Abstract
The hallmark of rheumatoid arthritis (RA) is the progressive destruction of articular joints, characterized by invasive synovial hyperplasia and pathological neovascularization. Here we report that PPI-2458, a member of the fumagillin class of irreversible methionine aminopeptidase-2 (MetAP-2) inhibitors, potently inhibits the proliferation of human fibroblast-like synoviocytes (HFLS-RA), derived from RA patients, with a growth inhibitory concentration 50 (GI50) of 0.04 nM and a maximum inhibition of >95% at 1 nM. Human umbilical vein endothelial cells (HUVEC) are similarly inhibited in proliferation by PPI-2458 (GI50, 0.2 nM). We developed a method to measure the level of MetAP-2 enzyme inhibition after exposure to PPI-2458 and demonstrate that growth inhibition of PPI-2458-sensitive HFLS-RA and HUVEC is linked to MetAP-2 enzyme inhibition, in a dose-dependent fashion. The secretion of several inflammatory mediators such as IL-6 and vascular endothelial growth factor from activated HFLS-RA was not inhibited by PPI-2458. The CNS toxicity profile of PPI-2458, determined by the incidence of seizures, is significantly improved over that of the parental compound TNP-470. In the rat model of peptidoglycan–polysaccharide-induced arthritis, PPI-2458 significantly attenuated paw swelling when therapeutically administered after the onset of chronic disease. We suggest that the mechanism of PPI-2458 action, highly selective and potent anti-proliferative activity on HFLS-RA and HUVEC in vitro, a significantly improved CNS toxicity profile, and marked attenuation of chronic disease in the rat peptidoglycan–polysaccharide arthritis model in vivo, positions this compound as a drug for the treatment of RA.
Rheumatoid arthritis (RA) is a chronic inflammatory disorder with unknown etiology, affecting ≈1% of the U.S. population. The disease is driven by a network of closely connected interdependent pathogenic mechanisms involving innate and adaptive immunity that ultimately leads to synovial inflammation and aggressive synovial hyperproliferation during the terminal destructive phase (1). The progressive destruction of the articular joints is the most prominent and unique feature of RA and distinguishes RA from other chronic forms of arthritis (2–4). These destructive changes are driven by invasive synovial hyperplasia and neovascularization (pannus formation), at the interface between synovium and intraarticular space, in a chronically inflamed microenvironment. During the process of synovial pannus formation, cells of the synovial intima [type A macrophage-like and type B fibroblast-like synoviocytes (FLS)], which form the cellular lining of the inner layer of the joint capsule wall, acquire an aggressive or “transformed” phenotype, undergoing rapid uncontrolled proliferation and invasion into the intraarticular space (3, 5). Furthermore, FLS actively contribute to the inflamed and destructive local microenvironment by secretion of a variety of mediators of inflammation and tissue degradation, such as IL-6 and matrix metalloproteinases (6–8). In response to IL-1 and tumor necrosis factor α (TNF-α), FLS also secrete angiogenic growth factors, such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor, which are essential for pathophysiological neovascularization in RA joints (9, 10). To study the pathophysiological mechanisms involved in chronic inflammatory disorders such as RA, in vitro tissue culture systems of activated HFLS from RA patients (HFLS-RA) have become relevant and useful models (2, 11).
Eukaryotic cells express two methionine aminopeptidase (MetAP) isoforms, MetAP-1 and -2, which are cotranslational regulators of protein synthesis (12–14). MetAPs remove the initiator methionine from growing polypeptide chains. They have the same general substrate specificity, but selective differences are determined by the penultimate residue to the initiator methionine (15, 16). Removal of the N-terminal methionine is a prerequisite for a variety of biological processes, such as activity, subcellular localization, and protein stability. The fungal metabolite fumagillin and several structural analogs such as TNP-470 (AGM-1470) selectively and irreversibly inhibit MetAP-2, via covalent modification of His-231 in the catalytic site of the enzyme (15, 17–19). This inhibition of the MetAP-2 enzyme activity provides the molecular link that triggers the in vitro growth arrest of human umbilical vein endothelial cells (HUVEC) in the late G1 phase of the cell cycle (20, 21). Fumagillin class compounds have also been proven to be potent angiogenesis inhibitors in vivo, and TNP-470 has advanced into human clinical trials for several oncology indications (22).
In this paper, we describe an irreversible inhibitor of MetAP-2, PPI-2458, and its potent antiproliferative activity on HFLS-RA and HUVEC. We demonstrate a CNS toxicity profile markedly improved over that of its parental compound, TNP-470, and further demonstrate significant in vivo activity in a rat model of RA. These features support the potential application of PPI-2458 as a drug for the treatment of RA.
Materials and Methods
Reagents. We synthesized PPI-2458 and a biotinylated analog of PPI-2458 and dissolved them in ethanol (10 mM). Dexamethasone and lipopolysaccharide were obtained from Sigma, TNF-α was obtained from Roche Applied Science, peptidoglycan–polysaccharide (PG-PS) was obtained from Lee Biomolecular Laboratories (San Diego), and [3H]thymidine was obtained from Amersham Pharmacia.
