Abstract
Chiari malformation type III is an extremely rare anomaly characterized by a small posterior fossa and a low occipital/high cervical encephalocele with herniation of the posterior fossa contents (that is, the cerebellum and/or the brainstem, occipital lobe, and fourth ventricle). We report a case of Chiari malformation type III in a neonate, discuss the etiopathogenetic and radiological features, and review the pertinent literature.
Introduction
Hans Chiari, Professor of Pathology at the German University in Prague, published a series on hindbrain herniations based on autopsy findings in 1891 (1). He described three classes of hindbrain anomalies, including Chiari malformation type III; he found this exclusively in patients with occipital and/or high cervical encephalocele, with herniated dysplastic posterior fossa contents, and other associated anomalies (2, 3)
Chiari malformation type III is the rarest of the Chiari malformations, and it is usually associated with a dismal prognosis—early death or severe disability in long-term survivors (4).
Case report
A two-day-old male child presented to our hospital emergency department with a ruptured occipital encephalocele. He was a full-term normal delivery, born of nonconsanguineous parents. There was neither any history of any medicinal intake nor any evidence of any maternal infection during pregnancy. The mother's ultrasound examination, during the eighth month of pregnancy, was normal. On examination, the child weighed 2.7 kg. There was no retrocollis or any other postural deformity. The child had a normal cry, with no episodes of apnea. There was no cranial nerve palsy, and eye movements were normal without any nystagmus. The tones of the limbs as well as reflexes were normal. On auscultation, the chest was clear, with normal vesicular breath sounds. On local examination, there was a soft multilobulated mass in the occipital region that was not covered by skin, but there was no cerebrospinal fluid leakage (Fig. 1). The hematological and biochemical parameters of the child were normal. Skull x-ray and cranial computed tomography (CT) scan showed a lacunar skull (Figure 2, Figure 3), and a large defect in the occipital region (approximately 3.64 × 3.16 cm) (Fig. 3). Magnetic resonance imaging (MRI) revealed a small posterior fossa; an occipital encephalocele with herniation of the occipital lobe, cerebellum, and fourth ventricle through the bony defect; a deep parietooccipitalis fissure; partial callosal agenesis; and a herniated part of brain stem into the cervical canal through the foramen magnum with the inferior tip between C5 and C6. There was no associated hydrocephalus or syringohydromyelia (Figure 4, Figure 5).
Figure 1.
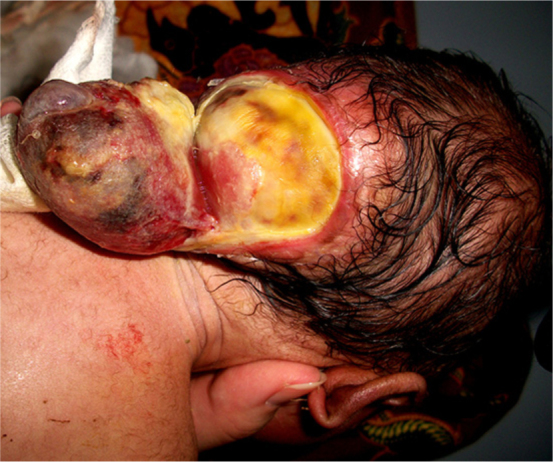
Neonate with Chiari malformation type III. 3D reformatted CT scan shows lacunar skull and a large defect in the occipital regional striae.
Figure 2.
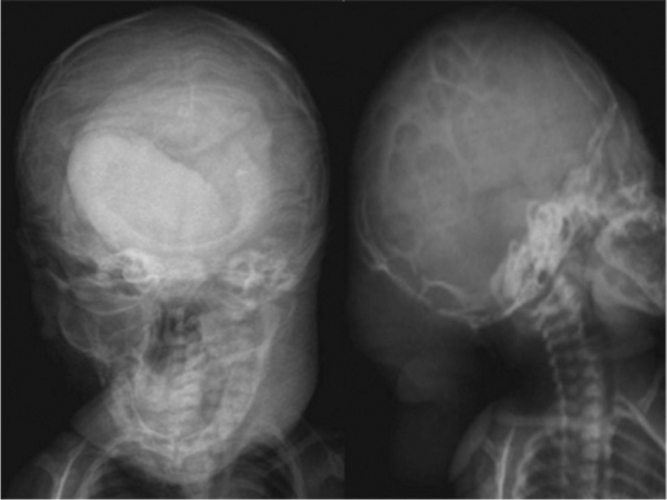
Neonate with Chiari malformation type III. Skull x-ray shows multiple defects on calvaria (lacunar skull).
