Abstract
Background
Anxiety is highly prevalent among patients with coronary heart disease (CHD), and there is growing evidence that high levels of anxiety are associated with worse prognosis. However, few studies have evaluated the efficacy of treating anxiety in CHD patients for reducing symptoms and improving clinical outcomes. Exercise and selective serotonin reuptake inhibitors have been shown to be effective in treating patients with depression, but have not been studied in cardiac patients with high anxiety.
Methods
The UNWIND trial is a randomized clinical trial of patients with CHD who are at increased risk for adverse events because of comorbid anxiety. One hundred fifty participants with CHD and elevated anxiety symptoms and/or with a diagnosed anxiety disorder will be randomly assigned to 12 weeks of aerobic exercise (3×/wk, 35 min, 70–85% VO2peak), escitalopram (5–20 mg qd), or placebo. Before and after 12 weeks of treatment, participants will undergo assessments of anxiety symptoms and CHD biomarkers of risk, including measures of inflammation, lipids, hemoglobin A1c, heart rate variability, and vascular endothelial function. Primary outcomes include post-intervention effects on symptoms of anxiety and CHD biomarkers. Secondary outcomes include clinical outcomes (cardiovascular hospitalizations and all-cause death) and measures of quality of life.
Conclusions
The UNWIND trial (ClinicalTrials.gov NCT02516332) will evaluate the efficacy of aerobic exercise and escitalopram for improving anxiety symptoms and reducing risk for adverse clinical events in anxious CHD patients.
Keywords: Anxiety, Stress, Coronary Heart Disease, Exercise, Selective Serotonin Reuptake Inhibitor
INTRODUCTION
Coronary heart disease (CHD) and anxiety disorders are both debilitating conditions that often co-exist and are associated with increased mortality, morbidity, and health care costs. CHD is a leading cause of mortality in the United States in men and women of every major ethnic group, affecting 370 million people annually1 at an estimated economic cost of $139.6 billion in both direct medical expenses and lost productivity2. Additionally, CHD may result in impaired quality of life and psychiatric comorbidity, including mood and anxiety disorders.
Anxiety disorders are the most commonly diagnosed form of mental illness and are responsible for one-third of the total expenditures for mental illness in the United States.3 Approximately half of those expenditures are due to the repeated use of health care services, since persons with anxiety disorders often solicit medical evaluation for symptoms that resemble physical illnesses. Data from the National Comorbidity Study-Replication suggest that the lifetime prevalence of any anxiety disorder is approximately one in three, more than any other psychiatric diagnostic category,4 and the prevalence of anxiety disorders has increased significantly over the past 2 decades.5
Although the precise prevalence of anxiety disorders among cardiac patients has not been established, it has been estimated that 11–14% of cardiac patients suffer from generalized anxiety disorder (GAD)6 and over 40% of patients with stable CHD may have elevated anxiety symptoms.7 While elevated anxiety symptoms are a criterion common to all anxiety disorders, diagnostic criteria can be quite heterogeneous. In addition to diagnosed anxiety disorders, subsyndromal anxiety symptoms also can impair individuals’ psychosocial functioning and can increase health care resource utilization.8 Anxiety represents a risk factor for lower health-related quality of life,9 increased risk of all-cause mortality,10 and a variety of physical health problems, including CHD.
Prognostic Importance of Anxiety
A number of studies have shown a prospective relationship between depression and adverse outcomes, prompting the American Heart Association to recommend depression as a risk factor for patients with CHD.11 Although less widely studied, anxiety is increasingly considered also to have important prognostic significance in CHD patients. A number of studies, albeit not all studies12–14, have found that elevated anxiety symptoms are associated with an increased mortality risk. For example, CHD patients with elevated anxiety assessed during hospitalization for cardiac catheterization showed a 2-fold increased risk of mortality, and this risk was independent of other predictors of risk, including depression.10 Similar findings have been reported by Frasure-Smith and colleagues who found that CHD patients with GAD assessed two months following hospital discharge for acute coronary syndrome showed a 2.3-fold increased risk of adverse cardiac events.7 Strik et al.15 reported a 2.8 fold increased risk of adverse events in acute post-MI patients in which anxiety was measured one month following hospital discharge. Similarly, a 2-fold increased risk of adverse events was found in patients with stable CHD undergoing cardiac rehabilitation16 and in patients with elevated anxiety during annual clinic visits;17 and patients with elevated anxiety symptoms in the coronary care unit were found to exhibit greater mortality within the first year post-AMI.18
Despite this evidence for a relationship between anxiety and adverse outcomes in CHD patients, the value of reducing anxiety in cardiac patients has not been established because of limited data from randomized control trials (RCTs) and a lack of evidence that treatment could improve clinical outcomes.
Pharmacologic Treatment for Anxiety
Many pharmacologic agents have been developed for treating different types of anxiety disorders, including barbiturates and benzodiazepines, azapirones (e.g., buspirone), tricyclics, selective serotonin reuptake inhibitors (SSRIs), and serotonin and norepinephrine reuptake inhibitors (SNRIs).19 SNRIs block the reuptake pump systems of serotonin and norepinephrine, inhibiting the reuptake of these neurotransmitters into the presynaptic neuron; SNRIs have a latency period of 2–4 weeks and low risk of dependency.19 The positive profile of SNRIs has led to the FDA approval of duloxetine for GAD20 and venlafaxine for GAD and social anxiety disorder (SAD).21 SSRIs block the reuptake of serotonin into the presynaptic neuron, and have a latency and risk profile similar to SNRIs. Further, SSRIs have proven to be effective for GAD, SAD, and panic disorder, thus multiple SSRIs have received FDA approval: paroxetine is approved for panic disorder, SAD, and GAD; sertraline is approved for SAD and panic disorder; fluoxetine is approved for panic disorder; and escitalopram is approved for GAD.21
Although these compounds are effective for treating a variety of anxiety conditions, to our knowledge, no studies have reported the effects of treating anxiety on clinical outcomes in anxious cardiac patients. SSRIs have been studied in cardiac populations with depression with mixed results. For example, the SADHART study reported that sertraline produced greater improvements in depressive symptoms compared to placebo controls, but only in the subset of patients with more severe depression,22 and SADHART-CHF found no difference in depressive symptoms or clinical outcomes following treatment with sertraline compared to placebo in depressed patients with heart failure.23 In a non-randomized substudy from the ENRICHD trial, antidepressant medications were associated with reduced mortality.24 The CREATE trial reported that citalopram, when combined with clinical management, improved depression compared to placebo.25
Several studies have examined the effects of escitalopram in depressed patients. MOOD-HF examined the effect of escitalopram on depression and anxiety in heart failure patients,26 but the study was stopped early due to ineffectiveness of the drug; changes in symptoms of anxiety were not reported.27 The DECARD study showed that escitalopram prevented depression28 and was well-tolerated in post-ACS patients.29 Finally, the REMIT study examined the effect of escitalopram in patients with stable CHD and evidence of mental-stress induced myocardial ischemia. Although not the primary endpoint, scores on the Spielberger State-Trait Anxiety Inventory (STAI)-State tended to decrease following treatment after adjusting for sex and baseline STAI-State scores.30 Because of the safety and tolerability of SSRIs in a cardiac population,31 their potential anxiolytic and anti-depressant effects, and the comorbidity between depression and anxiety, we believe that SSRIs, and specifically escitalopram, may be beneficial for treating anxiety in cardiac patients. To our knowledge, the UNWIND trial will be the first study to target cardiac patients with elevated anxiety using anxiolytic medication.
