Abstract
Benign prostatic hyperplasia, prostate cancer, and changes in the ratio of circulating testosterone and estradiol often occur concurrently in aging men and can lead to lower urinary tract (LUT) dysfunction. To explore the possibility of a fetal basis for the development of LUT dysfunction in adulthood, Tg(CMV-cre);Nkx3-1+/−;Ptenfl/+mice, which are genetically predisposed to prostate neoplasia, were exposed in utero and during lactation to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD, 1 μg/kg po) or corn oil vehicle (5 ml/kg) after a single maternal dose on 13 days post coitus, and subsequently were aged without further manipulation, or at 8 weeks of age were exposed to exogenous 17 β-estradiol (2.5 mg) and testosterone (25 mg) (T+E2) via slow release subcutaneous implants. In utero and lactational (IUL) TCDD exposure in the absence of exogenous hormone treatment reduced voiding pressure in adult mice, but otherwise had little effect on mouse LUT anatomy or function. By comparison, IUL TCDD exposure followed by exogenous hormone treatment increased relative kidney, bladder, dorsolateral prostate, and seminal vesicle weights, hydronephrosis incidence, and prostate epithelial cell proliferation, thickened prostate periductal smooth muscle, and altered prostate and bladder collagen fiber distribution. We propose a 2-hit model whereby IUL TCDD exposure sensitizes mice to exogenous-hormone-induced urinary tract dysfunction later in life.
Keywords: Lower urinary tract symptoms (LUTS), fetal basis of adult disease, TCDD, mouse, prostate, hydronephrosis.
Lower urinary tract symptoms (LUTS) are a group of medical symptoms experienced by men and women, especially during aging, that include excessive nighttime urination (nocturia), increased urinary frequency or urgency, painful urination, weak stream, difficulty in starting and stopping urination, incomplete bladder emptying, and incontinence. Most individuals will seek treatment for LUTS at some point in their lives and the annual estimated health care cost in the United States is in the billions of dollars (Miller et al., 2009).
The underlying basis for the development of LUTS is not completely known but is almost certainly multifactorial. LUTS frequently occur in men with benign prostatic hypertrophy (BPH), prostatic fibrosis, and other prostatic pathologies including inflammation and cancer (Bauman et al., 2014a; Bostwick et al., 1992; Bushman, 2009; Gharaee-Kermani and Macoska, 2013; Ma et al., 2012; Nicholson et al., 2012; Nicholson and Ricke, 2011; Rodriguez-Nieves and Macoska, 2013; Torkko et al., 2015; Wynder et al., 2015). Male LUTS also arise in old age when the ratio of circulating 17β-estradiol-to-testosterone increases (Bjornerem et al., 2004).
There is considerable variation in age of onset, progression, and severity of LUTS and the underlying basis for this variation is likely multifactorial. Recent studies in mice show that urinary function varies considerably across strains (Tam et al., 2015; Yu et al., 2014), genetic risk factors are strongly implicated in early onset or severe LUTS (Doehring et al., 1996; Partin et al., 1994; Pearson et al., 2003; Sanda et al., 1994), and dietary factors are also likely to play a role (Keil et al., 2015). Gene-environment interactions are implicated in many diseases, yet few studies have addressed whether environmental chemicals influence baseline urinary function, age of LUTS onset or severity.
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is a lipophilic and ubiquitous environmental contaminant that is a high-affinity aryl hydrocarbon receptor (AHR) ligand. Nearly all biological actions of TCDD in mammals are mediated by AHR activation (Gonzalez et al., 1996). The primary route of human TCDD exposure is through the diet. The cumulative dose (Van den Berg et al., 1998) of TCDD and closely related polychlorinated dibenzo-p-dioxin, dibenzofuran, and biphenyl AHR agonists is estimated at 65.8 pg toxic equivalents/day (USEPA, 2003). TCDD has a tendency to accumulate over an animal’s lifespan in part because of its long whole-body elimination half-life, which has been estimated to be 9–24 days in mice (Birnbaum, 1986; Gasiewicz et al., 1983) and 7–11 years in humans (Pirkle et al., 1989; Wolfe et al., 1994). TCDD exposure during the fetal and neonatal period causes delayed or incomplete prostate development in rats, mice, and monkeys (Arima et al., 2010; Gray et al., 1997; Lin et al., 2002; Mably et al., 1992; Simanainen et al., 2004; Theobald and Peterson, 1997) and can elicit lifelong changes in prostate biology (Fritz et al., 2005); however, whether it causes urinary complications or changes urinary function had not been assessed.
The Barker Hypothesis, also known as the Developmental Origins of Adult Disease (DOHaD) hypothesis, posits that an environmental chemical exposure reprograms a tissue thereby increasing its susceptibility to adult disease (Barker, 1990). In this study, we test the hypothesis that in utero and lactational (IUL) TCDD exposure exacerbates urinary dysfunction in adult mice. Because BPH/LUTS and prostate cancer often coexist in men (Bostwick et al., 1992; Ornstein et al., 1997; Sheldon et al., 1980), and because the ratio of circulating sex steroids changes as men age (Belanger et al., 1994), we recapitulated aspects of this complex environment by using a mouse strain susceptible to prostate neoplasia (Tg[CMV-cre];Nkx3-1+/ −;Ptenfl/+) (Abate-Shen et al., 2003) and by exposing mice in adulthood to exogenous testosterone and 17 β-estradiol (T + E2), which disrupt urinary function (Bernoulli et al., 2008; Keil et al., 2015; Nicholson et al., 2012, 2015; Noble, 1977; Ricke et al., 2006, 2008). Our results indicate that IUL TCDD exposure increases the severity of adult urinary dysfunction in TCDD and T + E2-treated mice, providing some of the first evidence that exposure to an environmental contaminant during a male’s fetal and neonatal development may adversely impact lifelong urinary health.
MATERIALS AND METHODS
Mice
C57BL/6J, B6.129S4-Ptentm1Hwu/J (Ptenfl, stock number 006440) and B6.C-Tg(CMV-cre)1Cgn/J (Tg[CMV-cre], stock number 006054) mice were from the Jackson Laboratory (Bar Harbor, Maine) and B6.129-Nkx3-1tm1Mmsmice were from the Mouse Models of Human Cancers Consortium Repository (Bhatia-Gaur et al., 1999; Lesche et al., 2002; Schwenk et al., 1995). Mice used in this study were of the genotype Tg(CMV-cre);Nkx3-1+/-;Ptenfl/+. Mice were housed in Udel Polysulfone microisolator cages; the room was on 12-h light and dark cycles; room temperature was typically 20.5 ± 1 °C; humidity was about 30%–70%; females were fed 5015 Mouse Diet (PMI Nutrition International, Brentwood, Missouri) from the start of timed breeding through weaning (PND 21); and mice were otherwise fed 8604 Teklad Rodent Diet (Harlan Laboratories, Madison, Wisconsin). Feed and water were available ad libitum, cages contained corn cob bedding, and pregnant and nursing females had cotton nesting material (Nestlets, Anacare, Bellmore, New York). All procedures were approved by the University of Wisconsin Animal Care and Use Committee and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Mice were mated overnight and separated the next day, 0 days post coitus (dpc). Pregnant females were given a single dose of TCDD (1 μg/kg, po) or corn oil (5 ml/kg) on 13 dpc which, because of its 11.0 day whole body elimination half-life in C57BL/6J mice (Gasiewicz et al., 1983), the genetic background for all mice in these experiments, results in an exposure that persists in male offspring through weaning. Though higher TCDD doses cause mouse ventral prostate agenesis by preventing formation of ventral prostatic buds during prostate development (Lin et al., 2003), the TCDD dose used in this study was selected because it is sufficiently low to allow ventral prostatic bud formation to occur in C57BL/6J mice (Abbott et al., 2003), thus enabling examination of the combined influences of IUL TCDD exposure and adult testosterone and 17 β-estradiol exposure on the ventral prostate. TCDD (98% purity) was purchased from Cambridge Isotope Laboratories (Andover, Massachusetts). Male offspring were aged to 8 weeks old (early adulthood) and a subset were implanted subcutaneously with compressed pellets of testosterone (T, 25 mg, Steraloids, Newport, Rhode Island) and 17 β−estradiol (E2, 2.5 mg and cholesterol, 22.5 mg, Sigma Aldrich, St. Louis, Missouri). The testosterone and 17 β−estradiol doses were selected to match reference studies and because they are sufficient to cause urinary dysfunction in C57BL/6J mice (Keil et al., 2015; Nicholson et al., 2015, 2012). Mice were maintained after surgery for up to 4.1 months but euthanized earlier if moribund or recommended for euthanasia by the veterinarian. Mice were euthanized by CO2 asphyxiation and cervical dislocation. Bladder volume was determined by measuring the width, length, and height with a calipers and calculating the volume as described previously (Nicholson et al., 2012). Bladder wet weight was determined after draining it of urine. The upper and lower urinary tracts including the kidneys, prostate lobes, seminal vesicles, and bladders were collected, weighted or measured, and embedded in paraffin for subsequent histological analysis. Hydronephrosis was identified in intact fixed kidney specimens by observing cysts within the kidney as we have described (Nicholson et al., 2012) and by noting the presence or absence of cysts and visible dilation of renal calyces and/or pelvis in longitudinally bisected fixed kidneys.