Figure 3.
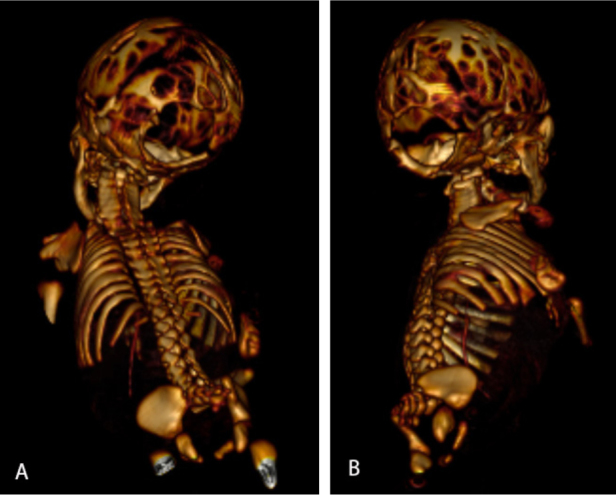
Neonate with Chiari malformation type III. 3D reformatted CT scan shows lacunar skull and a large defect in the occipital region.
Figure 4.
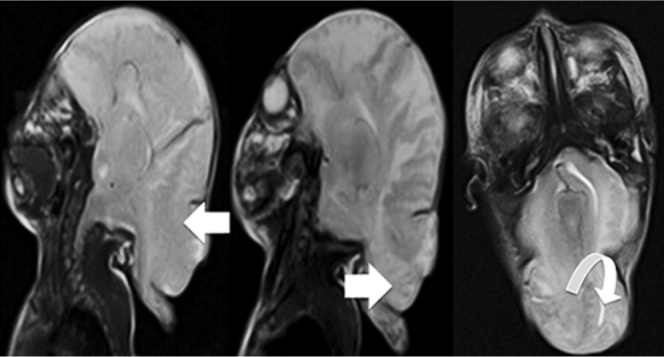
Neonate with Chiari malformation type III. T2-weighted sagittal and axial MRI scans of the patient show a small posterior fossa with encephalocele that contained a part of the occipital lobe (open arrow), cerebellum (closed arrow), and fourth ventricle (curved arrow).
Figure 5.
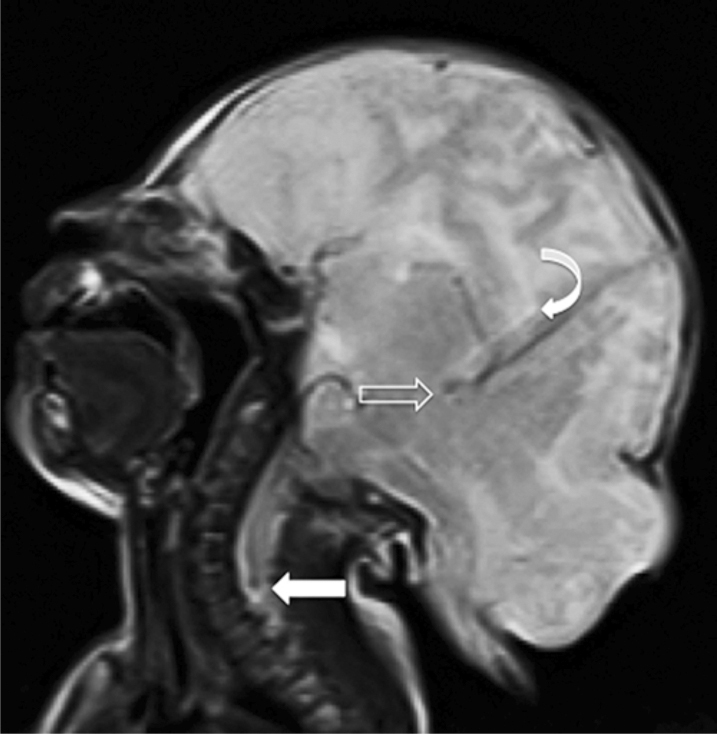
Neonate with Chiari malformation type III. T2-weighted mid-sagittal MRI scan of the patient shows a small posterior fossa, an deep parieooccipitalis fissure (open arrow), ad a partial callosal agenesis (curved arrow), and a caudal herniation of part of the brain stem through the foramen magnum, with inferior tip appearing between C5 and C6 (closed arrow).