Exercise Treatment for Anxiety
There is growing evidence that exercise may have beneficial effects on anxiety. Epidemiological studies have observed an inverse relationship between exercise and anxiety. For example, Goodwin reported that persons who indicated they exercise “regularly” were at reduced risk for being diagnosed with an anxiety disorder compared to their sedentary counterparts (odds ratios from 0.64 to 0.78 for exercisers).32 In another study of 19,288 participants in the Netherlands Twin Registry, individuals reporting 240 min a week of moderate exercise reported less anxiety and neuroticism compared with non-exercisers.33 Although encouraging, data from observational studies cannot prove that exercise was the cause of the reduced risk for an anxiety disorder; anxious persons also may be less likely to be physically active and engage in exercise.
There have been many exercise intervention trials with anxiety as an outcome, and while results have generally been positive, few studies have specifically targeted anxious individuals. For example, Wipfli and colleagues34 found that exercise was associated with an overall effect size of 0.48, indicating greater reductions in anxiety symptoms compared to no-treatment controls, and an effect size of 0.19 when exercise was compared to other established treatments for anxiety. In a review of 8 RCTs of patients with a broad range of anxiety disorders, Jayakody et al.35 noted that exercise seems to be effective as an adjunctive treatment for most anxiety disorders, but there were too few studies to provide meaningful conclusions. More recently, Stonerock et al.36 provided a comprehensive review of the literature on exercise and anxiety disorders. Examination of 12 RCTs suggested that the benefits of exercise were comparable to established psychiatric treatments and greater than placebo controls in select patient groups. However, most studies had significant methodological limitations, including small sample sizes, concurrent therapies, and inadequate assessment of adherence and fitness levels. Moreover, only two studies examined the anxiolytic effects of exercise in cardiac patients. One study reported more than a 69% reduction in anxiety among highly anxious participants in an exercise-based cardiac rehabilitation program37; however, there was no control group and exercise was only one component of the intervention. A second RCT that targeted cardiac patients with elevated anxiety reported greater reductions in symptoms of anxiety assessed by the STAI and the Profile of Mood States after 8 weeks of cardiac rehabilitation compared to community care controls.38,39 However, the cardiac rehabilitation group also received concurrent weekly 90-min group counseling sessions, so that the improvements could not be attributed to exercise training. Thus, while results from exercise studies are encouraging, the potential therapeutic benefits of exercise in CHD patients remain uncertain.
The UNWIND Trial
UNWIND is a single-site, randomized clinical trial designed to evaluate whether aerobic exercise can reduce anxiety in CHD patients and, if so, how it compares to the currently prescribed anxiolytic medication escitalopram. In addition to the primary endpoint of anxiety, we also will examine the effects of aerobic exercise and/or escitalopram on an array of CHD biomarkers, including autonomic regulation, vascular function, and CHD blood markers (e.g., interleukin-6 [IL-6], high-sensitivity C-reactive protein [hsCRP], HbA1c, and insulin). These biomarkers were selected because of their prognostic significance and potential responsiveness to treatment.
Heart rate variability (HRV) is widely recognized as an important index of autonomic regulation of the heart and prognostic indicator of risk and will serve as our primary biomarker of interest. Reduced 24-hour HRV independently predicts mortality in community samples40,41 and patients with CHD42,43.
Baroreceptor reflex sensitivity (BRS) is also an index of cardiac regulation by the parasympathetic nervous system (PSNS), with experimental models showing that low levels of BRS predict sudden cardiac death (SCD) due to ventricular fibrillation.44 Anxiety has been linked to reduced PSNS control of heart rate in several different populations, including patients with anxiety disorders.45,46 Elevated sympathetic nervous system (SNS) activity is another pathophysiological aspect of autonomic dysregulation contributing to the development of CHD that is thought to contribute to increased cardiovascular risk associated with anxiety.47 SSRIs appear to reduce HRV48, and there is evidence that regular exercise improves HRV, as well as BRS, in middle-aged and elderly sedentary subjects and patients with CHD.49,50
Endothelial dysfunction plays a vital role in the development, progression, and clinical manifestations of atherosclerosis.51,52 It has been related to a wide range of CHD risk factors, and has been shown to be prognostic in cardiac patients.53 Impaired FMD has been linked to elevated anxiety symptoms in CHD patients.54 Several small interventional studies have shown that FMD may be improved by exercise training in cardiac patients.55,56
Inflammation is widely considered to play a central role in the development and progression of CHD. C-reactive protein (CRP) is a highly sensitive marker of underlying systemic inflammation.57 An elevated level of CRP is an independent risk factor for MI and stroke.58,59 Interleukin-6 (IL-6) is an inflammatory cytokine that may be the initial event leading to an increase in CRP levels, and recent meta-analyses indicate that IL-6 may be more strongly related than CRP to the promotion of atherosclerosis.60 Pitsavos et al.61 observed a significant dose-response relationship between the severity of anxiety symptoms and CRP, and Frasure-Smith et al.62 reported that HRV was correlated with inflammatory markers and suggested that interventions targeting regulation of both autonomic control and inflammation may be especially worthwhile.
