Abstract
The utility of computed tomography (CT) has not been studied in the initial evaluation of a patient with suspected spontaneous Clostridial myonecrosis. Here, we present a patient with acute lymphoblastic leukemia (ALL) and neutropenia who developed spontaneous Clostridium perfringens myonecrosis after induction chemotherapy. Although suspected, the patient’s symptoms and physical exam findings were not specific for Clostridial myonecrosis. CT confirmed the diagnosis and helped direct surgical intervention.
Introduction
Spontaneous Clostridial myonecrosis in children is most commonly associated with neutropenia and often presents with vague abdominal symptoms, with rapidly progressing muscular pain, fever, and sepsis. Classically, physical exam reveals focal pain, skin discoloration, and crepitus at the site of muscular involvement. However, these findings are often absent, making the diagnosis of Clostridial myonecrosis challenging. Timely diagnosis is crucial, as parenteral antibiotics and targeted surgical intervention are associated with greater rates of survival (1).
Case report
The patient, a previously healthy 14-year-old girl, was diagnosed with ALL nearly one month before admission. At that time, she was started on induction chemotherapy, including Vincristine, Daunorubicin, Prednisone, and PEG-Asparaginase. On the morning of admission, routine outpatient laboratory studies revealed pancytopenia with an absolute neutrophil count of 210/mm3. That afternoon, she developed acute-onset left-lower-extremity pain and subsequently presented to the emergency department for evaluation. Within 4 hours of symptom onset, she complained of worsening bilateral leg, hip, and back pain. She also complained of progressive weakness of both lower extremities and an inability to bear weight. She was tachycardic, febrile, and hypotensive, with systolic pressures in the low 80s. Physical exam demonstrated nonspecific swelling and significant tenderness of the left thigh without localizing findings such as skin discoloration or crepitus. Initial laboratory studies confirmed pancytopenia; however, there were significant decreases in platelet count compared to her outpatient laboratory studies performed earlier that morning (37,000/mm3 in the emergency department compared to 112,000/mm3 as an outpatient the morning of admission). Additionally, her fibrinogen was low (78 mg/dL), and thrombin time was elevated (19 seconds). She was given an IV fluid bolus and started on gentamycin and ceftazadime.
A left-lower-extremity Doppler ultrasound examination was initially performed to exclude a deep venous thrombosis; the result was negative. Given the acute onset of thigh pain, rapidly developing sepsis, neutropenia, and laboratory studies suggestive of disseminated intravascular coagulation, a necrotizing deep-soft-tissue infection was considered. The General Surgery service was consulted for immediate operative intervention. Localizing the area of involvement was not possible due to the lack of external findings on physical examination. Additionally, the patient was also unable to localize to a specific muscle compartment due to her altered mental status. Cross-sectional imaging was therefore used to identify involved muscle compartments and to allow for planning of the operative intervention. CT revealed a large amount of intramuscular gas within multiple muscle compartments of the left hemipelvis, bilateral thighs, and bilateral calves, highly concerning for myonecrosis (Figure 1, Figure 2). Blood cultures drawn at admission demonstrated moderate Clostridium perfringens.
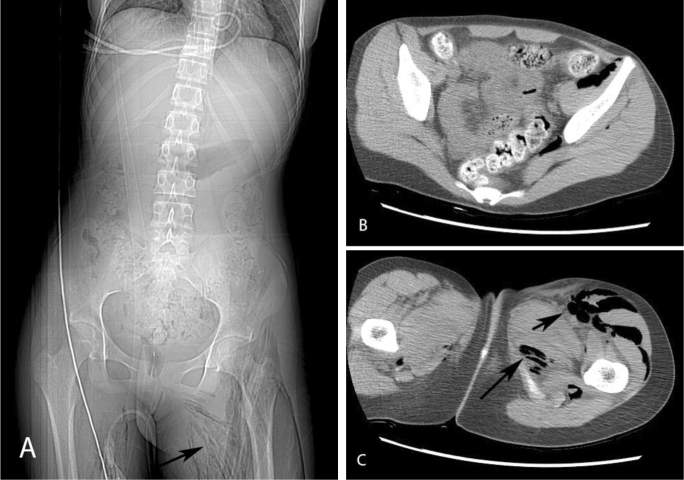
Figure 1.
14-year-old female with spontaneous Clostridium perfringens myonecrosis. Axial noncontrast CT of the abdomen and pelvis. Scout image reveals intramuscular gas involving the bilateral thigh musculature (arrow) (A). Axial images of the pelvis (B) and thighs (C) show intramuscular gas in the left iliacus, piriformis, quadriceps, and adductor muscles (arrows). Minimal gas is also seen in the right adductor musculature (C).
