Abstract
Adverse early life experience is thought to increase an individual's susceptibility to mental health disorders, including anxiety and affective disorders, later in life. Our previous studies have shown that post-weaning social isolation of female rats during a critical period of development sensitizes an anxiety-related serotonergic dorsal raphe nucleus (DR) system in adulthood. Therefore, we investigated how post-weaning social isolation, in combination with a challenge with the anxiogenic drug, N-methyl-beta-carboline-3-carboxamide (FG-7142; a partial inverse agonist at the benzodiazepine allosteric site on the GABAA receptor), affects home cage behavior and serotonergic gene expression in the DR of female rats using in situ hybridization histochemistry. Juvenile female rats were reared in isolation or groups of three for a 3-week period from weaning (postnatal day (PD) 21 to mid-adolescence (PD42)), after which all rats were group-reared for an additional 16 days until adulthood. Among vehicle-treated rats, isolation-reared rats had decreased tryptophan hydroxylase 2 (tph2) mRNA expression in ventral and ventrolateral subdivisions of the DR, a pattern observed previously in a rat model of panic disorder. Isolation-reared rats, but not group-reared rats, responded to FG-7142 with increased duration of vigilance and arousal behaviors. In addition, FG-7142 decreased tph2 expression, measured 4 h following treatment, in multiple subregions of the DR of group-reared rats but had no effect in isolation-reared rats. No treatment effects were observed on 5-HT1A receptor or serotonin transporter gene expression. These data suggest that adolescent social isolation alters tph2 expression in specific subregions of the DR and alters the effects of stress-related stimuli on behavior and serotonergic systems.
Keywords: dorsal raphe nucleus, FG-7142, htr1a, in situ hybridization histochemistry, isolation-rearing, tph2
Adverse early-life experience, major stressful life experiences during adulthood, and genetic composition all appear to play important roles in determining vulnerability to anxiety and affective disorders (Caspi et al., 2003; Teicher et al., 2006). Isolation-rearing of rodents during adolescence is thought to model the behavioral consequences of adverse experiences during development in humans. During adolescence emotive behaviors mature, proper social and coping behaviors are acquired, and stress-related forebrain regions are organized (Andersen, 2003). Exposure to stress during this period can have long-term effects on behavior and its underlying neural mechanisms. Our previous studies have shown that isolation-rearing of male rats from pre- to mid-adolescence (postnatal (PD) 21–42) increases fear reactivity and anxiety-related behavior compared to group-reared controls (Lukkes et al., 2009b). This social isolation paradigm allows examination of how isolation stress during a critical period of development may manifest in physiologic and behavioral changes during early adulthood, a time when symptoms of many psychiatric disorders emerge (Andersen, 2003; Teicher et al., 2006).
According to the National Institute of Mental Health, 18.1% of American adults suffer from anxiety disorders, and women are 60% more likely to experience an anxiety disorder in their lifetime than are males. Despite the evidence that females have an increased risk for the development of neuropsychiatric illnesses, most studies have focused on males, and females have been insufficiently studied. Although the neural mechanisms underlying vulnerability to anxiety and affective disorders are undoubtedly complex, the serotonergic systems arising from the dorsal raphe nucleus (DR) in the brainstem appear to play an important role (Graeff et al., 1996; Hale et al., 2012; Maier and Watkins, 2005).
In the few studies that have investigated the effects of post-weaning social isolation on behavioral measures in female rodents, findings have been inconsistent. The limited number of studies that have used female rats suggest that isolation rearing and/or social deprivation appear to increase anxiety-like behavior of female rats (e.g.;Arakawa, 2007; Leussis and Andersen, 2008). However, other studies have failed to find effects of adolescent social isolation of female rats on anxiety-like behavior (Weiss et al., 2004). Methodological differences amongst isolation studies, including differences in onset and duration of isolation and strain differences, may account for the lack of consistency in relation to the expression of heightened anxiety states.
The DR is a brainstem nucleus that consists of topographically organized subsets of serotonergic neurons, including a subpopulation of neurons that projects to forebrain circuits that modulate anxiety-related behaviors and anxiety states (Lowry et al, 2005). Due to both the projections from and the inputs to the DR, these unique connections suggest that specific subregions of the DR may play an important role in the control of anxiety-related physiologic and behavioral responses (Maier and Watkins, 2005; Lowry et al, 2005). Therefore, altered expression of the brain-specific, rate-limiting enzyme in serotonin (5-hydroxytryptamine; 5-HT) synthesis, tryptophan hydroxylase 2 (TPH2), the 5-HT1A receptor (HTR1A), and the serotonin transporter (SLC6A4) within the DR, might contribute to the vulnerability to stress-related neuropsychiatric disorders (Lowry et al., 2008; Zill et al., 2004). For instance, increases in neuronal TPH2 mRNA expression and TPH immunoreactivity have been described in specific subregions of the DR in human depressed suicide patients, specifically within the caudal part of the DR (Bach-Mizrachi et al., 2008; Bach-Mizrachi et al., 2006; Boldrini et al., 2005; Bonkale et al., 2006; Underwood et al., 1999). Consistent with a potential role for TPH2 in vulnerability to anxiety disorders, in addition to affective disorders, allelic variants of TPH2 may be genetic predictors of panic disorder (Kim et al., 2009; Maron et al., 2007), susceptibility to panic attacks (Maron et al., 2008), and comorbidity between panic disorder and mood disorders (Campos et al., 2011; Mossner et al., 2006; Yoon et al., 2008).
Allelic variants of HTR1A, encoding the 5-HT1A receptor, also may be genetic predictors of panic disorder, while a functional promoter polymorphism in HTR1A has been associated with anxiety and depression traits (Strobel et al., 2003), and has been found in a subset of panic patients with agoraphobia (Lesch and Gutknecht, 2004; Rothe et al., 2004). Finally, allelic variants of regulatory regions of SLC6A4, including the serotonin transporter-linked polymorphic region (5-HTTLPR), may be genetic predictors of stress-related neuropsychiatric disorders, suicide risk among depressed patients, and responses to antidepressant treatment (Caspi et al., 2003; Hariri and Holmes, 2006; Hoefgen et al., 2005; Lesch et al., 1996; Yu et al., 2002).
Interactions among these factors may also be important, as a three-way interaction between the 5-HTTLPR polymorphism, allelic variation in HTR1A and negative life events has been described for major depressive disorder and anxiety disorders (Zhang et al., 2009). Together, these genetic association studies are consistent with the hypothesis that brainstem serotonergic systems play an important role in the vulnerability to anxiety and affective disorders.
Previous studies have found that maternal separation of male rat pups results in altered tph2 and slc6a4 mRNA expression in the DR and vulnerability to social defeat-induced increases of tph2 and slc6a4 mRNA expression in the DR in adulthood compared to animal facility reared control rats. The latter effects were specifically observed in the ventrolateral part of the DR, (Gardner et al., 2009a; Gardner et al., 2009b), a region associated with control of anxiety states (Hale and Lowry, 2011). These data suggest that adverse early-life stress can alter both baseline serotonergic gene expression and vulnerability to stress-induced alterations in serotonergic gene expression in the DR during adulthood, which might be underlying mechanisms leading to vulnerability to stress-related neuropsychiatric disorders. Our previous studies have found that isolation-rearing of female rats during a critical period of development sensitizes specific subpopulations of serotonergic neurons in the DR to the anxiogenic drug, N-methyl-beta-carboline-3-carboxamide (FG-7142; a partial inverse agonist at the benzodiazepine allosteric site on the GABAA receptor(FG-7142; a partial inverse agonist at the benzodiazepine allosteric site on the GABAA receptor; Lukkes et al., 2012b). Specifically, when challenged with FG-7142 as adults, isolates responded with increased c-Fos expression in serotonergic neurons in the mid-rostrocaudal and caudal DR, including the dorsal, caudal, ventrolateral, and interfascicular subregions, relative to vehicle-injected controls, whereas group-reared rats did not (Lukkes et al., 2012b). In order to test the hypothesis that isolation-rearing of female rats during a critical period of development also alters baseline or stress-induced expression of serotonergic genes in these regions, we investigated the interactions among adverse early-life experience and administration of the anxiogenic/panicogenic drug FG-7142 during adulthood on tph2, slc6a4, and htr1a mRNA expression in specific subregions of the DR of female rats.