We hypothesize that both exercise training and escitalopram will reduce anxiety symptoms to a greater extent than placebo; exercise training will improve CHD biomarkers of risk, including autonomic regulation, vascular endothelial function, and inflammation, more than either escitalopram or placebo; and improvements in CHD biomarkers will be mediated by reductions in symptoms of anxiety. We also will explore potential moderators of treatment (e.g., anxiety diagnoses, CHD severity) as well as the longer-term benefits of treatment by documenting medical events over a follow-up period of up to 4 years. The findings from this RCT will have important implications for anxiety assessment and management in CHD patients.
METHODS
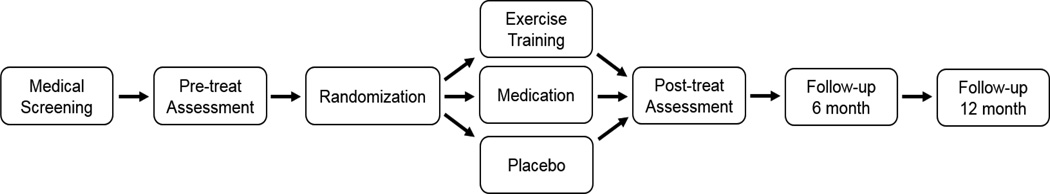
One hundred fifty participants with CHD and anxiety will be randomized with 2:2:1 allocation to 1 of 3 groups: (1) aerobic exercise (N = 60); (2) escitalopram (N = 60); or (3) placebo pill (N = 30). Participants will be evaluated on clinical, behavioral, and physiological dimensions at baseline and after a 12-week intervention. Assessments of anxiety, health behaviors, and quality of life also will be obtained 6- and 12-months post-intervention. Additional measures of quality of life also will be obtained along with annual follow-up interviews to document clinical events. The study design is depicted in Figure 1. The study is registered at www.clinicaltrials.gov (ID: NCT02516332).
Figure 1. Study Design.
Figure 1 depicts the study design.
Participants
UNWIND will actively recruit women and minorities, with at least 50% women and 25% minorities.
Inclusion Criteria
Men and women age 40 years or older with documented CHD will be selected for study. Participants will have an anxiety symptom severity score of ≥8 (mild-moderate) on the anxiety section of the Hospital Anxiety and Depression Scale (HADS-A) ; a subgroup of participants also will have a DSM-5 diagnosis of an Anxiety Disorder (See Table S1 for a more complete description). Because of the importance of treating debilitating symptoms and not merely psychiatric diagnoses,63 we will target patients with elevated symptoms of anxiety regardless of whether they meet diagnostic criteria for anxiety disorders.
Exclusion Criteria
Medical exclusions will include an MI or coronary revascularization procedure within the last 3 months, unstable angina, severe left ventricular dysfunction or decompensated heart failure, unrevascularized left main coronary artery stenosis >50%, and conditions that would preclude randomization to either escitalopram or exercise. Patients with a primary psychiatric diagnosis other than Anxiety Disorder will be excluded along with patients who are taking other medications that would preclude assignment to either drug or exercise conditions (e.g., clonidine, anticonvulsants, and MAO inhibitors).
Screening Procedures
All eligible participants will undergo a psychiatric screen to determine anxiety symptom severity and a medical screen to verify safe participation in any treatment group.
Psychiatric Screen
The Anxiety and Depression Detector (ADD)64 and the Patient Health Questionnaire (PHQ-9)65 will be used to screen for anxiety and depression symptom severity. The ADD is a 5-item instrument that has been used to detect anxiety (and depression) in medical settings and is an effective screening tool for anxiety disorders (e.g., panic disorder, social phobia, and GAD). It is an established overall measure of distress and is likely to reflect a diagnosis of at least one of these conditions or significantly elevated levels of anxiety. Participants must obtain a score of ≥1; the ADD is correlated with the Overall Anxiety Severity and Impairment Scale,66 which also has been used to detect the presence of significant anxiety. The PHQ-9 is a brief, reliable, and valid measure of depression severity, which will be used to examine the comorbidity between anxiety and depression in participants.
Medical Screen
Each participant will receive a screening physical examination to assess blood pressure, routine blood tests, including metabolic and lipid panels, insulin, and HbA1c, health behaviors, including smoking and alcohol use, and presence of significant cognitive impairment.
Treatment Conditions
Participants will be randomly assigned to 1 of the 3 treatment conditions for 12 weeks. Randomization will occur after each subject has completed the assessment protocol and will adhere to standard procedures for randomized clinical trials.67 Participants will be stratified by sex (male/female), history of myocardial infarction (yes/no), diagnosed anxiety disorder (yes/no), and age (<60/≥60 years).
Aerobic Exercise
Participants will exercise three times per week under medical supervision at a designated cardiac rehabilitation facility. Exercise intensity will be at a level of 70–85% of VO2 peak as determined at the time of the baseline exercise treadmill test. Each exercise session will consist of 10 min of gradual warm-up exercises followed by 35 min of continuous walking, biking, or jogging at the target intensity, and 5 min of cool down exercises for a total a 50 min.
Escitalopram/Placebo Pill
Treatment in the escitalopram and placebo pill arms will involve the same protocol. Subjects will have regular face-to-face visits and phone contact with a prescribing psychiatrist, who will be blinded to treatment condition. Participants will start on 5 mg once per day of either escitalopram or placebo. Daily doses will be titrated to 10 mg at week 2 and to 15 mg or placebo equivalent at week 3 if there is no change or only minimal improvement in anxiety. At week 4, if there is no change or only minimal improvement in anxiety, and no or minimal side effects, a maximum daily dose of 20 mg or placebo equivalent will be prescribed. Supportive measures will be used to help manage medication side effects, and in such cases, doses may be decreased. Further, the STAI-State will be assessed weekly to assess changes in symptoms and determine the rate of change in anxiety symptoms over the course of the trial.