Spontaneous void spot assay
Void spot assays (VSAs) were conducted and analyzed as described previously (Bjorling et al., 2015; Keil et al., 2015; Nicholson et al., 2015; Yu et al., 2014). Mice were removed from their cages and singly housed in a clean, empty cage lined with 3 MM Whatman filter paper (Fisher Scientific No.057163W). Assays were conducted during a 4-h period in the same quiet location and time of day while mice had access to food but not water. Filter papers were imaged using UV light on a transilluminator and analyzed using Image J Software (Version 1.46r). Voiding behaviors were examined for 3–14 mice per treatment group.
Cystometry
Mice were anesthetized with urethane (1.25 g/kg, ip), catheters were placed and secured as described previously (Keil et al., 2015) and saline infusion (0.8 ml/h) was initiated 45 min later. Voiding was recorded when a stable pattern was reached and measurements were made from at least 6 voiding events per mouse with 5 mice per treatment group. Measured parameters analyzed include bladder capacity, intercontractile interval (defined as the length of time between voids), and peak to baseline pressure associated with each voiding contraction (a contraction expelling urine from the body) or nonvoiding contraction (an intravesicular pressure spike without expulsion of urine from the body).
Bladder bath assay
In vitro bladder bath studies were conducted as described previously (Tengowski et al., 1997) with modifications described by Keil et al. (2015). Tissues were maintained at a tension of 1 g for 75 min prior to experimentation. Tension was recorded using the AxoScope Application of pCLAMP Software (Molecular Devices, Sunnyvale, California). Bladder hemisections were then equilibrated for approximately 1 h in Krebs solution and increasing concentrations (0–10 μM) of the cholinergic agonist carbachol were added stepwise thereafter. Tissues were then washed with carbachol-free Krebs solution to return the tension to baseline and a maximal response was generated by adding 60 mM KCl to baths. The average bladder hemisection response was determined for each mouse and 3 mice per treatment group were evaluated.
Immunohistochemistry
Tissue sections were deparaffinized with xylene and rehydrated with graded ethanol. Immunofluorescence staining was conducted as described previously (Abler et al., 2011), while immunohistochemical staining was conducted as described (Bauman et al., 2014b; Nicholson et al., 2012; Ricke et al., 2008). Antibodies included: rabbit anti-KI67 (No.Ab15580, working dilution 1:200, Abcam, Cambridge, Massachusetts), mouse antismooth muscle alpha actin (MS-113-B0, working dilution 1:100, Thermo Scientific, Waltham, Massachusetts), mouse anti-e-cadherin (2101181, working dilution 1:250, BD Biosciences, San Jose, San Jose, CA), Dylight 488-conjugated goat anti-mouse IgG (No.115-487-003, working dilution 1:250, Jackson ImmunoResearch Laboratory, West Grove, Pennsylvania) and Dylight 549-conjugated goat anti-rabbit IgG (No.111-507-003, working dilution 1:250, Jackson ImmunoResearch). Tissue sections were visualized and imaged using an Eclipse E600 compound microscope (Nikon Instruments Inc., Melville, New York) equipped with NIS elements imaging software (Nikon Instruments Inc).
Cell proliferation indices were determined by counting KI67-positive and KI67-negative cells in two 200× fields per prostate tissue section (5 mice per group) and mean labeling indices (KI67-positive/total cells) were separately determined for prostate epithelium and stroma. Similarly, to determine smooth muscle thickness, prostate were assessed for smooth muscle content via immunohistochemistry. Smooth muscle thickness was measured by an optical micrometer from 2 nonadjacent tissue sections per mouse (5 mice per group). Twenty measurements were made from a 100× field and an additional 20 measurements were made from a 10× field. The mean was determined for all measurements for each mouse and used to determine the average for corn oil + T + E2 and TCDD + T + E2 groups.
Picrosirius red staining and quantification of birefringent collagen
Tissues were processed and analyzed as described previously (Bauman et al., 2014a). Briefly, formalin-fixed, paraffin-embedded tissue sections (5 μm thick) were deparaffinized in xylene, hydrated, and sequentially stained with Weigert’s iron hematoxylin and Sirius red F3BA. Sections were dehydrated, mounted and imaged under bright field and polarized light. Background was subtracted using Image J Color Corrector plugin and intensity of red, yellow, orange, and green hues quantified. Prostate tissue sections from 5 mice were analyzed per treatment group. To determine how the picrosirius red staining pattern changed as a function of T + E2 treatment time, tissue sections from some mice, euthanized prior to the expected endpoint of the experiment because they were moribund or recommended for euthanasia by a veterinarian, were also examined. Picrosirius red staining patterns were therefore analyzed across experimental groups independent of T + E2 treatment time, and within experimental groups divided on the basis of subchronic (6–12 weeks) or chronic (16–18 weeks) exposure to T + E2.
Statistics
Statistical analysis was performed using R version 2.13.1 and Graphpad Prism 5.04 (Graphpad Software, La Jolla, California). Homogeneity of variance was determined using Levene’s or Bartlet’s test and data were transformed if necessary to normalize variance. Student’s t test was used to identify significant differences (P ≤ .05) between treatment groups for most comparisons. Chi-squared analysis was used to assess differences in hydronephrosis and paraphimosis incidence. Litter independence was observed when collecting and analyzing data.
RESULTS
IUL TCDD Exposure Increases Relative Bladder, Seminal Vesicle, and Dorsolateral Prostate Weight in Mice Exposed as Adults to Exogenous T+E2
Male LUTS often occur with BPH, can arise in conjunction with prostate cancer, and at a life stage when the ratio of circulating E2 to testosterone is increasing (Bjornerem et al., 2004; Bostwick et al., 1992; Kristal et al., 2008). We used mice to model this complex environment and to determine if IUL TCDD exposure exacerbates the acquired urinary dysfunction in adulthood. Tg(CMV-cre);Nkx3-1+/ −;Ptenfl/+ male mice, which are genetically susceptible to prostate neoplasia (Abate-Shen et al., 2003), were exposed via a single maternal dose to vehicle or TCDD (1 μg/kg) on 13 dpc and subcutaneously implanted with compressed T and E2 pellets at 8 weeks of age. Mice were euthanized 4 months after T and E2 pellet implantation. We previously demonstrated that exogenous T + E2 exposure increased mouse bladder weight (Nicholson et al., 2012) and bladder weight is also increased by surgically induced urethral obstruction (Austin et al., 2004). We found here that IUL TCDD exposure amplifies the hormone-induced increase in relative bladder weight (Figs. 1A and B). TCDD significantly (P < .05) decreased body weight, significantly increased relative bladder, dorsolateral prostate (DLP), and seminal vesicle (SV) weight and caused a nearly significant (P = .06) increase in relative bladder volume, but had no significant effect on relative anterior prostate (AP) or ventral prostate (VP) weight (Table 1). The incidence of penile paraphimoses (trapping of foreskin behind the prepuce) was not significantly increased by IUL TCDD exposure in T + E2-treated mice (Table 1) but was far greater than we previously observed in T + E2 implanted wild-type mice (Nicholson et al., 2012). Frank prostate carcinoma was not observed in any group. These results support the notion that IUL TCDD exposure magnifies some T + E2-induced changes in mouse LUT anatomy without causing frank prostate cancer.