2. Experimental Procedures
2.1. Animals
Female Sprague Dawley rats arrived on postnatal day (PD) 21 (day of weaning; Harlan Laboratories, Indianapolis, IN, USA) and were housed according to treatment groups in clear polycarbonate cages (26 cm width × 47.63 cm length × 20.32 cm height; Alternative Design, Siloam Springs, AR, USA) containing an approximately 2.5 cm-deep layer of bedding (Cat. No. 7090; Teklad Sani-Chips; Harlan Laboratories). Thirty-six weanlings were received from 6 litters and equal numbers of weanlings from each litter were assigned to each treatment group. Food (Cat. No. 8640; Teklad 22/5 Rodent Diet, Harlan Laboratories) and tap water, stored in 16 oz reduced-height water bottles (Cat. No. WB16RH; Alternative Designs) with screw lids (Cat. No. FSPCST2.5; AnCare Corp., Bellmore, NY, USA), were available ad libitum. Rats were maintained on a 12 hr: 12 hr reverse light cycle (lights off at 6 A.M.) at room temperature (22 °C). All animal care was conducted in accordance with the guidelines of the Guide for the Care and Use of Laboratory Animals, Eighth Edition (Institute for Laboratory Animal Research, The National Academies Press, Washington, D.C., 2011) and approved by the University of Colorado Institutional Animal Care and Use Committee. All efforts were made to minimize the number of animals used and their suffering.
2.2. Adolescent social isolation procedure
The adolescent social isolation procedure employed in these studies was based on a previously published protocol of social isolation/re-socialization (Lukkes et al., 2009a; Lukkes et al., 2009b; Lukkes et al., 2009c). On PD21 (weaning age corresponding to early adolescence), female Sprague Dawley rats were housed either individually or in groups of 3 as described above for 3 weeks during a time of early-adolescent (PD21) to mid-adolescent (PD42) development. After 3 weeks of isolation or group housing, rats were weighed and group-housed (3 rats/cage) according to treatment (isolates with isolates, n = 18; group-housed with unfamiliar group-reared rats, n = 18) for a further 16 days (PD42–PD58). This re-socialization following the critical isolation period (early- to mid-adolescence) allowed the rats to complete their development from mid-adolescence to early adulthood. At the end of the isolation/re-socialization procedure, when rats were in early adulthood (PD58), they were used in the experiments. Behavioral testing was performed during the dark phase from 8:00 a.m. to 6:00 p.m. Testing was done using a randomized design that ensured rats in any given treatment group were not preferentially tested at one time of day. Fig. 1 is a diagram illustrating the experimental timeline.
Figure 1.
Diagram illustrating the experimental timeline and sequence of procedures. Abbreviations: HC, home cage; PD, postnatal day; veh, vehicle.
2.3. Social interaction test
Following the isolation/re-socialization procedure (on PD58) female rats were habituated to an open-field arena. Rats were placed individually for 5 min in a plastic black oval open-field (97.8 cm × 70.17 cm × 31.8 cm deep). This occurred during the active phase of their circadian cycle (between the hours of 8 a.m. and 1 p.m.) under dim red light. One day following habituation to the open-field, we exposed rats to a 5 minute social interaction test. This involved placement of the rat in the same open-field, illuminated with dim red light (~11 lux), with an unfamiliar, size-and age-matched female conspecific. At the start of the test, rats were placed on opposite sides of the test arena towards the center facing each other. The partner rat was another female Sprague Dawley rat of similar weight (no more than 10 g difference), housed in groups of 3/cage, that had never been in contact with the experimental rat. Each partner rat interacted with a rat from each treatment group and was used no more than four times for behavioral tests. Video footage of the social interaction test was then scored by an observer blind to treatment, using Noldus The Observer XT (version 10.0 software, Noldus Information Technology, Leesburg, VA, USA). The frequency and duration of social interaction (SI) was measured. SI was defined as interaction with the partner rat initiated by the experimental rat, and the following behaviors were scored and classified as active SI: sniffing, following, grooming, kicking, mounting, jumping on, wrestling and boxing with, and crawling over or under the partner. In addition to the measures of SI, the frequency and duration of approach, freezing, head-weaving, and grooming were scored during the 5-min test. Immediately following the 5 min SI test, the stage of estrus was determined by examining vaginal cytology with light microscopy. Diestrus was characterized by the presence of leukocytes, proestrus by round nucleated cells, and estrus by cornified, irregularly shaped cells with degenerate nuclei (Marcondes et al, 2001). In each housing condition, all stages of the estrous cycle were represented.
2.4. FG-7142 injection
Twenty-four hours following the SI test, rats previously exposed to post-weaning social isolation or group housing received injections of either N-methyl-beta-carboline-3-carboxamide (FG-7142; a partial inverse agonist at the benzodiazepine allosteric site on the GABAA receptor; n = 18; 7.5 mg/kg, i.p.; Cat No. 0554, Tocris Bioscience, Ellisville, MO, USA) in vehicle (0.9% saline containing 40% 2-hydroxypropyl-β-cyclodextrin (HBC); Cat No. 332607, Sigma-Aldrich, St. Louis, MO, USA) or vehicle alone (n = 18) and were replaced in their home cages. This dose has been shown to increase anxiety-like behavior using a number of behavioral measures 30 to 60 min following administration of FG-7142 (Abrams et al., 2005; Otter et al., 1997). Furthermore, this dose of FG-7142 (7.5 mg/kg) has been shown to increase c-Fos expression in multiple anxiety-related brain regions and increase serotonergic metabolism within multiple stress-related brain regions (Abrams et al., 2005; Evans et al., 2006; Salchner et al., 2006; Singewald et al., 2003; Singewald and Sharp, 2000).
Each cage was placed under a video camera, and home cage behavior was recorded from 30 min before the injection to 2 hrs after the injection. Home cage behavior was scored for 10 min beginning 30 min prior to injection and again from 30 to 40 min post-injection, corresponding to the time of peak behavioral effects of FG-7142 in the home cage (Abrams et al., 2005). Since there were 3 rats per cage, rats were marked for identification during behavioral scoring. Behavior was scored using the continuous sampling method by an observer blind to treatment using Noldus The Observer XT ver. 10.0. The frequency and duration of behaviors scored were burying, drinking-related behavior, feeding-related behavior, grooming, inactivity, locomotion, rearing, and spontaneous non-ambulatory motor activity (SNAMA; visual scanning of environment, head movements associated with sniffing, shifts in body position, non-ambulatory limb movements, and behaviors associated with risk assessment or anxiety-like behaviors in rats in the home cage; Abrams et al., 2005).
2.5. Brain removal and sectioning
Four hours following injection of FG-7142 or vehicle (a time point when stress-induced increases in gene expression are evident; Gardner et al., 2009a; Gardner et al., 2009b; Harbuz and Lightman, 1989), rats were rapidly decapitated. The brains were removed, frozen using dry ice ventral side down on a flat piece of aluminum foil to avoid distortion of the brain dimensions, and stored at −80 °C. Brains were then blocked using a rat brain matrix (RBM-4000C, ASI Instruments, Warren, MI, USA) to ensure a correct coronal plane; the cortex was removed and the midbrain was sectioned in series of seven between −7.580 mm and −8.672 mm bregma (Paxinos and Watson, 1998) at 12 μm using a cryostat, thaw-mounted onto VistaVision HistoBond microscope slides (VWR Scientific, West Chester, PA, USA), and stored at −80 °C until in situ hybridization histochemistry (ISHH) was performed. Three separate series of slides, consisting of sections throughout the DR at 84 μm intervals, were used, one each for detection and analysis of tph2 mRNA, slc6a4 mRNA, and htr1a mRNA, respectively.
