Abstract
Microtubule-associated protein tau is abnormally hyperphosphorylated and aggregated into neurofibrillary tangles in brains of individuals with Alzheimer's disease (AD) and other tauopathies. Tau pathology is critical to pathogenesis and correlates to the severity of dementia. However, the mechanisms leading to abnormal hyperphosphorylation are unknown. Here, we demonstrate that human brain tau was modified by O-GlcNAcylation, a type of protein O-glycosylation by which the monosaccharide β-N-acetylglucosamine (GlcNAc) attaches to serine/threonine residues via an O-linked glycosidic bond. O-GlcNAcylation regulated phosphorylation of tau in a site-specific manner both in vitro and in vivo. At most of the phosphorylation sites, O-GlcNAcylation negatively regulated tau phosphorylation. In an animal model of starved mice, low glucose uptake/metabolism that mimicked those observed in AD brain produced a decrease in O-GlcNAcylation and consequent hyperphosphorylation of tau at the majority of the phosphorylation sites. The O-GlcNAcylation level in AD brain extracts was decreased as compared to that in controls. These results reveal a mechanism of regulation of tau phosphorylation and suggest that abnormal hyperphosphorylation of tau could result from decreased tau O-GlcNAcylation, which probably is induced by deficient brain glucose uptake/metabolism in AD and other tauopathies.
Alzheimer's disease (AD) is characterized by the presence of two major brain lesions: neuritic (senile) plaques and neurofibrillary tangles (NFTs). NFTs are composed of paired helical filaments (PHFs), which comprise pathological filamentous aggregations of abnormally hyperphosphorylated tau (1–3). Tau is a cytosolic phosphoprotein, the function of which is to stimulate and stabilize microtubule assembly from tubulin subunits. All six isoforms of adult brain tau are hyperphosphorylated and aggregated into PHFs in AD brain (4, 5). To date, >30 phosphorylation sites have been identified in PHF-tau; only some of these sites are phosphorylated and at a much lower extent in the normal brain (6, 7). Normal tau contains 2–3 moles of phosphates per mole of protein, whereas tau in AD brain contains 3- to 4-fold more phosphates (8, 9).
Many studies have demonstrated that the abnormal hyperphosphorylation of tau is crucial to the loss of its biological function of stimulating microtubule assembly, to its disassociation from microtubules, to its gain of toxicity, and to its aggregation into PHFs in AD brain (for reviews, see refs. 6 and 10). Hence, the abnormal hyperphosphorylation of tau is critical to the molecular pathogenesis of AD and other related neurodegenerative disorders called tauopathies. The correlation between the numbers of neurofibrillary tangles in patients' brains and the severity of dementia symptoms (11, 12) further indicates the pivotal role of tau pathology in AD. The recent discovery of tau mutations that cause hereditary frontotemporal dementia and Parkinsonism linked to chromosome 17 (FTDP-17) demonstrates that tau abnormality itself is sufficient to produce neurodegeneration and dementia (for review, see ref. 13). However, the exact mechanisms leading to abnormal hyperphosphorylation of tau are still unknown.
It was discovered recently that bovine tau is modified by O-GlcNAcylation (14), a novel type of O-glycosylation by which the monosaccharide β-N-acetylglucosamine (GlcNAc) attaches to serine/threonine residues via an O-linked glycosidic bond. In contrast to classical N- or O-glycosylation, O-GlcNAcylation dynamically modifies nucleoplasmic and cytoplasmic proteins and is analogous to protein phosphorylation (for review, see ref. 15). A reciprocal relationship between O-GlcNAcylation and phosphorylation has been observed both at the global cellular protein level and at specific sites of particular proteins (for review, see ref. 16). These findings together raise the intriguing possibility that human tau may also be O-GlcNAcylated and that O-GlcNAcylation might regulate tau phosphorylation.
In this study, we found that human brain tau was O-GlcNAcylated and that O-GlcNAcylation regulated phosphorylation of tau in a site-specific manner. Reduced glucose (Glc) uptake/metabolism as seen in AD brain resulted in decreased O-GlcNAcylation and, consequently, hyperphosphorylation of tau in an animal model. These studies suggest a unique mechanism of regulation of tau phosphorylation and of the abnormal hyperphosphorylation of tau occurring in AD brain.
Materials and Methods
Human Brain Tissue. Frontal cortex from frozen human brains of 19 AD cases [12 male and 7 female, age = 73.4 ± 12.9 (mean ± SD) years, postmortem delay = 4.6 ± 1.9 h] and 15 controls (9 male and 6 female, age = 76.5 ± 9.1 years, postmortem delay = 6.6 ± 2.0 h) was used in this study. The tissues were obtained from The Brain Tissue Resource Center of McLean Hospital, Belmont, MA, and both AD and control cases were confirmed histopathologically. The tissue was homogenized in cold buffer consisting of 50 mM Tri·HCl (pH 7.0), 100 mM GlcNAc, 2.0 mM benzamidine, 1.0 mM PMSF, and 2.0 μg/ml each of aprotinin, leupeptin, and pepstatin. After centrifugation at 100,000 × g at 4°C for 20 min, the supernatants were used for affinity purification of tau or radioimmuno-dot-blot assay of protein O-GlcNAcylation level.