Assessments
Anxiety
Anxiety diagnosis will be assessed by structured clinical interviews. A clinical psychologist will administer modules from the Structured Clinical Interview for DSM-5 Disorders (SCID)68 and the 14-item Hamilton Anxiety Rating Scale (HAMA)69. The primary outcome measure will be scores on the HADS-A.70 Other psychiatric self-report anxiety assessments will include the Anxiety Sensitivity Index (ASI),71 the General Anxiety Disorder 7-item questionnaire (GAD-7),72 and the STAI-Trait.73 The STAI-State will be used to assess ongoing treatment response during the 12-week intervention.
Ancillary Self-Report Measures
Because symptoms of depression are likely to coexist with anxiety and major depressive disorder is an exclusion criterion, depressive symptoms will be assessed with the Beck Depression Inventory-II (BDI-II).74 Together, these assessments will afford a comprehensive analysis of each participant’s severity of anxiety symptoms and the comorbidity with depression.
In addition to measuring anxiety and depression, areas considered to reflect quality of life and coping will be evaluated by measuring general health,75 perceived stress,76 resilience,77 optimism,78 self-efficacy, and perceived social support.79,80 Further, we will assess a number of health behaviors that may affect participants’ health including the Godin Leisure-Time Exercise questionnaire81 to evaluate exercise habits, the Pittsburgh sleep questionnaire82 to examine sleep patterns, the Morisky Medication Adherence Scale83 to document medication adherence, and a dietary habits questionnaire84 to examine nutrition habits.
CHD Biomarkers
We will assess autonomic regulation by measuring heart rate variability (HRV) and baroreflex sensitivity (BRS), vascular endothelial function by measuring flow-mediated dilation (FMD) of the brachial artery, and a panel of CHD blood markers including measures of inflammation (hsCRP and IL-6), lipids (total cholesterol, HDL-, LDL-, and VLDL-cholesterol), and metabolic markers (glucose, insulin, and HbA1c). Further, to assess biologic markers of stress, urine samples will be collected over a 24-hour period, and will be assayed for norepinephrine, epinephrine, and creatinine.
Physical Activity
Graded treadmill exercise testing will be conducted at baseline and at the conclusion of treatment to evaluate functional capacity. To obtain a measure of average daily physical activity, a 3-axis wrist accelerometer (Actigraph™) will be worn for 7 continuous days. These clinical, biological, and psychometric measures will be completed by participants at both pre- and post-treatment assessments. Supplementary Table S1 provides a detailed description of the measures to be obtained in the UNWIND study.
Follow-up
Participants will be followed up at 6- and 12-months post-randomization (i.e. 3 and 9 months after completion of the 3-month intervention). At the 6-month follow-up, participants will receive assessments for anxiety and quality of life, and they will report exercise habits, any psychiatric treatments, psychopharmacologic medications, and medical events. At the 12-month follow-up, anxiety will be evaluated by the HADS-A and medical events will be reported. At both follow-up visits, participants will be queried as to whether they participated in any supplemental programs to reduce anxiety, including other medications and exercise. This naturalistic follow-up has been used in prior work85–87 and yields important information regarding maintenance of lifestyle habits over time, which will be used to help interpret any group differences in anxiety and cardiovascular biomarkers after 1 year. We also will document a number of clinical end points, including all-cause mortality, cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, and hospitalization for angina or heart failure during annual follow-up examinations for up to 5 years. The schedule for the assessments is presented in Table 1.
Table 1.
Assessment Schedule
| MEASURE | Medical Screen |
Pre- Treatment (baseline) |
Randomization | Treatment | Post- Treatment (l2 week) | Follow up (6 month) |
Follow up (12 month) |
|---|---|---|---|---|---|---|---|
| ASSESSMENT | |||||||
| Informed consent | X | ||||||
| History and Physical Exam | X | ||||||
| Demographics questionnaire | X | ||||||
| Anxiety Assessments | |||||||
| ADD (≥ 1) | X | ||||||
| HADS-A(≥ 8) | X | X | X | X | |||
| SCID | X | X | X | ||||
| HAM-A | X | X | X | ||||
| ASI | X | X | X | ||||
| GAD-7 | X | X | X | ||||
| STAI-T | X | X | X | ||||
| STAI-S | X weekly | ||||||
| Depression Assessments | |||||||
| PHQ-9 (<15) | X | ||||||
| BDI-II | X | X | X | X | |||
| Quality of Life Assessments | |||||||
| ESSI | X | X | X | ||||
| PSSS | X | X | X | ||||
| LOT-R | X | X | X | ||||
| RES | X | X | X | ||||
| GHQ | X | X | X | ||||
| GSE | X | X | X | ||||
| PST | X | X | X | ||||
| PSQ | X | X | X | ||||
| Diet | X | X | X | ||||
| Morisky | X | X | X | ||||
| CHD Biomarkers | |||||||
| HRV | X | X | |||||
| BRS | X | X | |||||
| FMD | X | X | |||||
| Blood Pressure | X | X | |||||
| Blood/urine | X | X | |||||
| Aerobic Fitness | |||||||
| Exercise Treadmill Test | X | X | |||||
| Actigraphy 7 day | X | X | |||||
| Godin | X | X | X | ||||
| Clinical Events | X | X |
Abbreviations: ADD, Anxiety and Depression Detector; HADS-A, Hospital Anxiety and Depression Scale – Anxiety; SCID, Structured Clinical Interview for DSM-5 Disorders; HAM-A, Hamilton Anxiety Rating Scale – Anxiety; ASI, Anxiety Sensitivity Index; GAD-7, General Anxiety Disorder-7 item; STAI, Spielberger State-Trait Anxiety Inventory; PHQ9, Personal Health Questionnaire; BDI-II, Beck Depression Inventory-II; ESSI, ENRICHD Social Support Abbreviations: ADD, Anxiety and Depression Detector; HADS-A, Hospital Anxiety and Depression Scale – Anxiety; SCID, Structured Clinical Interview for DSM-5 Disorders; HAM-A, Hamilton Anxiety Rating Scale – Anxiety; ASI, Anxiety Sensitivity Index; GAD-7, General Anxiety Disorder-7 item; STAI, Spielberger State-Trait Anxiety Inventory; PHQ9, Personal Health Questionnaire; BDI-II, Beck Depression Inventory-II; ESSI, ENRICHD Social Support
Data analysis
The basic analytic strategy is a linear model carried out in the MPlus modeling software,88 with treatment group contrasts as factors, and ethnicity, age, sex, and pre-treatment measure of the outcome variable as the adjustment covariables. The intent-to-treat principle will be followed in all models, using full information maximum likelihood available in MPlus to manage missing data. For the case of the primary outcome, HADS-A, the model will include post-treatment HADS-A score as the response, with the predictors including two planned contrast variables representing 1) the two active treatments (exercise and escitalopram) vs. placebo and 2) exercise vs. escitalopram, with ethnicity, sex, age, and pre-treatment level of the HADS-A as the adjustment covariables. The primary biomarker outcome, HRV, will be evaluated similarly.