Proliferation Assays. HFLS-RA, derived from RA patients after synovectomy, were purchased from Cell Applications (San Diego). HUVEC and normal human dermal fibroblasts (NHDF-Ad) were purchased from Cambrex (Walkersville, MD). HFLS-RA and NHDF-Ad were routinely cultured in synoviocyte growth medium (Cell Applications) and HUVEC in endothelial growth medium (Cambrex). For proliferation assays, 8,000 cells (16,000 cells/ml) were seeded in 48-well plates (in triplicate). After 48 h, PPI-2458 was added, and fresh medium containing drug was then added every 48 h for a period of 4 (HUVEC) or 7 (HFLS-RA and NHDF-Ad) days. After 6 days (3 days for HUVEC), 1 μCi/well (1 Ci = 37 GBq) [3H]thymidine was added for the final 24 h of incubation. To determine the recovery of HFLS-RA proliferation after incubation with PPI-2458 (10-9, 10-8, and 10-7 M) for 7 days, cells were washed once with drug-free medium and incubated in either drug-free medium or PPI-2458 containing medium for another 7 days. After 13 days, 1 μCi/well [3H]thymidine was added for the final 24 h of incubation. Cell proliferation was determined by the amount of incorporated [3H]thymidine, by using liquid scintillation counting.
Quantitation of VEGF and IL-6 Secretion. To measure the amount of VEGF and IL-6 in supernatants of HFLS-RA, 50,000 cells (200,000 cells/ml) were seeded in wells of a 24-well plate (in triplicate). After 24 h, synoviocyte growth medium was replaced with Ham's F-12 medium containing 1% FBS to arrest cell growth. For VEGF measurements, lipopolysaccharide (10 μg/ml) was added, in the presence or absence of different concentrations of PPI-2458 or dexamethasone, for 60 h. The culture supernatant was collected and the VEGF concentration determined by ELISA (Immuno-Biological Laboratory, Gunma, Japan). For IL-6 measurements, TNF-α (1 ng/ml) was added, in the presence or absence of different concentrations of PPI-2458 or dexamethasone, for 14 h. The amount of secreted IL-6 was determined by using Pierce/Perbio Searchlight Proteome Array technology.
Western Blot Analysis. For Western blot analysis, 20 μg of cellular protein was used. For protein detection in cell lysates, we used MetAP-2 polyclonal antibody CM33 (Zymed), proliferating cell nuclear antigen (PCNA) monoclonal antibody PC-10 (Santa Cruz Biotechnology), and MetAP-1 polyclonal antibody (Mediomics, St. Louis).
MetAP-2 Assay. We have developed an ELISA that measures the amount of free MetAP-2 not covalently bound by PPI-2458 in cell culture samples. In this assay, 10–20 μg of cellular protein was incubated with a biotinylated analog of PPI-2458. This analog covalently binds to the catalytic site of MetAP-2 that has not already been derivatized by PPI-2458. After a 1-h incubation period, the biotinylated MetAP-2–inhibitor complex was captured on a plate with immobilized streptavidin (Pierce). After 1 h, the plates were washed, and the immobilized biotinylated MetAP-2-inhibitor complex was then detected with an anti-MetAP-2 antibody (0.5 μg/ml). After 1-h incubation, horseradish peroxidase-conjugated goat anti-rabbit IgG was added and incubated for 1 h. After several washing steps, 100 μl of TMB substrate [3.5′′–5.5′′ tetramethylbenzidine and peroxidase solution (1:1), Kirkegaard & Perry Laboratories] was added for 10 min. The reaction was stopped by adding 100 μl of 18 M H2SO4. Analysis was performed by determining the absorption of each well at 450 nm by using a Labsystems (Chicago) Multiskan plate spectrophotometer. Human recombinant MetAP-2 (Mediomics), prebound to the biotinylated PPI-2458 analog, was used to generate the standard curve.
Evaluation of Incidence of Seizures. Twenty-four male and 24 female Sprague–Dawley rats (Taconic Farms) were divided equally into six groups, including four per sex per group, for a total of eight rats per treatment group. Treatment groups included untreated controls, vehicle-treated controls, TNP-470 treated at either 6 or 60 mg/kg, and PPI-2458 treated at either 6 or 60 mg/kg. TNP-470 and PPI-2458 were formulated daily with 14% hydroxyl propyl β-cyclodextrin as vehicle. Formulations were administered daily for 14 consecutive days, i.v. via the tail vein, by using a 25-gauge butterfly infusion set (Terumo, Tokyo). Animals were observed daily after dosing for clinical signs of toxicity including aberrant behavior, coat condition/grooming, and CNS effects (seizures, circling, etc.).
PG-PS Antigen-Induced Arthritis. Female Lewis rats (101–121 g) were received from Charles River Breeding Laboratory. Rats were housed two per cage and allowed to acclimate to the animal facility for 3 days after reception. Food and water were available ad libitum. PG-PS (25 mg/kg) was injected i.p. on day 1. Treatment with vehicle (1% propylene glycol/5% dextrose), dexamethasone, or PPI-2458 started on day 15. Dexamethasone (1 mg/kg) was administered s.c. every second day (qod). Vehicle and PPI-2458 (0.25, 1, 5, and 50 mg/kg) were administered orally (po) qod. Paw swelling was monitored by using a plethysmometer (Stoelting) according to instrument specifications. The volumes of the two hind paws were measured and averaged on days 1, 4, 5, 6, 8, 11, 13, 15, 17, 19, 21, 23, 25, 27, 29, and 31. Eight animals were assigned to each group, except the vehicle control (n = 4). All animal studies were approved by the Praecis Pharmaceuticals Incorporated Institutional Animal Care and Use Committee.