2.6. In situ hybridization histochemistry (ISHH) procedure
A 583-base pair fragment of tph2 cDNA was transcribed and radiolabeled with [35-S]-uridine-5'-triphosphate (Cat. No. 5610301, MP Biomedicals, Santa Ana, CA, USA) using T7 RNA-polymerase (Promega, Madison, WI, USA) to create a cRNA probe complementary to bases 761 to 1343 of rat tph2 mRNA. The probe was cleaned with an RNeasy® kit (Cat. No. 74104, Qiagen, Valencia, USA), and used in an ISHH assay as described previously (Donner and Handa, 2009; Donner et al., 2012). To detect slc6a4 mRNA, a synthetic 50-base antisense oligonucleotide (5′-ACT GCA GAG TAC CCA TTG GAT ATT TGG CTA GGC TCT GCC CTG TCC GCT GT-3′, Integrated DNA Technologies, Coralville, IA, USA), complementary to bases 207–256 of rat slc6a4 mRNA (previously reported as bases 77–126 Fujita et al., 1993; Hansen and Mikkelsen, 1998), based on the published sequence of the rat serotonin transporter cDNA clone (Blakely et al., 1991) of slc6a4 mRNA (Rattus norvegicus solute carrier family 6 (neurotransmitter transporter, serotonin), member 4, mRNA; GenBank Accession no., NM_013034.3) was used as previously described (Gardner et al., 2009a). To detect htr1a mRNA, two synthetic anti-sense oligonucleotides, one 49-base oligonucleotide (5'-ACG AAG TTC CTA AGC TGG TGC CTG CTC CCT TCT TTT CCA CCT TCC TGA C-3', Integrated DNA Technologies) complementary to bases 810–858 of rat htr1a mRNA and one 47-base oligonucleotide (5'-GCC TCA CTG CCC CAT TAG TGC ACG GAG TCC CCA CCG CCC TGT TCT CA-3', Integrated DNA Technologies) complementary to bases 923–969 of rat htr1a mRNA, were used (Hansen and Mikkelsen, 1998). The oligonucleotides were labeled at the 3' end with [35-S]-deoxyadenosine-5'-triphosphate (Cat. No. 5620001, MP Biomedicals, Santa Ana, CA, USA) using terminal deoxynucleotidyl transferase (20 U/μl, Cat. No. EP0161, Fermentas, Glen Burnie, MD, USA) for 1 h at 37 °C, cleaned using a QIAquick® nucleotide removal kit (Cat. No. 28304, Qiagen, Valencia, CA, USA), and used in an ISHH assay as described previously (Gardner et al., 2009a; Gardner et al., 2009b; Hansen and Mikkelsen, 1998). Air-dried slides were apposed to a BioMax MR autoradiography film (Cat. No. 871 5187, Carestream Health, Rochester, NY, USA) for 6.5 (tph2), 11 (slc6a4), or 17 (htr1a) days, respectively.
2.7. Semi-quantitative analysis of gene expression
Digital autoradiographic images were analyzed with ImageJ (NIH, Bethesda, MD, USA) by an experimenter blind to the treatment groups. This program was used to measure “Mean gray value × area” (representative of tph2, slc6a4, or htr1a mRNA expression) using matrices in the shape of each subdivision of the brainstem DR. Gray value was measured in arbitrary units, generated by Image J, representing the relative darkness of each of the pixels measured. Only pixels within the predefined sampling area that were equal to or greater than a predetermined gray value threshold were included in the measurement. Mean gray value was defined as the sum of the gray values of all the pixels in the selection that were above threshold, divided by the number of pixels above threshold. Area (mm2) was defined as the area within each subdivision (as defined by the matrix) that fell above the gray value threshold. The final measurement, then, was “Mean gray value (of pixels in the sampling area that were above threshold) multiplied by area (of pixels in the sampling area that were above threshold, mm2)”. The gray value threshold was chosen so as to include as much signal and as little background as possible for all sections and was kept consistent throughout the analysis of each gene. Based on Gardner et al. (Gardner et al., 2005) and Abrams et al. (Abrams et al., 2004), a total of 14 rostro-caudal sections, designated levels +7 to −6 (Fig. 2), containing 5 major subdivisions of the DR (dorsal raphe nucleus, dorsal part (DRD), −7.580 to −8.336 mm bregma; dorsal raphe nucleus, ventral part (DRV), −7.580 to −8.504 mm bregma; dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray (DRVL/VLPAG), −7.832 to −8.504 mm bregma; dorsal raphe nucleus, caudal part (DRC), −8.420 to −8.672 mm bregma; dorsal raphe nucleus, interfascicular part (DRI), −8.420 to −8.672 mm bregma) were analyzed. An average value was computed for the DRVL/VLPAG using values from both the left and right hemisphere. The background of each image, measured in the surrounding midbrain tegmentum, was subtracted from each value for tph2 and slc6a4. Since htr1a is expressed extensively outside of the DR, the background for this gene was measured in the white matter tracts present in each section (specifically the superior cerebellar peduncle). The rostrocaudal levels were assigned based on the pattern of slc6a4 mRNA expression (Gardner et al., 2009a), so that assignments were identical for analysis of the slc6a4, tph2, and htr1a mRNA expression; this was critical for htr1a mRNA expression as htr1a expression is not restricted to serotonergic neurons (Kirby et al., 2003), and the more diffuse pattern of htr1a mRNA makes is more difficult to assign rostrocaudal levels of the DR. Throughout the 14 rostrocaudal levels, values for each of the 5 DR subdivisions were averaged for tph2, slc6a4, and htr1a mRNA expression for each rat; in addition, mean tph2, slc6a4, and htr1a expression levels in the entire DR were also calculated for each rat, and these mean values were used for statistical comparisons among treatment groups.
Figure 2.
Neuroanatomic atlas of rat tph2 mRNA expression in the midbrain dorsal raphe nucleus (DR) from a female rat in the present study. Displayed (from left to right) are 5 representative coronal sections (12 μm) taken from one rat that were used to measure tph2 mRNA expression in all subdivisions of the DR from rostral (−7.580 mm bregma, designated level +7) to caudal (−8.672 mm bregma, designated level −6). Photomicrographs are autoradiographic images revealing the localization of hybridized 35S-labeled cRNA probe complementary to tph2 mRNA. Dashed lines indicate borders between subdivisions of the DR. Abbreviations: DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL, dorsal raphe nucleus, ventrolateral part; VLPAG, ventrolateral periaqueductal gray. Scale bar: 1 mm.
2.9. Statistical Analysis
Mean data were analyzed using PASW (version 19.0, SPSS Inc., Chicago, IL, USA) after elimination of statistical outliers using Grubbs' test (Grubbs, 1969). Social interaction behavior and home cage behavior before injection of vehicle or drug were analyzed using independent Student's t-tests. Home cage behavior after injection of vehicle or drug was analyzed using a two factor ANOVA for each behavior separately, with rearing condition and drug treatment as between-subjects factors. When appropriate, planned pairwise comparisons were conducted using Fisher's protected least significant difference (LSD) tests to determine behavior-specific treatment effects on either duration or frequency of behavior. Gene expression was analyzed using a linear mixed model analysis, with subdivision as the repeated-measure, and rearing condition and drug treatment as the between-subjects factors. Where appropriate, Fisher's protected LSD tests were applied for each of the 7 subdivisions of the DR to reveal subdivision-specific treatment effects on gene expression. A two factor ANOVA was used to compare treatment effects on the average tph2, slc6a4, or htr1a mRNA expression in the entire DR. Pearson's correlation was used to determine the r for the correlation between genes. Significance was accepted at p < 0.05. Values are shown as the mean + the standard error of the mean (S.E.M).
3. Results
3.1. Social interaction test in a familiar dark open-field
No differences between treatment groups were observed in total social interaction (SI) time (mean SI time (sec) of group-reared, 41.9 ± 4.9, and isolation-reared rats, 47.8 ± 3.9). Also, no differences between treatment groups were observed in the total freezing time (mean freezing time (sec) of group-reared, 3.2 ± 0.9 and isolation-reared rats, 2.5 ± 0.7). Similarly, no differences between treatment groups were observed in the total duration of rearing, head-weaving, and self-grooming (data not shown). Furthermore, no treatment differences were observed in the total frequency of approaches, freezing, head-weaving, SI bout, and self-grooming (data not shown).
3.2. Home cage behavior
3.2.1. Pre-injection behavior
No differences between treatment groups were observed in either the total duration or frequency of any home cage behavior scored during the 10 min period beginning 30 min prior to injection of FG-7142 or vehicle (Table 1).
Table 1.
Duration and frequency of home cage behavior in group- and isolation-reared rats prior to injection of vehicle or FG-7142
| Behavior |
Duration
|
Frequency
|
||
|---|---|---|---|---|
| Group | Isolate | Group | Isolate | |
| Burying | 0.0±0.0 | 0.1±0.1 | 0.0±0.0 | 0.0±0.0 |
| Drinking-related behavior | 27.4±6.4 | 29.4±6.6 | 2.6±0.4 | 2.6±0.5 |
| Feeding-related behavior | 72.1±24.4 | 81.7±24.7 | 2.3±0.5 | 4.4±1.0 |
| Inactivity | 19.9±8.5 | 11.6±8.4 | 1.1±0.4 | 1.1±0.5 |
| Locomotion | 22.7±2.0 | 18.7±1.7 | 16.5±1.3 | 16.3±1.3 |
| Rearing | 235.2±24.3 | 228.1±23.9 | 22.8±1.6 | 22.2±2.0 |
| Self-grooming | 84.8±11.4 | 98.7±15.0 | 9.4±0.8 | 10.3±0.7 |
| SNAMA | 76.4±15.2 | 53.3±6.8 | 14.2±1.1 | 12.6±1.2 |
| Social contact | 12.3±3.3 | 13.1±3.4 | 4.0±0.6 | 3.9±0.7 |
Values are presented as mean±S.E.M.