Transfection and Treatments of PC12 Cells. PC12 cells that stably express the largest isoform of human brain tau, tau441, were generated as described (17). The cells were differentiated with 50 ng/ml nerve growth factor for 6 days before harvesting for tau purification or treatment with pharmacological compounds. After treatments, the cells were lysed with buffer containing 50 mM Tris·HCl (pH 7.4), 150 mM NaCl, 1.0% SDS, 10 mM β-mercaptoethanol, 1.0 mM orthovanadate, 1.0 mM NaF, 50 mM β-phosphoglycerol, 1.0 mM PMSF, 10 mM benzamidine, 1.0 mM EGTA, 1.0 mM EDTA, and 100 mM GlcNAc, followed by heating in boiling water for 5 min. In some experiments, cells were lysed at 37°C in microtubule-stabilizing buffer consisting of 80 mM Pipes (pH 6.8), 1.0 mM MgCl2, 2.0 mM EGTA, 0.1 mM EDTA, 0.1% Triton X-100, 30% glycerol, 0.5 μM okadaic acid, 100 mM GlcNAc, and protease inhibitors. The microtubule-bound and -unbound tau was collected from the pellets and supernatants of centrifugation at 15,000 × g for 15 min at room temperature.
Purification of Tau. Tau was immunoaffinity-purified from extracts of human brain homogenates or cell lysates by using a Seize X Protein G affinity kit (Pierce) and monoclonal tau antibody Tau-1 (from L. Binder of Northwestern University, Chicago) or 43D (generated in our laboratory at the New York State Institute for Basic Research in Developmental Disabilities) according to the manufacturer's instruction. Tau purified with this method was free of IgG and other proteins.
Rat Brain Slices. Metabolically active brain slices (400 μm × 400 μm) were prepared from 6.5-month-old male Wistar rats and incubated in oxygenated artificial cerebrospinal fluid (CSF), as described (18). Okadaic acid (OA) and O-(2-acetamido-2-deoxy-d-glucopyranosylidene)amino-N-phenyl carbonate (PUGNAc) were added individually or in combination to the artificial CSF during incubation. The slices were then homogenized and analyzed by Western blots.
Starvation of Mice. Eight-week-old male C57BL/6NJCL mice (Charles River Breeding Laboratories) were housed singly in cages with grid floor to prevent coprophagy. After starvation for 48 h by removal of food, but not water, from the cages, the mice were killed and the brains were removed. The cerebral cortices were immediately dissected and homogenized for Western blot analysis. All experiments involving animals were approved by the New York State Institute for Basic Research in Developmental Disabilities Animal Welfare Committee, and the “Principles of Laboratory Animal Care” (National Institutes of Health Publication 86-23, revised 1985) and U.S. Law on the Protection of Animals were followed.
Analyses of Levels of O-GlcNAcylation and Phosphorylation of Tau. Levels of O-GlcNAcylation and tau phosphorylation were determined by using quantitative Western blots developed with 125I-labeled secondary antibodies, and the radio-immunoreactivity was quantitated by a PhosphorImager, as described (18). mAbs RL2 (Affinity Bioreagents, Golden, CO) and CTD110.6 (Covance, Richmond, CA) were used for O-GlcNAc detection. For blots presented in Fig. 1c, duplicate blots were treated with either 0.1 M NaOH at 45°C for 4 h to remove O-GlcNAc (β-elimination) or H2O for control treatment before incubation with the primary antibody. For data presented in Fig. 1d, β-elimination was carried out in test tubes by treating tau with 0.1 M NaOH at 25°C before Western blot analysis. Levels of tau phosphorylation at each specific site were determined by using phosphorylation-dependent and site-specific tau antibodies from BioSource International (Camarillo, CA), and total tau level was determined by using phosphorylation-independent tau antibodies R134d or 43D produced in our laboratory at the New York State Institute for Basic Research in Developmental Disabilities. The protein O-GlcNAcylation level of human brains was measured by radioimmuno-dot-blot assay (19) using RL2 as the primary antibody. The human brain O-GlcNAcylation data were analyzed with nonlinear regression software stata 7.0 (Stata, College Station, TX).
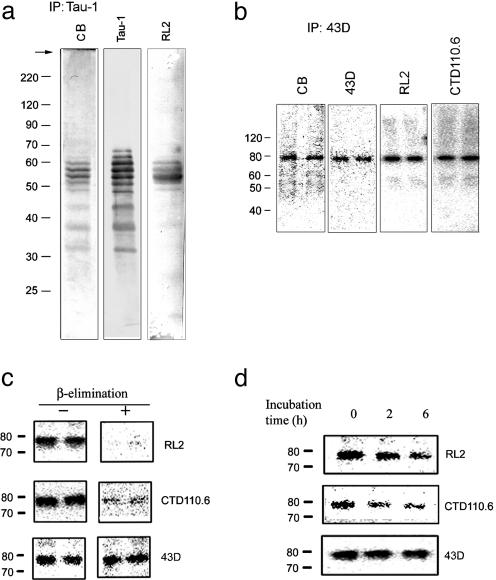
Fig. 1.