Results
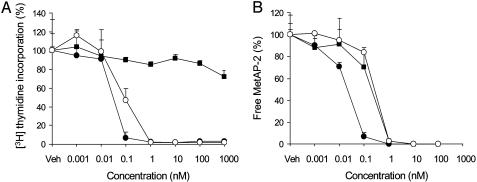
PPI-2458 Is a Potent Inhibitor of HFLS-RA Proliferation. PPI-2458 is a structural analog of the fumagillin class of irreversible MetAP-2 inhibitors (Fig. 1). We determined the antiproliferative activity of PPI-2458 on HFLS-RA in a 7-day proliferation assay (doubling time of HFLS-RA is 52 h). PPI-2458 potently inhibited HFLS-RA proliferation with a GI50 of 0.04 nM and a maximum inhibition of >95% at 1 nM (Fig. 2A). In contrast, NHDF-Ad, which closely resemble normal FLS, are relatively resistant to PPI-2458-mediated growth inhibition, with a maximum inhibition of 25% at 1 μM (Fig. 2 A). To investigate whether PPI-2458 exerts its antiproliferative activity through a cytostatic mechanism similarly to TNP-470, rather than cytotoxicity, we analyzed HFLS-RA after 7 days of PPI-2458 treatment by staining with Annexin V, a marker for early apoptosis, and trypan blue and propidium iodide exclusion. No increase in the number of Annexin V-stained HFLS-RA was observed at 100 nM of PPI-2458 (Fig. 5A, which is published as supporting information on the PNAS web site), and no cellular uptake of either dye was detected at drug concentrations up to 10 μM (data not shown). These data are also consistent with the observation that the growth inhibitory activity of PPI-2458 on HFLS-RA is completely reversible. Removal of the drug for a 7-day period resulted in full restoration of cell proliferation, except at the highest concentration tested (100 nM) (Fig. 5B).
Fig. 1.
Chemical structures of fumagillin, TNP-470, and PPI-2458.
Fig. 2.
PPI-2458-induced growth inhibition in HFLS-RA (•) and HUVEC (○), but not NHDF-Ad (▪) (A), is linked to MetAP-2 enzyme inhibition (B). (A) HFLS-RA and NHDF-Ad (8 × 103 cells) and HUVEC (8 × 103 cells) were incubated with increasing concentrations (10-12 to 10-6 M) of PPI-2458 for 7 and 4 days, respectively. For the final 24 h, 1 μCi/well of [3H]thymidine was added, and proliferation was determined by [3H]thymidine incorporation. The results are representative of at least five independent experiments. (B) The MetAP-2 assay was performed with cell lysates from HFLS-RA, NHDF-Ad, and HUVEC treated exactly as described in A, except that no [3H]thymidine was added. The amount of free MetAP-2 in each cell lysate was measured as described in Materials and Methods. Total amounts of MetAP-2 in PPI-2458-free cell lysates (=100%) were 47 pg/mg of cellular protein in HFLS-RA, 53 pg/mg in NHDF-Ad, and 7 pg/mg in HUVEC.
Fumagillin class compounds, such as TNP-470, have previously been shown to have potent antiproliferative activity on endothelial cells in vitro (15, 20, 23, 24). Our data are in full agreement with these reports, in that PPI-2458 potently inhibited HUVEC proliferation with a GI50 of 0.2 nM and a maximum inhibition of >95% at 1 nM (Fig. 2 A).
MetAP-2 Enzyme Inhibition in HFLS-RA Is Directly Correlated with Growth Inhibition. To determine whether the growth inhibitory activity of PPI-2458 is correlated to the level of MetAP-2 enzyme inhibition, we have developed a quantitative assay to determine the amount of free MetAP-2 (not covalently bound to the inhibitor PPI-2458) in cell lysates. This assay takes advantage of the fact that a biotinylated analog of PPI-2458 can bind to the catalytic site of the MetAP-2 enzyme only if this site has not already been derivatized by PPI-2458. This allows for the direct measurement of MetAP-2 enzyme inhibition in PPI-2458 treated cells. In vitro activity assays confirmed that MetAP-2 catalyzed cleavage of synthetic tripeptide substrates was inhibited by the biotinylated PPI-2458 analog (data not shown). This is consistent with previous reports that fluorescently tagged fumagillin covalently modifies His-231 in the catalytic site of MetAP-2 and inhibits its enzymatic activity (19). As shown in Fig. 2B, the growth inhibition of HFLS-RA and HUVEC is directly correlated with the degree of MetAP-2 enzyme inhibition, in that the GI50 for HFLS-RA and HUVEC proliferation and the IC50 for MetAP-2 enzyme inhibition are similarly achieved at 0.04 and 0.2 nM of PPI-2458, respectively. These data suggest a critical regulatory function for MetAP-2 in HFLS-RA and HUVEC proliferation. Despite a similar dose–response curve for MetAP-2 enzyme inhibition in NHDF-Ad (IC50 0.2 nM) (Fig. 2B), these cells were resistant to PPI-2458-induced growth inhibition.
