Abstract
Pregnancy is associated with a compensatory increase in beta cell mass. It is well established that somatolactogenic hormones contribute to the expansion both indirectly by their insulin antagonistic effects and directly by their mitogenic effects on the beta cells via receptors for prolactin and growth hormone expressed in rodent beta cells. However, the beta cell expansion in human pregnancy seems to occur by neogenesis of beta cells from putative progenitor cells rather than by proliferation of existing beta cells. Claes Hellerström has pioneered the research on beta cell growth for decades, but the mechanisms involved are still not clarified. In this review the information obtained in previous studies is recapitulated together with some of the current attempts to resolve the controversy in the field: identification of the putative progenitor cells, identification of the factors involved in the expansion of the beta cell mass in human pregnancy, and the relative roles of endocrine factors and nutrients.
Keywords: Beta cells, neogenesis, pregnancy, proliferation, somatolactogenic hormones
Introduction
Pregnancy represents a unique physiological condition with profound changes in the hormonal regulation of metabolism in order to provide sufficient nutrients to the fetus. The increased food intake increases the demand for insulin, which is further reinforced by the elevated levels of the somatolactogenic hormones (prolactin (PRL), growth hormone (GH), placental lactogen (PL), and placental growth hormone (GH-V)). The increased demand for insulin is in normal pregnancy compensated for by an expansion of the beta cell mass. If this does not occur, gestational diabetes will develop. Maternal hyperglycemia is transmitted to the fetus and may induce a premature insulin secretion resulting in macrosomia as well as an up to 8-fold increased risk of developing type 2 diabetes (1). It is therefore pertinent to unravel the mechanisms involved in beta cell adaptation to pregnancy. Through history several investigators have described an increased islet cell mass in various species, and recently several transcriptional and proteomic characterizations of islets from pregnant mice and rats have been performed (2–5) (for a review see Nielsen et al., in press). The scope of this review is to focus on some of the challenges that still remain in the understanding of the mechanisms involved in beta cell adaptation to pregnancy.
Role of hormones and nutrients
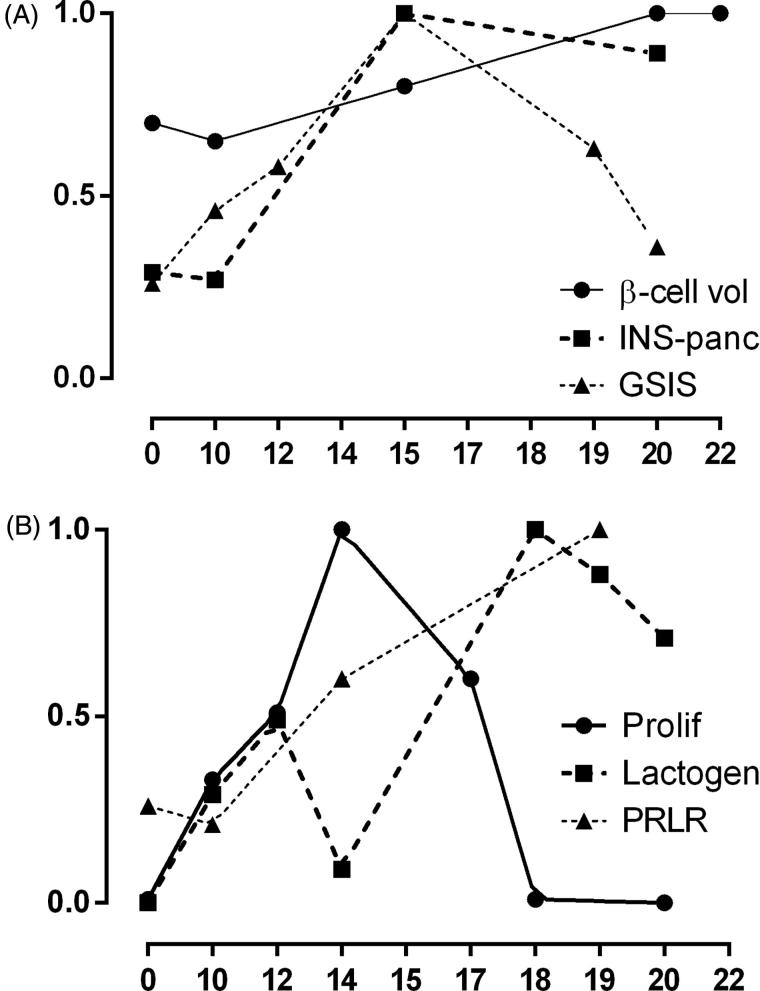
The first systematic quantitative study of the influence of pregnancy and lactation on the endocrine pancreas in mice was published by Claes Hellerström in 1963 (6). The islet volume at gestational day 20 was increased with about 25% compared with virgin mice. The numerical distribution of the islets was asymmetric in both groups, with the majority of islets in the small size class. In pregnant mice the number of islets in the large size class was increased. As the ratio between beta and alpha cells was increased in the pregnant mice it was concluded that the enlargement of the endocrine pancreas was mainly due to proliferation of the beta cells. On day 14 of gestation a significant increase in nuclear size of the beta cells was found, suggesting an increased insulin synthesis activity. This was not seen at day 20, suggesting that the glucose load was reduced at the end of the pregnancy or that the increased