O-GlcNAcylation of human brain tau. (a) Immunoaffinity-purified tau from human brain was resolved by 10% SDS/PAGE and then stained with Coomassie blue (CB) or immunolabeled with antibodies Tau-1 to tau or RL2 to O-GlcNAc. (b) Recombinant human tau441 immunoaffinity-purified from PC12 cells was analyzed by 10% SDS/PAGE and stained with CB, antibodies 43D to tau, or RL2 and CTD110.6 to O-GlcNAc. (c) Tau441 from PC12 cells was analyzed by Western blots (+) with or (-) without β-elimination on the blots. (d) Tau441 was analyzed by Western blots after β-elimination in test tubes for 0, 2, and 6 h.
Results
Human Brain Tau Is Modified by O-GlcNAcylation. To elucidate whether O-GlcNAcylation regulates tau phosphorylation in human brain and contributes to the abnormal hyperphosphorylation of tau in AD, we first examined whether human brain tau is O-GlcNAcylated. We purified tau from normal human brain by immunoaffinity chromatography using antibody Tau-1. A comparison of Coomassie blue staining with Western blots developed with Tau-1 revealed several bands of tau and no detectable contamination of other proteins in the affinity-purified tau (Fig. 1a). Five of the six tau bands were stained by RL2, which recognizes O-GlcNAcylated proteins (20), suggesting that human brain tau is O-GlcNAcylated. To confirm this, we immunoprecititated O-GlcNAcylated proteins from human brain extracts with antibody RL2 and another monoclonal O-GlcNAc antibody, CTD110.6 (21) and found the presence of tau bands in the immunoprecipitates by Western blots (data not shown). Interestingly, the RL2- and CTD110.6-immunoprecipitated tau was also stained by antibodies that recognize phosphorylated tau at their specific epitopes (data not shown), indicating that the same tau molecules can be modified both by O-GlcNAc and phosphates at different sites.
We similarly studied recombinant human tau, tau441, affinity-purified from tau–overexpressing PC12 cells by using mAb 43D specific to human tau. The purified tau441 was stained by antibody RL2 and CTD110.6, indicating that tau441-expressed in PC12 cells is also O-GlcNAcylated (Fig. 1b). Tau441 was tagged with FLAG and myc; hence, it had an apparent molecular mass of ≈80 kDa. FLAG or myc tags were not modified with O-GlcNAc, because they did not contain potential consensus sequences for O-GlcNAcylation. To confirm human tau O-GlcNAcylation, we carried out β-elimination, which is commonly used to remove O-GlcNAcylation (22). β-Elimination on blots markedly reduced immunostaining of tau by antibodies RL2 and CTD110.6 (Fig. 1c). The reduced immunostaining was not caused by a loss of tau, because immunostaining of tau with anti-tau antibody 43D was unchanged. Using mild β-elimination conditions in test tubes, which did not degrade tau protein as shown by Western blots developed with 43D, we observed a time-dependent decrease in immunostaining of tau with RL2 and CTD 110.6 (Fig. 1d). These results together confirmed the O-GlcNAcylation of human tau.
O-GlcNAcylation Regulates Tau Phosphorylation in PC12 Cells. We then investigated whether O-GlcNAcylation regulates phosphorylation of tau in differentiated PC12 cells that stably overexpress tau441. For these studies, we treated cells with streptozotocin (STZ) and PUGNAc to elevate protein O-GlcNAcylation. These two compounds are cell-permeable, selective inhibitors of β-N-acetylglucosaminidase, the enzyme that removes O-GlcNAc from proteins (23, 24). Because the phosphorylation level of tau in differentiated PC12 cells is low, which made it difficult to observe the effects of O-GlcNAcylation on phosphorylation of tau, we elevated tau phosphorylation by treating the cells with 0.1 μM OA, a commonly used protein phosphatase inhibitor (18, 25). As expected, OA treatment resulted in hyperphosphorylation of tau at several protein phosphatase 2A-sensitive sites (Fig. 2c Left). Treatment of PC12 cells with STZ (10 mM) or PUGNAc (50 μM) for 3 h significantly induced O-GlcNAcylation of tau and other cellular proteins to the similar extents, as determined by O-GlcNAc–specific antibodies (Fig. 2 a and b). Although these treatments did not significantly alter tau level in PC12 cells, they reduced the phosphorylation level of tau at Ser-199, Ser-202, Thr-205, Thr-212, Ser-214, Ser-262, and Ser-396, but not Ser-404 (Fig. 2c). In the absence of OA, decreased phosphorylation of tau was also observed at some of these sites after treatments with STZ or PUGNAc (data not shown). These changes were not caused by cell toxicity, because MTT assay did not reveal significant cytotoxicity under these conditions (data not shown).
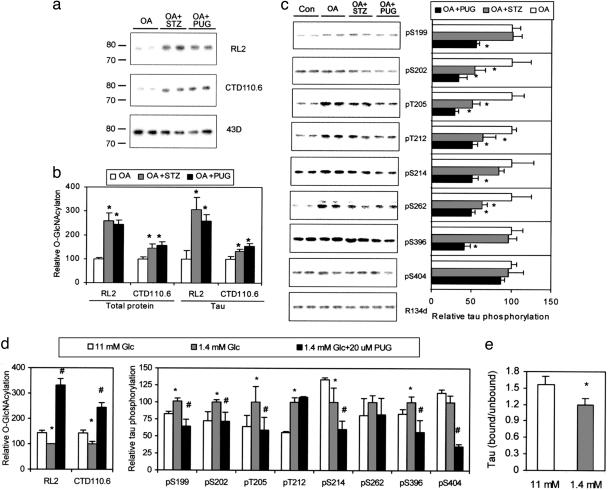
Fig. 2.