3.2.2. Post-injection behavior
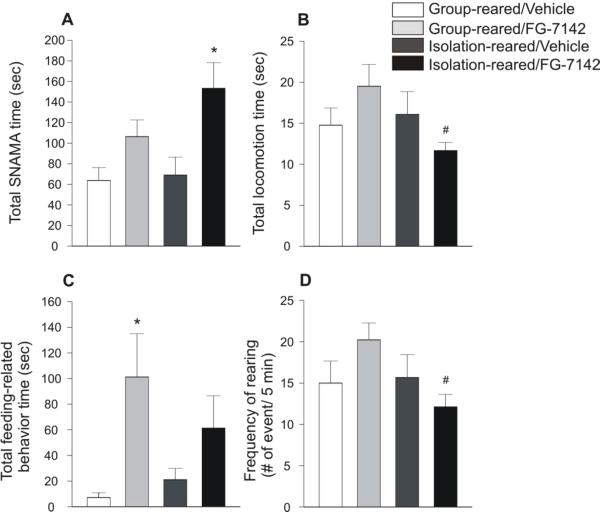
Isolation-rearing altered home cage behavior measured 30 to 40 min following administration of FG-7142 (Fig. 3). There was a main effect of drug (F(1,5) = 12.112, p < 0.01) on total duration of SNAMA (Fig. 3A), but no main effect of rearing condition (F(1,5) = 1.057, p = 0.321) nor an interaction between drug × rearing condition (F(1,5) = 2.322, p = 0.137). Post hoc analysis revealed that among isolation-reared rats, FG-7142-injected rats had increased duration of SNAMA compared to vehicle-injected controls (p < 0.01), while no such effect of FG-7142 was observed in group-reared rats, although the comparison approached statistical significance (p = 0.110). Overall, regression analysis suggested that there was no association between estrous cycle and SNAMA duration (β = 0.235, p = 0.134).
Figure 3.
Graphs illustrating the duration and frequency of selected home cage behaviors 30 to 40 min following injection of vehicle or FG-7142 (a partial inverse agonist at the benzodiazepine allosteric site on the γ-aminobutyric acid (GABA)A receptor, 7.5 mg/kg, i.p.). Graphs represent A) the duration of spontaneous non-ambulatory motor activity (SNAMA), B) the duration of locomotion, C) the duration of feeding-related behavior, and D) the frequency of rearing. Data are presented as means (+ S.E.M.). *p < 0.05 versus vehicle-treated controls within the same housing condition; #p < 0.05 versus group-reared rats within the same drug condition; Fisher's protected least significant difference (LSD) test. Sample size, n = 9 for all treatment groups. Abbreviations: SNAMA, spontaneous non-ambulatory motor activity.
There was a significant interaction between drug × rearing condition (F(1,5) = 9.494, p < 0.01) on total locomotion time (Fig. 3B). Post hoc analysis revealed that among FG-7142-injected rats, isolation-reared rats had decreased duration of locomotion compared to group-reared rats.
There was a main effect of drug (F(1,5) = 4.578, p < 0.05) on total duration of feeding-related behavior (Fig. 3C), but no main effect of rearing condition (F(1,5) = 0.001, p = 0.973) nor an interaction between drug × rearing condition (F(1,5) = 3.058, p = 0.090). Post hoc analysis revealed that among group-reared rats, FG-7142-injected rats had increased duration of feeding-related behavior compared to vehicle-injected controls, while no such effect of FG-7142 was observed in isolation-reared rats.
There was a significant interaction between drug × rearing condition (F(1,5) = 7.324, p < 0.05) on frequency of rearing behavior (Fig. 3D). Post hoc analysis revealed that among FG-7142-injected rats, isolation-reared rats had decreased frequency of rearing behavior compared to group-reared rats.
3.3. tph2 mRNA expression
There was a main effect of drug on average tph2 mRNA expression across the entire DR (Fig. 4A; F(1, 29) = 7.567, p = 0.010), but no effect of rearing condition (F(1, 29) = 0.002, p = 0.968) nor an interaction between drug × rearing condition (F(1, 29) = 3.386, p = 0.076). Post hoc analysis revealed that among group-reared rats, FG-7142 decreased average tph2 mRNA expression across the entire DR (Fig. 4A). Group-rearing also resulted in FG-7142-induced decreases in tph2 mRNA expression across subdivisions of the DR (Fig. 4B–G; drug × rearing condition × subdivision, F(4,15) = 7.567, p < 0.05). Post hoc tests showed that among group-reared rats, FG-7142-treated rats had decreased tph2 mRNA expression in the all subregions studied, excluding the DRI (Fig. 4B–G). There were also effects of rearing condition on tph2 mRNA expression; among vehicle-treated rats, those exposed to isolation-rearing had decreased tph2 mRNA in the DRV and DRVL/VLPAG (Fig. 4C,E). Overall, regression analysis indicated that there was no association between estrous cycle and tph2 mRNA expression (β = −0.228, p = 0.197).
Figure 4.
Graphs illustrating the effects of adolescent social isolation or group-rearing followed by subsequent administration of either FG-7142 (7.5 mg/kg) or vehicle on the expression of tph2 mRNA in the dorsal raphe nucleus (DR). Shown is the mean (+ S.E.M.) tph2 mRNA expression in the whole DR (A) and in each of the 5 subdivisions of the DR studied (B-F). (G) Representative autoradiograms of tph2 mRNA expression at −8.168 mm bregma (designated level 0) in each treatment group. *p<0.05 versus vehicle-treated controls within the same housing condition; #p < 0.05 versus group-reared rats within the same drug condition; Fisher's protected least significant difference (LSD) test. Sample size: n = 9, group-reared/vehicle-treated; n = 8, group-reared/FG-7142-treated; n = 9, isolation-reared/vehicle-treated; n = 7, isolation-reared/FG-7142-treated. Abbreviations: DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL, dorsal raphe nucleus, ventrolateral part; VLPAG, ventrolateral periaqueductal gray. Scale bar: 1 mm.
3.4. slc6a4 mRNA expression
There were no effects of drug or rearing condition and no interaction between drug × rearing condition on average slc6a4 mRNA expression across the entire DR (data not shown; drug, F(1, 32) = 0.214, p = 0.646; rearing condition, F(1, 32) = 0.897, p = 0.351; drug × rearing condition, F(1, 32) = 0.260, p = 0.614). There were also no effects of drug or rearing condition and no interaction between drug × rearing condition on slc6a4 expression across subdivisions of the DR (drug, F(1,33) = 0.793, p = 0.380; rearing condition, F(1, 33) = 0.016, p = 0.900; drug × subdivision, F(1, 31) = 1.319, p = 0.285; rearing condition × subdivision, F(4, 25) = 0.617, p = 0.645; drug × rearing condition, F(4, 25) = 0.629, p = 0.434; drug × rearing condition × subdivision, F(4, 15) = 0.143, p = 0.965). Overall, regression analysis indicated that there was no association between estrous cycle and slc6a4 mRNA expression (β = −0.100, p = 0. 921).
3.5. hrt1a mRNA expression
There were no effects of drug or rearing condition and no interaction between drug × rearing condition on mean htr1a mRNA expression across the entire DR (data not shown; drug, F(1, 32) = 2.863, p = 0.100; rearing condition, F(1, 32) = 0.195, p = 0.662; drug × rearing condition, F(1, 32) = 0.807, p = 0.376). There were also no effects of drug or rearing condition and no interaction between drug × rearing condition on htr1a expression across subdivisions of the DR (drug, F(1, 33) = 2.575, p = 0.119; rearing condition, F(1, 33) = 0.090, p = 0.766; drug × subdivision, F(1, 31) = 0.798, p = 0.536; rearing condition × subdivision, F(4, 25) = 0.651, p = 0.630; rearing condition × drug, F(4, 25) =, p =;drug × rearing condition × subdivision, F(4, 15) = 0.832, p = 0.515), although a drug × rearing condition interaction approached statistical significance (F(4, 25) = 3.507, p = 0.071). Overall, regression analysis indicated that there was no association between estrous cycle and htr1a mRNA expression (β = −0.133, p = 0.476).