FIG. 1.
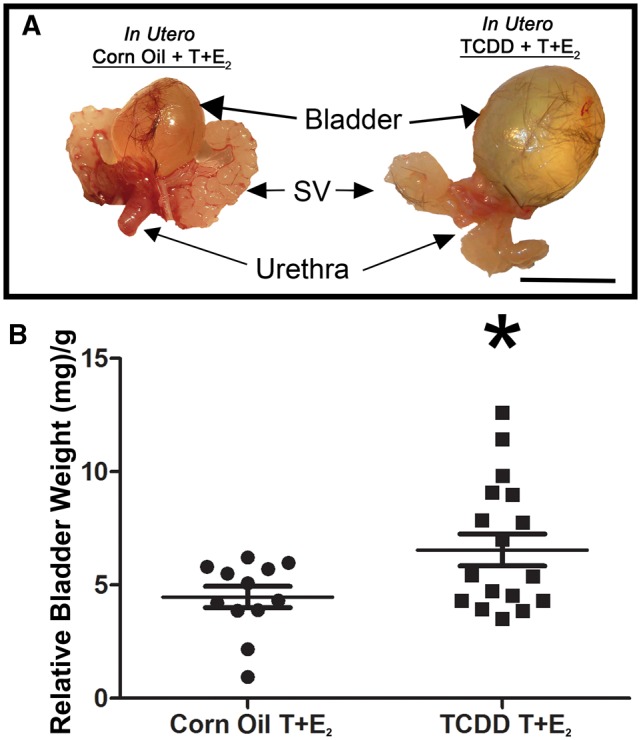
IUL TCDD exposure causes urinary dysfunction in male mice exposed in adulthood to T+E2. Male mice were exposed to TCDD (a single 1 μg/kg maternal dose on 13 dpc) or corn oil (vehicle control), implanted sc with compressed testosterone (T) and 17β−estradiol (E2) pellets at 8 wk of age, and analyzed 4–18 wk later. (A) Lower urinary tracts from representative mice are shown. (B) Relative bladder weight is increased by IUL TCDD exposure in T+E2 treated mice. Results are mean ± SE, n ≥ 13/group. The asterisk indicates a significant difference (P < .05) between groups. Abbreviations: IUL: In utero and lactational; TCDD, tetrachlorodibenzo-p-dioxin; SV: seminal vesicle. Color image is available in the online version of the article.
TABLE 1.
Effects of IUL TCDD Exposure on Genitourinary Responses to Adult T+E2 Exposure
| Body Weight (g) | Bladder Volume (mm3/g) | Bladder Weight (mg/g) | Ventral Prostate (mg/g) | Anterior Prostate (mg/g) | Dorsolateral Prostate (mg/g) | Seminal Vesicles (mg/g) | Penile Paraphimosis (Incidence) | |
|---|---|---|---|---|---|---|---|---|
| Corn Oil T+E2 | 28.2 ± 0.7 (12) | 25 ± 8 (9) | 4.5 ± 0.5 (12) | 0.9 ± 0.1 (12) | 1.2 ± 0.1 (12) | 2.0 ± 0.3 (12) | 4.0 ± 0.7 (12) | 45% (22) |
| TCDD T+E2 | 24.3 ± 1.0* (18) | 44 ± 7 (15) | 6.7 ± 0.7* (17) | 1.1 ± 0.2 (16) | 1.2 ± 0.2 (16) | 3.2 ± 0.4* (16) | 7.9 ± 0.8* (15) | 29% (21) |
Results are mean ± SE (or incidence), with the number of replicates shown in parentheses.
*Significantly different from control (P < .05).
IUL TCDD Exposure Increases Prostate Epithelial Proliferation, Prostate Smooth Muscle Thickness, and Alters Stromal Collagen Composition in Mice Exposed in Adulthood to Exogenous T+E2
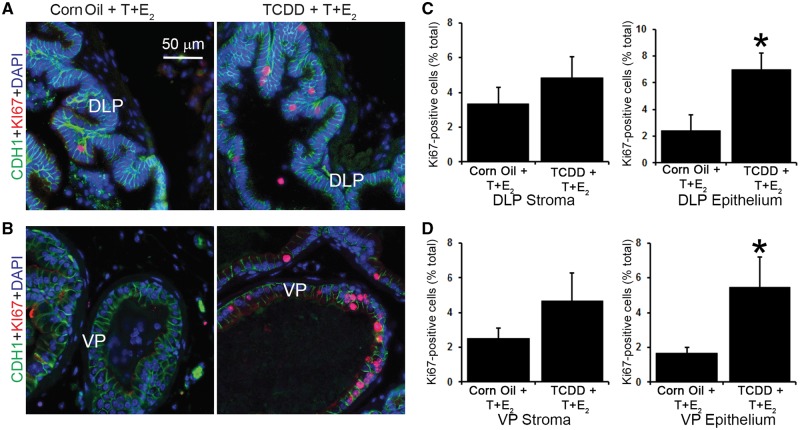
Prostate proliferative cell growth is a hallmark of BPH, which led us to test whether IUL TCDD exposure increased prostate cell proliferation in mice treated with T + E2. KI67 labeling indices were determined in DLP and VP. IUL TCDD exposure significantly increased KI67 labeling in both DLP epithelium and VP epithelium but no significant differences were observed in either DLP stroma or VP stroma (Figure 2).
FIG. 2.
IUL TCDD exposure increases hormone-induced dorsolateral and ventral prostate epithelial cell proliferation in male mice exposed in adulthood to T+E2. Immunofluorescence staining for E-cadherin (CDH1, green), KI67 (red), and DAPI (blue) was conducted in (A) dorsolateral (DLP) and (B) ventral (VP) prostates of corn oil + T+E2- and TCDD + T+E2-exposed mice. The KI67 proliferative index was calculated for (C) DLP and (D) VP stroma and epithelium. Results are the mean ± SE of 5 mice. An asterisk indicates a significant difference (P < .05) between groups.