Regulation of tau phosphorylation by O-GlcNAcylation in differentiated PC12 cells that stably expressed human tau441.(a) Tau was first immunoaffinity-purified by using phospho-independent tau antibody 43D from PC12 cells treated with OA alone or in combination with either STZ or PUGNAc and then examined by using Western blots developed with O-GlcNAc-specific antibodies RL2 and CTD110.6 or with 43D. (b) The O-GlcNAcylation levels of lysate proteins and immunoaffinity-purified tau were quantitated by 125I-Western blots developed with RL2 and CTD110.6. *, P < 0.05 versus OA group. (c) (Left) The level and phosphorylation state of tau were analyzed by 125I-Western blots developed with phosphorylation-independent tau antibody R134d and phosphorylation-dependent and site-specific tau antibodies, respectively, as indicated. (Right) Quantitations of these blots after normalization with the R134d blot are shown. Mean ± SD of three to four separate experiments is shown. *, P < 0.05 versus OA group. (d) PC12 cells were cultured in medium containing normal (11 mM) or low (1.4 mM) concentration of Glc with or without PUGNAc for 2 days, and then the levels of protein O-GlcNAcylation and tau phosphorylation of the lysates were determined by 125I-Western blots. (e) The microtubule-bound tau and -unbound tau were quantitated by 125I-Western blots. Data in d are the quantitations of the blots after normalization with R134d blot. Mean ± SD of three to four separate experiments is shown in d and e. *, P < 0.05 versus 11 mM Glc group; #, P < 0.05 versus 1.4 mM Glc group.
Protein O-GlcNAcylation in the cell is regulated mainly by intracellular Glc concentration that in turn determines intracellular level of UDP-GlcNAc, which is the GlcNAc donor for O-GlcNAcylation. Therefore, we investigated the effect of low Glc on O-GlcNAcylation and phosphorylation of tau. When PC12 cells were cultured in medium containing 1.4 mM instead of the normal 11 mM Glc, we observed decreased O-GlcNAcylation and, concurrently, increased tau phosphorylation at Ser-199, Ser-202, Thr-205, Thr-212, and Ser-396, but not Ser-214, Ser-262, or Ser-404 (Fig. 2d). The addition of PUGNAc to the low-Glc medium prevented low-Glc–induced reduction of O-GlcNAcylation and hyperphosphorylation of tau at most of the phosphorylation sites. The microtubule-binding tau was also decreased in low-Glc-cultured cells (Fig. 2e), suggesting that O-GlcNAcylation might have a functional role in tau's biological activity.
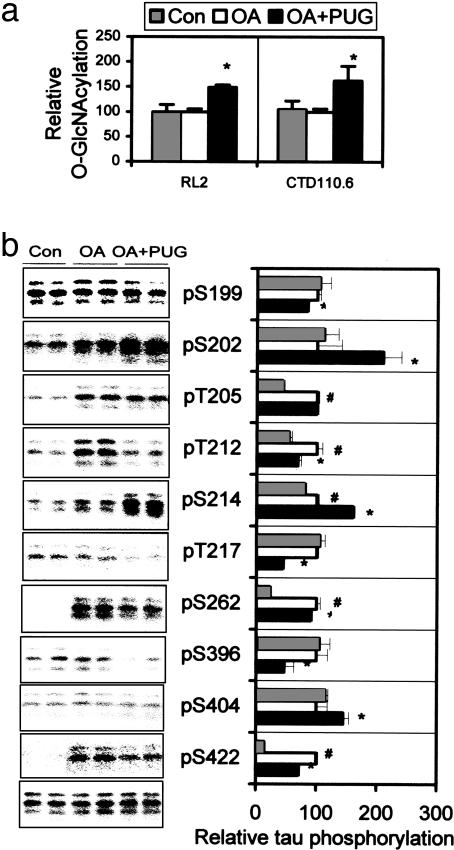
O-GlcNAcylation Regulates Tau Phosphorylation in Brain Slices. To investigate whether O-GlcNAcylation regulates phosphorylation of tau in mammalian brain, we treated metabolically active slices from adult rat brains with 0.1 μM OA alone or in combination with 0.2 mM PUGNAc and determined the level of O-GlcNAcylation and phosphorylation of tau in extracts of the brain slices. We found that PUGNAc increased protein O-GlcNAcylation level by ≈50% (Fig. 3a) and decreased phosphorylation of tau at Ser-199, Thr-212, Thr-217, Ser-262, Ser-396, and Ser-422 (Fig. 3b, compare OA+PUGNAc group with OA group). In contrast to PC12 cells, PUGNAc treatment enhanced tau phosphorylation at Ser-202, Ser-214, and Ser-404 and had no effect at Thr-205 in brain slices (compare Figs. 2c and 3b). In the absence of OA, tau was minimally phosphorylated at several of these sites (Fig. 3b), and therefore, the effect of PUGNAc on tau phosphorylation was not determined.
Fig. 3.
