Abstract
The Acari are of significant economic importance in crop production and human and animal health. Acaricides are essential for the control of these pests, but at the same time, the number of available pesticides is limited, especially for applications in animal production. The Acari consist of two major groups, the mites that demonstrate a wide variety of life strategies, i.e., herbivory, predation and ectoparasitism, and ticks which have evolved obligatory hematophagy. The major sites of chemoreception in the acarines are the chelicerae, palps and tarsi on the forelegs. A unifying name, the “foretarsal sensory organ” (FSO), is proposed for the first time in this review for the sensory site on the forelegs of all acarines. The FSO has multiple sensory functions including olfaction, gustation, and heat detection. Preliminary transcriptomic data in ticks suggest that chemoreception in the FSO is achieved by a different mechanism from insects. There are a variety of laboratory and field bioassay methods that have been developed for the identification and characterization of attractants but minimal techniques for electrophysiology studies. Over the past three to four decades, significant progress has been made in the chemistry and analysis of function for acarine attractants in mites and ticks. In mites, attractants include aggregation, immature female, female sex and alarm pheromones; in ticks, the attraction–aggregation–attachment, assembly and sex pheromones; in mites and ticks host kairomones and plant allomones; and in mites, fungal allomones. There are still large gaps in our knowledge of chemical communication in the acarines compared to insects, especially relative to acarine pheromones, and more so for mites than ticks. However, the use of lure-and-kill and lure-enhanced biocontrol strategies has been investigated for tick and mite control, respectively, with significant environmental advantages which warrant further study.
Keywords: Mites, Ticks, Chemical communication, Bioassay, Attractants, Control
1. Introduction
Mites and ticks are two different but evolutionary related groups of arthropods found in the subphylum Chelicerata, in the class Arachnida and in the subclass Acari. The feeding habits are quite different between mites and ticks. Mites exhibit diverse feeding behaviors as herbivores, predators, and blood and keratin feeding parasites. In contrast, ticks are obligatory blood feeders on humans and animals. Mites and ticks are also of significant economic and health importance. For example, herbivorous mites can cause plant damage resulting in decreased crop production [1]. In contrast, predatory mites can be used as biocontrol agents in crop production to control herbivorous mites while parasitic mites and ticks cause painful skin irritations producing stress and reducing meat, milk, wool, and leather production. They also vector pathogens of importance to plants, insects, humans, and animals [2]. Ticks for example vector the microbial agents of more animal diseases than any other arthropod [3]. The acarines are of special significance because of their significant economic, nuisance, and health impacts; their ancient origin, which goes back to the Devonian period; the unique biology and diversity of mites versus ticks; and the importance of their control as pests [2,3]. Also the Acari in general are an understudied taxon within the Arthropoda especially compared to insects.
The acarines have a unique chemosensory system. Mites and ticks have four pairs of walking legs. The first pair of these legs is used in chemoreception in many ways like the insect antennae (acarines lack antennae). The foretarsal sensory organ, most commonly called the Haller's organ in ticks, is located on the foretarsi of the first pair of legs in mites and ticks. At the gross anatomical level, and based on origin, this organ has little similarity to the insect antennae and is unique to the acarines. Foretarsal organs are involved in the detection of food, pheromones, aggregation chemicals, host kairomones, plant allomones, fungal allomones, and arthropod repellents [4–9]. Despite the importance of attractants in development, reproduction, and the ecology of mites and ticks, little is understood about the types of chemicals recognized, mechanism for chemoreception, and the relative importance of the Haller's organ and its similarity of function versus other chemosensory organs like the chelicerae and palps of acarines.
Unfortunately, the mostly medical versus agricultural significance of ticks versus mites, respectively; the lack of synergistic research efforts between these two taxonomic groups; gaps in data collection; conflicting results; and overlapping-repetitive studies have limited research progress in chemical communication in the acarines as a whole and the use of this information in control. This has been further exacerbated by the challenges associated with acarine morphology and biology (segmental reductions and difficulty of rearing), and the much smaller size of mites versus that of ticks. A review of chemical communication for the acarines as a whole should be valuable to future research, and the use of this knowledge to develop control solutions. Comprehensive reviews already exist on tick pheromones and chemical communication [7,9] and on tick repellents [10,11]. There also are reviews on mite pheromones [12], on control of insects and mites in certain crops and produce [13–15], and control of individual mite species [16–19]. This is the first review of their chemosensory systems, bioassay methods, chemistry, chemical ecology, and control.
2. Chemosensory structure and function
Any use of chemical attractants for control will depend on chemical detection. An understanding of chemosensory structures and mechanisms of chemosensation is a critical aspect for pesticide development.
2.1. Mite sensory structures
Chemosensory stimuli are detected in mites by receptors located on the palps and the foretarsi of the first pair of legs. Chemoreceptors on the palps are localized to the palpal organ (Fig. 1). The mite palpal organ is located on the tip of each palp, consisting of 10–30 sensilla innervated by 6–7 bipolar sensory neurons. The sensory sensilla represent five morphological types of basiconic sensilla: single walled multipore sensilla with the pore configuration in longitudinal grooves (olfactory receptors), double walled pore sensilla (contact chemomechanoreceptors), two types of double walled pore sensilla with distinct internal radial spoke structures (chemo-thermoreceptors), and fine apical processes and cones without pores (cold and humidity receptors) [6]. Similar morphological types of sensilla are found in tick chemosensory structures also located on the palps and the foretarsi of the first pair of legs [4,6]. The structure and organization of the palpal organ in mites remains somewhat uniform across mite families, with the exception of endoparasitic mites in the family Rhinonyssidae. Most endoparasitic mites do not actively host seek but are transferred from one host to another through close, direct contact. The loss of host seeking behavior is reflected in the structure and organization of the palpal organ with a reduction in both sensilla number and diversity of morphological sensilla types. This reduction in chemosensory structures is a common adaptation observed in species that transition from ectoparasites to endoparasites [20].