3.7. Correlations between genes and behavior
Among vehicle-treated rats, there was a positive correlation between total SI time and tph2 mRNA expression in the DRD & DRC (r = 0.592, p < 0.05; Fig. 5).
Figure 5.
Graph illustrating the positive correlation between mean tph2 mRNA expression in the combined DRD/DRC and the total social interaction (SI) time. Only data from vehicle-treated rats is shown. Open circles, group-reared/vehicle-treated; filled circles isolation-reared/vehicle-treated.
3.8. Gene expression correlations
Using data from all four treatment groups, there was a positive correlation between mean htr1a mRNA expression and mean tph2 mRNA expression across the entire DR (r = 0.356, p < 0.05; Fig. 6). The mean htr1a mRNA expression was correlated with mean tph2 mRNA expression in the DRVL/VLPAG subregion of the DR, while mean slc6a4 mRNA expression was correlated with mean tph2 mRNA expression in the DRI subdivision of the DR (Table 2).
Figure 6.
Graph illustrating a positive correlation between mean htr1a mRNA expression and mean tph2 mRNA expression across all subdivisions of the dorsal raphe nucleus. Data are mean gray value × area for each gene. Open circles, group-reared/vehicle-treated; open triangles, group-reared/FG-7142-treated; filled circles, isolation-reared/vehicle-treated; filled triangles, isolation-reared/FG-7142-treated.
Table 2.
Pearson correlation coefficients for comparison of mRNA expression for each gene
| Subregion | htr1a vs slc6a4 | htr1a vs tph2 | slc6a4 vs tph2 |
|---|---|---|---|
| DRD | r=−0.114 | r=0.390 | r=0.054 |
| DRV | r=−0.109 | r=0.408 | r=0.121 |
| DRC | r=0.148 | r=0.368 | r=0.071 |
| DRVL/VLPAG | r=0.008 | r=0.592* | r=0.422 |
| DRI | r=−0.018 | r=0.413 | r=−0.606* |
values are presented as mean±S.E.M.
p<0.01, Bonferroni pairwise comparisons
4. Discussion
Adolescent isolation-rearing of female rats, relative to group-rearing, had no effect on anxiety-like behaviors in the SI test, however, isolation-reared rats, in contrast to group-reared rats, did respond with increased arousal and vigilance behaviors in a home cage environment, associated with decreased exploration, following challenge with the anxiogenic drug FG-7142. These data suggest that while adolescent social isolation may not result in a chronic anxiety-like state, as assessed in the SI test, it sensitizes rats to behavioral responses to subsequent anxiogenic or panicogenic stimuli in adulthood. Consistent with this hypothesis, isolation-reared and group-reared rats differed in the amount of tph2 mRNA expression, under baseline conditions, in the DRVL/VLPAG, a subregion implicated in vulnerability to panic-like responses (Johnson et al., 2007; Johnson et al., 2008; Johnson et al., 2011). There were no rearing or FG-7142-induced effects on slc6a4 or htr1a mRNA expression, suggesting that tph2 is particularly sensitive to developmental and environmental influences.
As assessed using the SI test, adolescent isolation-rearing, relative to group-rearing, had no effect on anxiety-like behaviors. These findings are consistent with previous studies using female isolates, which failed to demonstrate increased anxiety-like behaviors in the SI test during adulthood (Lukkes et al., 2012c). Furthermore, no differences between group- and isolation-reared rats in home cage behavior prior to administration with FG-7142 were observed. Together, these studies suggest that adolescent social isolation, at least under the conditions of these studies, does not result in a chronic anxiety-like state as measured in standard conflict anxiety behavioral paradigms.
Adolescent isolation-rearing did result in a sensitization to arousal and vigilance behaviors, associated with decreased exploration, in a home cage environment following administration of FG-7142. Isolation-reared rats, but not group-reared rats, responded to FG-7142 with increased SNAMA, which is a common behavioral response in a home cage environment or photocell cages following administration of anxiogenic and panicogenic drugs with diverse pharmacologic properties, including the adenosine receptor antagonist caffeine, the serotonin 5-HT2A/2C receptor agonist m-chlorophenyl piperazine, and the α2-adrenoreceptor antagonist yohimbine (Abrams et al., 2005), as well as anxiogenic or panicogenic neuropeptides such as corticotropin-releasing factor (Butler et al., 1990). As mentioned above, FG-7142 is a partial inverse agonist at the benzodiazepine allosteric site on the GABAA receptor, with its highest affinity for the α1 subunit; FG-7142 has its highest efficacy for modulation of GABA-induced chloride flux mediated at the α1 subunit-containing GABAA receptor (for review, see: Evans and Lowry, 2007). Consistent with its pharmacologic profile, FG-7142 has pro-conflict actions across a number of anxiety-related behavioral paradigms, and increases cardioacceleratory sympathetic responses (for review, see: Evans and Lowry, 2007). The FG-7142-induced increase in SNAMA behavior in isolates was associated with decreases in exploratory behaviors such as locomotion and rearing. Sensitization to FG-7142 following adolescent social isolation may relate to the panicogenic effects of this drug (Bueno et al., 2005a; Bueno et al., 2005b; Bueno et al., 2007; for review, see Evans and Lowry, 2007; Jenck et al., 1995; Singewald et al., 2003; Singewald and Sharp, 2000). Although we did not assess panic-like responses in this study, changes in tph2 mRNA expression following adolescent social isolation are consistent with the hypothesis that adverse early-life experience results in vulnerability to a panic-like syndrome (see below).
Social isolates had decreased tph2 mRNA expression, under baseline conditions, in the DRVL/VLPAG and caudal DRV, subregions of the DR that are selectively sensitive to panicogenic agents such as CO2 and sodium lactate (Johnson et al., 2005; Johnson et al., 2008), and are implicated in vulnerability to panic-like responses (Hale et al., 2011; Johnson et al., 2007; Johnson et al., 2008; Johnson et al., 2011). In previous studies, we have found that social defeat in male rats increases tph2 mRNA expression, but only in rats previously exposed to neonatal maternal separation, and only in the DRVL/VLPAG subregion of the DR, suggesting that adverse early-life experience results in vulnerability to stress-induced increases in DRVL/VLPAG tph2 mRNA expression during adulthood. Although little is known about the effects of maternal separation on subsequent panic-like responses in rats, recent studies have shown that female rats previously exposed to neonatal maternal separation show an increased hypercapnic ventilator response to the panicogenic agent CO2 as adults (Dumont et al., 2011; Genest et al., 2007). Furthermore, recent studies in mice have shown that an unstable maternal environment (cross-fostering during PD2–PD5) results in increased sensitivity to CO2 during development and adulthood, relative to normally-reared mice (D'Amato et al., 2011). Finally, in humans, events involving childhood separation from caregivers or an unstable parental environment are associated with heightened CO2 sensitivity and increased risk for panic disorder in adulthood (Battaglia et al., 1995; Klein, 1995).
We have found that DRVL/VLPAG and caudal DRV serotonergic neurons are selectively activated in adult male rats following challenge with panicogenic stimuli, such as i.v. sodium lactate or hypercapnia (Johnson et al., 2004; Johnson et al., 2008; Johnson et al., 2005). Furthermore, we have shown that in a rat model of vulnerability to panic-like responses following i.v. administration of sodium lactate, DRVL/VLPAG and caudal DRV serotonergic neurons are highly activated following sodium lactate challenge in normal rats, but fail to be activated in panic-prone rats (Johnson et al., 2008). Finally, we have shown that amygdala priming (i.e. repeated, daily injections of subthreshold doses of urocortin 1 into the basolateral amygdala), which induces a chronic anxiety-like state associated with vulnerability to panic-like responses to sodium lactate (Rainnie et al., 2004; Sajdyk et al., 1999), results in altered tph2 mRNA expression selectively within DRVL/VLPAG and caudal DRV serotonergic neurons (Donner et al., 2012). Together, these findings suggest that DRVL/VLPAG and caudal DRV serotonergic neurons normally function to inhibit panic-like physiologic responses, and dysregulation of these groups of cells results in vulnerability to panic-like physiologic responses. These data are consistent with the original proposal by Deakin and Graeff (1991), over two decades ago, that a periventricular serotonergic system projecting to the dorsal periaqueductal gray inhibits panic (Graeff, 1991; Graeff, 2004). Decreased serotonergic neurotransmission in targets of DRVL/VLPAG and caudal DRV serotonergic neurons, as would be predicted by the decreased tph2 mRNA expression under baseline conditions observed in this study, would be expected to result in a vulnerability to panic-like physiologic responses. Specifically examining the effects of adolescent isolation-rearing on panic-like physiologic and behavioral responses is an important objective for future studies.