Regulation of tau phosphorylation by O-GlcNAcylation in metabolically active rat brain slices. (a) Rat brain slices were incubated with artificial cerebrospinal fluid alone or plus either OA or OA and PUGNAc. Then the O-GlcNAcylation level of the tissue homogenates was determined by 125I-Western blots developed with RL2 and CTD110.6. (b Left) The level and phosphorylation state of tau in the brain slice homogenates were analyzed by 125I-Western blots developed with R134d (bottom blot) and several phosphorylation-dependent tau antibodies as indicated, respectively. (b Right) The quantitation of these blots after normalization with total tau is shown as mean ± SD. *, P < 0.05 versus OA group; #, P < 0.05 versus control group.
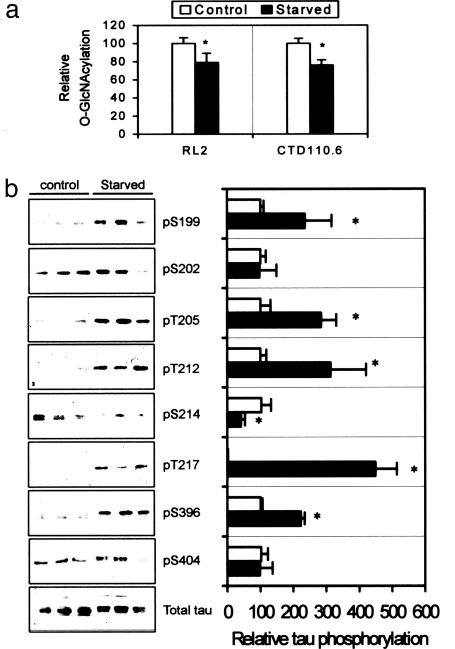
Decreased Glc Uptake/Metabolism Produces Tau Hyperphosphorylation. To determine whether decreased brain Glc uptake/metabolism as seen in AD brain can lead to abnormal hyperphosphorylation of tau via decreased O-GlcNAcylation, we used an animal model of starved mice. The mice were starved for 48 h to reduce brain supply of Glc, which led to decreased intracellular concentration of UDP-GlcNAc and, consequently, protein O-GlcNAcylation. The reduction of protein O-GlcNAcylation in the brains of starved mice was confirmed by Western blot analysis (Fig. 4a). We found that at the majority of the phosphorylation sites studied, starvation-induced decrease in O-GlcNAcylation resulted in an increase in tau phosphorylation (Fig. 4b). These data suggest an overall inverse regulation of tau phosphorylation by O-GlcNAcylation and further support our hypothesis that abnormal hyperphosphorylation of tau could result from the deficit of Glc uptake/utilization via decreased tau O-GlcNAcylation in the brain.
Fig. 4.
Effect of starvation on O-GlcNAcylation and phosphorylation of tau in mice. (a) Protein O-GlcNAcylation level of brain homogenates of mice after starvation for 48 h was analyzed by 125I-Western blots with anti-O-GlcNAc antibodies. (b Left) The level and phosphorylation state of tau in the brain homogenates were analyzed by 125I-Western blots developed with R134d (total tau) and several phosphorylation-dependent tau antibodies, respectively, as indicated. No significant immunostaining with antibodies against pS262 or pS422 was detected (data not shown). (b Right) The quantitation of these blots after normalization with total tau is shown as mean ± SD. The tau phosphorylation levels of control were defined as 100 for all sites, except that of pT217 was defined as 1. *, P < 0.05 versus control groups.
The effect of decreased brain Glc uptake/metabolism on protein phosphorylation was not limited to tau protein. Starvation-induced decrease in O-GlcNAcylation also resulted in an increase in phosphorylation of neurofilaments in mouse brain (see Fig. 6, which is published as supporting information on the PNAS web site), but not of high molecular weight microtubule-associated proteins MAP1b and MAP2 (data not shown).
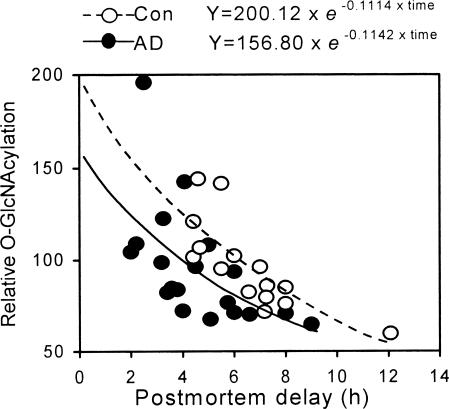
Protein O-GlcNAcylation Is Decreased in AD Brain. Glc uptake and metabolism are impaired in AD brain, and this impairment appears to be a cause of neurodegeneration (26, 27), but whether protein O-GlcNAcylation is compromised in AD brain is not known. To learn the pathophyiological significance of the regulation of tau phosphorylation by O-GlcNAcylation, we determined the O-GlcNAcylation level of cytosolic proteins of 19 AD and 15 control brains. Because the O-GlcNAcylation level was found to decline with postmortem delay of human brain tissue, we used nonlinear regression to analyze the difference in O-GlcNAcylation between the two groups, which achieved an excellent fit (adjust R2 = 0.951). We found that the O-GlcNAcylation level in AD brains was 22% lower than that in controls (156.80 versus 200.12 at the y intercept, i.e., at the time of death, P < 0.05) (Fig. 5). These data indicated that protein O-GlcNAcylation is compromised in AD brain. Furthermore, we found that unlike normal tau that is O-GlcNAcylated (Fig. 1), PHF-tau isolated from AD brain was not stained by succinylated wheat germ agglutinin, a lectin that specifically stains O-GlcNAcylated proteins, or by anti–O-GlcNAc antibodies (data not shown), suggesting that hyperphosphorylated PHF-tau is less or not O-GlcNAcylated.