MetAP-2 Protein Levels Are Not Down-Regulated by PPI-2458. We performed Western blot analysis to determine the cellular levels of MetAP-2 in PPI-2458 growth-inhibited HFLS-RA and resistant NHDF-Ad. After prolonged exposure to PPI-2458, MetAP-2 protein levels were moderately up-regulated in HFLS-RA (Fig. 6A, which is published as supporting information on the PNAS web site), but only slightly affected in NHDF-Ad (Fig. 6B). After prolonged exposure of HUVEC to PPI-2458, MetAP-2 protein levels were up-regulated at concentrations ≥1 nM (Fig. 6C). This finding is consistent with previous data that demonstrated an increase in cellular MetAP-2 protein levels in endothelial cells after incubation with fumagillin (24). No changes in the protein levels of MetAP-1, the second Met-AP isoform expressed in eukaryotic cells, were detected in NDHF-Ad after exposure to PPI-2458 (Fig. 6B). In contrast, MetAP-1 levels in HUVEC were markedly down-regulated after prolonged exposure to PPI-2458, with a sharp decrease occurring between 0.1 and 1 nM of drug, the dose range where 50% growth inhibition and MetAP-2 enzyme inhibition were achieved (Fig. 6C). No change in MetAP-1 protein levels was observed after exposure of up to 100 nM PPI-2458 for 24 h (data not shown), consistent with data showing no change of MetAP-1 protein levels in endothelial cells after exposure to up to 100 nM fumagillin for 24 h (24). MetAP-1 was also down-regulated in a dose-dependent manner in HFLS-RA, although to a lesser extent than in HUVEC (Fig. 6A).
Down-Regulation of PCNA Protein Expression After MetAP-2 Enzyme Inhibition in HFLS-RA. PCNA was originally characterized as a polymerase δ and ε processivity factor, and more recently as a cellular platform for multiple proteins involved in DNA processing and cell cycle regulation (25). In HFLS-RA and HUVEC, protein levels of PCNA were markedly down-regulated after PPI-2458 treatment, with a sharp decrease also occurring between 0.1 and 1 nM of drug (Fig. 6 A and C). This similarity identifies the down-regulation of PCNA protein expression as a downstream consequence of MetAP-2 enzyme inhibition in HFLS-RA and HUVEC and provides a molecular link to the proposed mechanism of a G1 cell cycle arrest. There was no detectable down-regulation of PCNA protein levels in PPI-2458-resistant NHDF-Ad (Fig. 6B).
Secretion of Inflammatory Mediators from Stimulated HFLS-RA Are Not Inhibited by PPI-2458. To investigate whether the inhibition of secretion of inflammatory mediators from stimulated HFLS-RA represents a secondary mechanism of PPI-2458 activity, we quantified the secretion of IL-6 and VEGF from stimulated HFLS-RA in vitro. Even at the highest concentration of PPI-2458 tested (100 nM), IL-6 secretion from TNF-α stimulated HFLS-RA was not inhibited (Fig. 7A, which is published as supporting information on the PNAS web site). IL-6 secretion was inhibited, however, in a dose-dependent fashion, by dexamethasone (Fig. 7A). Similarly, PPI-2458 did not inhibit the secretion of the angiogenic factor VEGF from lipopolysaccharide-stimulated HFLS-RA, whereas dexamethasone inhibited the secretion of VEGF in a dose-dependent fashion (Fig. 7B), as reported (9, 10).
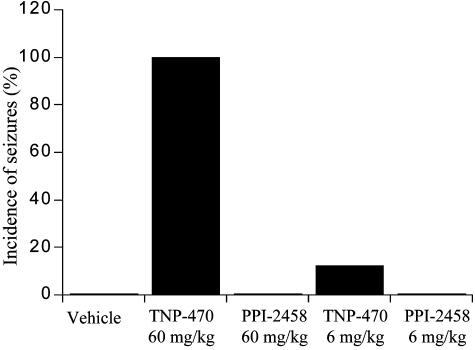
The CNS Toxicity Profile of PPI-2458 Is Significantly Improved over That of TNP-470. PPI-2458 is a structure based on the fumagillin class of compounds that include TNP-470. The clinical development of TNP-470, however, has been severely limited due to dose-limiting CNS toxicity (26–29). PPI-2458 was designed to maintain the potent antiproliferative activity of this class while improving the CNS toxicity profile. The purpose of this experiment was to examine the CNS toxicity profile of PPI-2458 compared to that of TNP-470. No clinical signs of toxicity were observed in animals administered vehicle or untreated. The most significant clinical observation noted was the occurrence of seizures in animals treated with the high dose (60 mg/kg) of TNP-470, beginning after the second dose (Fig. 3). One hundred percent of animals in this dose level of TNP-470 experienced seizures during the study. Clinical signs of seizure occurred within minutes after the dose and included ataxia, twitching, piloerection, salivation, muscle tetanus, wet dog shakes, pawing at the air with front paws, and exophthalmos. These animals were also noted to be easily excited and to have a heightened startle response. Seizures were initially mild with quick recovery, progressing to violent, with recoveries delayed as long as 20 min by study termination. Animals that received TNP-470 at the dose of 60 mg/kg were also noted to be easily excited and to have a heightened startle response. Only one of eight animals (12.5%) treated with TNP-470 at the dose of 6 mg/kg was observed to undergo a seizure in response to drug administration (Fig. 3). This response was not observed until after the 10th dose of TNP-470. In contrast, no animals in the PPI-2458-treated groups (6 or 60 mg/kg) were observed to experience seizures after administration of drug (Fig. 3).