In our patient, it happened that during early embryogenesis, failure of dorsal induction encompassed the neural tube defects, and incomplete distension of the telencephalic and the rhombencephalic ventricles resulted in Chiari malformation type III.
Discussion
Congenital and developmental abnormalities of the central nervous system (CNS) have been classified by van der Knaap and Valk according to the timing (that is, weeks of gestational age, or WGA) of the disorder, as the major determinant of the type of malformation. Malformations associated with disorders of dorsal induction take place between the third and fourth gestational weeks and include Chiari malformations. Dorsal induction is the notochondral process extending from the primitive knot to the cranial pole of the embyro, inducing the formation of the neural plate. The neural plate gives rise to the neural tube, the precursor of the brain and spinal cord. Failure of dorsal induction encompasses the neural tube defects (2, 5). The pathoembryology and pathophysology of Chiari malformation type III are best explained with the theory of McLone and Knepper. In this theory, both the open neural-tube defect and incomplete spinal occlusion allow CSF leaks through the spinal defects into the intrauterine environment. Ventricular distention is required to induce both neural and calvarial development; without this ventricular CSF driving force, the posterior fossa never fully develops. With later, rapid growth of the rhombencephalon, the cerebellum is forced in both a cephalad and caudad direction, along with the brainstem. Polymicrogyria, enlargement of the mass, low-lying tentorium and torcular herophili, and base-of-skull anomalies are produced by the same lack of ventricular distention in the telencephalon (1, 3, 7).
As enthusiasm for genotype-phenotype studies focused on the cerebellum continues to spread, our knowledge of the molecular cascades underlying neural-tube closure and posterior-fossa development will expand accordingly. Recent reports suggest that a transcription factor expressed in the head mesenchyme, FOXC1, is required for normal skull development and normal cerebellar development. Variations in the amino-acid sequence of FOXC1 can result in a spectrum of phenotypes in cerebellar morphology via defective mesenchymal signaling. FOXC1 is a reasonable candidate for further study, as it is expressed near the time of the neural-tube closure, and it is localized to the mesenchyme, where skull, brainstem, and cerebellar development could all be influenced by variability in its expression levels. However, definitive mechanisms underlying Chiari malformations development still remain to be elucidated (7). Despite growing evidence of genetic influences in familial Chiari malformation, the underlying culprit genes have not been fully identified. The PAX family of genes encodes for transcription factors that play a role in pattern formation during embryogenesis in vertebrates. PAX1, a highly conserved gene mapped to chromosome 20p11.2, is involved in segmentation and vertebral development during embryogenesis. Other studies have implicated PAX2 and FGF2 mutations, with PAX3 and PAX6 as potential culprit genes in the formation of this malformation (8).
Chiari malformation type III requires a small posterior fossa and a cervical and/or occipital encephalocele associated with herniated, dysplastic, posterior fossa contents. In addition to the encephalocele, radiographic findings similar to those of Chiari malformation type II are present. The midbrain is deformed in all patients, the corpus callosum is partially or completely dysgenic (77%), the petrous temporal bone and clivus are scalloped (56%), the tonsils are herniated (43%), and hydrocephalus and hydromyelia are present in 22% and 30% of patients.The contents of the encephalocele vary in size and degree of dysplasia. At least a portion of the cerebellum herniates in all patients, and in one third of patients this portion may be accompanied by a variable amount of the occipital lobe. A dysmorphic ventricle may be trapped within the encephalocele (2, 4, 5).
Our patient demonstrated several features that support Chiari malformation type III. Skull x-ray and CT scan showed lacunar skull (”lacunar skull” signifies a dysplasia of the membranous bone with well-defined lucent areas in the calvaria that correspond to nonossified fibrous bone). The lacunae are bounded by normally ossified bone. The appearance resolves spontaneously by age 6 months and is not related to the degree of concurrent hydrocephalus (9).
MRI has become the imaging study of choice for the diagnosis of Chiari malformation type III (1). In our case, MRI revealed a small posterior fossa; an occipital encephalocele with herniation of the occipital lobe, cerebellum, and fourth ventricle through the bony defect; and partial callosal agenesis. A part of the brain stem was herniated into the cervical canal through the foramen magnum; its inferior tip was located between C5 and C6. There was no associated hydrocephalus or syringohydromyelia. Due to the wide opening of the encephalocele sac, there was no impact on the neural tissue, and the CSF flow through the foramen of Luschka and Magendi was not impaired. Thus, hydrocephalus did not develop (3).