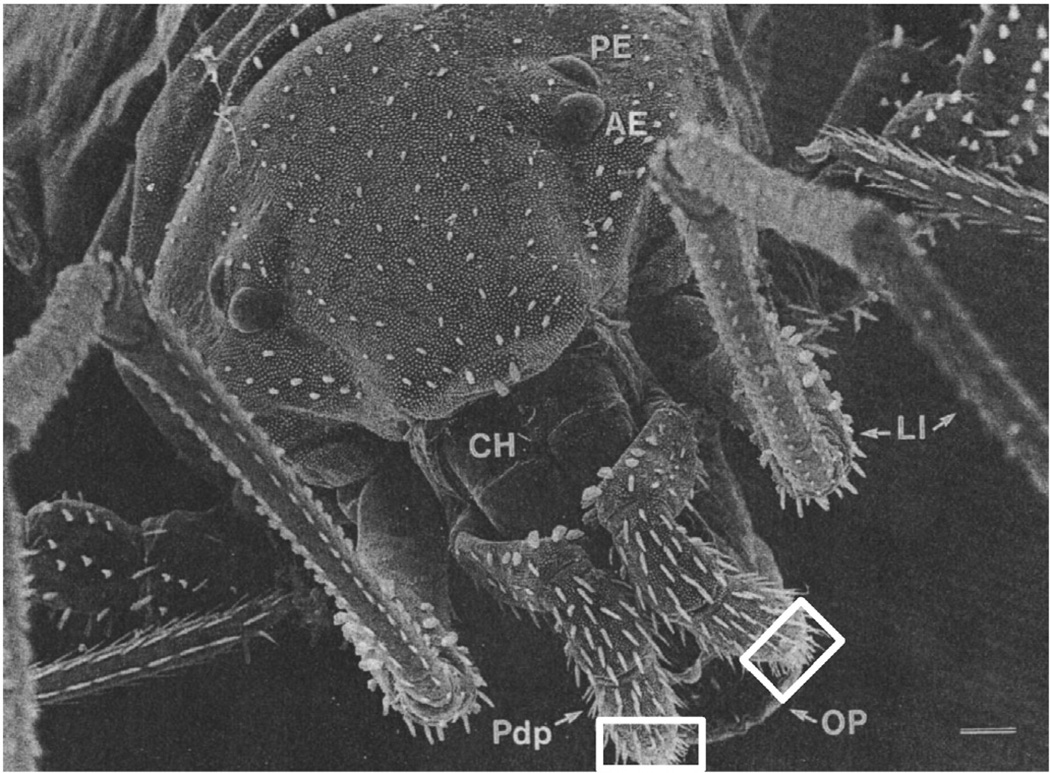
Fig. 1.
The palpal organ (boxed), located on the tip of the palp pedipalp of the Neocarus texanus mite (AE = anterior eye, CH = chelicera, OP = ovipositor, LI = leg I, PE = posterior eye, PdP = pedipalp), used with permission from Wiley-Liss © 1999 Alberti and Coons.
The mite tarsal complex (Fig. 2) is a localization of chemoreceptors on the foretarsi of the first pair of legs, and is homologous to the Haller's organ found in ticks. The tarsal complex consists of 9–28 sensilla innervated by 6–15 bipolar sensory neurons [6]. These sensilla represent similar morphological types to those found in the palpal organ [21]. Topography of the tarsal complex, the number of sensilla, and morphological types are dependent on the phylogenetic history of the mite and degree of specialization [22]. Endoparasitic mites also exhibit reductions of the tarsal complex similar to those observed in the palpal organ [20].
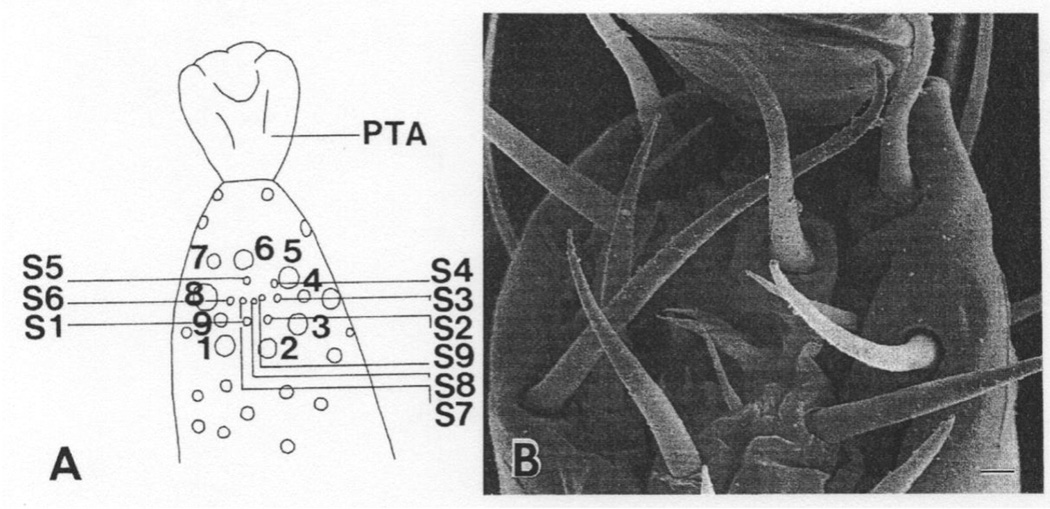
Fig. 2.
(A, B) Sensillar organization in the tarsal complex, located on the foretarsus of the first pair of legs of the Varroa jacobsoni mite (1–9 = edge bristles, PTA = ambulacrum, S1–S9 = pit sensilla), used with permission from Wiley-Liss © 1999 Alberti and Coons.
Information describing the mite palpal organ and tarsal complex is limited and primarily consists of morphological descriptions of sensilla structure and organization. This is primarily due to the difficulties associated with the minute size of mites. The apparent similarities in location and gross structure between mite and tick chemosensory systems suggest that ticks could be an ideal model for functional and mechanistic studies of chemoreception which are just not possible with mites because of their small size. Drawing comparisons between mites and ticks could help relate structure to function; so far, there has been minimal comparative work, especially at the molecular level and in understanding detailed functions.
2.2. Tick sensory structures
Chemosensation in ticks is mainly ascribed to the Haller's organ (Fig. 3). The Haller's organ is a localization of chemoreceptors on the foretarsi of the first pair of legs [23]. Similar to mites, ticks also have chemoreceptors present on their palps (Fig. 4), which are not well characterized. Research on chemosensation in ticks has primarily focused on determining the structure and function of the Haller's organ. The Haller's organ consists of two main parts, the posterior capsule and the anterior pit. The anterior pit contains 6–7 sensilla innervated by 2–9 bipolar sensory neurons. The posterior capsule contains 4–13 sensilla, each innervated by 4–5 bipolar sensory neurons. In addition to the sensilla, there are other pleomorph structures present in both the anterior pit and posterior capsule [4]. In ixodid ticks, 13 different structural types of sensilla have been identified with 11 of these 13 also found in argasid ticks [24]. These sensilla represent four morphological types of basiconic sensilla, similar to those described in mites: single walled multipore sensilla with evenly distributed plugged pores (olfactory receptors), double walled pore sensilla with longitudinal grooves (contact chemo-mechanoreceptors), double walled pore sensilla with internal cutical spoke wheel arrangements (chemo-thermoreceptors), and apical processes without pores (hypothesized to function as cold and humidity receptors) [25,26].