In addition to treatment effects, we will test the hypothesis that pre- to post-treatment change in HADS-A scores will mediate the improvements in intermediate CHD biomarkers. We also will explore possible treatment-specific mediators (e.g., change in VO2 mediating change in HRV for the Exercise group). We will explore possible moderators of the treatment effect using interaction terms including sex, race, participant expectations, age, and initial severity of anxiety (e.g., DSM-5 diagnosis for an Anxiety Disorder).
Power Analysis
The primary effect of interest is the treatment group difference on anxiety as measured by HADS-A scores. Power and sample size were estimated under the following assumptions: an alpha of .05, a linear model with age, sex, ethnicity, and baseline HADS-A score as covariates, a conservative estimate of the R-squared of .20 for the full model predicting post-treatment HADS-A, a 15% attrition rate, and two planned contrasts: active treatment vs. placebo and exercise vs. escitalopram. The comparison of primary interest will be active treatments vs. placebo. We estimate that a sample size of 150 (127 after attrition) will yield .80 power to detect at least a .45 SD difference between the active treatments and Placebo. In the general linear model, the exercise vs. escitalopram test will be only slightly less powered in being able to detect a .50 SD difference.
Funding source
The study is supported by a grant (HL125522) from the National Heart, Lung, and Blood Institute. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents.
DISCUSSION
Despite the prevalence and prognostic significance of anxiety in CHD populations, there have been few RCTs specifically targeting anxious CHD patients. Moreover, most of the previous studies have significant methodological limitations. A previous systematic review by our group36 revealed that limitations include appropriate control groups, adequate sample size, blinding of assessors, monitoring of exercise adherence and volume, documentation of aerobic training effects, and selection of well-validated instruments to assess anxiety before and after treatment. UNWIND improves upon these methodologic limitations by providing well-validated anxiety assessments both pre- and post-intervention, wherein the assessments will be both self-report and clinician interview; using a prescribed exercise program in which exercise will be supervised and cardiorespiratory fitness changes will be measured by expired gas analyses; and assessors blinded to participants’ treatment group assignment. We also will examine potential mechanisms by which exercise and escitalopram may reduce anxiety and improve autonomic regulation, vascular function, and inflammatory markers.
SSRIs have been used safely for the treatment of clinical depression in cardiac patients, with equivocal efficacy. While SSRIs have been shown to be effective in treating anxiety in non-CHD populations, to our knowledge there have been no RCTs examining the efficacy of SSRIs for treating anxiety in CHD patients. Because some cardiac patients may be reluctant to take additional medications, and psychotropic medications may not be effective for everyone or may produce unwanted side effects, there continues to be a need to identify alternative approaches for treating anxiety in cardiac patients. Evidence suggests that exercise may be one such approach,36 which we will evaluate in the UNWIND study.
Summary
UNWIND will examine the impact of a 12-week intervention of exercise, escitalopram, or placebo on anxiety symptoms and CHD biomarkers among individuals with cardiac disease and elevated anxiety. We hypothesize that: (1) Both exercise training and escitalopram will reduce anxiety symptoms to a greater extent than placebo; (2) Exercise training will improve CHD biomarkers of risk, including autonomic regulation, vascular endothelial function, and inflammation, more than escitalopram or placebo; and (3) Improvements in CHD biomarkers will be mediated by reductions in symptoms of anxiety. We also will explore potential moderators of treatment (e.g., specific anxiety diagnoses, sex, and race) as well as the longer-term benefits of treatment by documenting medical events over a follow-up period of up to 4 years. The results of UNWIND will inform patients, providers, and policy makers in judging whether exercise may be an effective treatment option for cardiac patients with elevated anxiety.
Supplementary Material
Acknowledgments
We want to thank the members of our Data and Safety Monitoring Board, Leo Pozuelo MD, Diane Catellier PhD, and David Sheps, MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2015 Dec 16; doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011 Mar 1;123(8):933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 3.DuPont RL, Rice DP, Miller LS, Shiraki SS, Rowland CR, Harwood HJ. Economic costs of anxiety disorders. Anxiety. 1996;2(4):167–172. doi: 10.1002/(SICI)1522-7154(1996)2:4<167::AID-ANXI2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 4.Judd LL, Kessler RC, Paulus MP, Zeller PV, Wittchen HU, Kunovac JL. Comorbidity as a fundamental feature of generalized anxiety disorders: results from the National Comorbidity Study (NCS) Acta Psychiatr Scand Suppl. 1998;393:6–11. doi: 10.1111/j.1600-0447.1998.tb05960.x. [DOI] [PubMed] [Google Scholar]
- 5.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication.(vol 62, pg 593, 2005) Arch Gen Psychiat. 2005 Jul;62(7):768–768. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 6.Tully PJ, Cosh SM. Generalized anxiety disorder prevalence and comorbidity with depression in coronary heart disease: A meta-analysis. J Health Psychol. 2013 Dec;18(12):1601–1616. doi: 10.1177/1359105312467390. [DOI] [PubMed] [Google Scholar]
- 7.Frasure-Smith N, Lesperance F. Depression and anxiety as predictors of 2-year cardiac events in patients with stable coronary artery disease. Arch Gen Psychiatry. 2008 Jan;65(1):62–71. doi: 10.1001/archgenpsychiatry.2007.4. [DOI] [PubMed] [Google Scholar]
- 8.Kessler RGP. Neuropsychopharmacology Chapter 67 The economic burden of anxiety and stress disorders Neuropsychopharmacology:. Vol The Fifth Generation of Progress. In: Davis Kenneth L, Charney Dennis, Coyle Joseph T, Nemeroff Charles., editors. American College of Neuropsychopharmacology. 2002 2002. [Google Scholar]