number of beta cells was sufficient to maintain euglycemia. The islet volume was still increased by 25% at day 20. In the rat an increased nuclear size was maintained until term, suggesting a sustained high insulin synthesis (7). Increased insulin content and secretory response were found in islets isolated from late pregnant rats (8,9). Data compiled from previous studies have shown a marked increase in the beta cell mass during pregnancy until term (Figure 1A) (10) and a corresponding increase in pancreatic insulin content (Figure 1A) (11). Sorenson and collaborators have demonstrated an increased proliferation of the islet cells during pregnancy in rats (Figure 1B) (12). Interestingly, there was a decline in proliferation late in pregnancy. There was also a decrease in the glucose-stimulated insulin secretion (GSIS) of the perfused pancreas (Figure 1A) (12). In contrast, the levels of lactogenic activity reflecting the secretion of the two placental lactogens PL-1 and PL-2 and pituitary PRL were high until term (Figure 1B) (12), as was the level of prolactin receptor (PRLR) mRNA (Figure 1B) and growth hormone receptor (GHR) mRNAs (data not shown) in the pancreas of pregnant rats (13). It has been suggested that the decreased proliferation may be due to the increased levels of progesterone (14) and/or glucocorticoids in late pregnancy (15), findings that have been supported by in vitro studies in isolated islets. It may be that the very high PL levels prevent the dimerization of the PRLR that is required for signal transduction. Alternatively, the insulin demand may decrease in late pregnancy as it has been reported that the blood glucose is lower close to term (10) and therefore attenuates the metabolic pressure on the beta cells, as already pointed out by Hellerström in 1963 (6). Restricted carbohydrate intake during pregnancy has been found to prevent the increased glucose sensitivity of the beta cells (16), and it has been shown that insulin treatment or food restriction during pregnancy reduces the mitogenic effect of PL on beta cells (17,18). Thus, hyperglycemia seems to play a central role in the proliferative response of the beta cells, and in fact glucose metabolism in the beta cells has been demonstrated to promote beta cell replication (19). The first step in glucose metabolism in beta cells is glucokinase followed by ATP production and closure of K/ATP channels and membrane depolarization. Interestingly, pregnancy is associated with an increase in glucokinase synthesis induced by lactogenic hormones (20), resulting in a lowering in the threshold for glucose-induced insulin secretion. Support for the role of glucokinase in beta cell proliferation comes from studies of activating glucokinase mutations in patients with congenital neonatal hyperinsulinemic hypoglycemia that have hyperplastic islets (21). Thus, the increased demand for insulin reflected in hyperglycemia may be compensated for by an increased glucose metabolism in the beta cells, resulting in increased proliferation and insulin production. This was already predicted by Hellerström in his extensive review from 1977 where he stated: ‘It therefore appears that endocrine factors may be of minor significance for the islet growth in pregnancy’ (22). The role of the lactogenic hormones may be to amplify the stimulating effect of glucose on beta cell growth and function and to provide energy for the growth of the fetus.
Figure 1.