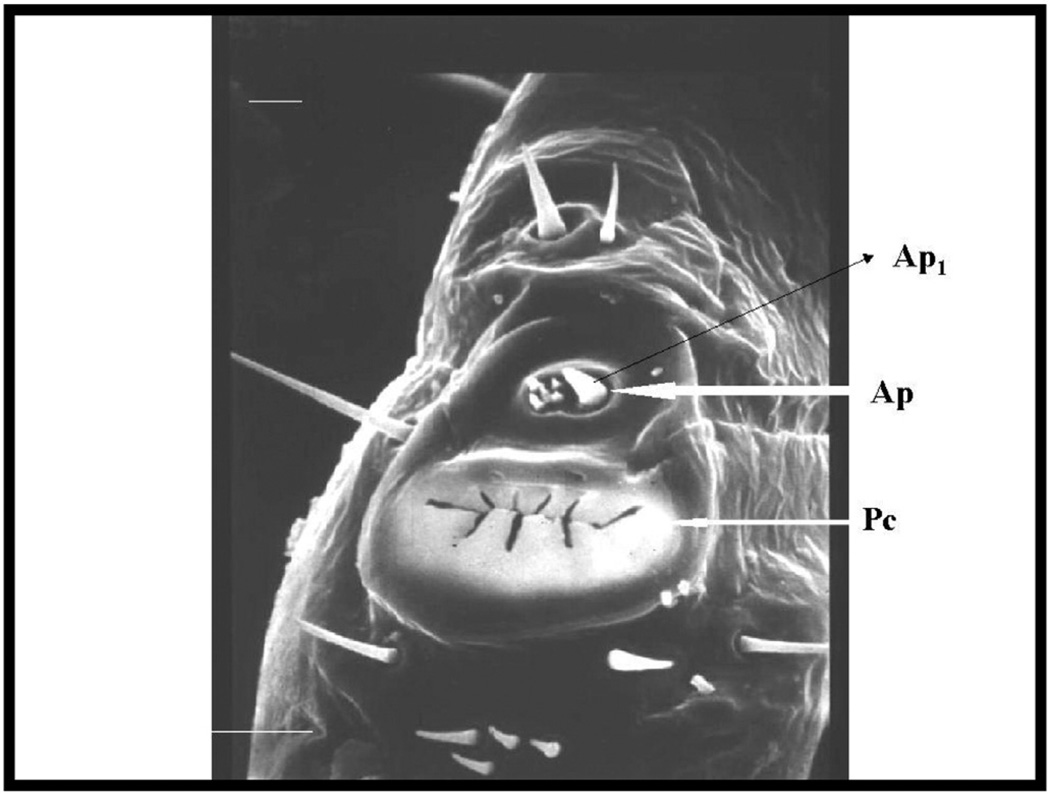
Fig. 3.
The Haller's organ, located on the first tarsus of the first pair of legs of the American dog tick, Dermacentor variabilis (Ap = anterior pit, Ap1 = large setiform sensillum, Pc = posterior capsule) used with permission from Cambridge © 2004 Sonenshine.
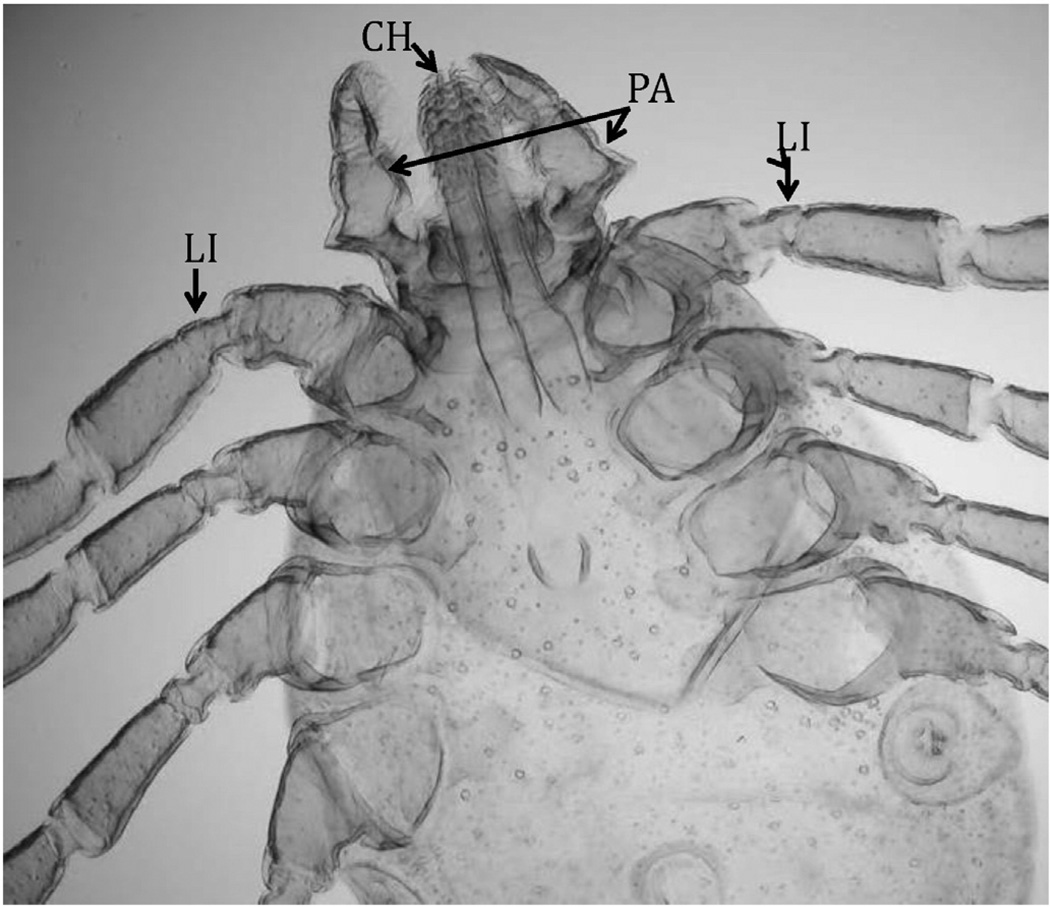
Fig. 4.
Mouthparts of the tick Hyalomma savignyi with possibly chemo-mechanosensory functions (CH = chelicerae, LI = leg I, PA = palps) [Sonenshine, unpublished].
The structure and organization of the Haller's organ is diverse across ixodid and argasid genera, though there is uniformity among species of the same genus. The Haller's organ also consistently contains a combination of chemoreceptors, chemo-mechanoreceptors, and presumed chemo-thermoreceptors. The unique placement of these structures on the tarsi of the first pair of legs may advantageously allow for the coordination of chemo-, mechano-, and thermoreception. Of the chemoreceptors present in the Haller's organ, very few are specialized, which suggests that the detection of varying compositions and concentrations of compounds induce tick host seeking and mating behavior [26,27]. The ratio of signals from more generalized sensilla may provide the tick with a systematic method of host identification and pheromone recognition [28]. Coordinating signals from chemo-, mechano-, and thermoreceptors may increase the accuracy of host detection and location as well as courtship and mating. Since mites have similar numbers and morphological types of sensilla present in the tarsal complex and papal organ, it can be hypothesized that mites also rely on a similar coordination of sensory signals for locating food sources, mating, and other activities.
Since the Haller's organ is only found in ticks, with homologous organs present in mites (most commonly called the tarsal complex), the distinct combination of different sensory receptors and the mechanisms of sensory detection of the acarines may not be present in other organisms, and may be especially different from that of well-studied insects. Comparative morphological data of sensillar organization in acarines and lower arthropods suggests that all types of chemosensory sensilla have evolved from a common multifunctional presensillum [29]. With clear similarities in the morphological types of sensilla between ticks and mites, it is likely that both groups have evolved chemosensory sensilla from the same presensillum. The general reference to the Haller's organ on the front legs of ticks versus that of the tarsal complex for mites unfortunately is misleading, suggesting differences in origin and function, when most likely this is not the case. We are recommending a unifying name for the acarines, which also denotes function for both mites and ticks, i.e., the foretarsal sensory organ (FSO).