Fig. 5.
Level of O-GlcNAcylation in AD and control (Con) brains. The O-GlcNAcylation level of cytosolic proteins from 19 AD and 15 control brains was determined by radioimmuno-dot-blots with antibody RL2 and plotted against the postmortem delay of the brains. Equations of nonlinear analysis of each group are shown on the top, where Y is relative O-GlcNAcylation level, and time is postmortem delay (h).
Discussion
In this study, we have found that tau in human brain is O-GlcNAcylated. Because a reciprocal regulation between O-GlcNAcylation and phosphorylation has been observed in several other proteins (for review, see ref. 16), our finding led us to investigate whether O-GlcNAcylation regulates tau phosphorylation, the dysregulation of which is a key event in the pathogenesis of AD and other tauopathies. Our observation that tau phosphorylation is indeed regulated by O-GlcNAcylation in cultured neuron-like cells and mammalian brains suggests a unique mechanism of regulation of tau phosphorylation. Consistent with our finding, a preliminary study showed, by using lectin blots, that in cultured neuroblastoma Kelly cells, O-GlcNAc mainly modified the less-phosphorylated tau, whereas the highly phosphorylated tau was devoid of O-GlcNAc (28).
Bovine tau contains >12 O-GlcNAcylation sites and an average stoichiometry of 4 moles of O-GlcNAc per mole of protein (14). This finding suggests that, like tau phosphorylation, the majority of the O-GlcNAcylation sites are modified at substoichiometric levels. Because >30 phosphorylation sites and 7–10 moles of phosphates per mole of tau have been observed in PHF-tau (6–9), O-GlcNAcylation probably regulates only some but not all phosphorylation sites of tau. We have demonstrated that tau O-GlcNAcylation negatively regulates its phosphorylation at Ser-199, Ser-202, Thr-205, Thr-212, Ser-214, Ser-262, and Ser-396, but not Ser-404 in PC12 cells. However, in metabolically active rat brain slices and mouse brain, the phosphorylation of tau at Ser-202, Ser-214, and Ser-404 is either positively regulated or not affected by O-GlcNAcylation. The exact cause of this discrepancy is unknown. It is possibly because of the different compositions of tau isoforms in these systems. Interestingly, each of these three sites is near a site whose phosphorylation is negatively regulated by O-GlcNAcylation, i.e., Ser-202 is proximal to Ser-199, Ser-214 is proximal to Thr-212 and Thr-217, and Ser-404 is proximal to Ser-396. These data reveal the intriguing possibility that phosphorylation of tau might be negatively regulated both by O-GlcNAcylation of the same sites and phosphorylation of the proximal sites. This hypothesis is supported by our recent observations that phosphorylation of tau at Ser-214 markedly inhibited the subsequent phosphorylation of tau at Thr-212 and Thr-217, and phosphorylation at Ser-409 inhibited subsequent phosphorylation of tau at Ser-404 (F.L., K.I., I.G.-I. and C.-X.G., unpublished observation).
The exact O-GlcNAcylation sites of tau are unknown. Our studies in rat brain slices and mouse brains indicated that O-GlcNAcylation negatively regulates phosphorylation of tau at Ser-199, Thr-205, Thr-212, Thr-217, Ser-262, Ser-396, and Ser-422, suggesting that these sites are probably O-GlcNAcylated. Consistent with this prediction, Ser-262 is a favorable substrate for O-GlcNAcylation in vitro (14). Tau residues Ser-202, Ser-214, and Ser-404, the phosphorylation of which is positively regulated by O-GlcNAcylation, might not be modified by O-GlcNAc; O-GlcNAcylation of the sites proximal to these residues might change tau's conformation such that these non-GlcNAcylated sites become more favorable substrates for phosphorylation.
Brain Glc uptake and metabolism are impaired in AD, and this impairment appears to be a cause, rather than a consequence, of neurodegeneration (26, 27). However, the molecular mechanism involved in this impairment is not understood. In the animal model of starved mice, we mimicked the impaired brain Glc uptake/metabolism by starvation and found a decrease in O-GlcNAcylation and consequent hyperphosphorylation of tau in the brains. We also discovered that O-GlcNAcylation is indeed compromised in AD brain. These findings suggest a mechanism by which decreased brain Glc uptake/metabolism produces neurofibrillary degeneration in AD through the promotion of abnormal hyperphosphorylation of tau. In starved mice, decreased activities of protein phosphatase 2A, glycogen synthase kinase-3β, and cyclin-dependent kinase 5 have been reported (29). The decrease in protein phosphatase 2A activity, which can lead to hyperphosphorylation of tau (18), could contribute to tau hyperphosphorylation in the starved animals.