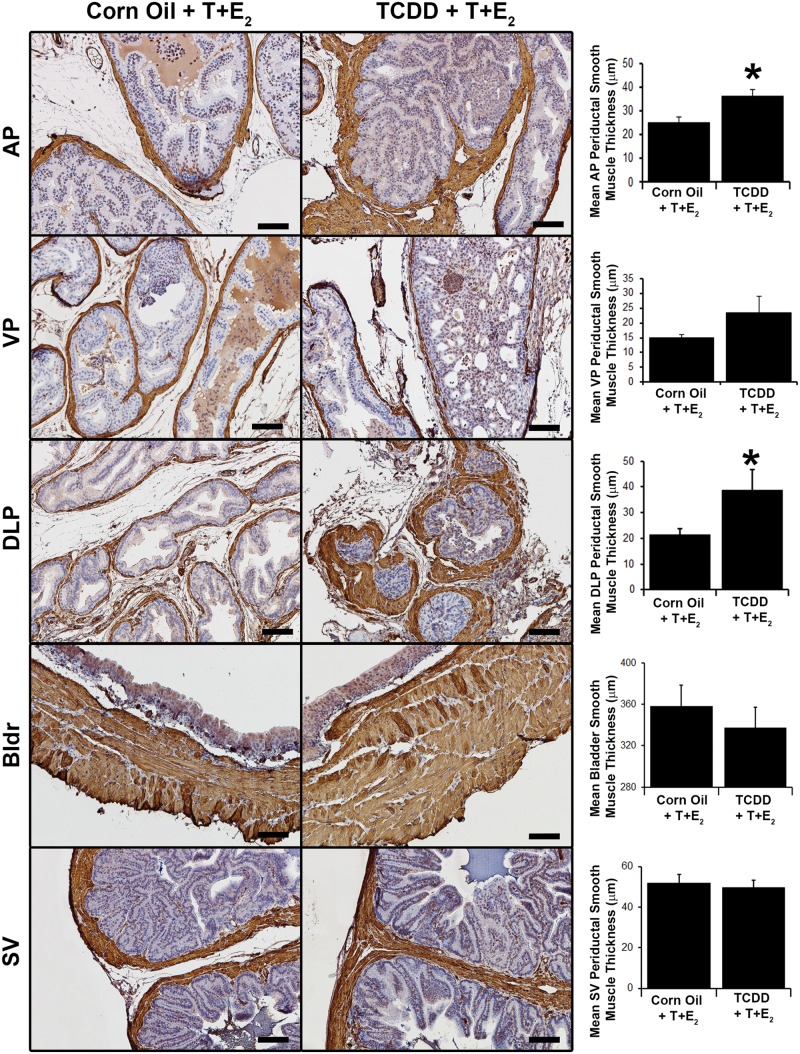
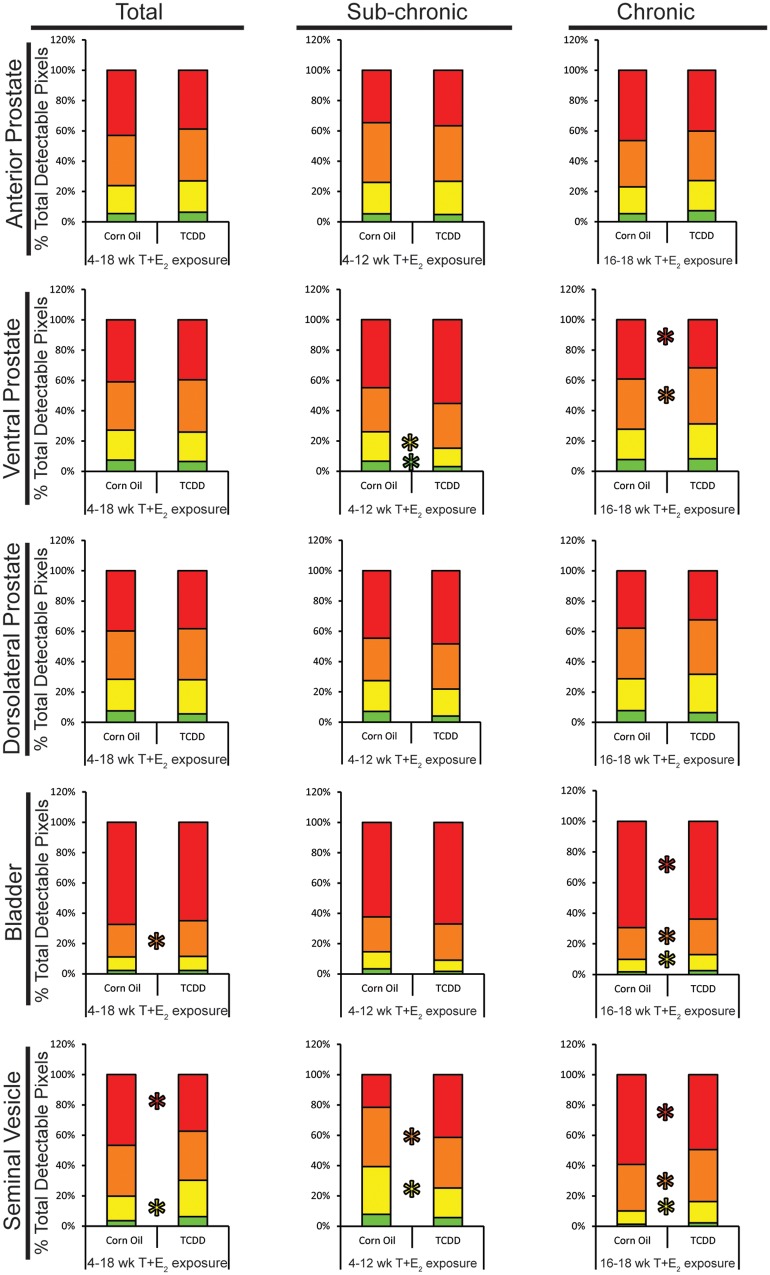
Human BPH has been characterized by increased prostate stromal cell number and fibrosis (Bauman et al., 2014a; Ma et al., 2012; Nicholson et al., 2013). Therapeutic efficacy of alpha adrenergic receptor antagonists in managing LUTS also supports a role of smooth muscle in moderating urinary function. Histochemical and immunohistochemical staining were used to test whether IUL TCDD exposure alters prostatic smooth muscle thickness and collagen distribution in T + E2-exposed mice. In hormone-exposed mice, following IUL TCDD exposure, we observed a significant increase in the thickness of the prostatic smooth muscle within the DLP and AP but not in the VP, SV, or bladder detrusor muscle (Figure 3). Prostate and bladder collagen fibers were assessed quantitatively (Figure 4) from images of picrosirius red-stained tissue sections viewed under polarized light (Supplementary Figure 1). Stained collagen fibers are birefringent under these conditions and their polarization spectra (red, yellow, orange, green) are determined by collagen fiber size, identity, and packing (Dayan et al., 1989; Nicoletti et al., 1995). Collagen birefringence in prostate, SV and bladder was quantified across all T + E2 exposed mice and subsequently separated into subchronic (6–12 weeks) and chronic (16–18 weeks) T + E2 exposure groups. There were no significant differences in prostate birefringent pixel density between treatment groups when evaluated across all mice independent of T + E2 exposure duration and no differences in DLP and AP when separately assessed after subchronic or chronic T + E2 exposure. However, in VP, IUL TCDD exposure was associated with less green and yellow pixels after subchronic T + E2 exposure and more orange and less red pixels after chronic T + E2 exposure (Figure 4). IUL TCDD exposure reduced yellow and orange pixels in SV after subchronic T + E2 exposure and increased yellow and orange but decreased red pixels in SV after chronic T + E2 exposure (Figure 4). IUL TCDD exposure increased orange bladder pixels when all mice were counted independent of T + E2 exposure duration. No difference was observed after subchronic T + E2 treatment. However, IUL TCDD increased yellow and orange pixels and significantly reduced red bladder pixels after chronic T + E2 exposure (Figure 4). These results demonstrate that IUL TCDD exposure impacts adult prostate smooth muscle thickness and prostate, SV, and bladder collagen distribution in mice with adult T + E2 exposure. Our results also indicate that collagen fiber distribution is remodeled between subchronic and chronic T + E2 exposure.
FIG. 3.
IUL TCDD exposure increases prostate smooth muscle thickness in mice exposed in adulthood to T+E2. (A) Immunohistochemical staining was used to visualize smooth muscle alpha actin in anterior (AP), ventral (VP), dorsolateral (DLP) prostates, seminal vesicles (SV), and bladders (Bldr) of corn oil + T+E2- and TCDD + T+E2-exposed mice and smooth muscle thickness was measured. Results are mean ± SE of 5 mice. An asterisk indicates a significant difference (P < .05) between groups. Scale bar = 100 μm. Color image is available in the online version of the article.
FIG. 4.
IUL TCDD exposure changes collagen fiber distribution in the lower urinary tract in mice exposed as adults to T+E2. Picrosirius red stained-sections of anterior (AP), ventral (VP), and dorsolateral (DLP) prostates, seminal vesicles (SV) and bladders of corn oil + T+E2- and TCDD + T+E2-exposed mice were imaged under polarized light and red, orange, yellow, and green pixel densities were quantified. Results in the “Total” column combined data from all mice receiving 4–18 wk T+E2 exposure, while the “Subchronic” column includes only mice with 6–12 wk T+E2 exposure, and the “Chronic” column includes mice with 16–18 wk T+E2 exposure. Results are mean ± SE of at least 5 mice. An asterisk indicates a significant difference (P < .05) between groups.