2.3. Mechanism of chemosensation
Acarines use chemosensory sensilla present on their palps and tarsi to identify olfactory and gustatory chemicals in the environment. Olfactory sensilla detect volatilized chemicals while gustatory sensilla detect chemicals through direct contact [20,27]. The presence of both receptor types in acarine chemosensory structures makes the differentiation between spatial and direct contact chemical responses very difficult. It is also hypothesized that the molecular mechanism involved in the binding of chemical molecules and neuron depolarization in olfactory and gustatory receptor cells share a similar signal cascade mechanism, though the exact proteins involved are still unknown [Carr, Sonenshine and Roe, unpublished].
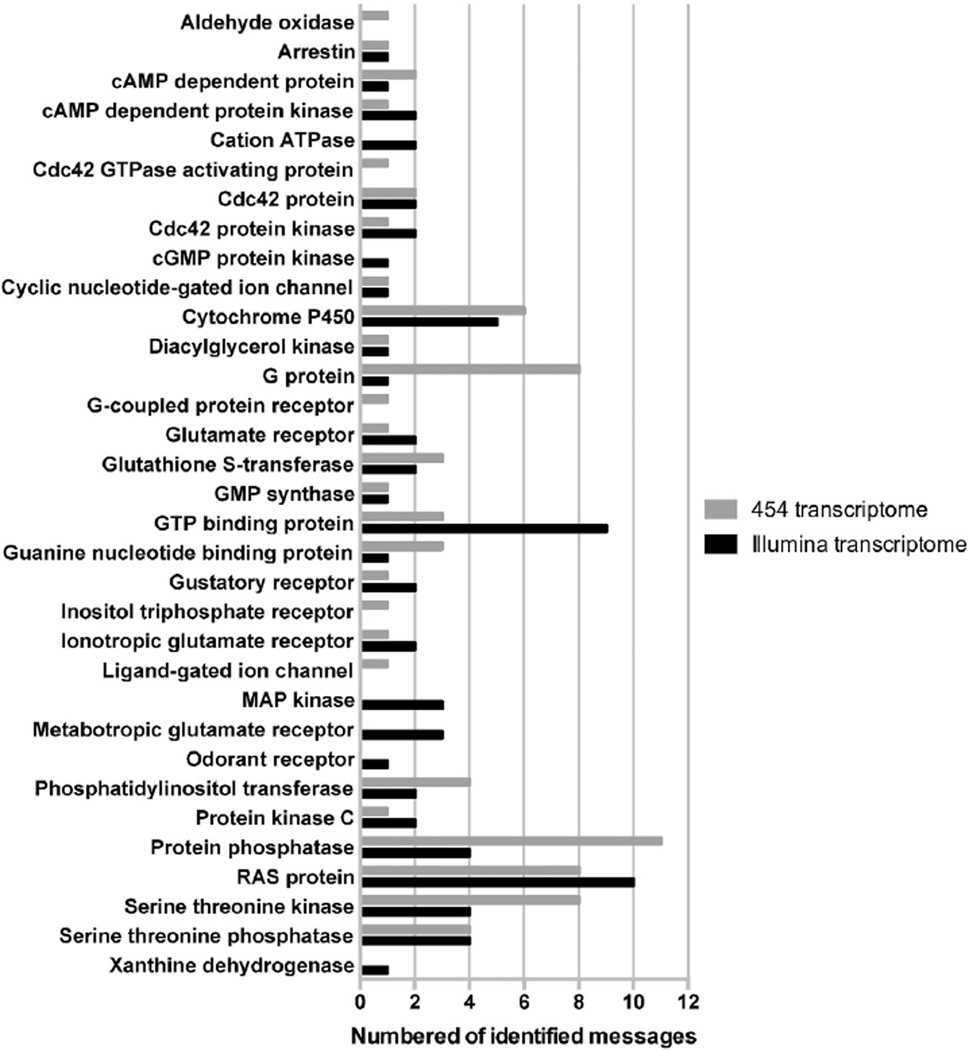
Current research investigating these olfactory and gustatory mechanisms is primarily focused on ticks. The published mite genome is the only data currently available that could potentially provide information about chemosensation mechanisms in mites, but presently no chemosensory proteins have been identified [30]. For ticks, multiple transcriptome data sets have been generated, specifically for the Haller's organ in our laboratory. The analysis of this transcriptome data is still in process but already has resulted in the identification of probable olfactory and gustatory messages (Fig. 5). Transcriptomes of the Haller's organ from males of the American dog tick, Dermacentor variabilis, have identified specific G protein-coupled receptors (GPCRs) found in the front legs, but absent from the hind legs, which likely are acting as olfactory and gustatory receptors. We have also identified from this same work multiple messages involved in the GPCR signal cascade mechanism and enzymes involved in the degradation of chemical molecules in the sensillar lymph described for other organisms [31,32], including cytochrome P450, glutathione S-transferase, protein kinase C, and serine threonine kinase [Carr, Sonenshine and Roe, unpublished]. It is thought that the degradation enzymes may help prevent overstimulation of chemoreceptors, which could potentially lead to receptor cell death. Degradation enzymes break down odor molecules into alternative structures that no longer bind to chemoreceptors and/or can be easily removed from the sensillar lymph [32]. Despite finding multiple messages involved in sensory signal initiation, regulation, and cytoprotection, none of the specialized odorant binding proteins found in insects have been identified in ticks [Carr, Sonenshine and Roe, unpublished]. One possible explanation is that ticks are using lipocalins to transport chemicals from the sensillar lymph to GPCRs instead of specialized odorant binding proteins. Lipocalins may give ticks the ability to respond to a variety of chemicals in a single sensillum at one time. Additional research is needed to confirm the GPCR signal cascade mechanism as the chemosensory mechanism in the Haller's organ of ticks. Since palps also contain known chemoreceptors, similar research will be needed to understand their function at the molecular level. At the same time, comparative work is needed with the mite genome to determine if the mechanism of chemoreception is a common feature for the acarines. Understanding chemosensory systems at the molecular level can be useful in the reverse engineering or screening of novel chemical attractants.
Fig. 5.
The number of each type of probable olfactory and gustatory messages identified in both the 454 and Illumina Dermacentor variabilis Haller's organ transcriptomes that are found in the Ixodes scapularis genome and have annotations and pfam domains [Carr and Roe, unpublished].
3. Bioassay methods for acarine attractants
3.1. Laboratory bioassays
Laboratory bioassays are the first experiments needed for attractant identification and to assess their importance in acarine biology or to determine their utility for pest control. Bioassays also are an easy, fast, and inexpensive approach for screening chemical libraries for attractants and evaluating different formulations. Bioassays for testing acarine attractants are basically repellency bioassays (recently reviewed for ticks by Bissinger and Roe [10,11]) but in reverse. When provided with the opportunity to choose between a test compound and a control substrate, movement towards the test compound is indicative of an attractive behavioral response [33]. On the other hand, repellency assays describe repellent behavioral responses as movements away from test compounds.