Fig. 3.
Incidence of seizures after PPI-2458 and TNP-470 administration. Sprague–Dawley rats were given PPI-2458 or TNP-470 at doses of 6 or 60 mg/kg i.v. for 14 consecutive days. The animals were observed daily after dosing for clinical signs of toxicity, and the incidence of seizures was recorded for each treatment group (n = 8; four per sex).
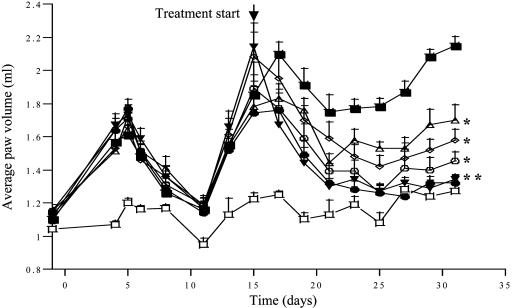
PPI-2458 Has Potent Antiinflammatory Activity in the PG-PS-Induced Model of Arthritis. The ability of PPI-2458 to ameliorate joint swelling and inflammation, measured by paw swelling of the hind limbs, was tested in the rat model of PG-PS antigen-induced arthritis. PG-PS-induced arthritis follows a biphasic mode, with an early acute, predominantly neutrophil-driven phase, which develops within 24–48 h and persists for 4–5 days. This phase is then followed by a chronic T cell-driven phase, characterized by chronic inflammation and progressive erosive synovitis. The treatment of rats with PPI-2458 (0.25, 1, 5, and 50 mg/kg po qod) was started at day 15 after the chronic destructive phase of the disease was established and terminated on day 31. PPI-2458 significantly and dose-dependently attenuated the chronic inflammatory response. Paw volume measurements at day 31 demonstrated that administration of 0.25 mg/kg qod of PPI-2458 achieved ≈50% reduction in paw swelling compared to the PG-PS group treated with vehicle, and 50 mg/kg qod PPI-2458 achieved almost complete protection, similarly to dexamethasone (Fig. 4). Similar efficacy of PPI-2458 was observed when treatment was started on day 7, immediately after the early acute phase of the disease (data not shown).
Fig. 4.
PPI-2458 decreases paw volumes (swelling) in PG-PS-induced arthritis in rats. Arthritis was induced in female Lewis rats by a single injection of PG-PS (25 mg/kg, i.p.) (▪). Treatment was started at day 15, after the onset of chronic disease. Rats were dosed po/qod with vehicle (□), dex (1 mg/kg, po) (▾) or PPI-2458 at 0.25 mg/kg, po (Δ) 1 mg/kg, po (224), 5 mg/kg, po (o), 50 mg/kg, po (•). At day 31, the rats were killed, and paw volumes were measured as described in Materials and Methods. Each point on the graph represents mean ± SEM (n = 8, except vehicle: n = 4). **, P < 0.01, *, P < 0.05 compared with PG-PS-treated rats.
Discussion
The common cellular target of the fumagillin class of molecules is MetAP-2. The selective inhibition of the MetAP-2 enzyme is believed to be the underlying molecular mechanism for the potent antiangiogenic activity of this class of compounds. In stark contrast to endothelial cells, however, the proliferation of a wide range of primary human cell types is not affected by the fumagillin class of molecules (23). Here we report that PPI-2458, a structural analog of fumagillin, potently and selectively inhibits the proliferation of HFLS-RA (GI50 0.04 nM), one of only a few primary cell types identified to date that are exquisitely sensitive to growth inhibition caused by this class of molecules. In RA, activated FLS with a transformed phenotype are the dominant cell type involved in synovial hyperplasia and progressive joint destruction. PPI-2458-dependent growth inhibition of HFLS-RA in vitro is fully reversible, and the cells completely recover after removal of the drug. This suggests that the balance between MetAP-2-PPI-2458 turnover and the level of newly synthesized MetAP-2 determines the time of recovery. To determine the functional significance of MetAP-2 enzyme inhibition in cells either sensitive or resistant to PPI-2458, we developed a MetAP-2 assay that measures the amount of active cellular MetAP-2, which is not covalently bound by the PPI-2458 inhibitor. We demonstrate both in HFLS-RA and HUVEC that growth inhibition is directly proportional to the amount of enzyme inhibited and shows a linear dose dependency. Their GI50s are similar to the concentration of PPI-2458 required to achieve 50% MetAP-2 enzyme inhibition in both cell types. Similar data have previously been reported for HUVEC exposed to TNP-470 (15, 20, 23). The MetAP-2 inhibition curve in PPI-2458-resistant NHDF-Ad closely resembles that of HFLS-RA and HUVEC, indicating that MetAP-2 enzyme inhibition is achieved equally in these three cell types. These results strongly suggest that MetAP-2 enzyme inhibition is the critical first step in the growth inhibition of PPI-2458-sensitive HFLS-RA and HUVEC. The relative level of cellular MetAP-2 protein, however, does not appear to be critical to this process. Exposure of HFLS-RA to PPI-2458 increases, albeit moderately, the level of MetAP-2 protein. A similar pattern has been reported for human microvascular endothelial cells after exposure to fumagillin (24) and has been observed in our laboratory after exposure of HUVEC to PPI-2458. Furthermore, MetAP-2 is expressed in most cell types tested (15, 24). Therefore, differential expression most likely does not account for the selective drug sensitivity. The second MetAP isoform, MetAP-1, is constitutively expressed in all eukaryotic cells. MetAP-1 protein levels are moderately affected by PPI-2458 in HFLS-RA and are dose-dependently down-regulated in HUVEC after prolonged exposure. Therefore, MetAP-1 appears to be involved in HFLS-RA proliferation control but has a more prominent function in the proliferation of HUVEC. The mammalian homolog of MetAP-2 in yeast, MAP2, was cloned as a suppressor of the slow-growth phenotype of a map1 null strain, suggesting overlapping substrate specificity in yeast (30). Furthermore, yeast map1 and map2 null strains have significantly slower growth rates, which is consistent with the cytostatic effect observed in mammalian cells sensitive to MetAP-2 inhibition (30).