IUL TCDD Exposure Is Associated With Hydronephrosis in Mice Exposed as Adults to Exogenous T+E2
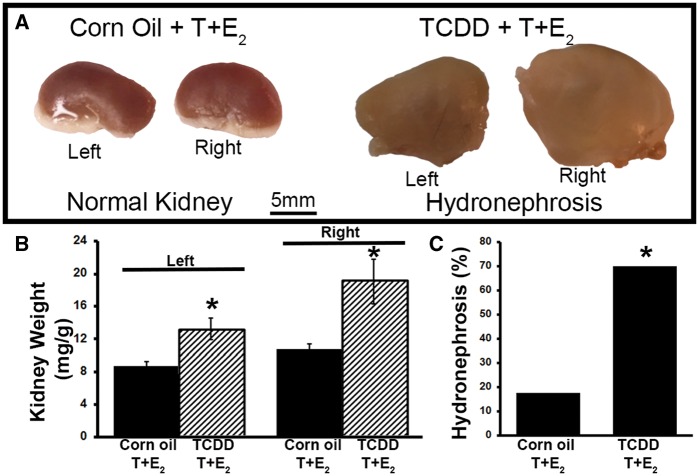
Acute urinary retention and hydronephrosis are potential manifestations of severe bladder outlet obstruction in mice and men (Izard and Nickel, 2011; Nicholson et al., 2012). As an index of urinary dysfunction in mice, we tested whether IUL TCDD exposure increased relative kidney weight and the incidence of frank hydronephrosis in steroid-implanted mice (Figs. 5A–C). IUL TCDD exposure significantly increased both left and right relative kidney weight (Figure 5B) and frank hydronephrosis incidence (Figure 5C) in mice also exposed to T + E2. Early-life TCDD exposure has been shown previously to cause hydronephrosis in mice (Moore et al., 1973). However, in this study, neither IUL corn oil nor IUL TCDD exposure caused hydronephrosis in the absence of exogenous T + E2 (Supplementary Table 1). These results indicate the combined actions of IUL TCDD exposure and an adult change in circulating hormone concentration were needed to increase relative kidney weight and elicit an increased incidence of hydronephrosis.
FIG. 5.
IUL TCDD exposure increases relative kidney wet weight and hydronephrosis incidence in mice exposed in adulthood to T+E2. A, Representative kidneys collected from corn oil + T+E2- and TCDD + T+E2-exposed mice. Frank hydronephrosis is characterized by renal cysts and dilation of the renal pelvis and calyces, exists in the TCDD-exposed kidney. B, Relative kidney (left and right) wet weights from IUL TCDD-exposed + T+E2-treated mice compared to corn oil + T+E2 controls. Results are mean ± SE from 9-17 mice per treatment group. C, In IUL TCDD-exposed + T+E2-treated mice, the incidence of hydronephrosis was significantly higher compared to corn oil + T+E2 controls. An asterisk indicates a significant difference (P < .05) between groups. Color image is available in the online version of the article.
IUL TCDD Exposure Without Exposure to Exogenous T+E2 in Adulthood Has Little Effect Mouse Urinary Tract Wet Weights
Since IUL TCDD exposure appeared to increase voiding dysfunction incidence and severity in T + E2-exposed mice, we assessed the anatomical impact of IUL TCDD exposure without the second hit of adult T + E2 exposure. Under these conditions, body weight was significantly reduced, but there were no IUL TCDD exposure-related changes in adulthood in relative bladder volume or relative bladder, AP, DP, SV, or kidney weights (Supplementary Table 1). Neither frank hydronephrosis nor penile paraphimosis was observed in any of the mice. The only difference observed in the IUL TCDD exposure group was a significant decrease in relative VP weight. Overall, effects of IUL TCDD exposure without the second hit of adult T + E2 exposure were unremarkable.
IUL Exposure to TCDD Without Adult Exposure to Exogenous T+E2 Does Not Alter Spontaneous Voiding Behavior in Mice
To determine whether IUL TCDD exposure impacted adult baseline voiding behavior, adult male and female mice (≥ 42 days of age) underwent VSA testing, recently optimized by our group and others to assess spontaneous voiding function in free moving mice (Bjorling et al., 2015; Keil et al., 2014, 2015; Nicholson et al., 2015). Total urine spot number, urine area, mean spot size, area of primary void, % total urine area in primary void, % total urine area in corners, and % total urine area in center were analyzed. Most of the measured voiding parameters were unchanged by IUL TCDD exposure (Supplementary Table 2).
IUL TCDD Exposure Without Adult Exposure to Exogenous T+E2 Decreases Peak to Baseline Pressure of Voiding Contractions
To determine whether IUL TCDD exposure impacts bladder physiology, anesthetized cystometry was performed on male mice, ≥ 42 days old, to analyze mouse bladder pressure profiles associated with filling and emptying. Void intervals and the quantity and pressure of voiding and nonvoiding contractions were determined. Bladder volume, intervoid interval, peak-to-baseline pressures of voiding and nonvoiding contractions were not significantly changed by IUL TCDD (Supplementary Table 3). IUL TCDD exposure did increase adult body weight. When cystometry parameters were normalized to body weight, IUL TCDD exposure significantly decreased peak to baseline voiding pressure (Supplementary Table 3). These results indicate that IUL TCDD exposure may lead to changes in baseline bladder contractile activity in adulthood.
IUL TCDD Exposure Without Adult T+E2 Exposure Does Not Alter Bladder Contractility in Response to Cholinergic Stimulation
We next determined whether decreased voiding pressure observed during cystometry in male mice (≥ 42 days old) with IUL TCDD exposure (Supplementary Table 3) is in part due to decreases in bladder contractility. Bladder strips were affixed to force transducers and placed in organ bath media. Bladder contractile response to increasing concentrations of a cholinergic agonist, carbachol, was measured and quantified as percent of the maximum response elicited by potassium chloride. IUL TCDD exposure did not significantly change bladder sensitivity to cholinergic stimulation or maximal response to potassium chloride (Supplementary Figure 2).
DISCUSSION
Advancing age and prostate volume are significant risk factors for LUTS and urinary dysfunction, but why some men are affected at an earlier age than others, and which factors control disease severity is largely unknown. This study conducted in a mouse strain susceptible to prostate cancer and in the presence of hormone imbalance, is the first to demonstrate that IUL TCDD exposure worsens urinary dysfunction in adulthood. It supports a new concept that early life chemical exposures interact with genetic susceptibilities and other risk factors to shape a man’s lifelong urinary health. The 2 hit hypothesis supported by our results involves IUL TCDD exposure (hit No. 1) followed by hormonal changes later in life (hit No. 2), culminating in urinary dysfunction.
IUL TCDD exposure in the absence of adult exposure to exogenous hormones had very little effect on mouse urinary tract anatomy and physiology: spontaneous voiding behavior was not significantly changed, most cystometric parameters and bladder contractile response to cholinergic stimulation were unaffected, frank hydronephrosis was not observed, and kidney wet weight was unchanged. However, IUL TCDD exposure combined with adult T + E2 exposure exacerbated mouse urinary dysfunction: mouse bladders exhibited a greater relative wet weight and incidence of frank hydronephrosis and kidney wet weight were increased. These mice also developed increased relative DLP wet weight and abnormal LUT histology characterized by increased smooth muscle thickness, prostate cell proliferation, and changes in prostate collagen fiber distribution. Importantly, given that the TCDD half-life in C57Bl/6J mice is 11 days (Gasiewicz et al., 1983), it is estimated that 99% of the single embryonic TCDD dose was eliminated from male mice when endpoint data was collected. This argues that TCDD and T + E2 act at distinct life stages to cause adult urinary dysfunction. The molecular mechanism could be dependent on epigenetic or other persistent changes that when confronted with a “second hit” (ie, hormones) elicit a phenotype.