There are two primary types of laboratory bioassays used for testing attractants, the Petri dish design and olfactometer methods. The Petri dish assay uses treated filter paper to expose mites and ticks to test chemicals versus an appropriate control in an enclosed petri dish [34]. Two pieces of filter paper, one treated with a test chemical and the second a control, are placed along the bottom of a Petri dish. When test subjects are introduced into the Petri dish, pausing or arrestment of subjects on the treated surface would be indicative of an attractive behavioral response. Subjects arresting on the control surface would indicate that there was no attraction to the test chemical [35]. Comparing the number of mites or ticks on the treated surface versus the control can easily determine if a test chemical is an attractant and can be used as a measure of the effectiveness of the attractant. Additionally, conducting assays at different times after the application of the attractant can provide information on treatment persistence. The Petri dish design requires minimal supplies and space for execution, and is an effective method for identifying chemical attractants. The main disadvantages of this design are due to the fact that test subjects come in direct contact with chemically treated surfaces. Because ticks and mites crawl across treated filter paper, this design is incapable of differentiating between attraction to olfactory stimuli from contact or gustatory stimuli. Additionally, this design cannot differentiate between chemicals that are toxicants and those that are simply not attractive. If test chemicals are toxic, mites and ticks may perish after contact and remain on the treated surface for the duration of the experiment. Since attractive behavioral responses are determined by observing the location of test subjects, a test compound may falsely present as an attractant. Due to these limitations, the Petri dish design is best suited for rapid screening of test chemicals.
Chemicals identified as attractants using the Petri dish design should be further evaluated using olfactometers. The main advantage of an olfactometer is that mites and ticks do not come in direct contact with chemically treated surfaces and gustatory responses are excluded by this method. Olfactometers use airflow to expose subjects to test chemicals carried typically as a vapor in the air, making them capable of identifying only compounds detected by olfaction [36].
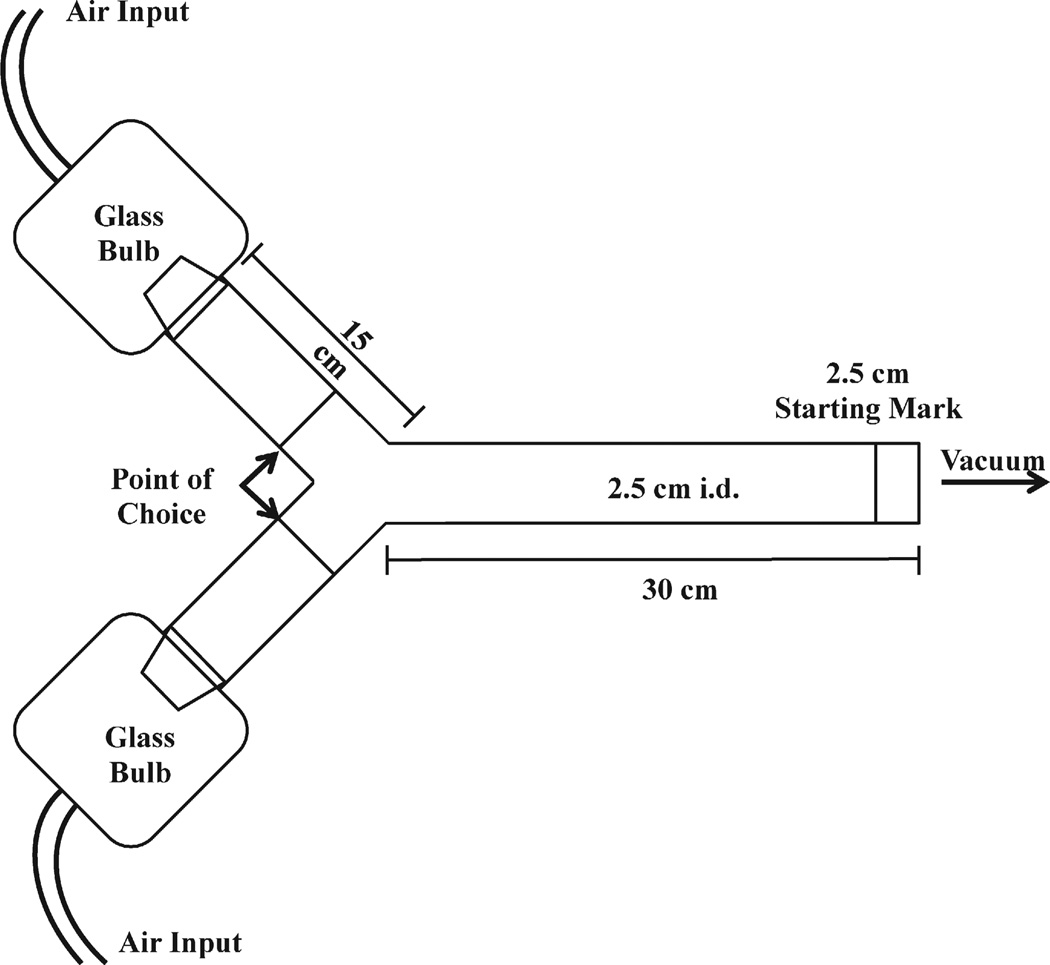
There are multiple designs of olfactometer instruments available for screening acarine attractants, though the most widely used is the Y-tube or two-way olfactometer. As illustrated in Fig. 6, the Y-tube is a glass tube that bifurcates into two distinct arms. Removable glass bulbs that fit onto the ends of each arm provide a location for the placement of a test chemical, on one arm, and the appropriate control on the other. Independent air input into each glass bulb carries the test and control odors into each arm, respectively, and then into the common adjoining tube where the air streams become mixed. A vacuum attached to the end of the common adjoining tube ensures unidirectional airflow. When test subjects are introduced into the common adjoining tube distal to the bifurcation, based on attraction to the test compound, they move to its source, entering into the arm associated with the test compound. Test subjects that remain in the common tube, or crawl into the arm associated with the control would indicate that there was no attraction to odors of the test chemical [37]. Documenting the location of mites and ticks in the Y-tube at the end of a timed interval determines if a test chemical is an olfactory attractant.
Fig. 6.
Image of olfactometer with two ports for use in laboratory bioassays of acarine attractants, used with permission from Wiley & Sons Inc. © 2012 Carr et al.
