Abstract
Cartesian diver microrespirometry was introduced by Claes Hellerström at the Department of Histology/Medical Cell Biology at Uppsala University, Sweden, to determine rates of oxygen consumption in islets of Langerhans. The theory behind this method is touched upon and the main findings described. Glucose-stimulated beta cell respiration significantly contributes to increased ATP generation, which is a prerequisite for stimulated insulin secretion and synthesis. This has had major implications for understanding the beta cell stimulus–secretion coupling.
Keywords: Cartesian diver, glucose, insulin secretion, islets of Langerhans, respiration
Introduction
The majority of our ATP production derives from respiration and oxidative phosphorylation. It is thus essential for research on cell metabolism to have access to techniques accurately determining oxygen consumption. The Cartesian diver technique was developed in the 1930s and 1940s at the Carlsberg Laboratory in Copenhagen to assess rates of oxygen utilization (1,2). The technique was at that time unsurpassed in sensitivity, allowing accurate measurements of respiration in samples of 10 μg living tissue or less. The name, Cartesian diver, was inspired by its similarity to a device putatively developed by Cartesius (René Descartes) for demonstrating buoyancy and ideal gas law. Accordingly, a small gas-filled glass vessel sinks or floats in a fluid-filled sealed container depending on the external pressure applied. Without external pressure, the capillary vessel with gas floats. Applying pressure to the sealed container compresses the gas phase in the capillary vessel and thereby increases its density by forcing liquid into it, thus making it sink. Although a tool for demonstrating a scientific principle, it had an entertaining value and became a popular toy for distraction among those who could afford it.
Cartesian diver theory
The simple principle of this procedure is that the density of a capillary vessel having an enclosed gas space increases as oxygen is consumed by the biological sample, and oxygen consumption rates can be calculated by monitoring this change in density. The apparatus consists of a capillary vessel holding the biological sample in a suitable buffer solution, a gas phase, and an oil seal at the top to prevent gas diffusion in and out of the vessel (2). The vessel (Cartesian diver) is placed in a closed container with a fluid of a given density that is connected to a pressure manometer. The container sits in a water bath that is under precise temperature control. One critical element for the use of the Cartesian diver is that there must be no CO2 present in the compartment with respiring cells since production of CO2 would offset O2 consumption. Therefore the vessel will contain a drop of KOH to absorb CO2 in addition to a drop of buffer containing the respiring tissue in the closed gas compartment below the neck oil seal. A side-drop below the main drop containing respiring tissue may be introduced as well. The side-drop can be mixed with the tissue-containing drop at a given moment, thus allowing a change in the composition of the buffer of the respiring cells. That experimental design allowed the discovery of the stimulatory effect of glucose on beta cell respiration.
Applying pressure to the sealed container with the diver will make the diver sink to the bottom. When reducing the pressure by adjusting the manometer, a state can be achieved at which the diver is buoyant at exactly the same position inside the container. Thus, the density of the diver at such a state of equilibrium will be the same as that of the liquid in the outside container. The manometer recording will reveal the outside pressure required to achieve the same density of the diver as that of the fluid in the container. When the living cells respire, the enclosed gas compartment below the neck oil seal will decrease due to oxygen consumption occurring without parallel release of gaseous CO2 due to its absorption in the KOH drop. This will cause liquid from the outside container to enter the top of the diver (above the oil seal), making the diver density go up. For the next recording, less pressure must be applied to the outside container by the manometer. The recorded value of the manometer will be different from the previous one, and subsequent recordings will give a plot that indicates the respiratory rate with time.