Figure 2.
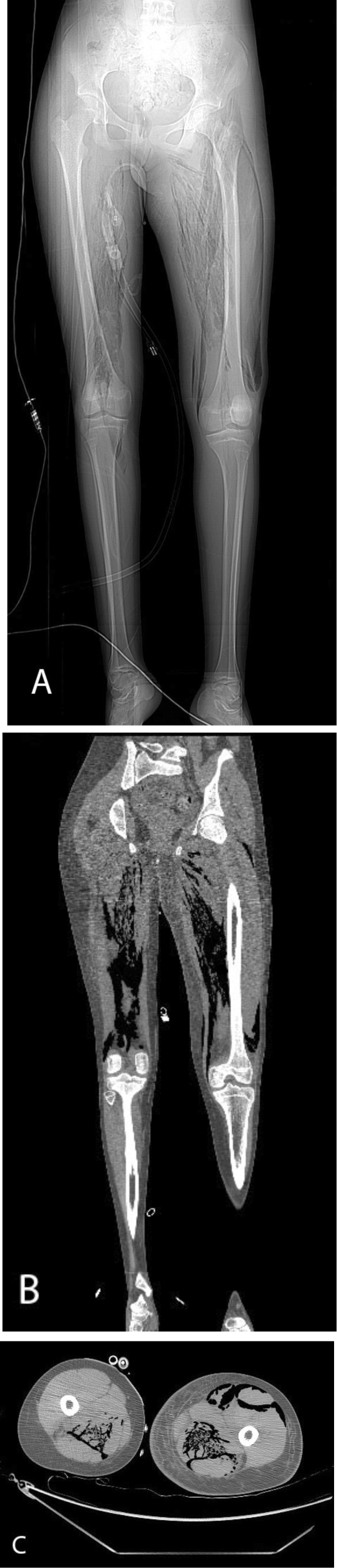
14-year-old female with spontaneous Clostridium perfringens myonecrosis. Axial and coronal images from a noncontrast CT of the lower extremities. Scout image reveals extensive intramuscular gas in the bilateral thigh musculature (A). Coronal (B) and axial (C) images confirm intramuscular gas within the bilateral posterior thigh compartments and the left anterior thigh compartment. Note the subcutaneous thigh edema (C).
The patient was brought emergently to the operating room for exploration and debridement of the necrotizing soft-tissue infection. Extensive longitudinal incisions along the lower extremities were made to allow access to the medial and lateral thigh compartments, and posterior compartment of the calf and hemipelvis. The skin, subcutaneous tissue, and superficial fascia were all intact with good blood supply. However, upon entrance into the deep muscle compartments of the thigh and calf, a sweet foul-smelling liquefactive necrosis was encountered, consistent with myonecrosis. The adductor magnus, adductor brevis, gracilis, gastrocnemius, and posterior hamstrings were involved (Fig. 3).
Figure 3.
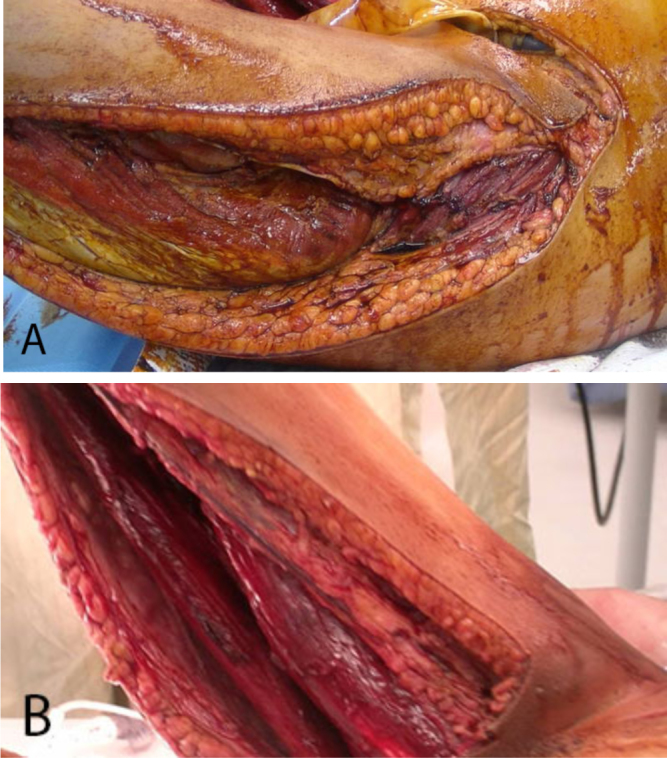
14-year-old female with spontaneous Clostridium perfringens myonecrosis. Intraoperative images of the lateral muscular compartment of the left thigh (A) and medial muscular compartment of the right thigh (after extensive debridement) (B). Sweet, foul-smelling liquefactive necrosis was encountered upon entrance to the compartments.
