting and susceptibility to insulin-dependent diabetes mellitus: an epidemiological approach. Genet Epidemiol. 1991;8:177–86. [DOI] [PubMed] [Google Scholar]
- 230.Pociot F, Nørgård K, Hobolth N, Andersen O, Nerup J.. A nationwide population-based study of the familial aggregation of insulin-dependent diabetes in Denmark. Danish Study Group of Diabetes in Childhood. Diabetologia. 1993;36:870–5. [DOI] [PubMed] [Google Scholar]
- 231.Tuomilehto J, Podar T, Tuomilehto-Wolf E, Virtala E.. Evidence for importance of gender and birth cohort for risk of IDDM in offspring of IDDM parents. Diabetologia. 1995;38:975–82. [DOI] [PubMed] [Google Scholar]
- 232.Comess LJ, Bennett PH, Burch TA, Miller M.. Congenital anomalies and diabetes in the Pima Indians of Arizona. Diabetes. 1969;18:471–7. [DOI] [PubMed] [Google Scholar]
- 233.Chung CS, Myrianthopoulos NC.. Factors affecting risks of congenital malformations. II. Effect of maternal diabetes on congenital malformations. Birth Defects. 1975;11:23–38. [PubMed] [Google Scholar]
- 234.Stoll C, Alembik Y, Dott B, Roth MP.. Study of Down syndrome in 238,942 consecutive births. Ann Genet. 1998;41:44–51. [PubMed] [Google Scholar]
- 235.Narchi H, Kulaylat N.. High incidence of Down's syndrome in infants of diabetic mothers. Arch Dis Child. 1997;77:242–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Pelz J, Kunze J.. Down's syndrome in infants of diabetic mothers. Arch Dis Child. 1998;79:199–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Nelson M, Lessell S, Sadun AA.. Optic nerve hypoplasia and maternal diabetes mellitus. Arch Neurol. 1986;43:20–5. [DOI] [PubMed] [Google Scholar]
- 238.Van Allen MI, Myhre S.. New multiple congenital anomalies syndrome in a stillborn infant of consanguinous parents and a prediabetic pregnancy. Am J Med Genet. 1991;38:523–8. [DOI] [PubMed] [Google Scholar]
- 239.Endo A, Ingalls TH.. Chromosomal anomalies in embryos of diabetic mice. Arch Environ Health. 1968;16:316–25. [DOI] [PubMed] [Google Scholar]
- 240.Yamamoto M, Endo A, Watanabe G, Ingalls TH.. Chromosomal aneuploidies and polyploidies in embryos of diabetic mice. Arch Environ Health. 1971;22:468–75. [DOI] [PubMed] [Google Scholar]
- 241.Eriksson UJ, den Bieman M, Prins JB, van Zutphen LFM.. Differences in susceptibility for diabetes-induced malformations in separated rat colonies of common origin. 4th FELASA Symposium. Lyon, France: Fondation Marcel Mérieux; 1990. p. 53–7. [Google Scholar]
- 242.Cederberg J, Eriksson UJ.. Decreased catalase activity in malformation-prone embryos of diabetic rats. Teratology. 1997;56:350–7. [DOI] [PubMed] [Google Scholar]
- 243.Wyman A, Pinto AB, Sheridan R, Moley KH.. One-cell zygote transfer from diabetic to nondiabetic mouse results in congenital malformations and growth retardation in offspring. Endocrinology. 2008;149:466–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ejdesjo A, Wentzel P, Eriksson UJ.. Genetic and environmental influence on diabetic rat embryopathy. Am J Physiol Endocrinol Metab. 2011;300:E454–67. [DOI] [PubMed] [Google Scholar]
- 245.Ejdesjo A, Wentzel P, Eriksson UJ.. Influence of maternal metabolism and parental genetics on fetal maldevelopment in diabetic rat pregnancy. Am J Physiol Endocrinol Metab. 2012;302:E1198–209. [DOI] [PubMed] [Google Scholar]
- 246.Cederberg J, Galli J, Luthman H, Eriksson UJ.. Increased mRNA levels of Mn-SOD and catalase in embryos of diabetic rats from a malformation-resistant strain. Diabetes. 2000;49:101–17. [DOI] [PubMed] [Google Scholar]
- 247.Jiang B, Kumar SD, Loh WT, Manikandan J, Ling EA, Tay SS, et al. . Global gene expression analysis of cranial neural tubes in embryos of diabetic mice. J Neurosci Res. 2008;86:3481–93. [DOI] [PubMed] [Google Scholar]
- 248.Fu J, Tay SS, Ling EA, Dheen ST.. High glucose alters the expression of genes involved in proliferation and cell-fate specification of embryonic neural stem cells. Diabetologia. 2006;49:1027–38. [DOI] [PubMed] [Google Scholar]
- 249.Liao DM, Ng YK, Tay SS, Ling EA, Dheen ST.. Altered gene expression with abnormal patterning of the telencephalon in embryos of diabetic Albino Swiss mice. Diabetologia. 2004;47:523–31. [DOI] [PubMed] [Google Scholar]
- 250.Zhao Z. Cardiac malformations and alteration of TGFbeta signaling system in diabetic embryopathy. Birth Defects Res B Dev Reprod Toxicol. 2010;89:97–105. [DOI] [PubMed] [Google Scholar]
- 251.Madri JA, Enciso J, Pinter E.. Maternal diabetes: effects on embryonic vascular development–a vascular endothelial growth factor-A-mediated process. Pediatr Dev Pathol. 2003;6:334–41. [DOI] [PubMed] [Google Scholar]
- 252.Pavlinkova G, Salbaum JM, Kappen C.. Wnt signaling in caudal dysgenesis and diabetic embryopathy. Birth Defects Res A Clin Mol Teratol. 2008;82:710–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Reece EA, Ji I, Wu YK, Zhao Z.. Characterization of differential gene expression profiles in diabetic embryopathy using DNA microarray analysis. Am J Obstet Gynecol. 2006;195:1075–80. [DOI] [PubMed] [Google Scholar]
