Introduction
Infants who experience extended intensive care stays may lose opportunities for learning to eat and may associate eating with pain or discomfort.1 Feeding difficulties affect 40% to 70% of infants with prematurity or complex medical issues.2 Following severe neonatal pain experiences some infants may present with food refusal or early satiety because they experience oral discomfort, odynophagia, dysphagia, or dyspepsia as a consequence of hyperalgesia, sensitization of peripheral nociceptors, and central nervous system activation of nonspecific arousal systems.3,4
Children born with life-threatening cardiac abnormalities who required corrective or palliative heart surgery are at high risk for oral feeding difficulties.5,6 When oral feeding does not advance in a timely fashion after cardiovascular surgery, infants may require gastrostomy feeding tubes with or without fundoplication.7
Drugs for chronic pain have demonstrated great promise in improving oral feeding outcomes in medically complicated children.8,9 The nerve-stabilizing medication gabapentin has been used for chronic neuropathic pain10 and reduced irritability in neurologically impaired children.11 Furthermore, gabapentin appeared to be an effective adjunct to move medically fragile toddlers from tube to oral feedings.8
In the current study, we hypothesized that feeding difficulties may be related to sensory hypersensitivities of the face and mouth, oropharynx, esophagus, and stomach. We aimed to assess the effect of gabapentin on voluntary oral intake in infants following surgery for congenital heart disease. We selected gabapentin because it is not associated with serious cardiovascular side effects.
Materials and Methods
We conducted a chart review in 15 infants in the heart intensive care unit treated with gabapentin for feeding difficulties from 2010 to 2011. All the infants had serious congenital cardiac disease and recent cardiac surgery. None were advancing on oral feeding, and in each case, the attending surgeon requested a feeding team consultation. Infants without feeding difficulties did not receive a feeding team consultation. We acquired oral feeding volumes for the 3 days before starting gabapentin and the 3 days following gabapentin. We collected demographic information including age, sex, and diagnoses (Table 1). Their mean age was 2.4 months (standard deviation = 2.7; mode 2.0 months; range 1.5-12 months). Children were treated with gabapentin 10 mg/kg/dose twice daily. If there was no sedation after the first doses, the dosing schedule was increased to 3 times daily. Infants were encouraged to nipple, and the remainder of the scheduled volume was gavaged through a nasogastric tube. We added the oral volume for 3 days before and for 3 days after initiation of gabapentin, and compared the totals for each infant. Results were expressed as mean ± standard deviation. We used the Wilcoxon rank test for paired samples to assess the effect of gabapentin. This study was approved by the Human Subjects Committees of Louisiana State University and the Children’s Hospital of New Orleans.
Table 1.
Demographic and Oral Intake Pre- and Post-Gabapentin.
| Sex | Age (Months) | Gestational Age (Weeks) | Diagnosis | Pre (mL) | Post (mL) |
|---|---|---|---|---|---|
| Female | 1.5 | Unavailable | TGV | 1514 | 1498 |
| Male | 12 | 39 | ASD, TGV | 0 | 23 |
| Female | 2 | 36 | Heart block | 1046 | 1655 |
| Female | 2 | 37 | ASD, VSD | 149 | 420 |
| Female | 2 | 40 | HLH | 211 | 568 |
| Male | 1.3 | 42 | Hypoplastic aortic arch | 128 | 214 |
| Female | 1.5 | 38 | VSD, TA | 562 | 1303 |
| Male | 1 | 39 | TOF, PA | 416 | 1520 |
| Male | 2 | 39 | TGA, TA, VSD | 212 | 1030 |
| Female | 2 | 37 | PA, VSD, ASD, Ebstein’s anomaly | 306 | 1230 |
| Male | 1.5 | 39 | TGA, PA, VSD | 1044 | 1165 |
| Female | 1.5 | 37 | HLH | 51 | 145 |
| Male | 1 | 39 | HLH | 127 | 310 |
| Female | 3 | 31 | TOF, PA | 227 | 622 |
| Female | 2.5 | 38 | HLH | 27 | 25 |
Abbreviations: TGV, transposition of the great vessels; ASD, atrial septal defect; VSD, ventricular septal defect; HLH, hypoplastic left heart; TOF, tetralogy of Fallot; TGA, transposition of the great arteries; TA, tricuspid atresia; PA, pulmonary valve atresia.
Results
Thirteen of 15 subjects improved oral intake with gabapentin, P < .01 (Z = −3.24), Wilcoxon rank test for paired data (Figure 1). Prior to gabapentin initiation, subjects averaged 401 ± 451 mL/day voluntary oral intake; after gabapentin subjects averaged 781 ±586 mL/day. There were no acute safety issues or sedation effects.
Figure 1.