These muscle bellies were debrided to apparent viable tissue, leaving large defects in the muscle groups. The patient was brought to the Intensive Care Unit; over the next several days, serial washouts and further debridement were performed. By postoperative day 4, the wound bed was felt to be adequately debrided, and a Vacuum-Assisted Closure device was applied to assist with wound management. Large areas of the overlying skin and subcutaneous tissue that had become nonviable due to the extensive infection and subsequent debridement of these areas also were excised. As the patient improved clinically, it was found that she had no sensory or voluntary motor function below either knee. She subsequently underwent bilateral above-knee amputations. Skin coverage was provided by pedicled myocutaneous flaps based on the popliteal artery. She was eventually transferred from the intensive care unit to the surgical floor, where her wounds healed well. She has begun physical therapy and rehabilitation and has done well at short-interval followup of two months.
Discussion
In this case, rapid onset of severe bilateral lower extremity pain with laboratory signs of sepsis and disseminated intravascular coagulation led to the early clinical suspicion of myonecrosis. However, early cross-sectional imaging with CT was crucial for identification of involved muscular compartments, aiding surgical localization (given the inability of the patient to localize her pain and the lack of physical examination findings). Prompt imaging allowed targeted surgical intervention, which was fundamental for the survival of the child.
Clostridial myonecrosis is a rare and devastating illness and has two major presentations—spontaneous and nonspontaneous. Historically, the vast majority of cases of Clostridial myonecrosis, also known as gas gangrene, were nonspontaneous and seen in the setting of penetrating trauma during wartime, with contamination of the deep muscle compartments from spores present in the soil. Traumatic gas gangrene is caused by Clostridium perfringens, an anaerobic spore-forming gram-positive bacillus. Patients present with rapid onset of severe pain, skin discoloration, fulminant tissue destruction, and gas formation in the soft tissues after penetrating trauma. If left untreated, sepsis, multi-organ failure, and death rapidly ensue.
Injuries sustained in the battlefield of the Civil War had a nearly 50% case fatality rate due to slow evacuation, limited surgical techniques, and unavailability of anesthesia and antibiotics. Therapeutic amputation was common for the treatment of gas gangrene. During World War I, gas gangrene complicated nearly 10% of penetrating injuries (2). World War II had a lower incidence of C. perfringens gas gangrene complicating penetrating trauma due to improved surgical techniques, availability of antibiotics, and more rapid evacuation of the wounded. Nevertheless, incidence of gas gangrene was higher among prisoners (delayed care), and among those wounded in bacterially fertile European battlefields (2, 3).
In contrast, spontaneous Clostridial myonecrosis occurs without trauma, is most often caused by Clostridium septicum, and has been described in specific clinical settings such as neutropenia, colonic malignancy, inflammatory bowel disease, bowel ischemia, and hemolytic-uremic syndrome (1, 4, 5). Spontaneous Clostridial myonecrosis is extremely devastating, with overall mortality rates ranging from 67% to 100%, with death occurring in the first 24–48 hours (2, 6). Among pediatric patients, a case review by Smith-Slatas et al described mortality rates of spontaneous C. septicum myonecrosis approaching 60% (1). Colonic pathology disrupts the colonic mucosal integrity, allowing C. septicum access to the blood, resulting in hematogenous metastasis of the aerotolerant organism to the muscle compartments (7). In the absence of primary colonic pathology, neutropenia increases the likelihood of translocation of C. septicum from the colon into the blood pool (8, 9).
The toxic effects of C. perfringens are well described. Two extracellular toxins, known as alpha toxin and theta toxin, exert the major pathophysiologic effects on tissues. Alpha toxin is a potent platelet agonist, resulting in capillary, venule, and arteriole occlusion and severely limiting blood flow to infected tissues. Theta toxin is a member of a group of cholesterol-dependent cytolysins. Theta toxin monomers, when in contact with cell membranes, oligomerize and form pores, causing cell lysis. Interestingly, Clostridial growth and proliferation is improved in acidic conditions, accelerated by alpha toxin, which occludes blood flow to hypoxic muscles (2, 3).