The mechanism(s) of MetAP-2-dependent proliferation control in PPI-2458-sensitive primary cell types remains to be elucidated. Our finding that the down-regulation of PCNA protein levels is proportional to the level of MetAP-2 enzyme inhibition suggests that PCNA is involved in the MetAP-2-dependent proliferation pathway. In normal cells, the total cellular level of PCNA remains relatively constant throughout the cell cycle, with some elevation in late G1/early S phase. Therefore, the significant down-regulation of PCNA in growth-inhibited HFLS-RA and HUVEC after exposure to PPI-2458 is consistent with the proposed mechanism of a PPI-2458-induced G1 cell cycle arrest. The cyclin-dependent kinase inhibitor p21CIP/WAF, which inhibits cell cycle progression in G1, is physically associated with PCNA (31). Terminally differentiated cardiocytes depend on high p21CIP/WAF levels to maintain cell cycle arrest, which in turn are required to down-regulate PCNA (32). Furthermore, elevated p21CIP/WAF protein levels are detected in HUVEC after TNP-470-induced G1 arrest, thus suggesting a link between MetAP-2 inhibition and two key cell cycle regulators, PCNA and p21CIP/WAF (23, 33). It remains to be determined what that link is in molecular terms. All of our observations suggest a reversible control of proliferation directly or indirectly controlled by MetAP-2.
The pathogenesis of RA is perpetuated by the activity of a complex network of cytokines (34). In particular, the interactions of type A and B synoviocytes drive synovial hyperplasia through a network of autocrine and paracrine factors. Synovial macrophages predominantly secrete TNF-α and IL-1, whereas HFLS-RA mainly secrete IL-6 and the angiogenic factors VEGF and basic fibroblast growth factor. We show that the secretion of IL-6, likely the most abundant cytokine in arthritic joints, and VEGF from activated HFLS-RA is not inhibited by PPI-2458. These results distinguish PPI-2458 from several established antiinflammatory drugs used in the treatment of RA, such as glucocorticoids and anti-TNF-α therapeutics, whose mechanism of action specifically targets the proinflammatory cytokine network in RA. It is conceivable, however, that in vivo the antiproliferative activity of PPI-2458 on activated synoviocytes and the inhibition of neovascularization in the inflamed joint may consequently down-regulate the secretion of inflammatory mediators. The unique mechanism of action of PPI-2458 further provides the rationale for combination therapy with drugs that act through a different mechanism to achieve additional or synergistic drug effects. Interestingly, very high and selective MetAP-2 expression has recently been reported in germinal center B cells and malignant lymphomas of the germinal center (GC) phenotype (35). No specific function, however, has yet been assigned to MetAP-2 in these cells. It is intriguing that lymphoid follicles with GCs, organized ectopically around follicular dendritic cells of FLS origin, have been identified in inflamed synovial tissue of RA patients (36). Most B cells derived from these GCs show no mutations in their VH genes, which suggests that their activation occurs locally in the inflamed synovium (37, 38). Because these GCs form a local microenvironment for the production of antibodies, such as rheumatoid factor, MetAP-2 enzyme inhibition in GC B cells may provide further clinical benefit by inhibiting their differentiation and RA autoantibody production, thereby interfering with the local antigen-driven immune response.
The clinical development of the fumagillin class of compounds, such as TNP-470, has been severely limited due to cerebellar toxicity as the principal dose-limiting toxicity. Reported symptoms predominantly associated with this CNS toxicity included coordination disturbances, memory impairment, increased anxiety, and emotional liability (26–29). Therefore, PPI-2458 was designed to be significantly more polar than TNP-470 and other fumagillin class compounds, to maintain the potent antiproliferative and antiangiogenic activity while improving the CNS toxicity profile by limiting its blood–brain-barrier permeability. In our study, the incidence of seizures after PPI-2458 and TNP-470 administration served as a clinical symptom of CNS toxicity and demonstrated a markedly improved CNS toxicity profile of PPI-2458 over its parental compound TNP-470. Most significantly, all animals treated with the high dose (60 mg/kg) of TNP-470 experienced seizures beginning as soon as after the second dose, whereas no seizures were observed in animals treated with 60 mg/kg PPI-2458. This finding appears to validate our approach that the structure of PPI-2458, designed to increase the polarity of the molecule, effectively limits its blood–brain-barrier permeability and therefore drug exposure to the CNS.