Our 2-hit hypothesis is consistent with DOHaD, wherein fetal exposures to environmental toxicants predispose animals to adult diseases. Our studies involved a mouse strain (Tg[CMV-cre];Nkx3-1+/ −;Ptenfl/+) genetically susceptible to prostate neoplasia, an early life environmental toxicant (TCDD) exposure, and an experimentally established hormonal environment that mimics that of aging men. A mouse strain (Pten+/ −;Nkx3-1+/ −) closely related to the one used in the present study develops invasive prostate carcinoma at approximately 1 year of age (Kim et al., 2002). Although no obvious prostate cancer or metastases were observed in this study, severe urinary retention was evident. We previously found that a maternal TCDD exposure during pregnancy causes the prostate of male mouse offspring to retain androgen dependence into old age and causes prostatic hyperplasia when males reach senescence (510 days old) (Fritz et al., 2005). Furthermore, a maternal TCDD dose given to Rhesus macaques during pregnancy decreases prostate glandular content, increases prostatic periductal smooth muscle thickness, and causes prostatic fibrosis (ie, collagen deposition) in male offspring at 7 years of age (Arima et al., 2010; Korenaga et al., 2007). These endpoints are cardinal features of human BPH (Akduman and Crawford, 2001; Bauman et al., 2014a; Ma et al., 2012; Nicholson et al., 2013; Shapiro et al., 1992), offering a potential link between IUL TCDD exposure and BPH. Indeed we observed a significant increase in prostatic proliferation and an increase in prostate smooth muscle thickness in mice that had IUL TCDD exposure followed by adult hormone treatment. Furthermore, we found that IUL TCDD exposure changed prostatic collagen distribution, which is consistent with changes in prostatic collagen content in humans with BPH (Bauman et al., 2014a). Together these results support our assertion that IUL TCDD exposure in combination with genetic risk factors predisposes animals to BPH pathology later in life. Mechanisms by which environmental factors interact with genetic susceptibilities to confer individual risk are largely unknown and an area of future study. Our results indicate pathways involved in deposition or remodeling of smooth muscle and collagen are potential targets.
Results presented in this study combined with other experimental and epidemiological data point to a paradox in the field, in that factors such as TCDD may be both harmful and beneficial, with exposure age serving as the key determinant of outcome. Previous studies have shown that men with known or suspected adult TCDD exposure may be at a reduced risk of developing BPH (Gupta et al., 2006a,b). This is an important observation and distinct from this study involving IUL TCDD exposure. Although our results suggest IUL TCDD exposure may be a risk factor for adult urinary dysfunction, less toxic AHR agonists, such as selective AHR modulators, may be therapeutically beneficial if given to adult men with LUTS. Future studies in mice and men will lay the groundwork to resolve the apparent enigma presented by timing of TCDD exposure and benign urologic outcomes.
We showed that IUL TCDD exposure, followed by adult T + E2 treatment, increased kidney wet weight and incidence of frank hydronephrosis. It should be noted that others have demonstrated IUL TCDD exposure induces upper urinary tract dysfunction including fetal hydronephrosis (Aida-Yasuoka et al., 2014; Moore et al., 1973). We did not observe frank hydronephrosis in mice exposed to TCDD without adult exogenous hormone exposure. Additionally, exogenous T + E2 exposure without IUL TCDD exposure causes hydronephrosis in mice albeit at an incidence less than 10% (Nicholson et al., 2012). Together our data indicate that IUL TCDD exposure interacts with adult exposure to T+E2 to increase susceptibility to hydronephrosis later in life. Hydronephrosis is a potential complication of acute urinary retention from bladder outlet obstruction (Izard and Nickel, 2011). The fact that IUL TCDD exposure increased relative bladder weight in T + E2 exposed mice (previously shown to be increased by surgically induced urethral obstruction (Austin et al., 2004)), is supporting evidence for urethral obstruction and provides a plausible mechanism for increased hydronephrosis incidence in these mice.
We found that IUL TCDD exposure predisposed male mice to urinary dysfunction. To determine if aspects of urinary dysfunction were due to IUL TCDD exposure alone, we assessed the impact of IUL TCDD exposure on adult urinary anatomy and physiology without hormone treatment later in life. Aside from a decrease in relative VP wet weight, we did not observe any obvious IUL TCDD exposure-associated changes in anatomy or any significant impact on baseline urinary function, behavior, or bladder contractility. Although we did observe a decrease in peak to baseline voiding pressure, which suggested that early life TCDD exposure may predispose mice to increased sensitivity to bladder filling later in life. This could be construed as an aspect of LUTS observed in patients. We conclude that early life TCDD exposure may act with genetic risk factors and hormonal changes associated with aging to enhance male susceptibility to LUTS later in life.
Future studies will assess the impact of TCDD and hormones on wild-type mouse urinary tract anatomy and physiology. Although our study links TCDD exposure to a common urologic disease, it is unlikely that we can change our exposure to TCDD and related environmental AHR agonists, as these chemicals are ubiquitous and long lasting. Nonetheless, understanding how chemicals like TCDD modify disease risk, as well as understanding the molecular mechanisms by which TCDD confers enhanced susceptibility to disease later in life, may reveal therapeutic targets to slow or prevent disease progression. This study also points to hormonal regulation as a possible way to slow or prevent LUTS. Future research is needed to assess these possibilities.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
ACKNOWLEDGMENTS
The authors thank Dr. Dale Bjorling, UW-Madison, for his intellectual contributions and the UW-Madison George M. O’Brien Rodent Urinary Function Testing Facility for technical and intellectual contributions. Grant Sponsors: National Institutes of Health Grants R01DK093690 (W.A.R.), R01DK099328 (C.M.V.), U54 DK104310 (W.A.R., C.M.V.), ES001332 (R.E.P.), R25ES020720, and the University of Wisconsin-Madison Molecular and Environmental Toxicology Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
FUNDING
National Institute of Environmental Health Sciences, (Grant/Award Number: “R01ES001332,” “R25ES020720”) and National Institute of Diabetes and Digestive and Kidney Diseases, (Grant/Award Number: “R01DK093690,” “R01DK099328,” “U54DK104310”).