The vast majority of spontaneous cases of spontaneous Clostridial myonecrosis have been due to C. septicum, while spontaneous, nontraumatic Clostridial myonecrosis due to C. perfringens (as presented here) has remained extremely rare. Our review of the literature found only three cases in the pediatric population (10, 11, 12).
In one of these rare cases, Minutti et al describe a neutropenic 7-year-old boy with ALL, undergoing chemotherapy, who acutely presented with excruciating left-thigh pain, soft-tissue crepitus, and skin discoloration. Clostridial myonecrosis was suspected based on history, physical, and laboratory values, and the patient was immediately brought to the operating room for debridement of the involved muscular compartments. Fulminant muscular necrosis was encountered intraoperatively, and tissue cultures revealed heavy C. perfringens. Unfortunately, the patient was extremely hemodynamically unstable and died in the operating room (12).
In this illustrative example, cross-sectional imaging was not used to guide operative intervention, given the acuity of the patient presentation and the presence of localizing physical exam findings. Although skin discoloration and crepitus were present in the Minutti et al case, these findings are often not present on physical exam, making early diagnosis extremely challenging (1). Imaging played no role in the Minutti et al patient’s management and, if used, may only have served to delay definitive surgical management.
However, in the absence of localizing physical examination findings (as described in our case of spontaneous Clostridial myonecrosis), CT served a valuable role in guiding appropriate surgical management and may have improved the outcome for the child.
Footnotes
Published: August 4, 2013
References
- 1.Smith-Slatas CL, Bourque M, Salazar JC. Clostridium septicum infections in children: a case report and review of the literature. Pediatrics. 2006;117:e796–e805. doi: 10.1542/peds.2005-1074. [PubMed] [DOI] [PubMed] [Google Scholar]
- 2.Stevens DL, Aldape MJ, Bryant AE. Life-threatening clostridial infections. Anaerobe. 2011 doi: 10.1016/j.anaerobe.2011.11.001. [PubMed] [DOI] [PubMed] [Google Scholar]
- 3.Stevens DL, Bryant AE. The role of clostridial toxins in the pathogenesis of gas gangrene. Clin Infect Dis. 2002;35:S93–S100. doi: 10.1086/341928. [PubMed] [DOI] [PubMed] [Google Scholar]
- 4.Kiel N, Ho V, Pascoe A. A case of gas gangrene in an immunosuppressed Crohn's patient. World J Gastroenterol. 2011;17:3856–3858. doi: 10.3748/wjg.v17.i33.3856. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valentine EG. Nontraumatic gas gangrene. Ann Emerg Med. 1997;30:109–111. doi: 10.1016/s0196-0644(97)70121-5. [PubMed] [DOI] [PubMed] [Google Scholar]
- 6.Johnson S, Driks MR, Tweten RK. Clinical courses of seven survivors of Clostridium septicum infection and their immunologic responses to alpha toxin. Clin Infect Dis. 1994;19:761–764. doi: 10.1093/clinids/19.4.761. [PubMed] [DOI] [PubMed] [Google Scholar]
- 7.Bryant AE, Stevens DL. Clostridial myonecrosis: new insights in pathogenesis and management. Curr Infect Dis Rep. 2010;12:383–391. doi: 10.1007/s11908-010-0127-y. [PubMed] [DOI] [PubMed] [Google Scholar]
- 8.Bar-Joseph G, Halberthal M, Sweed Y, Bialik V, Shoshani O, Etzioni A. Clostridium septicum infection in children with cyclic neutropenia. J Pediatr. 1997;131:317–319. doi: 10.1016/s0022-3476(97)70175-6. [PubMed] [DOI] [PubMed] [Google Scholar]
- 9.Barnes C, Gerstle JT, Freedman MH, Carcao MD. Clostridium septicum myonecrosis in congenital neutropenia. Pediatrics. 2004;114:e757–e760. doi: 10.1542/peds.2004-0124. [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Keogh G, Unsworth I, Vowels M, Kern IB. Spontaneous Clostridium septicum myonecrosis in congenital neutropaenia. Aust N Z J Surg. 1994;64:574–575. doi: 10.1111/j.1445-2197.1994.tb02291.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 11.Sawhney R, Rees JH, Markowitz SK. Clostridial gas gangrene complicating leukemia. Abdom Imaging. 1994;19:451–452. doi: 10.1007/BF00206938. [PubMed] [DOI] [PubMed] [Google Scholar]
- 12.Minutti CZ, Immergluck LC, Schmidt ML. Spontaneous gas gangrene due to Clostridium perfringens. Clin Infect Dis. 1999;28:159–160. doi: 10.1086/517192. [PubMed] [DOI] [PubMed] [Google Scholar]