- 9.Comer JS, Blanco C, Hasin DS, et al. Health-related quality of life across the anxiety disorders: results from the national epidemiologic survey on alcohol and related conditions (NESARC) J Clin Psychiatry. 2011 Jan;72(1):43–50. doi: 10.4088/JCP.09m05094blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watkins LL, Koch GG, Sherwood A, et al. Association of anxiety and depression with all-cause mortality in individuals with coronary heart disease. Journal of the American Heart Association. 2013 Apr;2(2):e000068. doi: 10.1161/JAHA.112.000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lichtman JH, Froelicher ES, Blumenthal JA, et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation. 2014;129(12):1350–1369. doi: 10.1161/CIR.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 12.Ahern DK, Gorkin L, Anderson JL, et al. Biobehavioral variables and mortality or cardiac arrest in the Cardiac Arrhythmia Pilot Study (CAPS) The American journal of cardiology. 1990;66(1):59–62. doi: 10.1016/0002-9149(90)90736-k. [DOI] [PubMed] [Google Scholar]
- 13.Kornerup H, Zwisler A-DO, Prescott E Danrehab Group CD. No association between anxiety and depression and adverse clinical outcome among patients with cardiovascular disease: findings from the DANREHAB trial. Journal of psychosomatic research. 2011;71(4):207–214. doi: 10.1016/j.jpsychores.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Lane D, Carroll D, Ring C, Beevers DG, Lip GY. Mortality and quality of life 12 months after myocardial infarction: effects of depression and anxiety. Psychosomatic medicine. 2001;63(2):221–230. doi: 10.1097/00006842-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Strik JJMH, Denollet J, Lousberg R, Honig A. Comparing symptoms of depression and anxiety as predictors of cardiac events and increased health care consumption after myocardial infarction. Journal of the American College of Cardiology. 2003;42(10):1801–1807. doi: 10.1016/j.jacc.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Rothenbacher D, Hahmann H, Wusten B, Koenig W, Brenner H. Symptoms of anxiety and depression in patients with stable coronary heart disease: prognostic value and consideration of pathogenetic links. Eur J Cardiovasc Prev Rehabil. 2007;14(4):547–554. doi: 10.1097/HJR.0b013e3280142a02. [DOI] [PubMed] [Google Scholar]
- 17.Shibeshi WA, Young-Xu Y, Blatt CM. Anxiety worsens prognosis in patients with coronary artery disease. Journal of the American College of Cardiology. 2007;49(20):2021–2027. doi: 10.1016/j.jacc.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Wrenn KC, Mostofsky E, Tofler GH, Muller JE, Mittleman MA. Anxiety, anger, and mortality risk among survivors of myocardial infarction. Am J Med. 2013 Dec;126(12):1107–1113. doi: 10.1016/j.amjmed.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012 Jun;16(2):77–84. doi: 10.3109/13651501.2012.667114. [DOI] [PubMed] [Google Scholar]
- 20.Strawn JR, Prakash A, Zhang Q, et al. A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2015 Apr;54(4):283–293. doi: 10.1016/j.jaac.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz TL, Nihalani N, Simionescu M, Hopkins G. History repeats itself: pharmacodynamic trends in the treatment of anxiety disorders. Curr Pharm Des. 2005;11(2):255–263. doi: 10.2174/1381612053382214. [DOI] [PubMed] [Google Scholar]
- 22.Glassman AH, O'Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA : the journal of the American Medical Association. 2002 Aug 14;288(6):701–709. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- 23.O'Connor CM, Jiang W, Kuchibhatla M, et al. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010 Aug 24;56(9):692–699. doi: 10.1016/j.jacc.2010.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor CB, Youngblood ME, Catellier D, et al. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005 Jul;62(7):792–798. doi: 10.1001/archpsyc.62.7.792. [DOI] [PubMed] [Google Scholar]
- 25.Lesperance F, Frasure-Smith N, Koszycki D, et al. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease - The Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. Jama-J Am Med Assoc. 2007 Jan 24;297(4):367–379. doi: 10.1001/jama.297.4.367. [DOI] [PubMed] [Google Scholar]
- 26.Angermann CE, Gelbrich G, Stork S, et al. Rationale and design of a randomised, controlled, multicenter trial investigating the effects of selective serotonin re-uptake inhibition on morbidity, mortality and mood in depressed heart failure patients (MOOD-HF) Eur J Heart Fail. 2007 Dec;9(12):1212–1222. doi: 10.1016/j.ejheart.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Pellicori P, Clark AL. Clinical trials update from the European Society of Cardiology-Heart Failure meeting 2015: AUGMENT-HF, TITRATION, STOP-HF, HARMONIZE, LION HEART, MOOD-HF, and renin-angiotensin inhibitors in patients with heart and renal failure. Eur J Heart Fail. 2015 Sep;17(9):979–983. doi: 10.1002/ejhf.340. [DOI] [PubMed] [Google Scholar]
- 28.Hansen BH, Hanash JA, Rasmussen A, et al. Effects of escitalopram in prevention of depression in patients with acute coronary syndrome (DECARD) J Psychosom Res. 2012 Jan;72(1):11–16. doi: 10.1016/j.jpsychores.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Hanash JA, Hansen BH, Hansen JF, Nielsen OW, Rasmussen A, Birket-Smith M. Cardiovascular safety of one-year escitalopram therapy in clinically nondepressed patients with acute coronary syndrome: results from the DEpression in patients with Coronary ARtery Disease (DECARD) trial. J Cardiovasc Pharmacol. 2012 Oct;60(4):397–405. doi: 10.1097/FJC.0b013e3182677041. [DOI] [PubMed] [Google Scholar]
- 30.Jiang W, Velazquez EJ, Kuchibhatla M, et al. Effect of escitalopram on mental stress-induced myocardial ischemia: results of the REMIT trial. JAMA : the journal of the American Medical Association. 2013 May 22;309(20):2139–2149. doi: 10.1001/jama.2013.5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrade C, Kumar CB, Surya S. Cardiovascular mechanisms of SSRI drugs and their benefits and risks in ischemic heart disease and heart failure. Int Clin Psychopharmacol. 2013 May;28(3):145–155. doi: 10.1097/YIC.0b013e32835d735d. [DOI] [PubMed] [Google Scholar]