The discovery of the mechanism by which impaired brain Glc uptake/metabolism may cause neurofibrillary degeneration is consistent with the hypothesis that sporadic AD might be a metabolic disorder caused by decreased brain Glc uptake/metabolism (27, 30). Interestingly, the gene encoding β-N-acetylglucosaminidase, which dynamically removes O-GlcNAc from proteins, maps to a locus associated with AD (31, 32). Glc deprivation in cultured hippocampal neurons causes antigenic alterations similar to those seen in AD neurofibrillary tangles (33). In nonhuman primates, decreased cerebral Glc consumption is correlated positively with the extent of entorhinal damage (34) and memory impairment (35). Diabetes mellitus, which is characterized by deficient Glc uptake and utilization, doubles the likelihood of developing AD (36). Administration of Glc and insulin together enhances the memory of AD patients (37). Amyloid β-peptide, which is increased in AD brain, can cause deficient Glc uptake and utilization (38–40). Thus, our findings also provide a mechanism by which β-amyloid pathology links to tau pathology through brain Glc uptake/metabolism impairment, O-GlcNAcylation and tau hyperphosphorylation in AD brain. Finally, brain Glc uptake/metabolism could be a promising therapeutic target to prevent and treat AD and probably other tauopathies.
Supplementary Material
Acknowledgments
We thank Prof. F. Chen of Nanjing Medical University, Nanjing, China, for assistance with statistical analysis; Dr. F. J. Kieras of the New York State Institute for Basic Research in Developmental Disabilities for technical assistance; and Ms. M. Marlow and Ms. J. Mahoney of the New York State Institute for Basic Research in Developmental Disabilities for editorial suggestions. This work was supported in part by funds from the New York State Office of Mental Retardation and Developmental Disabilities; National Institutes of Health Grants AG16760, AG19158, and HD13563; and a fellowship from the Li Foundation.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AD, Alzheimer's disease; Glc, glucose; GlcNAc, β-N-acetylglucosamine; OA, okadaic acid; PHF, paired helical filament; PUGNAc, O-(2-acetamido-2-deoxy-d-glucopyranosylidene)amino-N-phenyl carbonate; STZ, streptozotocin.
References
- 1.Grundke-Iqbal, I., Iqbal, K., Quinlan, M., Tung, Y. C., Zaidi, M. S. & Wisniewski, H. M. (1986) J. Biol. Chem. 261, 6084-6089. [PubMed] [Google Scholar]
- 2.Ihara, Y., Nukina, N., Miura, R. & Ogawara, M. (1986) J. Biochem. (Tokyo) 99, 1807-1810. [DOI] [PubMed] [Google Scholar]
- 3.Lee, V. M.-Y., Balin, B. J., Otvos, L. & Trojanowski, J. Q. (1991) Science 251, 675-678. [DOI] [PubMed] [Google Scholar]
- 4.Goedert, M., Spillantini, M. G., Jakes, R., Rutherford, D. & Crowther, R. A. (1989) Neuron 3, 519-526. [DOI] [PubMed] [Google Scholar]
- 5.Goedert, M., Spillantini, M. G., Cairns, N. J. & Crowther, R. A. (1992) Neuron 8, 159-168. [DOI] [PubMed] [Google Scholar]
- 6.Buée, L., Bussiére, T., Buée-Scherrer, V., Delacourte, A. & Hof, P. R. (2000) Brain Res. Brain Res. Rev. 33, 95-130. [DOI] [PubMed] [Google Scholar]
- 7.Liu, F., Iqbal, K., Grundke-Iqbal, I. & Gong, C.-X. (2002) FEBS Lett. 530, 209-214. [DOI] [PubMed] [Google Scholar]
- 8.Ksiezak-Reding, H., Liu, W. K. & Yen, S. H. (1992) Brain Res. 597, 209-219. [DOI] [PubMed] [Google Scholar]
- 9.Köpke, E., Tung, Y.-C., Shaikh, S., Alonso, A. del C., Iqbal, K. & Grundke-Iqbal, I. (1993) J. Biol. Chem. 268, 24374-24384. [PubMed] [Google Scholar]
- 10.Iqbal, K., Alonso, A. del C., El-Akkad, E., Gong, C.-X., Haque, N., Khatoon, S., Pei, J.-J., Tsujio, I., Wang, J. Z. & Grundke-Iqbal, I. (2002) J. Mol. Neurosci. 19, 95-99. [DOI] [PubMed] [Google Scholar]
- 11.Arriagada, P. V., Growdon, J. H., Hedley-Whyte, E. T. & Hyman, B. T. (1992) Neurology 42, 631-639. [DOI] [PubMed] [Google Scholar]
- 12.Riley, K. P., Snowdon, D. A. & Markesbery, W. R. (2002) Ann. Neurol. 51, 567–577. [DOI] [PubMed] [Google Scholar]