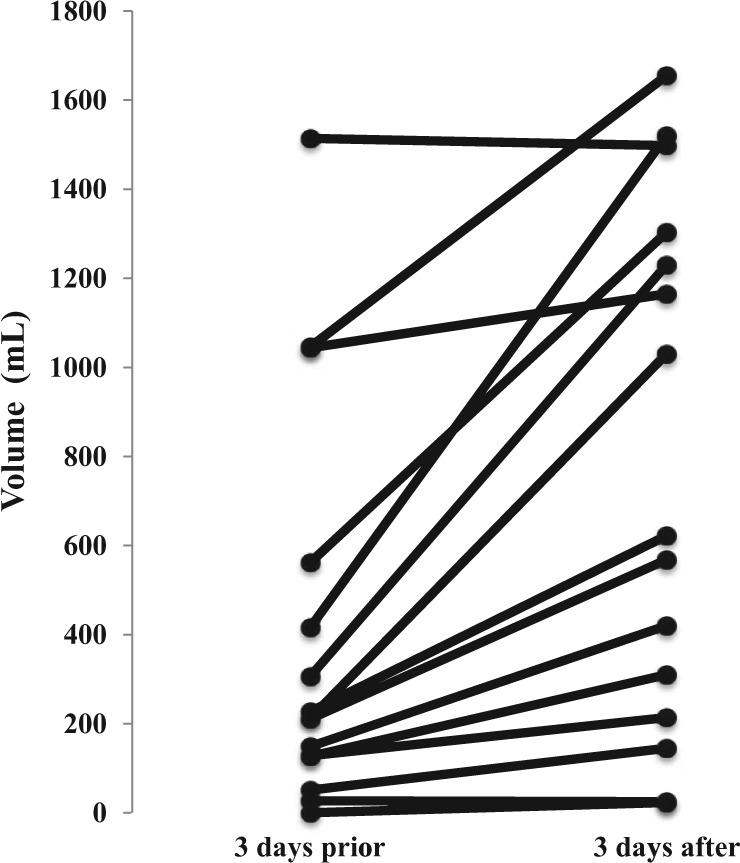
Effect of gabapentin on voluntary oral feeding volume in individual subjects.
Discussion
This study described an inpatient pharmacological approach to reduce discomfort associated with oral feeding in postoperative cardiac infants following the diagnosis of feeding problem (ICD 9 783.3). Some oral feeding problems may be due to chronic pain following multiple sensitizing events. Gabapentin may increase voluntary oral intake by reducing oropharyngeal, esophageal, or gastric discomfort associated with feeding.
There are several limitations to the current study. First, the small sample size makes it difficult to generalize about the effectiveness of this approach. Second, this is a retrospective chart review, rather than a randomized controlled trial with a placebo-control group, which somewhat limits the scientific rigor of the findings. One expects that feeding volumes would improve as infants recover from surgery and gain strength and endurance. The duration of the gabapentin treatment was also relatively brief, and it is unknown whether these initial improvements in oral intake would persist.
Despite the limitations, this preliminary study is the first to examine the effectiveness of gabapentin on oral feeding difficulties in hospitalized infants with feeding problems. Although there are clinical cues that establish acute pain in infants, there are no such markers for chronic pain. If a child refuses to walk, the clinician suspects pain in the foot, leg, or hip. If an infant refuses to eat, the clinician may suspect pain, or an expectation of pain associated with eating. Chronic pain is a probable cause for food refusal or early satiety in some medically complicated children. While there is no way to prove that infants are experiencing pain, the observed favorable response to gabapentin is consistent with pain reduction.9 A prospective, large randomized control trial comparing gabapentin to placebo to other chronic pain medicine (eg, amitriptyline) could help clarify the value of this pharmacologic approach.
Author Contributions
ASB, AMD and PH conceived of and designed the retrospective study. CFB, DC and PH performed the clinical chart reviews. ASB, AK, JM and PH analyzed and interpreted the data. ASB, AMD, CFB, DC, AK, JM, OA, and PH wrote the paper.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Zangen T, Ciarla C, Zangen S, et al. Gastrointestinal motility and sensory abnormalities may contribute to food refusal in medically fragile toddlers. J Pediatr Gastroenterol Nutr. 2003;37:287-293. [DOI] [PubMed] [Google Scholar]
- 2. Staiano A. Food refusal in toddlers with chronic diseases. J Pediatr Gastroenterol Nutr. 2003;37:287-293. [DOI] [PubMed] [Google Scholar]
- 3. Al-Chaer ED, Hyman PE. Visceral pain in infancy. In: Anand KJS, Stevens BJ, McGrath PJ, eds. Pain in Neonates and Infants. Edinburgh, Scotland: Elsevier; 2007:201-210. [Google Scholar]
- 4. Leslie AT, Akers KG, Martinez-Canabal A, Mello LE, Covolan L, Guinsburg R. Neonatal inflammatory pain increases hippocampal neurogenesis in rat pups. Neurosci Lett. 2011;501:78-82. doi: 10.1016/j.neulet.2011.06.047. [DOI] [PubMed] [Google Scholar]
- 5. Golbus JR, Wojcik BM, Charpie JR, Hirsch JC. Feeding complications in hypoplastic left heart syndrome after the Norwood procedure: a systematic review of the literature. Pediatr Cardiol. 2011;32:539-552. [DOI] [PubMed] [Google Scholar]
- 6. Jadcherla SR, Vijayapal AS, Leuthner S. Feeding abilities in neonates with congenital heart disease: a retrospective study. J Perinatol. 2009;29:112-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sachdeva R, Hussein E, Moss MM, et al. Vocal cord dysfunction and feeding difficulties after pediatric cardiovascular surgery. J Pediatr. 2007;151:312-315. [DOI] [PubMed] [Google Scholar]
- 8. Davis AM, Bruce AS, Mangiaracina C, Schultz T, Hyman PE. Moving from tube to oral feeding in medically fragile non-verbal toddlers. J Pediatr Gastroenterol Nutr. 2009;49:233-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hussain SZ, Hyman PE. Psychotropic medications for pediatric functional gastrointestinal disorders. J Pediatr Gastroenterol Nutr. 2014;59:280-287. [DOI] [PubMed] [Google Scholar]
- 10. Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237-251. [DOI] [PubMed] [Google Scholar]
- 11. Hauer JM, Wical BS, Charnas L. Gabapentin successfully manages chronic unexplained irritability in children with severe neurologic impairment. Pediatrics. 2007;119:519-522. [DOI] [PubMed] [Google Scholar]