Changes in beta cell area (β-cell vol) (10), pancreatic insulin content (INS-panc) (11), and glucose-stimulated insulin secretion (GSIS) (12) (A); and islet cell proliferation (prolif) (12), circulating lactogenic hormones (lactogen) (12), and pancreatic prolactin receptor mRNA (PRLR) (13) (B) during pregnancy in rats. Pregnancy days are shown on the x-axis, and the fraction of the maximal effect of each parameter is shown on the y-axis. Data from (10–13).
The challenge of human pregnancy
In human pregnancy there is also an increase in the beta cell mass, although only few studies have been performed. In 1978 van Assche and co-workers reported five cases with a 2-fold increase islet mass with an increased number of beta cells (23). In 2010 Peter Butler and co-workers published 18 cases and found a 1.4-fold increase in the beta cell area mainly due to an increased number of small islets and not larger islets and no increase in Ki67-positive beta cells, suggesting that in human pregnancy the beta cell adaptation occurs by neogenesis of beta cells from progenitor cells rather than proliferation of existing beta cells (24). The lack of proliferation of adult human beta cells has been suggested to be due to low expression of PRL receptors, and in fact gene expression profiles for mouse and human beta cells have confirmed that the expression level of PRLR is 40-fold higher in mouse beta cells than in human beta cells (25). However, this may not be the only reason, since a recent study has shown that over-expression of PRLR in human islets did not result in a mitogenic response to PRL (26). Whereas activation of STAT5 has been shown to stimulate proliferation in rat beta cells (27), activation of human STAT5 was not sufficient to stimulate human beta cell proliferation, but surprisingly over-expression of mouse STAT5 did stimulate human beta cell proliferation (26). Apparently there are species differences in the post-receptor PRL signaling pathway as well.
Role of beta cell neogenesis
Studies of pregnancy in other species like cow, pig, sheep, and dog have described the appearance of single beta cells or small clusters associated with ducts or in the acinar compartment, suggesting that neogenesis may contribute to the increased beta cell mass (see Nielsen et al., in press). Using a lineage-tracing approach, evidence for the recruitment of non-beta cell progenitors of beta cells in pregnant mice has been presented (28). In order to investigate if neogenesis of beta cells may contribute to the beta cell adaptation in rodent pregnancy we have recently studied the expression of neurogenin-3 (Ngn-3) that is transiently expressed during the embryonic development of the endocrine pancreas (29) in pancreata of pregnant mice. By immunochemical staining we found a 3.5-fold increase in expression mainly in the acinar compartment at pregnancy day 14 where it was 8-fold higher than in non-pregnant mice (30). Strikingly, this time point coincides with the maximal expression of Ngn-3 in the embryonic pancreas (29). The validity of Ngn-3 as a marker of post-natal beta cell neogenesis is controversial, but even if all the Ngn-3-positive cells differentiated to beta cells their quantitative contribution to the increased beta cell would be small compared to the contribution from the proliferation of the existing beta cells. In order to test if circulating factors in pregnancy contribute to neogenesis we employed the method for large-scale islet isolation from fetal rat pancreata in tissue culture developed by Claes Hellerström and co-workers in 1979 (31). At this late fetal period from gestational day 20 to 22 the number of beta cells is more than doubled mainly by formation of new beta cells from differentiation and proliferation of precursor cells (32). When we added serum from pregnant women in third trimester to cultures of dispersed fetal rat pancreas isolated at gestational day 21.5 we found a marked increase in Ngn-3 mRNA expression, in particular in the fibroblast-like monolayer (30). We are currently searching for factors in serum from pregnant women that influence beta cell growth and function (33).
Concluding remarks
Although much is known about beta cell adaptation to pregnancy, there are still unanswered questions that already were pointed out and investigated by Claes Hellerström decades ago. What is the relative contribution of the somatolactogenic hormones and the food intake to the health of the pregnant woman and her offspring? What is the relative contribution of proliferation and neogenesis of beta cells to the beta cell growth in human pregnancy? What is the identity of the progenitor cells? Can identification of factors that promote beta cell growth and function in pregnancy be useful in the treatment or prevention of gestational diabetes and other forms of diabetes?
Acknowledgements
The author is grateful for the longstanding collaboration and inspiration by the late Professor Claes Hellerström and Professor Arne Andersson.
Disclosure statement
The author reports no conflicts of interest.