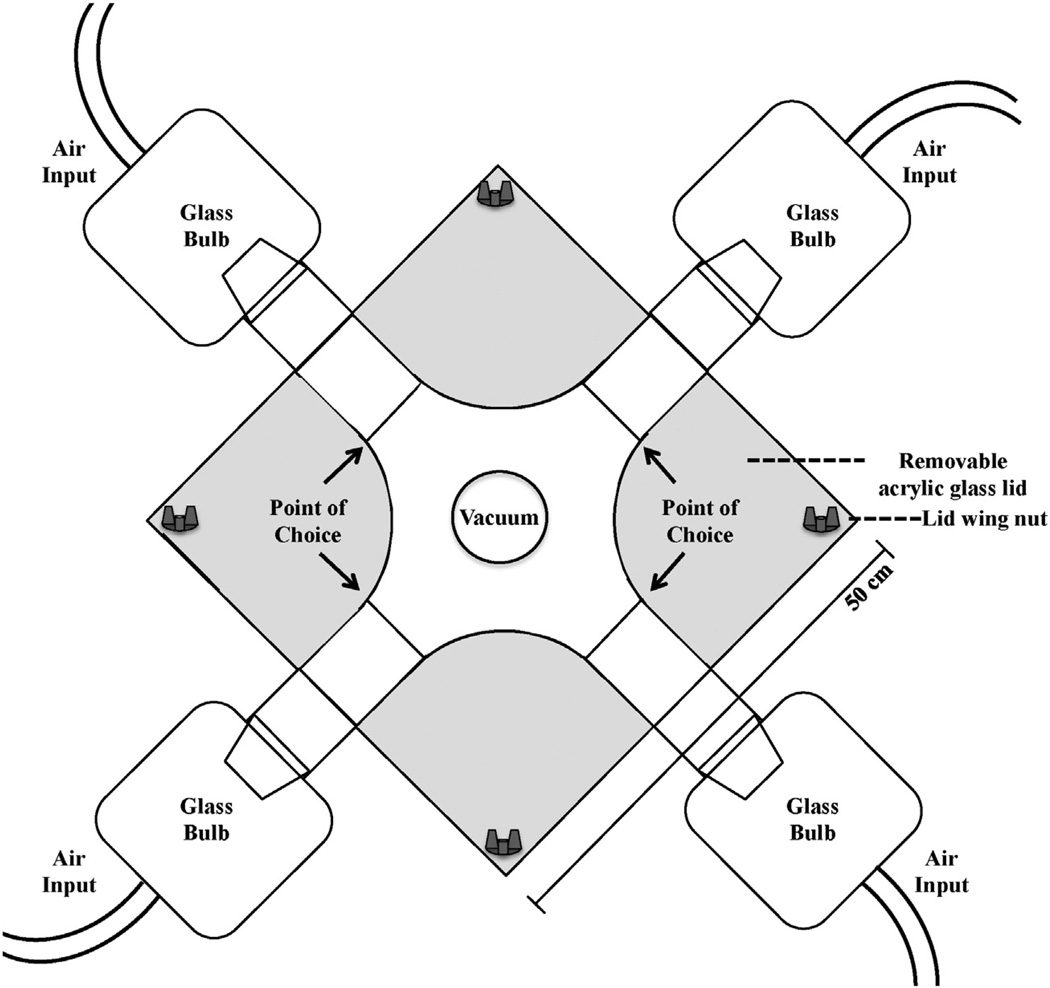
One modification of the Y-tube design is the addition of two extra arms, with associated glass bulbs, creating a 4-way olfactometer (Fig. 7). The 4-way olfactometer functions similarly to the Y-tube except that the four arms are all attached to a common central chamber rather than an adjoining tube. The common chamber is also the location for the attachment of the vacuum. An added advantage of the 4-way olfactometer is that mites and ticks can be exposed to more than one test odor during a single experiment. Comparing multiple chemicals in one trial can determine if there is a preference for one chemical over another. The 4-way olfactometer can help identify the most attractive chemical to ticks or mites from a group of acarine attractants.
Fig. 7.
Image of olfactometer with four ports for use in laboratory bioassays of acarine attractants.
The cost for running olfactometer experiments is higher than that for Petri dish assays, and requires either the purchase or construction of the olfactometer instrument. Additionally, olfactometer methods are more time consuming than the Petri dish design. The number of assays is limited by the number of olfactometer instruments available, and the instruments need to be cleaned between experiments while the Petri dish method using plastic plates is unlimited and disposable.
Other factors to consider in laboratory bioassays include selection of species, life stage, and the formulation and delivery method for test chemicals. Unfortunately, these variables make it difficult to draw conclusions from one study to the next, and more highly controlled research is needed for the identification and characterization of common attractants among the acarines.
3.2. Electrophysiological assays
Electrophysiological assays can provide information on specific chemosensory cell functions, and also potentially be used to detect and screen compounds of chemosensory activity. Electrodes inserted into chemoreceptors record action potentials produced when chemical molecules trigger receptor cell depolarization. Electrodes can be placed in areas to record multicellular function or attached to an individual sensillum for more specific recordings. This can be done utilizing either tungsten electrodes inserted into the sensillumor with glass electrodes placed over the tip of a spliced sensillum. Electrophysiology recordings can identify specific regions and potentially specific cells involved in the detection of attractants of varying types, as well as exposure to other stimulants, e.g., heat, humidity, and mechanical.
Unfortunately, electrophysiological assays have only been conducted in ticks and, even so problems with sensilla visualization have restricted most electrophysiological work to the anterior pit of the Haller's organ [38]. The strength and thickness of the cuticle covering the posterior capsule makes it difficult to cut away and gain visualization of the sensilla inside. Additionally, the nerve bundles originating from the anterior pit and posterior capsule are in close proximity to each other, so visualization of electrode placement is important to differentiate recordings from these two structures. Visualization of electrode placement is also needed to differentiate recordings obtained from chemosensory cells versus muscle cells. Steullet and Guerin [39, 40] performed recordings in the capsule of the Haller's organ. Steullet and Guerin [40] created a hand-drawn diagram of the posterior capsule, dividing the capsule surface into numbered regions and arbitrarily inserting electrodes into each region until recordings could be obtained. The electrodes were placed without good visualization of the sensilla and at unknown depths. Using these methods, it is unclear if the recordings obtained are from the posterior capsule or if they inadvertently were recording from the anterior pit or even from muscle. These difficulties associated with targeted electrode placement have limited the amount and value that comes from electrophysiology studies in ticks. To date, only a few published experiments have been conducted on ticks, and no work currently is available on mites. Future research is needed to develop better micro-dissecting tools, microscopy, and procedures for accurate visualization of the chemosensory sensilla and for better electrode placement. Additionally, more research is needed documenting the location of neurons within the tarsi, metatarsi, and tibia of the front pair of legs to aid in the discrimination between recordings from the anterior pit, posterior capsule, and muscle, and overall to obtain a better understanding of the function of the FSO in general.
3.3. Field bioassays
Field bioassays are critical for the evaluation of any final proof of concept for acarine attraction, especially associated with their practical use in control (discussed in more detail later). Since field bioassays are time consuming, labor intensive, and the results can be highly variable, they are typically conducted after laboratory bioassays. The ideal would be to have a laboratory assay system that would be a certain mimic of uses in the field. Bissinger et al. [41] for example, found an excellent correlation between tick Petri dish repellency assays and human subject tests in the field for two commercial products, DEET and BioUD. They concluded, at least for these two products that the laboratory assay was an acceptable predictor of their practical use in the field. However, it is difficult to argue, especially for new chemistries, that field studies simulating practical uses are not critical for a final proof of concept.
There are multiple experimental methods for conducting field bioassays, which range from basic designs to more complex ones. A basic approach would be to simply count the number of mites or ticks attracted to a chemical point source over a period of time. Chemical sources are often incorporated into trapping devices to determine the efficiency of capture. This basic design can easily determine if a chemical can function as an attractant in field applications. By examining capture rates over time, information is obtained about the duration of attractant activity [41,42]. While on the surface, such an assay may seem simple to conduct, the placement of treatments and controls can be problematic because acarine populations are not always evenly distributed in the landscape. Another iteration of this bioassay, and to deal with non-homogenous acarine distribution, employs ticks or mites physically marked and then released at different distances from a chemical source. The experiment can be replicated varying the distances between the release location and chemical source to determine the maximum effective range for capture; information on attraction in the form of a capture percentage for each distance tested can also be obtained [43]. This assay design has been used successfully with ticks, though not with mites because of the past difficulty in marking individuals for release. There are now examples in the insect literature where animal casein and albumin proteins have been used to mark insect parasitoids in laboratory experiments [44,45]. Additionally in field experiments, adult insects exposed to vegetation covered in either casein or albumin were capable of picking up the protein markers within 5 min and retaining them for approximately 2 days [45]. These and other marking systems could potentially be used in the future for mites and ticks.