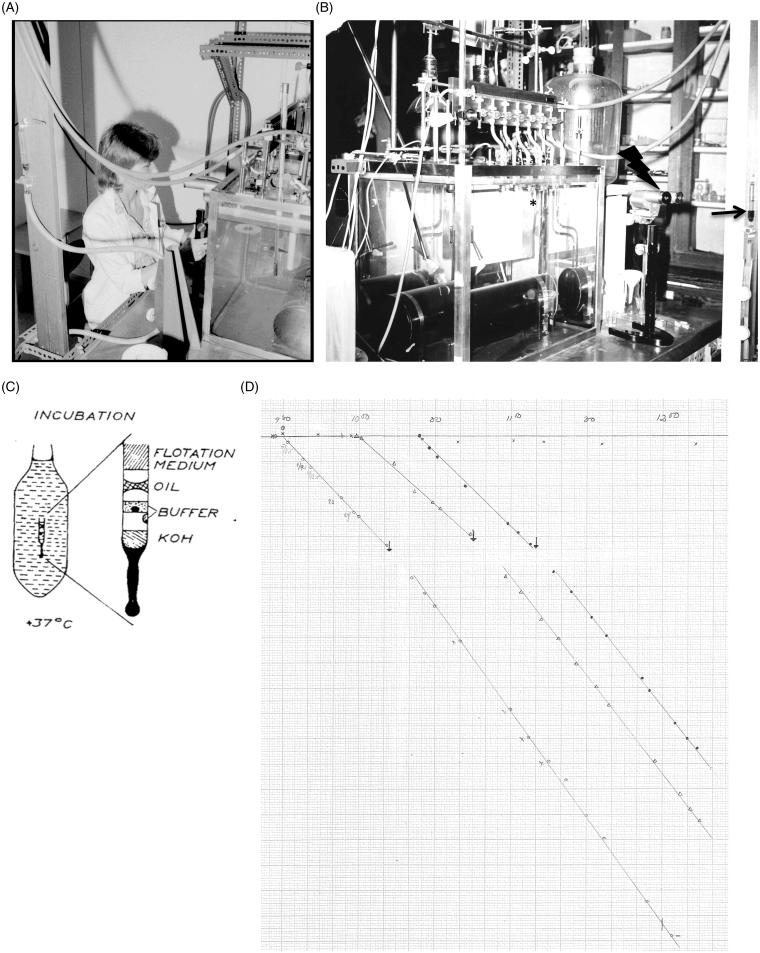
Figure 1(C) shows a schematic view of the experimental setup. The Cartesian diver is a capillary glass tube with an inner diameter of less than 1 mm. The foot is a solid glass structure with a drop of KOH on top. Further above is a side-drop that can be mixed with the drop above containing the islets (or other micro tissues) in a  -free buffer. Above is a drop of oil sealing the compartment below, and above the oil seal there is a third gaseous compartment. Its volume will vary depending on the applied pressure from outside (the manometer) and the oxygen consumption by the islets. Above the top gaseous compartment at the mouth of the diver there is fluid entering from the outer container (Figure 1C). The outer container has been sealed and is only in contact with a manometer (Figure 1B) that allows applying pressure on the container with the diver. Depending on the pressure applied, the diver will sink or float, and the change in buoyancy due to oxygen consumption can thus be recorded at equilibrium at exactly the same position of the diver in the container. For this purpose, a stereomicroscope is used (Figure 1B).
-free buffer. Above is a drop of oil sealing the compartment below, and above the oil seal there is a third gaseous compartment. Its volume will vary depending on the applied pressure from outside (the manometer) and the oxygen consumption by the islets. Above the top gaseous compartment at the mouth of the diver there is fluid entering from the outer container (Figure 1C). The outer container has been sealed and is only in contact with a manometer (Figure 1B) that allows applying pressure on the container with the diver. Depending on the pressure applied, the diver will sink or float, and the change in buoyancy due to oxygen consumption can thus be recorded at equilibrium at exactly the same position of the diver in the container. For this purpose, a stereomicroscope is used (Figure 1B).
Figure 1.
A: Person actively recording respiration by Cartesian diver microrespirometry (Ms Ing-Britt Hallgren). B: Apparatus at the department of Histology/Medical Cell Biology, Uppsala University, Sweden. The star indicates one of six containers with floatation medium that would contain the divers during experimentation. The arrow indicates the manometer that was in contact with the containers. The system was sealed so pressure could be applied to the container while simultaneously recorded. The lightning-bolt indicates the stereomicroscope that accurately could detect the position of the diver when in buoyant equilibrium. C: Schematic view of container containing floatation medium and diver. D: Original protocol showing manometer recordings with time at equilibrium, representing the change in pressure required to compensate for the consumption of oxygen. Three divers with human islets were maintained in a glucose-free medium until stimulated with 16.7 mM glucose by mixing with the side-drops (arrows). Respiratory rates could be determined in relation to islet mass (weight) after retrieval of islets at the end of the experiment.