In the PG-PS-induced arthritis model, PPI-2458 administration reversed joint swelling and inflammation. PPI-2458 markedly attenuated paw swelling in a dose-dependent manner with maximal protection at an orally administered dose of 50 mg/kg at day 31. TNP-470 has previously been reported to protect syngeneic female Louvain and female Lewis rats from collagen- and adjuvant-induced arthritis, respectively, at a s.c. daily dose of 27 mg/kg (39–41). A combination therapy of TNP-470 with taxol, a nonspecific antiproliferative agent that blocks cell cycle progression in G2, significantly reduced clinical symptoms of arthritis (40). This additive effect strongly supports the expected clinical benefit of a drug with both antiangiogenic and antiproliferative activity. Moreover, histological evaluation of joints from PG-PS-induced arthritic animals demonstrated significant reduction in histopathological indices of inflammatory cell infiltration, pannus formation, and bone and cartilage erosion in PPI-2458-treated animals. These data suggest that the protective activity of PPI-2458 in this model of RA may not be limited to either effects on fluid flux (edema) or direct antiproliferative activity (unpublished data). Therefore, we conclude that the highly selective, potent antiproliferative activity of PPI-2458 on HFLS-RA and HUVEC in vitro, a significantly improved CNS cytotoxicity profile, and the marked attenuation of established chronic disease in the rat PG-PS arthritis model in vivo support the potential application of PPI-2458 as a drug for the treatment of RA.
Supplementary Material
Abbreviations: RA, rheumatoid arthritis; FLS, fibroblast-like synoviocytes; HFLS-RA, human FLS from RA patients; HUVEC, human umbilical vein endothelial cells; MetAP, methionine aminopeptidase; NHDF-Ad, normal human dermal fibroblasts from adults; PCNA, proliferating cell nuclear antigen; PG-PS, peptidoglycan–polysaccharide; VEGF, vascular endothelial growth factor; GI50, growth inhibitory concentration 50; TNF-α, tumor necrosis factor α; qod, every second day; po, orally; GC, germinal center.
References
- 1.Firestein, G. S. (2003) Nature 423, 356-361. [DOI] [PubMed] [Google Scholar]
- 2.Firestein, G. S. (1996) Arthritis Rheum. 39, 1781-1790. [DOI] [PubMed] [Google Scholar]
- 3.Pap, T., Mueller-Ladner, U., Gay, R. E. & Gay, S. (2000) Arthritis Res. 2, 361-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Firestein, G. S. & Zvaifler, N. J. (2002) Arthritis Rheum. 46, 298-308. [DOI] [PubMed] [Google Scholar]
- 5.Iwanaga, T., Shikichi, M., Kitamura, H., Yanase, H. & Nozawa-Inoue, K. (2000) Arch. Histol. Cytol. 63, 17-31. [DOI] [PubMed] [Google Scholar]
- 6.Han, C. W., Choi, J. H., Kim, J. M., Kim, W. Y., Lee, K. Y. & Oh, G. T. (2001) Rheumatology 40, 267-273. [DOI] [PubMed] [Google Scholar]
- 7.Guerne, P. A., Zuraw, B. L., Vaughan, J. H., Carson, D. H. & Lotz, M. (1989) J. Clin. Invest. 83, 585-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Firestein, G. S., Paine, M. M. & Littman, B. H. (1991) Arthritis Rheum. 34, 1094-1105. [DOI] [PubMed] [Google Scholar]
- 9.Nagashima, M., Yoshino, S., Aono, H., Takai, M. & Sasano, M. (1999) Clin. Exp. Immunol. 116, 360-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagashima, M., Wauke, K., Hirano, D., Ishigami, S., Aono, H., Takai, M., Sasano, M. & Yoshino, S. (2000) Rheumatology 39, 1255-1262. [DOI] [PubMed] [Google Scholar]
- 11.Chicheportiche, Y., Chicheportiche, R., Sizing, I., Thompson, J., Benjamin, C. B., Ambrose, C. & Dayer, J.-M. (2002) Arthritis Res. 4, 126-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang, T. H., Teichert, U. & Smith, J. A. (1992) J. Biol. Chem. 267, 8007-8011. [PubMed] [Google Scholar]
- 13.Xuan, L. & Chang, Y. H. (1995) Biochim. Biophys. Acta 1260, 333-336. [DOI] [PubMed] [Google Scholar]
- 14.Xuan, L. & Chang, Y. H. (1996) Biochem. Biophys. Res. Commun. 227, 152-159. [DOI] [PubMed] [Google Scholar]
- 15.Turk, B., Griffith, E. C., Wolf, S., Biemann, K., Chang, Y.-H. & Liu, J. O. (1999) Chem. Biol. 6, 823-833. [DOI] [PubMed] [Google Scholar]
- 16.Chen, S., Vetro, J. A. & Chang, Y.-H. (2002) Arch. Biochem. Biophys. 398, 87-93. [DOI] [PubMed] [Google Scholar]
- 17.Griffith, E. C., Su, Z., Turk, B. E., Chen, S., Chang, Y.-H., Wu, Z., Biemann, K. & Liu, J. O. (1997) Chem. Biol. 4, 461-471. [DOI] [PubMed] [Google Scholar]
- 18.Sin, N., Meng, L., Wang, M. Q. W., Wen, J. J., Bornmann, W. G. & Crews, C. M. (1997) Proc. Natl. Acad. Sci. USA 94, 6099-6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffith, E. C., Su, Z., Niwayama, S., Ramsay, C. A., Chang, Y.-H. & Liu, J. O. (1998) Proc. Natl. Acad. Sci. USA 95 15183-15188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kusaka, M., Sudo, K., Kozai, Y., Marui, S., Fujita, T., Ingber, D. & Folkman, J. (1994) Br. J. Cancer 69, 212-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abe, J., Zhou, W., Taguchi, J., Kurokawa, K., Kumada, M. & Takuwa, Y. (1994) Cancer Res. 54, 3407-3412. [PubMed] [Google Scholar]
- 22.Milkowski, D. M. & Weiss, R. A. (1998) in Antiangiogenic Agents in Cancer Therapy, ed. Teicher, B. A. (Humana, Totowa, NJ), pp. 385-398.