- 13.Lee, V. M. Y., Goedert, M. & Trojanowski, J. Q. (2001) Annu. Rev. Neurosci. 24, 1121-1159. [DOI] [PubMed] [Google Scholar]
- 14.Arnold, C. S., Johnson, G. V., Cole, R. N., Dong, D. L., Lee, M. & Hart, G. W. (1996) J. Biol. Chem. 271, 28741-28744. [DOI] [PubMed] [Google Scholar]
- 15.Hart, G. W. (1997) Annu. Rev. Biochem. 66, 315-335. [DOI] [PubMed] [Google Scholar]
- 16.Comer, F. I. & Hart, G. W. (2000) J. Biol. Chem. 275, 29179-29182. [DOI] [PubMed] [Google Scholar]
- 17.Gong, C.-X., Liu, F., Wu, G., Rossie, S., Wegiel, J., Li, L., Grundke-Iqbal, I. & Iqbal, K. (2004) J. Neurochem. 88, 298-310. [DOI] [PubMed] [Google Scholar]
- 18.Gong, C.-X., Lidsky, T., Wegiel, J., Zuck, L., Grundke-Iqbal, I. & Iqbal, K. (2000) J. Biol. Chem. 275, 5535-5544. [DOI] [PubMed] [Google Scholar]
- 19.Liu, F., Zaidi, T., Grundke-Iqbal, I., Iqbal, K. & Gong, C.-X. (2002) Neuroscience 115, 829-837. [DOI] [PubMed] [Google Scholar]
- 20.Snow, C. M., Senior, A. & Gerace, L. (1987) J. Cell. Biol. 104, 1143-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comer, F. I., Vosseller, K., Wells, L., Accavitti, M. A. & Hart, G. W. (2001) Anal. Biochem. 293, 169-177. [DOI] [PubMed] [Google Scholar]
- 22.Roquemore, E. P., Chou, T.-Y. & Hart, G. W. (1994) Methods Enzymol. 230, 443-460. [DOI] [PubMed] [Google Scholar]
- 23.Haltiwanger, R. S., Grove, K. & Philipsberg, G. A. (1998) J. Biol. Chem. 273, 3611-3617. [DOI] [PubMed] [Google Scholar]
- 24.Roos, M. D., Xie, W., Su, K., Clark, J. A., Yang, X., Chin, E., Paterson, A. J. & Kudlow, J. E. (1998) Proc. Assoc. Am. Physicians 110, 422-432. [PubMed] [Google Scholar]
- 25.Fujiki, H. & Suganuma, M. (1993) Adv. Cancer Res. 61, 143-194. [DOI] [PubMed] [Google Scholar]
- 26.Jagust, W. J., Seab, J. P., Huesman, R. H., Valk, P. E., Mathis, C. A., Reed, B. R., Coxson, P. G. & Budinger, T. F. (1991) J. Cereb. Blood Flow Metab. 11, 323-330. [DOI] [PubMed] [Google Scholar]
- 27.Hoyer, S. (2004) Eur. J. Pharmacol. 490, 115-125. [DOI] [PubMed] [Google Scholar]
- 28.Lefebvre, T., Ferreira, S., Dupont-Wallois, L., Bussiere, T., Dupire, M. J., Delacourte, A., Michalski, J. C. & Caillet-Boudin, M. L. (2003) Biochim. Biophys. Acta 1619, 167-176. [DOI] [PubMed] [Google Scholar]
- 29.Planel, E., Yasutake, K., Fujita, S. C. & Ishiguro, K. (2001) J. Biol. Chem. 276, 34298-34306. [DOI] [PubMed] [Google Scholar]
- 30.Iqbal, K. & Grundke-Iqbal, I. (2000) Neurosci. News 3, 14-20. [Google Scholar]
- 31.Bertram, L., Blacker, D., Mullin, K., Keeney, D., Jones, J., Basu, S., Yhu, S., McInnis, M. G., Go, R. C., Vekrellis, K., et al. (2000) Science 290, 2302-2303. [DOI] [PubMed] [Google Scholar]
- 32.Gao, Y., Wells, L., Comer, F. I., Parker, G. J. & Hart, G. W. (2001) J. Biol. Chem. 276, 9838-9845. [DOI] [PubMed] [Google Scholar]
- 33.Cheng, B. & Mattson, M. P. (1992) Exp. Neurol. 117, 114-123. [DOI] [PubMed] [Google Scholar]
- 34.Meguro, K., Blaizot, X., Kondoh, Y., Le Mestric, C., Baron, J. C. & Chavoix, C. (1999) Brain 122, 1519-1531. [DOI] [PubMed] [Google Scholar]
- 35.Xavier-Blaizot, A., Meguro, K., Millien, I., Baron, J.-C. & Chavoix, C. (2002) J. Neurosci. 21, 9166-9170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ott, A., Stolk, R. P., van Harskamp, F., Pois, H. A., Hofman, A. & Bretele, R. M. M. (1999) Neurology 53, 1937-1942. [DOI] [PubMed] [Google Scholar]
- 37.Craft, S., Newcomer, J., Kanne, S., Dagogo-Jack, S., Cryer, P., Sheline, Y., Luby, J., Dagogo-Jack, A. & Alderson, A. (1996) Neurobiol. Aging 17, 123-130. [DOI] [PubMed] [Google Scholar]
- 38.Niwa, K., Kazama, K., Younkin, S. G., Carlson, G. A. & Iadecola, C. (2002) Neurobiol. Dis. 9, 61-68. [DOI] [PubMed] [Google Scholar]
- 39.Xie, L., Helmerhorst, E., Taddei, K., Plewright, B., Van Bronswijk, W. & Martins, S. (2002) J. Neurosci. 22, RC221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prapong, T., Buss, J., Hsu, W. H., Heine, P., Greenlee, H. W. & Etsuro Uemura, E. (2002) Exp. Neurol. 174, 253-258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.