Claes Hellerström and the Cartesian diver
There were several parallel developments that prompted Hellerström to adopt the Cartesian diver technique from the Carlsberg Laboratory in the 1960s. One highly debated issue at that time was whether glucose stimulated insulin secretion via a ‘glucoreceptor’ or via some intermediate linked to glucose metabolism. Furthermore, the discovery of the microdissection technique using ob/ob mice allowed preparation of viable beta cell-enriched islet material that could be used for functional and biochemical analysis (3). Finally, Bo Hellman and Sven Brolin with co-workers developed tools to analyze islet material for metabolic enzyme activities and metabolic intermediate levels, including those of ATP (4–7). These analyses required complementary determinations of oxygen consumption, and thus times were ready for establishing the Cartesian diver technique at the Department of Histology (now Medical Cell Biology) at Uppsala University. The apparatus constructed is shown in Figure 1(B). It contained a water bath that kept a very constant temperature of 37 °C. At the top front there were six containers (one is indicated by a star) containing the floatation fluid and divers. These were sealed and at their tops connected with a manometer (right, arrow) that could be used to adjust the pressure applied on the containers and thus the divers. There was also a stereomicroscope (marked by a lightning-bolt in Figure 1B) allowing accurate determination of the position of the diver when buoyant in the outer container and thus having the same density as that of the container’s fluid density at the given applied pressure via the manometer.
Findings
The important initial finding was that islet oxygen consumption increased when the glucose concentration of the incubation buffer was increased (8,9). This indicates that glucose not only increases the ATP/ADP ratio but also ATP generation, a finding that was considered intriguing at the time. The prevailing idea was that ATP/ADP ratios were autoregulated and if ATP utilization increased (drop in ATP/ADP) this would increase oxidative phosphorylation and respiration, whereas if ATP/ADP increased, this would inhibit respiration. This apparent peculiarity of beta cell glucose metabolism seems to have several metabolic explanations but nevertheless is of paramount importance for understanding the coupling between glucose metabolism and exocytosis. Thus, in extension of these important observations of glucose and beta cell metabolism, the ATP-dependent K+-channel was discovered, regulating the beta cell membrane potential and thus [Ca2+]i-dependent exocytosis (10). Other metabolic substrates found to increase islet oxygen consumption were citrate, oxaloacetate, d-mannose, d-fructose, l-leucine, 2-ketoisocaproic acid, and 2-aminonorbornane-2-carboxylic acid (BCH) (11–19). The fact that BCH stimulated islet oxygen consumption was particularly intriguing since it was a non-metabolizable leucine analogue and thus its insulin secretory effects were initially considered to support a receptor model for insulin secretion. The observed BCH-stimulation of islet oxygen consumption contradicted this notion but argued that increased metabolism was the cause of insulin secretion also in this setting. The increased metabolic flux was eventually found to depend on allosteric activation of glutamate dehydrogenase, which thereby fuels the tricarboxylic acid cycle and mitochondrial oxidative metabolism (20). Consequently, no discrepancy remained between glucose/amino acid metabolism and insulin secretion.
Willy Malaisse presented the fuel hypothesis of glucose-stimulated insulin release in 1979 (21). During the course of finalizing this Herculean effort he and Abdullah Sener became interested in islet microrespirometry. At that time I was a young PhD student at the Department of Medical Cell Biology, and Hellerström introduced me to this technique. This he did in a very friendly and inspiring manner, and in concert with Willy Malaisse’s interest in islet oxygen consumption determinations I found myself in a very productive and friendly collaboration with Claes Hellerström, Willy Malaisse, and Abdullah Sener, in which we (Hellerström and I) were able to make small contributions to certain aspects of the ‘Fuel Hypothesis’. I have very fond memories of those collaborative experiments occurring early in my career. The hypothesis was exciting, the technique—albeit ‘old-fashioned’—challenging and exhilarating, and the environment intellectually stimulating. My involvement in the collaboration between Claes Hellerström and Willy Malaisse had a major impact on my future development as a scientist.