In children, particularly young children, definitions based on milimeters of herniation are not particularly useful, given the variable distance between the foramen magnum and the arches of C1 and C2 and the variable size of the foramen magnum. Therefore, herniation has generally been referred to in terms of segments (that is, between the foramen magnum and C1 or beyond C1) (10). Other disorders can be assessed by the size of the foramen magnum. Previously, research studies have shown that some Chiari malformation patients have a wider foramen magnum, but recently a morphometric research showed no significant differences in the area of the foramen magnum between patients with Chiari malformation and healthy people (11). The mean anterior-posterior diameter of the foramen magnum was 3.1 cm, and the mean horizontal diameter was 2.7 cm (12). The measurement of diameter of the foramen magnum from our patient (2.96 cm anterior-posterior and 1.87 cm horizontal), showed that our patient's foramen magnum was not enlarged.
Operative approaches specific to Chiari malformation type III have not been extensively described. However, the same principles for open neural-tube defects should be applied to the encephalocele encountered in these patients. One major concern is avoiding injury or compression of the brainstem during surgical repair. Operative treatment on the defect frequently requires resection of some of the malformation in order to allow primary closure of the skin. In every case, the surgeon must be conservative when resecting nervous tissue, with the primary goal being complete coverage of the defect. However, in cases where the viable tissue is in the defect and prevents immediate closure, CSF diversion and delayed closure is an option for this difficult malformation (3, 4).
Footnotes
Published: July 31, 2013
References
- 1.Stevenson KL. Chiari Type II malformation: past, present, and future. Neurosurg Focus. 2004;16(2) doi: 10.3171/foc.2004.16.2.6. [PubMed] Article 5, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Woodruff William W. Fundamentals of neuroimaging. W. B. Saunders Company; Philadelphia: 1993. pp. 513–517. [Google Scholar]
- 3.Garg K, Malik N, Jaiswal AK, Behari S. Chiari III malformation with hypertelorism and microcephaly in a neonate: Case report and a review of the literature. J Pediatr Neurosci. 2008;3:169. www.pediatricneurosciences.com] [downloaded Friday, June 10, 2011, from. [Google Scholar]
- 4.Jaggi RS, Premsagr IC. Chiari malformation type III treated with primary closure. India Pediatric Neurosurgery. 2007;43:424–2007. doi: 10.1159/000106397. [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Agrawal A, Mittal A, Kohali GB, Sampley S, Gupta A. Chiari III malformation. Pediatr Neurosurg. 2011;47:309–331. doi: 10.1159/000335651. [DOI] [PubMed] [Google Scholar]
- 7.Juranek J, Salman MS. Anomalous development of brain structure and function in spina bifida myelomeningocele. Dev Disabil Res Rev. 2010;16(1):23–30. doi: 10.1002/ddrr.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schanker BD, Walcott BP, Nahed BV, Kahle KT, Li YM, Coumans J-V CE. Familial Chiari nalformation: Case series. Neurosurg Focus. 2011;31(3):e1. doi: 10.3171/2011.6.FOCUS11104. [PubMed] [DOI] [PubMed] [Google Scholar]
- 9.Glass RB, Fernbach SK, Norton KI, Choi PS, Naidich TP. The unfant skull: A vault of unformation. RadioGraphics. 2004;24:507–2004. doi: 10.1148/rg.242035105. [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Yassari R, Frim D. Evaluation and management of the Chiari malformation type 1 for the primary care pediatrician. Pediatric Clinics of North America. 2004;51:477–2004. doi: 10.1016/S0031-3955(03)00208-6. [PubMed] [DOI] [PubMed] [Google Scholar]
- 11.Study finds no difference in size of foramen magnum in Chiari kids. [downloaded Moday, March, 25, 2013 from www.conquerchiari.org].
- 12.Tubb RS, Griessenauer CJ, Loukas M, Shoja MM, Cohen-Gadol AA. Morphometric analysis of the foramen magnum; An anatomic study. Neurosurgery. 2010;66(2):385–388. doi: 10.1227/01.NEU.0000363407.78399.BA. [PubMed] [DOI] [PubMed] [Google Scholar]
Uncited Reference
- 6.Barnes Patrick D. Brain imaging. In: Blickman JG, Parker BR, Barnes PD, editors. Pediatric Radiology: The Requisites. 3rd edition. Mosby; Philadelphia: 2009. pp. 208–210. [Google Scholar]