Funding information
Work by the author was supported by grants from the Juvenile Diabetes Research Foundation International, European Union 6th Frame Work Program, European Foundation for the Study of Diabetes, Novo Nordisk Foundation, Danish Research Council for Health Sciences, Danish Diabetes Academy funded by the Novo Nordisk Foundation, and Danish Center for Fetal Programming funded by the Danish Research Council for Strategic Research.
References
- 1.Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, et al. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care. 2008;31:340–6. [DOI] [PubMed] [Google Scholar]
- 2.Rieck S, White P, Schug J, Fox AJ, Smirnova O, Gao N, et al. The transcriptional response of the islet to pregnancy in mice. Mol Endocrinol. 2009;23:1702–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schraenen A, de Faudeur G, Thorrez L, Lemaire K, Van Wichelen G, Granvik M, et al. mRNA expression analysis of cell cycle genes in islets of pregnant mice. Diabetologia. 2010;53:2579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim H, Toyofuku Y, Lynn FC, Chak E, Uchida T, Mizukami H, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med. 2010;16:804–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horn S, Kirkegaard JS, Hoelper S, Seymour PA, Rescan C, Nielsen JH, et al. Research resource: a dual proteomic approach identifies regulated islet proteins during beta-cell mass expansion in vivo. Mol Endocrinol. 2016;30:133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hellerström C. The influence of pregnancy and lactation on the endocrine pancreas of mice. Acta Soc Med Uppsal. 1963;68:17–28. [PubMed] [Google Scholar]
- 7.Hellman B. The islets of Langerhans in the rat during pregnancy and lactation, with special reference to the changes in the B/A cell ratio. Acta Obstet Gynecol Scand. 1960;39:331–42. [DOI] [PubMed] [Google Scholar]
- 8.Malaisse WJ, Malaisse-Lagae F, Picard C, Flament-Durand J.. Effects of pregnancy and chorionic growth hormone upon insulin secretion. Endocrinology. 1969;84:41–4. [DOI] [PubMed] [Google Scholar]
- 9.Green IC, Taylor KW.. Effects of pregnancy in the rat on the size and insulin secretory response of the islets of Langerhans. J Endocrinol. 1972;54:317–25. [DOI] [PubMed] [Google Scholar]
- 10.Marynissen G, Aerts L, Van Assche FA.. The endocrine pancreas during pregnancy and lactation in the rat. J Dev Physiol. 1983;5:373–81. [PubMed] [Google Scholar]
- 11.Sutter-Dub MT. Effects of pregnancy and progesterone and/or oestradiol on the insulin secretion and pancreatic insulin content in the perfused rat pancreas. Diabete Metab. 1979;5:47–56. [PubMed] [Google Scholar]
- 12.Parsons JA, Brelje TC, Sorenson RL.. Adaptation of islets of Langerhans to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology. 1992;130:1459–66. [DOI] [PubMed] [Google Scholar]
- 13.Moldrup A, Petersen ED, Nielsen JH.. Effects of sex and pregnancy hormones on growth hormone and prolactin receptor gene expression in insulin-producing cells. Endocrinology. 1993;133:1165–72. [DOI] [PubMed] [Google Scholar]
- 14.Sorenson RL, Brelje TC, Roth C.. Effects of steroid and lactogenic hormones on islets of Langerhans: a new hypothesis for the role of pregnancy steroids in the adaptation of islets to pregnancy. Endocrinology. 1993;133:2227–34. [DOI] [PubMed] [Google Scholar]
- 15.Weinhaus AJ, Bhagroo NV, Brelje TC, Sorenson RL.. Dexamethasone counteracts the effect of prolactin on islet function: implications for islet regulation in late pregnancy. Endocrinology. 2000;141:1384–93. [DOI] [PubMed] [Google Scholar]
- 16.Green IC, Taylor KW.. Insulin secretory response of isolated islets of Langerhans in pregnant rats: effects of dietary restriction. J Endocrinol. 1974;62:137–43. [DOI] [PubMed] [Google Scholar]