The primary advantage of field bioassays is to test attractants in practical field settings. Unfortunately, field conditions are also the main source of problems associated with field tests. Working in field settings limits the time frame for conducting experiments because of the seasonal distribution of wild acarine populations. Additionally, since wet weather decreases mite and tick activity, experiments cannot be conducted during rainstorms or on days immediately following rainfall. These time constraints can make it difficult to obtain sufficient, consistent experimental replicates. Changes in temperature from one day to the next, or even during a typical day, also affect vaporization of chemical compounds and acarine motor activity. Working in the field also exposes the researcher to wild populations of mites, ticks, and other animals, some of which produce a level of risk to the researcher, for example, exposing them to venoms or vector borne pathogens. Additionally, human subjects research requires special regulatory approvals that may be difficult and timely to obtain. Since there are no standardized methods for field bioassays, it is important that the experimental design is described in detail; otherwise replication of results between studies is difficult. More research is needed to develop standard methods, including both positive and negative controls, for the evaluation of attractants and repellents in field assays. This is especially relevant to cases where the goal is to develop a commercial use for compounds. More studies are also needed to develop correlations between lab bioassays and field tests for a variety of compounds to determine if reliable lab methods can be developed to effectively predict and minimize the need for field assays.
4. Chemistry of aggregation and assembly pheromones
Because of the common evolutionary history of the acarines, there likely is some level of uniformity in this group relative to the evolution of pheromones in general and their use in development and reproduction. The same is probable for environmental chemistries and the chemical ecology of the acarines. One of the challenges in the review of the work in this area is the naming of pheromones and other environmental compounds of importance to the acarines; to some degree the nomenclature has been affected by the many different life strategies of mites, the specialized obligatory hematophagy and reproductive strategies of ticks, which is quite different from mites, and likely some degree of isolation of work between the mite versus tick scientific communities. Because there has been no coordination in nomenclature across the acarines, in some cases the terminology appears to be contradictory or confusing. Since the nomenclature for specific acarine systems has already been established in the scientific literature, we have chosen to adhere to this nomenclature to minimize confusion, but it is clear that an effort is needed, as we go forward with new research, to identify similar acarine features versus those that are novel and specific to particular life strategies (or species), and to develop more unifying approaches by which we reference compounds which affect acarine behavior and development. Understanding the known chemistry of acarine attraction is the first step in developing strategies for their use as a pesticide and novel chemical or functional mimics.
4.1. Mite aggregation pheromone
The aggregation pheromone causes clustering of conspecific male and female mites of all developmental stages to protected and viable habitats while not on a host. Currently only one aggregation pheromone, lardolure (Table 1), has been identified in the acariform mites Caloglyphus polyphyllae and Lardoglyphus konoi [46]. However, there are many mite sex pheromones that have a dual aggregation function; this makes the identification of solely aggregation pheromones problematic. Despite the limited availability of information describing mite aggregation pheromones, a reasonable role for this pheromone is the clustering of conspecifics into a well-protected habitat to reduce the mortality associated with unfavorable environments. It is difficult to draw additional conclusions or make comparisons to ticks.
Table 1.
Mite pheromones and chemical components that elicit attractive or aggregative behavioral responses.
| Pheromone | Attractive components | Mite speciesa | Reference | Formula | Structure |
|---|---|---|---|---|---|
| Mite aggregation pheromone | Lardolure |
C. polyphyllae L. konoi |
[46] | C15H30O2 | 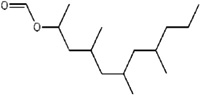 |
| Mite alarm pheromone | Neral | S. elongata | [93,94] | C10H16O | 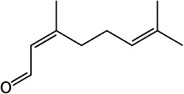 |
| Neryl formate |
D. farinae D. pteronyssinus |
[93,95] | C11H18O2 | 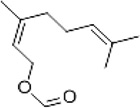 |
|
| Mite female sex pheromone | (2R,3R)-Epoxyneral | Caloglyphus sp. | [78] | C10H16O2 | 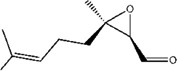 |
| 2-Hydroxy-6-methylbenzaldehyde |
A. immobilis A. ovatus C. hughesi D. farinae |
[73,74,75,76] | C8H8O2 | 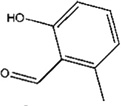 |
|
| α-Acaridial | R. robini | [80] | C10H14O2 | 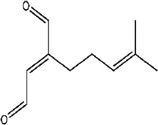 |
|
| β-Acaridial | Caloglyphus sp. | [35] | C10H14O2 | 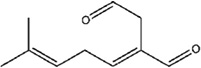 |
|
| Rosefuran | Caloglyphus sp. | [79] | C10H14O | 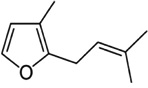 |
|
| S-Isorobinal | R. setosus | [81] | C10H12O2 | 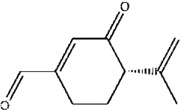 |
|
| Mite female sex pheromone | S-Isopiperitenone | T. similis | [82] | C10H14O | 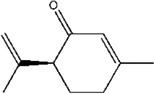 |
| Undecane | C. rodriguezi | [78] | C11H24 | ||
| Mite immature female pheromone | Citronellol | T. urticae | [66] | C10H20O | 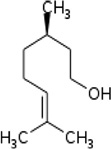 |
| Farnesol | T. urticae | [67] | C15H26O | ||
| Nerolidol | T. urticae | [68] | C15H26O |
Acarus immobilis, Aleuroglyphus ovatus, Caloglyphus polyphyllae, Caloglyphus rodriguezi, Cosmoglyphus hughesi, Dermatophagoides farinae, Dermatophagoides pteronyssinus, Lardoglyphus konoi, Rhizoglyphus robini, Rhizoglyphus setosus, Schwiebea elongata, Tetranychus urticae, Tyrophagus similis.
4.2. Tick attraction–aggregation–attachment pheromone
Attraction–aggregation–attachment pheromone (AAAP) (Table 2) is a tick pheromone secreted by feeding adult males to attract non-feeding conspecific immature and mature males and females, forming feeding clusters on bovine or large ungulate hosts [47]. AAAP is only produced by a subset of species in the genus Amblyomma that have a preference for large animal hosts. AAAP alerts unfed questing ticks of nearby feeding ticks and works in combination with host kairomones to orient unfed ticks towards the host [9]. Currently AAAP is known to be produced by A. cajennense, A. gemma, A. hebraeum, A. lepidum, A. marmoreum and A. variegatum [48]. The chemical composition of AAAP varies by tick species, but the primary active chemical component typically consists of 1–2 phenolic fatty acids. AAAP of A. variegatum, the tropical bont tick, is the most studied. It is composed of methyl salicylate, O-nitrophenol, and nonanoic acid (Table 2) in a 2:1:8 ratio [49]. A. hebraeum ticks also exhibit attractive behavioral responses to the AAAP components methyl salicylate and O-nitrophenol [50,51]. AAAP in combination with carbon dioxide, produced by the host, creates a strong bioactive mixture capable of attracting host-seeking ticks from a distance of 5 to 10 m in field experiments [50,52]. Attraction of A. variegatum increased from 35 to 90% when 6.6 mg of synthetic AAAP was tested in the presence of carbon dioxide gas delivered from 50 and 500 g of dry ice [47].