- 254.Sato N, Sugimura Y, Hayashi Y, Murase T, Kanou Y, Kikkawa F, et al. . Identification of genes differentially expressed in mouse fetuses from streptozotocin-induced diabetic pregnancy by cDNA subtraction. Endocr J. 2008;55:317–23. [DOI] [PubMed] [Google Scholar]
- 255.Pani L, Horal M, Loeken MR.. Rescue of neural tube defects in Pax-3-deficient embryos by p53 loss of function: implications for Pax-3- dependent development and tumorigenesis. Genes Dev. 2002;16:676–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Morgan SC, Lee HY, Relaix F, Sandell LL, Levorse JM, Loeken MR.. Cardiac outflow tract septation failure in Pax3-deficient embryos is due to p53-dependent regulation of migrating cardiac neural crest. Mech Dev. 2008;125:757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Fu J, Tay SS, Ling EA, Dheen ST.. Aldose reductase is implicated in high glucose-induced oxidative stress in mouse embryonic neural stem cells. J Neurochem. 2007;103:1654–65. [DOI] [PubMed] [Google Scholar]
- 258.Pavlinkova G, Salbaum JM, Kappen C.. Maternal diabetes alters transcriptional programs in the developing embryo. BMC Genomics. 2009;10:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Salbaum JM, Kappen C.. Neural tube defect genes and maternal diabetes during pregnancy. Birth Defects Res A Clin Mol Teratol. 2010;88:601–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Liang J, Gui Y, Wang W, Gao S, Li J, Song H.. Elevated glucose induces congenital heart defects by altering the expression of tbx5, tbx20, and has2 in developing zebrafish embryos. Birth Defects Res A Clin Mol Teratol. 2010;88:480–6. [DOI] [PubMed] [Google Scholar]
- 261.Nordquist N, Luthman H, Pettersson U, Eriksson UJ.. Linkage study of embryopathy-polygenic inheritance of diabetes-induced skeletal malformations in the rat. Reprod Toxicol. 2012;33:297–307. [DOI] [PubMed] [Google Scholar]
- 262.Vijaya M, Manikandan J, Parakalan R, Dheen ST, Kumar SD, Tay SS.. Differential gene expression profiles during embryonic heart development in diabetic mice pregnancy. Gene. 2013;516:218–27. [DOI] [PubMed] [Google Scholar]
- 263.Kappen C, Salbaum JM.. Gene expression in teratogenic exposures: a new approach to understanding individual risk. Reprod Toxicol. 2014;45:94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Hill AL, Phelan SA, Loeken MR.. Reduced expression of Pax-3 is associated with overexpression of cdc46 in the mouse embryo. Dev Genes Evol. 1998;208:128–34. [DOI] [PubMed] [Google Scholar]
- 265.Cai H, Smola U, Wixler V, Eisenmann-Tappe I, Diaz-Meco M, Moscat J, et al. . Role of diacylglycerol-regulated protein kinase C isotypes in growth factor activation of the Raf-1 protein kinase. Mol Cell Biol. 1997;17:732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Conway S, Henderson D, Copp A.. Pax3 is required for cardiac neural crest migration in the mouse: evidence from the splotch (Sp2H) mutant. Development. 1997;124:505–14. [DOI] [PubMed] [Google Scholar]
- 267.Wentzel P, Gareskog M, Eriksson UJ.. Decreased cardiac glutathione peroxidase levels and enhanced mandibular apoptosis in malformed embryos of diabetic rats. Diabetes. 2008;57:3344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, et al. . Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205:2409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Salbaum JM, Kappen C.. Diabetic embryopathy: a role for the epigenome? Birth Defects Res A Clin Mol Teratol. 2011;91:770–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Salbaum JM, Kappen C.. Responses of the embryonic epigenome to maternal diabetes. Birth Defects Res A Clin Mol Teratol. 2012;94:770–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Ge ZJ, Liang XW, Guo L, Liang QX, Luo SM, Wang YP, et al. . Maternal diabetes causes alterations of DNA methylation statuses of some imprinted genes in murine oocytes. Biol Reprod. 2013;88:117 DOI: 10.1095/biolreprod.112.105981. [DOI] [PubMed] [Google Scholar]
- 272.Ge ZJ, Liang QX, Luo SM, Wei YC, Han ZM, Schatten H, et al. . Diabetic uterus environment may play a key role in alterations of DNA methylation of several imprinted genes at mid-gestation in mice. Reprod Biol Endocrinol. 2013;11:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Shyamasundar S, Jadhav SP, Bay BH, Tay SS, Kumar SD, Rangasamy D, et al. . Analysis of epigenetic factors in mouse embryonic neural stem cells exposed to hyperglycemia. PLoS One. 2013;8:e65945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Wei D, Loeken MR.. Increased DNA methyltransferase 3b (Dnmt3b)-mediated CpG island methylation stimulated by oxidative stress inhibits expression of a gene required for neural tube and neural crest development in diabetic pregnancy. Diabetes. 2014;63:3512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]