Subsequent developments in the field
As mentioned above, the field exploded with the characterization and cloning of the ATP-dependent K+-channel, which controls the beta cell membrane potential and thus Ca2+-dependent exocytosis (10). A molecular link between glucose metabolism and insulin granule exocytosis was thus established. A key enzyme relevant for glucose utilization can be found in glucokinase (22). Consequently, genetic changes perturbing beta cell glucokinase activity will result in disturbed glucose tolerance or maturity-onset diabetes in the young (23). A significant contribution of beta cell mitochondrial oxidative metabolism to the coupling between ambient glucose and increased ATP production has also been established (24). Curiously, glucose does not only stimulate insulin secretion via its metabolism but also via a true ‘glucoreceptor’, the sweet taste receptor T1R3 expressed on the beta cell plasma membrane (25,26). This reinforces the complexity of the stimulus–secretion coupling in beta cells involving both cell surface receptor and metabolic events. A recent development has been the introduction of technology from Seahorse Bioscience using the XF analyzer to determine accurately the oxygen consumption and mitochondrial coupling in beta cells (27,28). Accordingly, oxygen-sensing fluorophores are employed to record oxygen concentrations, and thus changes in respiration with or without cell permeabilization can be assessed. Despite the dramatic increase in our knowledge of the underlying events coupling glucose metabolism with insulin secretion, major gaps in knowledge still exist. For example, what processes can explain the increased ATP consumption in glucose-stimulated beta cells? These could be biosynthetic needs, changes in ion fluxes, metabolic turnover—particularly in that of lipid metabolism—and the exocytotic process as such. However, no comprehensive effort to assess the relative importance of these possibilities has been made. Another lingering enigma is the cause and consequence of the metabolic defects commonly observed in type 2 diabetic beta cells. Recent focus has been targeted on understanding the regulation of beta cell proliferation and the exocytotic process, but our gain in understanding disturbances in beta cell metabolism occurring in type 2 diabetes is limited. Potential aberrations could lie in glycolysis, mitochondrial metabolism, and lipid metabolism, but our present knowledge is incomplete and would clearly benefit from future studies elucidating this topic (29,30).
Acknowledgements
The author is grateful to Professors Arne Andersson, Bo Hellman, Anders Tengholm, and Nils Welsh for insightful comments.
Declaration of interest
The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
The study was supported by the Swedish Diabetes Fund and by the Family Ernfors Fund.
References
- 1.Linderstrom-Lang K. On the theory of the cartesian diver microrespirometer. CR Lab Carlsberg, ser chim. 1943;24:334–98. [Google Scholar]
- 2.Holter H. Technique of the Cartesian diver. CR Lab Carlsberg, ser chim. 1943;24:399–478. [Google Scholar]
- 3.Hellerstroem C. A method for the microdissection of intact pancreatic islets of mammals. Acta Endocrinol (Copenh). 1964;45:122–32. [PubMed] [Google Scholar]
- 4.Wettermark G, Borglund E, Brolin SE.. A regenerating system for studies of phosphoryl transfer from ATP. Anal Biochem. 1968;22:211–18. [DOI] [PubMed] [Google Scholar]
- 5.Andersson A, Borglund E, Brolin S.. Effects of glucose on the content of ATP and glycogen and the rate of glucose phosphorylation of isolated pancreatic islets maintained in tissue culture. Biochem Biophys Res Commun. 1974;56:1045–51. [DOI] [PubMed] [Google Scholar]
- 6.Hellman B, Idahl LA.. [Control of ATP levels in stimulated pancreatic B-cells]. Acta Diabetol Lat. 1969;6(Suppl 1):597–611. [PubMed] [Google Scholar]
- 7.Hellman B. Methodological approaches to studies on the pancreatic islets. Diabetologia. 1970;6:110–20. [DOI] [PubMed] [Google Scholar]
- 8.Hellerstrom C. Oxygen consumption of isolated pancreatic islets of mice studied with the cartesian-diver micro-gasometer. Biochem J. 1966;98:7C–9C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hellerstrom C. Effects of carbohydrates on the oxygen consumption of isolated pancreatic islets of mice. Endocrinology. 1967;81:105–12. [DOI] [PubMed] [Google Scholar]