- 32.Goodwin RD. Association between physical activity and mental disorders among adults in the United States. Prev Med. 2003 Jun;36(6):698–703. doi: 10.1016/s0091-7435(03)00042-2. [DOI] [PubMed] [Google Scholar]
- 33.De Moor MH, Beem AL, Stubbe JH, Boomsma DI, De Geus EJ. Regular exercise, anxiety, depression and personality: a population-based study. Prev Med. 2006 Apr;42(4):273–279. doi: 10.1016/j.ypmed.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Wipfli BM, Rethorst CD, Landers DM. The anxiolytic effects of exercise: A meta-analysis of randomized trials and dose-response analysis. J Sport Exercise Psy. 2008 Aug;30(4):392–410. doi: 10.1123/jsep.30.4.392. [DOI] [PubMed] [Google Scholar]
- 35.Jayakody K, Gunadasa S, Hosker C. Exercise for anxiety disorders: systematic review. British journal of sports medicine. 2013 Jan 7; doi: 10.1136/bjsports-2012-091287. [DOI] [PubMed] [Google Scholar]
- 36.Stonerock GL, Hoffman BM, Smith PJ, Blumenthal JA. Exercise as Treatment for Anxiety: Systematic Review and Analysis. Ann Behav Med. 2015 Aug;49(4):542–556. doi: 10.1007/s12160-014-9685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lavie CJ, Milani RV. Prevalence of anxiety in coronary patients with improvement following cardiac rehabilitation and exercise training. The American journal of cardiology. 2004 Feb 1;93(3):336–339. doi: 10.1016/j.amjcard.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 38.Oldridge N, Guyatt G, Jones N, et al. Effects on quality of life with comprehensive rehabilitation after acute myocardial infarction. The American journal of cardiology. 1991;67(13):1084–1089. doi: 10.1016/0002-9149(91)90870-q. [DOI] [PubMed] [Google Scholar]
- 39.Oldridge N, Streiner D, Hoffmann R, Guyatt G. Profile of mood states and cardiac rehabilitation after acute myocardial infarction. Medicine and science in sports and exercise. 1995;27(6):900–905. [PubMed] [Google Scholar]
- 40.Dekker JM, Crow RS, Folsom AR, et al. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Atherosclerosis Risk In Communities. Circulation. 2000 Sep 12;102(11):1239–1244. doi: 10.1161/01.cir.102.11.1239. [DOI] [PubMed] [Google Scholar]
- 41.Tsuji H, Larson MG, Venditti FJ, Jr, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996 Dec 1;94(11):2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 42.Rich MW, Saini JS, Kleiger RE, Carney RM, Tevelde A, Freedland KE. Correlation of Heart-Rate Variability with Clinical and Angiographic Variables and Late Mortality after Coronary Angiography. American Journal of Cardiology. 1988 Oct 1;62(10):714–717. doi: 10.1016/0002-9149(88)91208-8. [DOI] [PubMed] [Google Scholar]
- 43.Kleiger RE, Miller JP, Bigger JT, Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987 Feb 1;59(4):256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz PJ, Billman GE, Stone HL. Autonomic mechanisms in ventricular fibrillation induced by myocardial ischemia during exercise in dogs with healed myocardial infarction. An experimental preparation for sudden cardiac death. Circulation. 1984;69(4):790–800. doi: 10.1161/01.cir.69.4.790. [DOI] [PubMed] [Google Scholar]
- 45.Licht CMM, de Geus EJC, van Dyck R, Penninx BWJH. Association between Anxiety Disorders and Heart Rate Variability in The Netherlands Study of Depression and Anxiety (NESDA) Psychosomatic Medicine. 2009 Jun;71(5):508–518. doi: 10.1097/PSY.0b013e3181a292a6. [DOI] [PubMed] [Google Scholar]
- 46.Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biol Psychiatry. 1996 Feb 15;39(4):255–266. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- 47.Dimsdale JE. What Does Heart Disease Have to Do With Anxiety? J Am Coll Cardiol. 2010 Jun 29;56(1):47–48. doi: 10.1016/j.jacc.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 48.Lederbogen F, Gernoth C, Weber B, et al. Antidepressive treatment with amitriptyline and paroxetine: comparable effects on heart rate variability. J Clin Psychopharmacol. 2001 Apr;21(2):238–239. doi: 10.1097/00004714-200104000-00018. [DOI] [PubMed] [Google Scholar]
- 49.Schuit AJ, van Amelsvoort LG, Verheij TC, et al. Exercise training and heart rate variability in older people. Med Sci Sports Exerc. 1999 Jun;31(6):816–821. doi: 10.1097/00005768-199906000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Levy WC, Cerqueira MD, Harp GD, et al. Effect of endurance exercise training on heart rate variability at rest in healthy young and older men. Am J Cardiol. 1998 Nov 15;82(10):1236–1241. doi: 10.1016/s0002-9149(98)00611-0. [DOI] [PubMed] [Google Scholar]
- 51.Brevetti G, Silvestro A, Schiano V, Chiariello M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation. 2003;108(17):2093–2098. doi: 10.1161/01.CIR.0000095273.92468.D9. [DOI] [PubMed] [Google Scholar]
- 52.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23(2):168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 53.Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. Journal of the American College of Cardiology. 1994;24(6):1468–1474. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 54.Munk PS, Isaksen K, Bronnick K, Kurz MW, Butt N, Larsen AI. Symptoms of anxiety and depression after percutaneous coronary intervention are associated with decreased heart rate variability, impaired endothelial function and increased inflammation. International journal of cardiology. 2012 Jun 28;158(1):173–176. doi: 10.1016/j.ijcard.2012.04.085. [DOI] [PubMed] [Google Scholar]
- 55.Luk TH, Dai YL, Siu CW, et al. Effect of exercise training on vascular endothelial function in patients with stable coronary artery disease: a randomized controlled trial. Eur J Prev Cardiol. 2012 Aug;19(4):830–839. doi: 10.1177/1741826711415679. [DOI] [PubMed] [Google Scholar]