REFERENCES
- Abate-Shen C, Banach-Petrosky W. A, Sun X, Economides K. D, Desai N, Gregg J. P, Borowsky A. D, Cardiff R. D, Shen M. M. (2003). Nkx3.1;Pten mutant mice develop invasive prostate adenocarcinoma and lymph node metastases. Cancer Res. 63, 3886–3890. [PubMed] [Google Scholar]
- Abbott B. D, Lin T. M, Rasmussen N. T, Albrecht R. M, Schmid J. E, Peterson R. E. (2003). Lack of expression of EGF and TGF-α in the fetal mouse alters formation of prostatic epithelial buds and influences the response to TCDD. Toxicol. Sci. 76, 427–436. [DOI] [PubMed] [Google Scholar]
- Abler L. L, Keil K. P, Mehta V, Joshi P. S, Schmitz C. T., Vezina C. M. (2011). A high-resolution molecular atlas of the fetal mouse lower urogenital tract. Dev. Dyn. 240, 2364–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aida-Yasuoka K, Yoshioka W, Kawaguchi T, Ohsako S, Tohyama C. (2014). A mouse strain less responsive to dioxin-induced prostaglandin E2 synthesis is resistant to the onset of neonatal hydronephrosis. Toxicol. Sci. 141, 465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akduman B., Crawford E. D. (2001). Terazosin, doxazosin, and prazosin: Current clinical experience. Urology 58, 49–54. [DOI] [PubMed] [Google Scholar]
- Arima A, Kato H, Ise R, Ooshima Y, Inoue A, Muneoka A, Kamimura S, Fukusato T, Kubota S, Sumida H, et al. (2010). In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induces disruption of glands of the prostate and fibrosis in rhesus monkeys. Reprod. Toxicol. 29, 317–322. [DOI] [PubMed] [Google Scholar]
- Austin J. C, Chacko S. K, DiSanto M, Canning D. A., Zderic S. A. (2004). A male murine model of partial bladder outlet obstruction reveals changes in detrusor morphology, contractility and myosin isoform expression. J. Urol. 172, 1524–1528. [DOI] [PubMed] [Google Scholar]
- Barker D. J. (1990). The fetal and infant origins of adult disease. BMJ 301, 1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman T. M, Nicholson T. M, Abler L. L, Eliceiri K. W, Huang W, Vezina C. M, Ricke W. A. (2014a). Characterization of fibrillar collagens and extracellular matrix of glandular benign prostatic hyperplasia nodules. PLoS One 9, e109102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman T. M, Vezina C. M, Huang W, Marker P. C, Peterson R. E, Ricke W. A. (2014b). Beta-catenin is elevated in human benign prostatic hyperplasia specimens compared to histologically normal prostate tissue. Am. J. Clin. Exp. Urol. 2, 313–322. [PMC free article] [PubMed] [Google Scholar]
- Belanger A, Candas B, Dupont A, Cusan L, Diamond P, Gomez J. L., Labrie F. (1994). Changes in serum concentrations of conjugated and unconjugated steroids in 40- to 80-year-old men. J. Clin. Endocrinol. Metab. 79, 1086–1090. [DOI] [PubMed] [Google Scholar]
- Bernoulli J, Yatkin E, Konkol Y, Talvitie E. M, Santti R., Streng T. (2008). Prostatic inflammation and obstructive voiding in the adult Noble rat: Impact of the testosterone to estradiol ratio in serum. Prostate 68, 1296–1306. [DOI] [PubMed] [Google Scholar]
- Bhatia-Gaur R, Donjacour A. A, Sciavolino P. J, Kim M, Desai N, Young P, Norton C. R, Gridley T, Cardiff R. D, Cunha G. R, et al. (1999). Roles for Nkx3.1 in prostate development and cancer. Genes Dev. 13, 966–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum L. S. (1986). Distribution and excretion of 2,3,7,8-tetrachlorodibenzo-p-dioxin in congenic strains of mice which differ at the Ah locus. Drug Metab. Dispos. 14, 34–40. [PubMed] [Google Scholar]
- Bjorling D. E, Wang Z, Vezina C. M, Ricke W. A, Keil K. P, Yu W, Guo L, Zeidel M. L, Hill W. G. (2015). Evaluation of voiding assays in mice: Impact of genetic strains and sex. Am. J. Physiol. Renal. Physiol. 308, F1369–F1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornerem A, Straume B, Midtby M, Fonnebo V, Sundsfjord J, Svartberg J, Acharya G, Oian P, Berntsen G. K. (2004). Endogenous sex hormones in relation to age, sex, lifestyle factors, and chronic diseases in a general population: The Tromso study. J. Clin. Endocrinol. Metab. 89, 6039–6047. [DOI] [PubMed] [Google Scholar]
- Bostwick D. G, Cooner W. H, Denis L, Jones G. W, Scardino P. T., Murphy G. P. (1992). The association of benign prostatic hyperplasia and cancer of the prostate. Cancer 70, 291–301. [DOI] [PubMed] [Google Scholar]
- Bushman W. (2009). Etiology, epidemiology, and natural history of benign prostatic hyperplasia. Urol. Clin. North Am. 36, 403–415. [DOI] [PubMed] [Google Scholar]
- Dayan D, Hiss Y, Hirshberg A, Bubis J. J., Wolman M. (1989). Are the polarization colors of picrosirius red-stained collagen determined only by the diameter of the fibers? Histochemistry 93, 27–29. [DOI] [PubMed] [Google Scholar]
- Doehring C. B, Sanda M. G, Partin A. W, Sauvageot J, Juo H, Beaty T. H, Epstein J. I, Hill G, Walsh P. C. (1996). Histopathologic characterization of hereditary benign prostatic hyperplasia. Urology 48, 650–653. [DOI] [PubMed] [Google Scholar]
- Fritz W. A, Lin T. M, Moore R. W, Cooke P. S, Peterson R. E. (2005). In utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure: Effects on the prostate and its response to castration in senescent C57BL/6J mice. Toxicol. Sci. 86, 387–395. [DOI] [PubMed] [Google Scholar]
- Gasiewicz T. A, Geiger L. E, Rucci G, Neal R. A. (1983). Distribution, excretion, and metabolism of 2,3,7,8-tetrachlorodibenzo-p-dioxin in C57BL/6J, DBA/2J, and B6D2F1/J mice. Drug Metab. Dispos. 11, 397–403. [PubMed] [Google Scholar]
- Gharaee-Kermani M, Macoska J. A. (2013). Promising molecular targets and biomarkers for male BPH and LUTS. Curr. Urol. Rep. 14, 628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F. J, Fernandez-Salguero P, Ward J. M. (1996). The role of the aryl hydrocarbon receptor in animal development, physiological homeostasis and toxicity of TCDD. J. Toxicol. Sci. 21, 273–277. [PubMed] [Google Scholar]
- Gray L. E, Ostby J. S., Kelce W. R. (1997). A dose-response analysis of the reproductive effects of a single gestational dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin in male long evans hooded rat offspring. Toxicol. Appl. Pharmacol. 146, 11–20. [DOI] [PubMed] [Google Scholar]
- Gupta A, Ketchum N, Roehrborn C. G, Schecter A, Aragaki C. C., Michalek J. E. (2006a). Serum dioxin, testosterone, and subsequent risk of benign prostatic hyperplasia: A prospective cohort study of air force veterans. Environ. Health Perspect. 114, 1649–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Schecter A, Aragaki C. C., Roehrborn C. G. (2006b). Dioxin exposure and benign prostatic hyperplasia. J. Occup. Environ. Med. 48, 708–714. [DOI] [PubMed] [Google Scholar]
- Izard J, Nickel J. C. (2011). Impact of medical therapy on transurethral resection of the prostate: Two decades of change. BJU Int. 108, 89–93. [DOI] [PubMed] [Google Scholar]
- Keil K. P, Abler L. L, Altmann H. M, Bushman W, Marker P. C, Li L, Ricke W. A, Bjorling D. E., Vezina C. M. (2014). Influence of animal husbandry practices on void spot assay outcomes in C57BL/6J male mice. Neurourol. Urodyn. doi: 10.1002/nau.22692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil K. P, Abler L. L, Altmann H. M, Wang Z, Wang P, Ricke W. A, Bjorling D. E, Vezina C. M. (2015). Impact of a folic acid-enriched diet on urinary tract function in mice treated with testosterone and estradiol. Am. J. Physiol. Renal. Physiol. 308, F1431–F1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. J, Cardiff R. D, Desai N, Banach-Petrosky W. A, Parsons R, Shen M. M., Abate-Shen C. (2002). Cooperativity of Nkx3.1 and Pten loss of function in a mouse model of prostate carcinogenesis. Proc. Natl. Acad. Sci. U.S.A. 99, 2884–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenaga T, Fukusato T, Ohta M, Asaoka K, Murata N, Arima A, Kubota S. (2007). Long-term effects of subcutaneously injected 2,3,7,8-tetrachlorodibenzo-p-dioxin on the liver of rhesus monkeys. Chemosphere 67, S399–S404. [DOI] [PubMed] [Google Scholar]
- Kristal A. R, Schenk J. M, Song Y, Arnold K. B, Neuhouser M. L, Goodman P. J, Lin D. W, Stanczyk F. Z., Thompson I. M. (2008). Serum steroid and sex hormone-binding globulin concentrations and the risk of incident benign prostatic hyperplasia: Results from the prostate cancer prevention trial. Am. J. Epidemiol. 168, 1416–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesche R, Groszer M, Gao J, Wang Y, Messing A, Sun H, Liu X, Wu H. (2002). Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis 32, 148–149. [DOI] [PubMed] [Google Scholar]