- 10.Rorsman P, Braun M.. Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol. 2013;75:155–79. [DOI] [PubMed] [Google Scholar]
- 11.Hellerstrom C. Effects of glucosamine on the respiration of pancreatic islet B-cells. Acta Endocrinologica. 1968;58:558–64. [PubMed] [Google Scholar]
- 12.Hellerström C, Westman S, Marsden N, Turner D.. Oxygen consumption of the beta-cells in relation to insulin release The structure and metabolism of the pancreatic islets ed. Oxford: Pergamon Press; 1970. [Google Scholar]
- 13.Hellerstrom C, Gunnarsson R.. [Bioenergetics of islet function: oxygen utilization and oxidative metabolism in the beta-cells]. Acta Diabetol Lat. 1970;7(Suppl 1):127–58. [PubMed] [Google Scholar]
- 14.Malaisse WJ, Sener A, Malaisse-Lagae F, Welsh M, Matthews DE, Bier DM, et al. The stimulus-secretion coupling of amino acid-induced insulin release. Metabolic response of pancreatic islets of L-glutamine and L-leucine. J Biol Chem. 1982;257:8731–7. [PubMed] [Google Scholar]
- 15.Malaisse WJ, Sener A, Welsh M, Malaisse-Lagae F, Hellerstrom C, Christophe J.. Mechanism of 3-phenylpyruvate-induced insulin release. Metabolic aspects. Biochem J. 1983;210:921–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malaisse-Lagae F, Welsh M, Lebrun P, Herchuelz A, Sener A, Hellerstrom C, et al. The stimulus-secretion coupling of amino acid-induced insulin release. Secretory and oxidative response of pancreatic islets to L-asparagine. Diabetes. 1984;33:464–9. [DOI] [PubMed] [Google Scholar]
- 17.Sener A, Welsh M, Lebrun P, Garcia-Morales P, Saceda M, Malaisse-Lagae F, et al. Mechanism of 3-phenylpyruvate-induced insulin release. Secretory, ionic and oxidative aspects. Biochem J. 1983;210:913–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellerstrom C, Andersson A, Welsh M.. Respiration of the pancreatic B-cell: effects of glucose and 2-aminonorbornane-2-carboxylic acid. Horm Metab Res Suppl. 1980;(Suppl 10):37–43. [PubMed] [Google Scholar]
- 19.Welsh M, Hellerstrom C, Andersson A.. Respiration and insulin release in mouse pancreatic islets. Effects of L-leucine and 2-ketoisocaproate in combination with D-glucose and L-glutamine. Biochim Biophys Acta. 1982;721:178–84. [DOI] [PubMed] [Google Scholar]
- 20.Sener A, Malaisse WJ.. L-leucine and a nonmetabolized analogue activate pancreatic islet glutamate dehydrogenase. Nature. 1980;288:187–9. [DOI] [PubMed] [Google Scholar]
- 21.Malaisse WJ, Sener A, Herchuelz A, Hutton JC.. Insulin release: the fuel hypothesis. Metabolism. 1979;28:373–86. [DOI] [PubMed] [Google Scholar]
- 22.Garfinkel D, Garfinkel L, Meglasson MD, Matschinsky FM.. Computer modeling identifies glucokinase as glucose sensor of pancreatic beta-cells. Am J Physiol. 1984;247:R527–36. [DOI] [PubMed] [Google Scholar]
- 23.Vionnet N, Stoffel M, Takeda J, Yasuda K, Bell GI, Zouali H, et al. Nonsense mutation in the glucokinase gene causes early-onset non-insulin-dependent diabetes mellitus. Nature. 1992;356:721–2. [DOI] [PubMed] [Google Scholar]
- 24.Sharoyko VV, Abels M, Sun J, Nicholas LM, Mollet IG, Stamenkovic JA, et al. Loss of TFB1M results in mitochondrial dysfunction that leads to impaired insulin secretion and diabetes. Hum Mol Genet. 2014;23:5733–49. [DOI] [PubMed] [Google Scholar]
- 25.Malaisse WJ. Insulin release: the receptor hypothesis. Diabetologia. 2014;57:1287–90. [DOI] [PubMed] [Google Scholar]
- 26.Nakagawa Y, Ohtsu Y, Nagasawa M, Shibata H, Kojima I.. Glucose promotes its own metabolism by acting on the cell-surface glucose-sensing receptor T1R3. Endocr J. 2014;61:119–31. [DOI] [PubMed] [Google Scholar]
- 27.Fred RG, Kappe C, Ameur A, Cen J, Bergsten P, Ravassard P, et al. Role of the AMP kinase in cytokine-induced human EndoC-betaH1 cell death. Mol Cell Endocrinol. 2015;414:53–63. [DOI] [PubMed] [Google Scholar]
- 28.Andersson LE, Valtat B, Bagge A, Sharoyko VV, Nicholls DG, Ravassard P, et al. Characterization of stimulus-secretion coupling in the human pancreatic EndoC-betaH1 beta cell line. PLoS One. 2015;10:e0120879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou CY, Gong Y, Liang J.. Metabolic signaling of insulin secretion by pancreatic beta-cell and its derangement in type 2 diabetes. Eur Rev Med Pharmacol Sci. 2014;18:2215–27. [PubMed] [Google Scholar]
- 30.Halban PA, Polonsky KS, Bowden DW, Hawkins MA, Ling C, Mather KJ, et al. Beta-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care. 2014;37:1751–8. [DOI] [PMC free article] [PubMed] [Google Scholar]