The regionally specific pattern of altered tph2 mRNA expression in female rats exposed to adolescent social isolation, selectively in the DRVL/VLPAG and DRV serotonergic neurons, in this study is identical to the regionally specific pattern of altered tph2 mRNA expression following amygdala priming (Donner et al., 2012). In the amygdala priming model, the chronic anxiety-like state is dependent on disinhibition of projection neurons in the basolateral amygdala, following a reduction of local GABAergic inhibition (Rainnie et al., 2004). These data suggest that social isolates have an increased excitability of the amygdala, which in turn selectively alters tph2 mRNA expression in DRVL/VLPAG and DRV serotonergic neurons that are heavily innervated by the amygdala (Donner et al., 2012; Peyron et al., 1998). Consistent with this hypothesis, we have found that adolescent isolation-rearing results in a decrease in c-Fos immunostaining in a parvalbumin-expressing subset of local GABAergic interneurons within the basolateral amygdala (Lukkes et al., 2012a), a subset of GABAergic neurons within the basolateral amygdala that has been demonstrated to be stress-sensitive (Reznikov et al., 2008).
No effect of post-weaning social isolation was observed on tph2 mRNA expression, under baseline conditions, in the DRD and DRC, subregions of the DR that project to forebrain regions implicated in conflict anxiety (Hale et al., 2011; Hale et al., 2012). The observed lack of effect on tph2 mRNA expression in the DRD/DRC corresponds with the null effect of isolation-rearing on anxiety-like behavior in the SI test. A strong correlation was observed between SI time and tph2 mRNA expression within the DRD/DRC in vehicle-injected group- and isolation-reared rats. This correlation suggests that increased tph2 mRNA expression in the DRD/DRC is correlated with an anxiolytic behavioral phenotype.
Group-reared, but not isolation-reared rats, responded to FG-7142 injection with decreased tph2 mRNA expression in all subregions of the DR, excluding the DRI. These data suggest that excitation of serotonergic neuronal firing, which would be predicted following inhibition of local GABAergic signaling following FG-7142 injection, may result in a decrease in tph2 mRNA expression. Defining the relationship between serotonergic neuronal firing and tph2 mRNA expression is an important objective for future studies. Conversely, female rats exposed to adolescent social isolation were desensitized to the inhibitory effects of FG-7142 on tph2 mRNA expression in all subregions where they occurred in group housed rats, including the panic-related DRVL/VLPAG and DRV subregions. This finding parallels previous work in which serotonergic neurons on the DRVL/VLPAG and DRV subregions of normal controls respond to a panicogenic agent (sodium lactate), whereas panic-prone rats do not (Johnson et al., 2008).
In contrast to the basal differences in tph2 mRNA expression in isolates and group-reared rats, there were no basal differences in slc6a4 or htr1a mRNA expression in these groups. A similar dissociation between control of tph2 and slc6a4 mRNA expression in the DRVL/VLPAG and DRV was found in a model of amygdala priming (Donner et al., 2012). Briefly, amygdala priming increased tph2 mRNA expression selectively in the DRV and DRVL/VLPAG subregions of the DR, while it had no effect on slc6a4 mRNA expression in these subregions (Donner et al., 2012). Characterizing mechanisms underlying selective control of tph2 mRNA expression, in the absence of changes in slc6a4 or htr1a mRNA expression, in two animal models thought to involve disinhibition of the amygdala is an important goal for future studies.
5. Conclusions
Together, these data demonstrate that adolescent isolation-rearing of female rats decreases tph2 mRNA expression in DRVL/VLPAG and DRV serotonergic neurons, changes that are potentially associated with vulnerability to panic-like physiologic responses. In contrast, we did not observe this effect of adolescent social isolation on tph2 mRNA expression in males (Lukkes et al., unpublished). These sex dependent, adverse early life experience-induced alterations in tph2 mRNA expression may be an underlying neural mechanism contributing to the increased vulnerability to anxiety disorders observed in females, relative to males. Future studies should directly assess panic-like physiologic responses in the adolescent social isolation model.
Acknowledgements
C.A. Lowry was supported by a 2007 NARSAD Young Investigator Award and is currently supported by an NSF CAREER Award (NSF-IOS #0845550) and a 2010 NARSAD Young Investigator Award. The project described was supported by award numbers F32MH084463 (JLL) and R01MH086539 (CAL) from the NIMH. Dr. Lowry has consulted for Enlight Biosciences. The authors certify that they have no other actual or potential conflicts of interest in relation to this article, nor do they have a financial relationship with the organization that sponsored the research. The authors have full control of all primary data and agree to allow the journal to review the data if requested.
Index of Abbreviations
- 5-HT
5-hydroxytryptamine; serotonin
- 5-HTTLPR
serotonin transporter-linked polymorphic region
- FG-7142
N-methyl-beta-carboline-3-carboxamide
- htr1a
rodent 5-hydroxytryptamine (serotonin) receptor 1A
- HTR1A
human 5-hydroxytryptamine (serotonin) receptor 1A
- CORT
corticosterone
- dlPAG
dorsolateral periaqueductal gray
- DR
dorsal raphe nucleus
- DRD
dorsal raphe nucleus, dorsal part
- DRI
dorsal raphe nucleus, interfascicular part
- DRV
dorsal raphe nucleus, ventral part
- DRVL
dorsal raphe nucleus, ventrolateral part
- HBC
2-hydroxypropyl-β-cyclodextrin
- ISHH
in situ hybridization histochemistry
- LSD
least significant difference
- PD
postnatal day
- slc6a4
rodent solute carrier family 6 (serotonin transporter), member 4, gene
- SLC6A4
human solute carrier family 6 (serotonin transporter), member 4, gene
- SI
social interaction
- SNAMA
spontaneous non-ambulatory motor activity
- tph2
rodent tryptophan hydroxylase 2 gene
- TPH2
human tryptophan hydroxylase 2 gene
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A, Lowry CA. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience. 2005;133:983–997. doi: 10.1016/j.neuroscience.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Abrams JK, Johnson PL, Hollis JH, Lowry CA. Anatomical and functional topography of the dorsal raphe nucleus. Ann. N. Y. Acad. Sci. 2004;1018:46–57. doi: 10.1196/annals.1296.005. [DOI] [PubMed] [Google Scholar]
- Arakawa H. Ontogeny of sex differences in defensive burying behavior in rats: effect of social isolation. Aggress. Behav. 2007;33:38–47. doi: 10.1002/ab.20165. [DOI] [PubMed] [Google Scholar]
- Bach-Mizrachi H, Underwood MD, Kassir SA, Bakalian MJ, Sibille E, Tamir H, Mann JJ, Arango V. Neuronal tryptophan hydroxylase mRNA expression in the human dorsal and median raphe nuclei: major depression and suicide. Neuropsychopharmacology. 2006;31:814–824. doi: 10.1038/sj.npp.1300897. [DOI] [PubMed] [Google Scholar]
- Bach-Mizrachi H, Underwood MD, Tin A, Ellis SP, Mann JJ, Arango V. Elevated expression of tryptophan hydroxylase-2 mRNA at the neuronal level in the dorsal and median raphe nuclei of depressed suicides. Mol. Psychiatry. 2008;13:507–513. doi: 10.1038/sj.mp.4002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia M, Bertella S, Politi E, Bernardeschi L, Perna G, Gabriele A, Bellodi L. Age at onset of panic disorder: influence of familial liability to the disease and of childhood separation anxiety disorder. Am. J. Psychiatry. 1995;152:1362–1364. doi: 10.1176/ajp.152.9.1362. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Berson HE, Fremeau RT, Jr., Caron MG, Peek MM, Prince HK, Bradley CC. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991;354:66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Mann JJ, Arango V. More tryptophan hydroxylase in the brainstem dorsal raphe nucleus in depressed suicides. Brain Res. 2005;1041:19–28. doi: 10.1016/j.brainres.2005.01.083. [DOI] [PubMed] [Google Scholar]