- 17.Nieuwenhuizen AG, Schuiling GA, Moes H, Koiter TR.. Role of increased insulin demand in the adaptation of the endocrine pancreas to pregnancy. Acta Physiol Scand. 1997;159:303–12. [DOI] [PubMed] [Google Scholar]
- 18.Nieuwenhuizen AG, Schuiling GA, Seijsener AF, Moes H, Koiter TR.. Effects of food restriction on glucose tolerance, insulin secretion, and islet-cell proliferation in pregnant rats. Physiol Behav. 1999;65:671–7. [DOI] [PubMed] [Google Scholar]
- 19.Porat S, Weinberg-Corem N, Tornovsky-Babaey S, Schyr-Ben-Haroush R, Hija A, Stolovich-Rain M, et al. Control of pancreatic beta cell regeneration by glucose metabolism. Cell Metab. 2011;13:440–9. [DOI] [PubMed] [Google Scholar]
- 20.Weinhaus AJ, Stout LE, Bhagroo NV, Brelje TC, Sorenson RL.. Regulation of glucokinase in pancreatic islets by prolactin: a mechanism for increasing glucose-stimulated insulin secretion during pregnancy. J Endocrinol. 2007;193:367–81. [DOI] [PubMed] [Google Scholar]
- 21.Cuesta-Munoz AL, Huopio H, Otonkoski T, Gomez-Zumaquero JM, Nanto-Salonen K, Rahier J, et al. Severe persistent hyperinsulinemic hypoglycemia due to a de novo glucokinase mutation. Diabetes. 2004;53:2164–8. [DOI] [PubMed] [Google Scholar]
- 22.Hellerström C. Growth pattern of pancreatic islets in animals In: Volk BW, Wellmann KF, editors. The diabetic pancreas. London: Ballière Tidall; 1977. p. 61–97. [Google Scholar]
- 23.Van Assche FA, Aerts L, De Prins F.. A morphological study of the endocrine pancreas in human pregnancy. Brit J Obstet Gynaecol. 1978;85:818–20. [DOI] [PubMed] [Google Scholar]
- 24.Butler AE, Cao-Minh L, Galasso R, Rizza RA, Corradin A, Cobelli C, et al. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia. 2010;53:2167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benner C, van der Meulen T, Caceres E, Tigyi K, Donaldson CJ, Huising MO.. The transcriptional landscape of mouse beta cells compared to human beta cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC Genomics. 2014;15:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen H, Kleinberger JW, Takane KK, Salim F, Fiaschi-Taesch N, Pappas K, et al. Augmented Stat5 signaling bypasses multiple impediments to lactogen-mediated proliferation in human beta-cells. Diabetes. 2015;64:3784–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedrichsen BN, Richter HE, Hansen JA, Rhodes CJ, Nielsen JH, Billestrup N, et al. Signal transducer and activator of transcription 5 activation is sufficient to drive transcriptional induction of cyclin D2 gene and proliferation of rat pancreatic beta-cells. Mol Endocrinol. 2003;17:945–58. [DOI] [PubMed] [Google Scholar]
- 28.Abouna S, Old RW, Pelengaris S, Epstein D, Ifandi V, Sweeney I, et al. Non-beta-cell progenitors of beta-cells in pregnant mice. Organogenesis. 2010;6:125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rukstalis JM, Habener JF.. Neurogenin3: a master regulator of pancreatic islet differentiation and regeneration. Islets. 2009;1:177–84. [DOI] [PubMed] [Google Scholar]
- 30.Sostrup B, Gaarn LW, Nalla A, Billestrup N, Nielsen JH.. Co-ordinated regulation of neurogenin-3 expression in the maternal and fetal pancreas during pregnancy. Acta Obstet Gynecol Scand. 2014;93:1190–7. [DOI] [PubMed] [Google Scholar]
- 31.Hellerstrom CH, Lewis NJ, Borg H, Johnson R, Freinkel N.. Method for large-scale isolation of pancreatic islets by tissue culture of fetal rat pancreas. Diabetes. 1979;28:769–76. [DOI] [PubMed] [Google Scholar]
- 32.Hellerstrom C. The life story of the pancreatic B cell. Diabetologia. 1984;26:393–400. [DOI] [PubMed] [Google Scholar]
- 33.Nalla A, Ringholm L, Sostrup B, Hojrup P, Thim L, Levery SB, et al. Implications for the offspring of circulating factors involved in beta cell adaptation in pregnancy. Acta Obst Gynecol Scand. 2014;93:1181–9. [DOI] [PubMed] [Google Scholar]