- Lin T. M, Ko K, Moore R. W, Simanainen U, Oberley T. D, Peterson R. E. (2002). Effects of aryl hydrocarbon receptor null mutation and in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on prostate and seminal vesicle development in C57BL/6 mice. Toxicol. Sci. 68, 479–487. [DOI] [PubMed] [Google Scholar]
- Lin T. M, Rasmussen N. T, Moore R. W, Albrecht R. M., Peterson R. E. (2003). Region-specific inhibition of prostatic epithelial bud formation in the urogenital sinus of C57BL/6 mice exposed in utero to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 76, 171–181. [DOI] [PubMed] [Google Scholar]
- Ma J, Gharaee-Kermani M, Kunju L, Hollingsworth J. M, Adler J, Arruda E. M, Macoska J. A. (2012). Prostatic fibrosis is associated with lower urinary tract symptoms. J. Urol. 188, 1375–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mably T. A, Moore R. W., Peterson R. E. (1992). In utero and lactational exposure of male rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin. 1. Effects on androgenic status. Toxicol. Appl. Pharmacol. 114, 97–107. [DOI] [PubMed] [Google Scholar]
- Miller D. C, Saigal C. S., Litwin M. S. (2009). The demographic burden of urologic diseases in America. Urol. Clin. North Am. 36, 11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. A, Gupta B. N, Zinkl J. G., Vos J. G. (1973). Postnatal effects of maternal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Environ. Health Perspect. 5, 81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson T. M, Moses M. A, Uchtmann K. S, Keil K. P, Bjorling D. E, Vezina C. M, Wood R. W., Ricke W. A. (2015). Estrogen receptor-alpha is a key mediator and therapeutic target for bladder complications of benign prostatic hyperplasia. J. Urol. 193, 722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson T. M, Ricke E. A, Marker P. C, Miano J. M, Mayer R. D, Timms B. G, vom Saal F. S, Wood R. W., Ricke W. A. (2012). Testosterone and 17beta-estradiol induce glandular prostatic growth, bladder outlet obstruction, and voiding dysfunction in male mice. Endocrinology 153, 5556–5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson T. M, Ricke W. A. (2011). Androgens and estrogens in benign prostatic hyperplasia: Past, present and future. Differentiation 82, 184–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson T. M, Sehgal P. D, Drew S. A, Huang W., Ricke W. A. (2013). Sex steroid receptor expression and localization in benign prostatic hyperplasia varies with tissue compartment. Differentiation 85, 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti A, Heudes D, Hinglais N, Appay M. D, Philippe M, Sassy-Prigent C, Bariety J, Michel J. B. (1995). Left ventricular fibrosis in renovascular hypertensive rats. Effect of losartan and spironolactone. Hypertension 26, 101–111. [DOI] [PubMed] [Google Scholar]
- Noble R. L. (1977). The development of prostatic adenocarcinoma in Nb rats following prolonged sex hormone administration. Cancer Res. 37, 1929–1933. [PubMed] [Google Scholar]
- Ornstein D. K, Rao G. S, Smith D. S, Andriole G. L. (1997). The impact of systematic prostate biopsy on prostate cancer incidence in men with symptomatic benign prostatic hyperplasia undergoing transurethral resection of the prostate. J. Urol. 157, 880–883. discussion 883-884. [PubMed] [Google Scholar]
- Partin A. W, Page W. F, Lee B. R, Sanda M. G, Miller R. N, Walsh P. C. (1994). Concordance rates for benign prostatic disease among twins suggest hereditary influence. Urology 44, 646–650. [DOI] [PubMed] [Google Scholar]
- Pearson J. D, Lei H. H, Beaty T. H, Wiley K. E, Isaacs S. D, Isaacs W. B, Stoner E, Walsh P. C. (2003). Familial aggregation of bothersome benign prostatic hyperplasia symptoms. Urology 61, 781–785. [DOI] [PubMed] [Google Scholar]
- Pirkle J. L, Wolfe W. H, Patterson D. G, Needham L. L, Michalek J. E, Miner J. C, Peterson M. R, Phillips D. L. (1989). Estimates of the half-life of 2,3,7,8-tetrachlorodibenzo-p-dioxin in Vietnam veterans of operation ranch hand. J. Toxicol. Environ. Health 27, 165–171. [DOI] [PubMed] [Google Scholar]
- Ricke W. A, Ishii K, Ricke E. A, Simko J, Wang Y, Hayward S. W, Cunha G. R. (2006). Steroid hormones stimulate human prostate cancer progression and metastasis. Int. J. Cancer 118, 2123–2131. [DOI] [PubMed] [Google Scholar]
- Ricke W. A, McPherson S. J, Bianco J. J, Cunha G. R, Wang Y., Risbridger G. P. (2008). Prostatic hormonal carcinogenesis is mediated by in situ estrogen production and estrogen receptor alpha signaling. Faseb J. 22, 1512–1520. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Nieves J. A, Macoska J. A. (2013). Prostatic fibrosis, lower urinary tract symptoms, and BPH. Nat. Rev. Urol. 10, 546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanda M. G, Beaty T. H, Stutzman R. E, Childs B., Walsh P. C. (1994). Genetic susceptibility of benign prostatic hyperplasia. J. Urol. 152, 115–119. [DOI] [PubMed] [Google Scholar]
- Schwenk F, Baron U, Rajewsky K. (1995). A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 23, 5080–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro E, Hartanto V, Lepor H. (1992). Quantifying the smooth muscle content of the prostate using double-immunoenzymatic staining and color assisted image analysis. J. Urol. 147, 1167–1170. [DOI] [PubMed] [Google Scholar]
- Sheldon C. A, Williams R. D, Fraley E. E. (1980). Incidental carcinoma of the prostate: A review of the literature and critical reappraisal of classification. J. Urol. 124, 626–631. [DOI] [PubMed] [Google Scholar]
- Simanainen U, Haavisto T, Tuomisto J. T, Paranko J, Toppari J, Tuomisto J, Peterson R. E, Viluksela M. (2004). Pattern of male reproductive system effects after in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure in three differentially TCDD-sensitive rat lines. Toxicol. Sci. 80, 101–108. [DOI] [PubMed] [Google Scholar]
- Tam N. N, Zhang X, Xiao H, Song D, Levin L, Meller J, Ho S. M. (2015). Increased susceptibility of estrogen-induced bladder outlet obstruction in a novel mouse model. Lab. Invest. 95, 546–560. [DOI] [PubMed] [Google Scholar]
- Tengowski M. W, Bjorling D. E, Albrecht R. M, Saban R. (1997). Use of gold-labeled ovalbumin to correlate antigen deposition and localization with tissue response. J. Pharmacol. Toxicol. Methods 37, 15–21. [DOI] [PubMed] [Google Scholar]
- Theobald H. M, Peterson R. E. (1997). In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin: Effects on development of the male and female reproductive system of the mouse. Toxicol. Appl. Pharmacol. 145, 124–135. [DOI] [PubMed] [Google Scholar]
- Torkko K. C, Wilson R. S, Smith E. E, Kusek J. W, van Bokhoven A., Lucia M. S. (2015). Prostate biopsy markers of inflammation are associated with risk of clinical progression of benign prostatic hyperplasia: Findings from the MTOPS study. J. Urol. 194, 454–461. [DOI] [PubMed] [Google Scholar]
- USEPA (2003). Exposure and human health reassessment of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related compounds. United States Environmental Protection Agency, Office of Research and Development, National Center for Environmental Assessment. NAS review draft, http://cfpub.epa.gov/ncea/iris_drafts/dioxin/nas-review/index.cfm.
- Van den Berg M, Birnbaum L, Bosveld A. T, Brunstrom B, Cook P, Feeley M, Giesy J. P, Hanberg A, Hasegawa R, Kennedy S. W, et al. (1998). Toxic equivalency factors (TEFS) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ. Health Perspect. 106, 775–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe W. H, Michalek J. E, Miner J. C, Pirkle J. L, Caudill S. P, Patterson D. G., Jr, Needham L. L. (1994). Determinants of TCDD half-life in veterans of operation ranch hand. J. Toxicol. Environ. Health 41, 481–488. [DOI] [PubMed] [Google Scholar]
- Wynder J. L, Nicholson T. M, DeFranco D. B, Ricke W. A. (2015). Estrogens and male lower urinary tract dysfunction. Curr. Urol. Rep. 16, 534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Ackert-Bicknell C, Larigakis J. D, MacIver B, Steers W. D, Churchill G. A, Hill W. G, Zeidel M. L. (2014). Spontaneous voiding by mice reveals strain-specific lower urinary tract function to be a quantitative genetic trait. Am. J. Physiol. Renal. Physiol. 306, F1296–F1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.