- Bonkale WL, Turecki G, Austin MC. Increased tryptophan hydroxylase immunoreactivity in the dorsal raphe nucleus of alcohol-dependent, depressed suicide subjects is restricted to the dorsal subnucleus. Synapse. 2006;60:81–85. doi: 10.1002/syn.20278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno CH, Zangrossi H, Jr., Nogueira RL, Soares VP, Viana MB. Panicolytic-like effect induced by the stimulation of GABAA and GABAB receptors in the dorsal periaqueductal grey of rats. Eur. J. Pharmacol. 2005a;516:239–246. doi: 10.1016/j.ejphar.2005.04.045. [DOI] [PubMed] [Google Scholar]
- Bueno CH, Zangrossi H, Jr., Viana MB. The inactivation of the basolateral nucleus of the rat amygdala has an anxiolytic effect in the elevated T-maze and light/dark transition tests. Braz. J Med. Biol. Res. 2005b;38:1697–1701. doi: 10.1590/s0100-879x2005001100019. [DOI] [PubMed] [Google Scholar]
- Bueno CH, Zangrossi H, Jr., Viana MB. GABA/benzodiazepine receptors in the ventromedial hypothalamic nucleus regulate both anxiety and panic-related defensive responses in the elevated T-maze. Brain Res. Bull. 2007;74:134–141. doi: 10.1016/j.brainresbull.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Butler PD, Weiss JM, Stout JC, Nemeroff CB. Corticotropin-releasing factor produces fear enhancing and behavioral activating effects following infusion into the locus coeruleus. Neuroscience. 1990;10:176–183. doi: 10.1523/JNEUROSCI.10-01-00176.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos SB, Miranda DM, Souza BR, Pereira PA, Neves FS, Tramontina J, Kapczinski F, Romano-Silva MA, Correa H. Association study of tryptophan hydroxylase 2 gene polymorphisms in bipolar disorder patients with panic disorder comorbidity. Psychiatr. Genet. 2011;21:106–111. doi: 10.1097/YPG.0b013e328341a3a8. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- D'Amato FR, Zanettini C, Lampis V, Coccurello R, Pascucci T, Ventura R, Puglisi-Allegra S, Spatola CA, Pesenti-Gritti P, Oddi D, Moles A, Battaglia M. Unstable maternal environment, separation anxiety, and heightened CO2 sensitivity induced by gene-by-environment interplay. PLoS. ONE. 2011;6:e18637. doi: 10.1371/journal.pone.0018637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin JFW, Graeff FG. 5-HT and mechanisms of defence. Journal of Psychopharmacology. 1991;5:305–315. doi: 10.1177/026988119100500414. [DOI] [PubMed] [Google Scholar]
- Donner N, Handa RJ. Estrogen receptor beta regulates the expression of tryptophan-hydroxylase 2 mRNA within serotonergic neurons of the rat dorsal raphe nuclei. Neuroscience. 2009;163:705–718. doi: 10.1016/j.neuroscience.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner NC, Johnson PL, Fitz SD, Kellen KE, Shekhar A, Lowry CA. Elevated tph2 mRNA expression in a rat model of chronic anxiety. Depression and Anxiety. 2012;29:307–319. doi: 10.1002/da.21925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont FS, Biancardi V, Kinkead R. Hypercapnic ventilatory response of anesthetized female rats subjected to neonatal maternal separation: insight into the origins of panic attacks? Respir. Physiol Neurobiol. 2011;175:288–295. doi: 10.1016/j.resp.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Evans AK, Abrams JK, Bouwknecht JA, Knight DM, Shekhar A, Lowry CA. The anxiogenic drug FG-7142 increases serotonin metabolism in the rat medial prefrontal cortex. Pharmacol. Biochem. Behav. 2006;84:266–274. doi: 10.1016/j.pbb.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Evans AK, Lowry CA. Pharmacology of the beta-carboline FG-7,142, a partial inverse agonist at the benzodiazepine allosteric site of the GABA A receptor: neurochemical, neurophysiological, and behavioral effects. CNS. Drug Rev. 2007;13:475–501. doi: 10.1111/j.1527-3458.2007.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Shimada S, Maeno H, Nishimura T, Tohyama M. Cellular localization of serotonin transporter mRNA in the rat brain. Neurosci. Lett. 1993;162:59–62. doi: 10.1016/0304-3940(93)90559-4. [DOI] [PubMed] [Google Scholar]
- Gardner KL, Hale MW, Lightman SL, Plotsky PM, Lowry CA. Adverse early life experience and social stress during adulthood interact to increase serotonin transporter mRNA expression. Brain Res. 2009a;1305:47–63. doi: 10.1016/j.brainres.2009.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner KL, Hale MW, Oldfield S, Lightman SL, Plotsky P, Lowry CA. Adverse experience during early life and adulthood ineract to elevate tph2 mRNA expression in serotonergic neurons within the dorsal raphe nucleus. Neuroscience. 2009b;163:991–1001. doi: 10.1016/j.neuroscience.2009.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner KL, Thrivikraman KV, Lightman SL, Plotsky PM, Lowry CA. Early life experience alters behavior during social defeat: focus on serotonergic systems. Neuroscience. 2005;136:181–191. doi: 10.1016/j.neuroscience.2005.07.042. [DOI] [PubMed] [Google Scholar]
- Genest SE, Balon N, Laforest S, Drolet G, Kinkead R. Neonatal maternal separation and enhancement of the hypoxic ventilatory response in rat: the role of GABAergic modulation within the paraventricular nucleus of the hypothalamus. J. Physiol. 2007;583:299–314. doi: 10.1113/jphysiol.2007.135160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeff FG. Serotonin, the periaqueductal gray and panic. Neurosci Biobehav. Rev. 2004;28:239–259. doi: 10.1016/j.neubiorev.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Graeff FG. Neurotransmitters in the dorsal periaqueductal gray and animal models of panic anxiety. In: Briley M, File SE, editors. New concepts in anxiety. MacMillan Press; London: 1991. pp. 288–312. [Google Scholar]
- Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacol. Biochem. Behav. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- Grubbs FE. Procedures for Detecting Outlying Observations in Samples. Technometrics. 1969;11:1–21. [Google Scholar]
- Hale MW, Lowry CA. Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology (Berl) 2011;213:243–264. doi: 10.1007/s00213-010-2089-z. [DOI] [PubMed] [Google Scholar]
- Hale MW, Shekhar A, Lowry CA. Development by environment interactions controlling tryptophan hydroxylase expression. J. Chem. Neuroanat. 2011;41:219–226. doi: 10.1016/j.jchemneu.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale MW, Shekhar A, Lowry CA. Stress-related serotonergic systems: implications for symptomatology of anxiety and affective disorders. Cell. Mol. Neurobiol. 2012;32:695–708. doi: 10.1007/s10571-012-9827-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen HH, Mikkelsen JD. Long-term effects on serotonin transporter mRNA expression of chronic neonatal exposure to a serotonin reuptake inhibitor. Eur. J. Pharmacol. 1998;352:307–315. doi: 10.1016/s0014-2999(98)00349-5. [DOI] [PubMed] [Google Scholar]
- Harbuz MS, Lightman SL. Responses of hypothalamic and pituitary mRNA to physical and psychological stress in the rat. J. Endocrinol. 1989;122:705–711. doi: 10.1677/joe.0.1220705. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hoefgen B, Schulze TG, Ohlraun S, von WO, Hofels S, Gross M, Heidmann V, Kovalenko S, Eckermann A, Kolsch H, Metten M, Zobel A, Becker T, Nothen MM, Propping P, Heun R, Maier W, Rietschel M. The power of sample size and homogenous sampling: association between the 5-HTTLPR serotonin transporter polymorphism and major depressive disorder. Biol. Psychiatry. 2005;57:247–251. doi: 10.1016/j.biopsych.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Jenck F, Moreau JL, Martin JR. Dorsal periaqueductal gray-induced aversion as a simulation of panic anxiety: elements of face and predictive validity. Psychiatry Res. 1995;57:181–191. doi: 10.1016/0165-1781(95)02673-k. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Fitz SD, Hollis JH, Moratalla R, Lightman SL, Shekhar A, Lowry CA. Induction of c-Fos in `panic/defence'-related brain circuits following brief hypercarbic gas exposure. J. Psychopharmacol. 2011;25:26–36. doi: 10.1177/0269881109353464. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Hollis JH, Moratalla R, Lightman SL, Lowry CA. Acute hypercarbic gas exposure reveals functionally distinct subpopulations of serotonergic neurons in rats. J. Psychopharmacol. 2005;19:327–341. doi: 10.1177/0269881105053281. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Lightman SL, Lowry CA. A functional subset of serotonergic neurons in the rat ventrolateral periaqueductal gray implicated in the inhibition of sympathoexcitation and panic. Ann. N. Y. Acad. Sci. 2004;1018:58–64. doi: 10.1196/annals.1296.006. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Lowry CA, Truitt W, Shekhar A. Disruption of GABAergic tone in the dorsomedial hypothalamus attenuates responses in a subset of serotonergic neurons in the dorsal raphe nucleus following lactate-induced panic. J. Psychopharmacol. 2008;22:642–645. doi: 10.1177/0269881107082900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Truitt WA, Fitz SD, Lowry CA, Shekhar A. Neural Pathways Underlying Lactate-Induced Panic. Neuropsychopharmacology. 2007;33:2093–2107. doi: 10.1038/sj.npp.1301621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Lee HJ, Yang JC, Hwang JA, Yoon HK. A tryptophan hydroxylase 2 gene polymorphism is associated with panic disorder. Behav. Genet. 2009;39:170–175. doi: 10.1007/s10519-008-9254-8. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Pernar L, Valentino RJ, Beck SG. Distinguishing characteristics of serotonin and nonserotonin-containing cells in the dorsal raphe nucleus: Electrophysiological and immunohistochemical studies. Neuroscience. 2003;116:669–683. doi: 10.1016/s0306-4522(02)00584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RG. Is panic disorder associated with childhood separation anxiety disorder? Clin Neuropharmacol. 1995;18(suppl 2):S7–S14. [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Gutknecht L. Focus on The 5-HT1A receptor: emerging role of a gene regulatory variant in psychopathology and pharmacogenetics. Int. J. Neuropsychopharmacol. 2004;7:381–385. doi: 10.1017/S1461145704004845. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Andersen SL. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse. 2008;62:22–30. doi: 10.1002/syn.20462. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ, Shekhar A. Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Ann. N. Y. Acad. Sci. 2008;1148:86–94. doi: 10.1196/annals.1410.004. [DOI] [PubMed] [Google Scholar]
- Lukkes J, Vuong S, Scholl J, Oliver H, Forster G. Corticotropin-releasing factor receptor antagonism within the dorsal raphe nucleus reduces social anxiety-like behavior after early-life social isolation. J. Neurosci. 2009a;29:9955–9960. doi: 10.1523/JNEUROSCI.0854-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Burke AR, Zelin NS, Hale MW, Lowry CA. Post-weaning social isolation attenuates c-Fos expression in GABAergic interneurons in the basolateral amygdala of adult female rats. Physiol Behav. 2012a doi: 10.1016/j.physbeh.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Engelman GH, Zelin NS, Hale MW, Lowry CA. Post-weaning social isolation of female rats, anxiety-related behavior, and serotonergic systems. Brain Res. 2012b;1443:1–17. doi: 10.1016/j.brainres.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Engelman GH, Zelin NS, Hale MW, Lowry CA. Post-weaning social isolation of female rats, anxiety-related behavior, and serotonergic systems. Brain Res. 2012c;1443:1–17. doi: 10.1016/j.brainres.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Mokin MV, Scholl JL, Forster GL. Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm. Behav. 2009b;55:248–256. doi: 10.1016/j.yhbeh.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Watt MJ, Lowry CA, Forster GL. Consequences of post-weaning social isolation on anxiety behavior and related neural circuits in rodents. Front Behav. Neurosci. 2009c;3:18. doi: 10.3389/neuro.08.018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav. Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Maron E, Toru I, Must A, Tasa G, Toover E, Vasar V, Lang A, Shlik J. Association study of tryptophan hydroxylase 2 gene polymorphisms in panic disorder. Neurosci. Lett. 2007;411:180–184. doi: 10.1016/j.neulet.2006.09.060. [DOI] [PubMed] [Google Scholar]
- Maron E, Toru I, Tasa G, Must A, Toover E, Lang A, Vasar V, Shlik J. Association testing of panic disorder candidate genes using CCK-4 challenge in healthy volunteers. Neurosci. Lett. 2008;446:88–92. doi: 10.1016/j.neulet.2008.09.052. [DOI] [PubMed] [Google Scholar]
- Mossner R, Freitag CM, Gutknecht L, Reif A, Tauber R, Franke P, Fritze J, Wagner G, Peikert G, Wenda B, Sand P, Rietschel M, Garritsen H, Jacob C, Lesch KP, Deckert J. The novel brain-specific tryptophan hydroxylase-2 gene in panic disorder. J. Psychopharmacol. 2006;20:547–552. doi: 10.1177/0269881106059704. [DOI] [PubMed] [Google Scholar]
- Otter MH, Matto V, Soukand R, Skrebuhhova T, Allikmets L, Harro J. Characterization of rat exploratory behavior using the exploration box test. Methods Find. Exp. Clin. Pharmacol. 1997;19:683–691. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Fourth Edition Academic Press; San Diego: 1998. [Google Scholar]
- Peyron C, Petit J-M, Rampon C, Jouvet M, Luppi P-H. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J. Neurosci. 2004;24:3471–3479. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikov LR, Reagan LP, Fadel JR. Activation of phenotypically distinct neuronal subpopulations in the anterior subdivision of the rat basolateral amygdala following acute and repeated stress. J. Comp. Neurol. 2008;508:458–472. doi: 10.1002/cne.21687. [DOI] [PubMed] [Google Scholar]
- Rothe C, Gutknecht L, Freitag C, Tauber R, Mossner R, Franke P, Fritze J, Wagner G, Peikert G, Wenda B, Sand P, Jacob C, Rietschel M, Nothen MM, Garritsen H, Fimmers R, Deckert J, Lesch KP. Association of a functional 1019C>G 5-HT1A receptor gene polymorphism with panic disorder with agoraphobia. Int. J. Neuropsychopharmacol. 2004;7:189–192. doi: 10.1017/S1461145703004061. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav. Brain Res. 1999;100:207–215. doi: 10.1016/s0166-4328(98)00132-6. [DOI] [PubMed] [Google Scholar]
- Salchner P, Sartori SB, Sinner C, Wigger A, Frank E, Landgraf R, Singewald N. Airjet and FG-7142-induced Fos expression differs in rats selectively bred for high and low anxiety-related behavior. Neuropharmacology. 2006;50:1048–1058. doi: 10.1016/j.neuropharm.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol. Psychiatry. 2003;53:275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- Singewald N, Sharp T. Neuroanatomical targets of anxiogenic drugs in the hindbrain as revealed by Fos immunocytochemistry. Neuroscience. 2000;98:759–770. doi: 10.1016/s0306-4522(00)00177-9. [DOI] [PubMed] [Google Scholar]
- Strobel A, Gutknecht L, Rothe C, Reif A, Mossner R, Zeng Y, Brocke B, Lesch KP. Allelic variation in 5-HT1A receptor expression is associated with anxiety- and depression-related personality traits. J. Neural Transm. 2003;110:1445–1453. doi: 10.1007/s00702-003-0072-0. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Polcari A, McGreenery CE. Sticks, stones, and hurtful words: relative effects of various forms of childhood maltreatment. Am. J. Psychiatry. 2006;163:993–1000. doi: 10.1176/ajp.2006.163.6.993. [DOI] [PubMed] [Google Scholar]
- Underwood MD, Khaibulina AA, Ellis SP, Moran A, Rice PM, Mann JJ, Arango V. Morphometry of the dorsal raphe nucleus serotonergic neurons in suicide victims. Biol. Psychiatry. 1999;46:473–483. doi: 10.1016/s0006-3223(99)00043-8. [DOI] [PubMed] [Google Scholar]
- Weiss IC, Pryce CR, Jongen-Relo AL, Nanz-Bahr NI, Feldon J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav. Brain Res. 2004;152:279–295. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Yoon HK, Yang JC, Lee HJ, Kim YK. The association between serotonin-related gene polymorphisms and panic disorder. J Anxiety. Disord. 2008;22:1529–1534. doi: 10.1016/j.janxdis.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Yu YW, Tsai SJ, Chen TJ, Lin CH, Hong CJ. Association study of the serotonin transporter promoter polymorphism and symptomatology and antidepressant response in major depressive disorders. Mol. Psychiatry. 2002;7:1115–1119. doi: 10.1038/sj.mp.4001141. [DOI] [PubMed] [Google Scholar]
- Zhang K, Xu Q, Xu Y, Yang H, Luo J, Sun Y, Sun N, Wang S, Shen Y. The combined effects of the 5-HTTLPR and 5-HTR1A genes modulates the relationship between negative life events and major depressive disorder in a Chinese population. J. Affect. Disord. 2009;114:224–231. doi: 10.1016/j.jad.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Zill P, Buttner A, Eisenmenger W, Moller HJ, Bondy B, Ackenheil M. Single nucleotide polymorphism and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene in suicide victims. Biol. Psychiatry. 2004;56:581–586. doi: 10.1016/j.biopsych.2004.07.015. [DOI] [PubMed] [Google Scholar]