- 56.Edwards DG, Schofield RS, Lennon SL, Pierce GL, Nichols WW, Braith RW. Effect of exercise training on endothelial function in men with coronary artery disease. American Journal of Cardiology. 2004;93(5):617–620. doi: 10.1016/j.amjcard.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 57.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. Journal of Clinical Investigation. 2003;111(12):1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. The New England journal of medicine. 2002;347(20):1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 59.Zamani P, Schwartz GG, Olsson AG, et al. Inflammatory biomarkers, death, and recurrent nonfatal coronary events after an acute coronary syndrome in the MIRACL study. J Am Heart Assoc. 2013 Feb;2(1):e003103. doi: 10.1161/JAHA.112.003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sarwar N, Butterworth AS, Freitag DF, et al. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012 Mar 31;379(9822):1205–1213. doi: 10.1016/S0140-6736(11)61931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pitsavos C, Panagiotakos DB, Papageorgiou C, Tsetsekou E, Soldatos C, Stefanadis C. Anxiety in relation to inflammation and coagulation markers, among healthy adults: the ATTICA study. Atherosclerosis. 2006;185(2):320–326. doi: 10.1016/j.atherosclerosis.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 62.Frasure-Smith N, Lesperance F, Irwin MR, Talajic M, Pollock BG. The relationships among heart rate variability, inflammatory markers and depression in coronary heart disease patients. Brain Behavior and Immunity. 2009 Nov;23(8):1140–1147. doi: 10.1016/j.bbi.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 63.Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. The American journal of psychiatry. 2014 Apr;171(4):395–397. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- 64.Means-Christensen AJSC, Roy-Byrne PP, Craske MG, Stein MB. Using five questions to screen for five common mental disorders in primary care: diagnostic accuracy of the Anxiety and Depression Detector. General hospital psychiatry. 2006;28:108–118. doi: 10.1016/j.genhosppsych.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 65.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001 Sep;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Campbell-Sills L, Norman SB, Craske MG, et al. Validation of a brief measure of anxiety-related severity and impairment: the Overall Anxiety Severity and Impairment Scale (OASIS) J Affect Disord. 2009 Jan;112(1–3):92–101. doi: 10.1016/j.jad.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Friedman LM, Furberg C, DeMets DL. Fundamentals of clinical trials. 3rd. St. Louis: Mosby-Year Book; 1996. [Google Scholar]
- 68.First MBS, R L, Gibbon M, Williams JBW. Structured Clinical Interivew for DSM-IVTR Axis I Disorders (SCID-I), Research Version (SCID-I/P) New York: Biometrics Research; 2002. [Google Scholar]
- 69.Shear MK, Vander Bilt J, Rucci P, et al. Reliability and validity of a Structured Interview Guide for the Hamilton Anxiety Rating Scale (SIGH-A) Depress Anxiety. 2001;13(4):166–178. [PubMed] [Google Scholar]
- 70.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002 Feb;52(2):69– 77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 71.Taylor S, Zvolensky MJ, Cox BJ, et al. Robust dimensions of anxiety sensitivity: development and initial validation of the Anxiety Sensitivity Index-3. Psychol Assess. 2007 Jun;19(2):176–188. doi: 10.1037/1040-3590.19.2.176. [DOI] [PubMed] [Google Scholar]
- 72.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006 May 22;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 73.Spielberger CE, Gorsuch RL. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 74.Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 75.Gureje O, Obikoya B. The GHQ-12 as a screening tool in a primary care setting. Soc Psychiatry Psychiatr Epidemiol. 1990 Sep;25(5):276–280. doi: 10.1007/BF00788650. [DOI] [PubMed] [Google Scholar]
- 76.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 77.Smith BW, Dalen J, Wiggins K, Tooley E, Christopher P, Bernard J. The brief resilience scale: assessing the ability to bounce back. Int J Behav Med. 2008;15(3):194–200. doi: 10.1080/10705500802222972. [DOI] [PubMed] [Google Scholar]
- 78.Herzberg PY, Glaesmer H, Hoyer J. Separating optimism and pessimism: a robust psychometric analysis of the revised Life Orientation Test (LOT-R) Psychol Assess. 2006 Dec;18(4):433–438. doi: 10.1037/1040-3590.18.4.433. [DOI] [PubMed] [Google Scholar]
- 79.Blumenthal JA, Burg MM, Barefoot J, Williams RB, Haney T, Zimet G. Social support, type A behavior, and coronary artery disease. Psychosomatic medicine. 1987;49(4):331–340. doi: 10.1097/00006842-198707000-00002. [DOI] [PubMed] [Google Scholar]
- 80.Fiebiger W, Mitterbauer C, Oberbauer R. Health-related quality of life outcomes after kidney transplantation. Health Qual Life Outcomes. 2004;2:2. doi: 10.1186/1477-7525-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Godin G, Jobin J, Bouillon J. Assessment of leisure time exercise behavior by self-report: a concurrent validity study. Can J Public Health. 1986 Sep-Oct;77(5):359–362. [PubMed] [Google Scholar]
- 82.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989 May;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 83.Krousel-Wood M, Islam T, Webber LS, Re RN, Morisky DE, Muntner P. New medication adherence scale versus pharmacy fill rates in seniors with hypertension. The American journal of managed care. 2009 Jan;15(1):59–66. [PMC free article] [PubMed] [Google Scholar]
- 84.Block G, Gillespie C, Rosenbaum EH, Jenson C. A rapid food screener to assess fat and fruit and vegetable intake. Am J Prev Med. 2000 May;18(4):284–288. doi: 10.1016/s0749-3797(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 85.Babyak M, Blumenthal JA, Herman S, et al. Exercise treatment for major depression: Maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000 Sep-Oct;62(5):633–638. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 86.Hinderliter AL, Sherwood A, Craighead LW, et al. The long-term effects of lifestyle change on blood pressure: One-year follow-up of the ENCORE study. Am J Hypertens. 2014 May;27(5):734–741. doi: 10.1093/ajh/hpt183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoffman BM, Babyak MA, Craighead WE, et al. Exercise and pharmacotherapy in patients with major depression: one-year follow-up of the SMILE study. Psychosom Med. 2011 Feb-Mar;73(2):127–133. doi: 10.1097/PSY.0b013e31820433a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Muthen LK, Muthen B. Mplus User's Guide. 3rd. Los Angeles, CA: Muth,n and Muth,n; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.