- 23.Yeh, J.-R. J., Mohan, R. & Crews, C. M. (2000) Proc. Natl. Acad. Sci. USA 97, 12782-12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang, J., Lou, P. & Henkin, J. (2000) J. Cell. Biochem. 77, 465-475. [PubMed] [Google Scholar]
- 25.Maga, G. & Huebscher, U. (2003) J. Cell Sci. 116, 3051-3060. [DOI] [PubMed] [Google Scholar]
- 26.Kudelka, A. P., Levy, T., Verschraegen, C. F., Edwards, C. L., Piamsomboon, S., Termrungruanglert, W., Freedman, R. S., Kaplan, A. L., Kieback, D. G., Meyers, C. A., et al. (1997) Clin. Cancer Res. 3, 1501-1505. [PubMed] [Google Scholar]
- 27.Bhargava P., Marshall, J. L., Rizvi, N., Dahut, W., Yoe, J., Figuera, M., Phipps, K., Ong, V. S., Kato, A. & Hawkins, M. J. (1999) Clin. Cancer Res. 5, 1989-1995. [PubMed] [Google Scholar]
- 28.Logothetis, C. J., Wu, K. K., Finn, L. D., Daliani, D., Figg, W., Ghaddar, H. & Gutterman, J. A. (2001) Clin. Cancer Res. 7, 1198-1203. [PubMed] [Google Scholar]
- 29.Herbst, R. S., Madden, T. L., Tran, H. T., Blumenschein, G. R., Meyers, C. A., Seabrooke, L. F., Khuri, F. R., Puduvalli, V. K., Allgood, V., Fritsche, H. A., et al. (2002) J. Clin. Oncol. 20, 4440-4447. [DOI] [PubMed] [Google Scholar]
- 30.Li, X. & Chang, Y.-H. (1995) Proc. Natl. Acad. Sci. USA 92, 12357-12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gulbis, J. M., Kelman, Z., Huewitz, J., O' Donnell, M. & Kuriyan, J. (1996) Cell 87, 1-20. [DOI] [PubMed] [Google Scholar]
- 32.Engel, F. B., Hauck, L., Boehm, M., Nabel, E. G., Dietz, R. & von Harsdorf, R. (2003) Mol. Cell. Biol. 23, 555-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang, Y., Griffith, E. C., Sage, J., Jacks, T. & Liu, J. O. (2000) Proc. Natl. Acad. Sci. USA 97, 6427-6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choy, E. H. S. & Panayi, G. S. (2001) N. Engl. J. Med. 344, 907-916. [DOI] [PubMed] [Google Scholar]
- 35.Kanno, T., Endo, H., Takeuchi, K., Morishita, Y., Fukayama, M. & Mori, S. (2002) Lab. Invest. 82, 893-901. [DOI] [PubMed] [Google Scholar]
- 36.Lindhout, E., van Eijk, M., van Pel, M., Lindeman, J., Dinant, H. J. & de Groot, C. (1999) J. Immunol. 162, 5949-5956. [PubMed] [Google Scholar]
- 37.Kim, H.-J. & Berek, C. (2000) Arthritis Res. 2, 126-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clausen, B. E. (1998) Mol. Med. 4, 240-251. [PMC free article] [PubMed] [Google Scholar]
- 39.Peacock, D. J., Banquerigo, M. L. & Brahn, E. (1992) J. Exp. Med. 175, 1135-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oliver, S. J., Banquerigo, M. L. & Brahn, E. (1994) Cell. Immunol. 157, 291-299. [DOI] [PubMed] [Google Scholar]
- 41.Peacock, D. J., Banquerigo, M. L. & Brahn, E. (1995) Cell. Immunol. 160, 178-184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.