Table 2.
Tick pheromones and chemical components that elicit attractive or aggregative behavioral responses.
| Pheromone | Attractive components |
Tick speciesa | Reference | Formula | Structure |
|---|---|---|---|---|---|
| Tick attraction–aggregation–attachment pheromone |
O-Nitrophenol |
A. hebraeum A. variegatum |
[49,50,51,52] | C6H5NO3 | 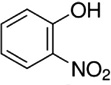 |
| Methyl salicylate |
A. hebraeum A. variegatum |
[49,50,51,52] | C8H8O3 | 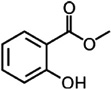 |
|
| Nonanoic acid | A. variegatum | [49,52] | C9H18O2 | ||
| Tick assembly pheromone | Adenine |
I. scapularis I. uriae |
[54,62] | C5H5N5 | 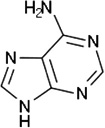 |
| Guanine |
I. ricinus I. scapularis I. uriae |
[54,58,62] | C5H5N5O | 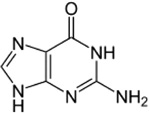 |
|
| Hematin | I. ricinus | [54,58] | C34H33N4O5Fe | 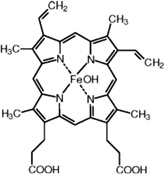 |
|
| Uric acid |
I. ricinus I. uriae |
[54,58] | C5H4N4O3 | 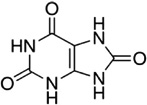 |
|
| Xanthine |
I. ricinus I. scapularis I. uriae |
[54,58,62] | C5H4N4O2 | 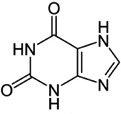 |
|
| Tick sex pheromone | 2,6-Dichlorophenol |
A. nitens A. americanum A. cajennense A. hebraeum A. variegatum D. andersoni D. variabilis H. anatolicum H. dromedarii R. appendiculatus R. microplus |
[9,26,38,85,86,87,88,89,90,91], | C6H3Cl2OH | 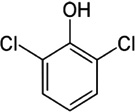 |
Amblyomma americanum, Amblyomma cajennense, Amblyomma hebraeum, Amblyomma variegatum, Anocentor (Dermacentor) nitens, Dermacentor andersoni, Dermacentor variabilis, Hyalomma anatolicum, Hyalomma dromedarii, Ixodes ricinus, Ixodes scapularis, Ixodes uriae, Rhipicephalus appendiculatus, Rhipicephalus microplus, Rhipicephalus sanguineus.
AAAP induces the formation of species specific aggregations on host animals. This pheromone benefits both emitting and responding ticks. Responding unfed ticks use AAAP to locate hosts and commence blood feeding. Also, blood feeding in a large aggregation may protect ticks from physical damage inflicted by the host by reducing the individual risk of injury by increasing the group size. The emitting adult males have the added benefit of possibly attracting adult females to the feeding aggregation for subsequent mating.
Despite differences between AAAP and the mite aggregation pheromone, i.e., the sex that emits the pheromone and the location of the aggregation on a host, both pheromones act to cluster conspecifics to increase their viability. The mite aggregation pheromone induces clustering to help protect mites from unfavorable environmental conditions while not on a host. AAAP induces clustering on hosts to increase access to blood meals and protect ticks from starvation. It is currently unknown if other genera of ticks, outside of Amblyomma, exhibit similar clustering behavior on hosts in response to aggregation pheromones. This phenomenon may also be present in parasitic mites that blood feed, but that is currently undetermined. AAAP has been successfully incorporated into Amblyomma tick control strategies. However, since the pheromone is species specific it is ineffective against other tick species. Investigating on-host aggregations in other genera of ticks and in mites could be useful in the identification of other attractive pheromone chemistries for use in acarine control.
4.3. Tick assembly pheromone
The assembly pheromone (Table 2) causes clustering of conspecific individuals in protected, viable habitats off the host [53]. The tick assembly pheromone differs from the mite aggregation pheromone and AAAP in the behavior elicited to form the off-host aggregation. Typical aggregation pheromones are volatile compounds that cause responding ticks to actively search for emitters and cluster at the source, forming an aggregation. The assembly pheromone is non-volatile, and is detected by ticks through direct contact. When ticks encounter the pheromone, locomotor activity is arrested. Assemblies are formed as a result of multiple ticks encountering the pheromone and all pausing together. Assembly pheromones are typically species specific and active against unfed larvae, nymphs, and adult male and female ticks. Though, there are exceptions, especially among argasid ticks [48]. Fed immature ticks are unresponsive to the assembly pheromone, but regain responsiveness after molting [54]. Assembly pheromones are produced by multiple ixodid and argasid species including A. cohaerens [33], Aponomma concolor [55], Argas persicus, A. reflexus, A. polonicus [56], Hyalomma dromedarii [57], I. holocylus [55], I. ricinus [58], I. scapularis [59], I. uriae [54], Ornithodoros moubata [60], Rhipicephalus appendiculatus [33], and R. evertsi [61]. Assembly pheromones are found in excreta, caste skins, and on the cuticle of larvae, nymphs, and adult male and female ticks [9,62]. In Petri dish bioassays, larval fecal deposits on filter paper caused 67% of tested I. uriae larvae to pause and aggregate on the treated filter paper. Similar results were observed with nymph feces causing 66% of tested I. uriae nymphs to pause and aggregate, 64% of tested I. uriae adult males paused in response to adult male feces, and 71% of tested I. uriae adult females in response to adult female feces [54]. I. ricinus ticks also exhibit similar behavior in response to fecal matter with 48% of tested adult males and females ceasing activity and clustering [58,63].
GC–MS analysis of fecal material, caste skins, and whole body tick extracts identified the primary components of assembly pheromone to be hematin, guanine, xanthine, uric acid, and several other purines [54,62] (Table 2). Synthetic mixtures of these components applied to filter paper caused both adult and immature males and females to pause on the treated surface and aggregate. For I. scapularis, a 25:1:1 mixture of guanine, xanthine and adenine, respectively, applied to filter paper caused 28–48% of tested adult and nymph males and females to pause and aggregate on the treated filter paper [62]. Similar responses were observed when I. uriae was tested against varying mixtures of guanine and uric acid [54].
The tick assembly pheromone regulates clustering of unfed individuals into well-protected habitats to reduce mortality caused by predation and exposure to unfavorable environments while not on a host, as well as to sites frequented by hosts [Sonenshine, personal communication]. The risk of desiccation is the highest for ticks when they are not on a host. Clustering of immature and mature ticks helps decrease water loss by establishing a group transpiration rate. When the group transpiration rate is distributed among aggregation members, it typically results in lower individual transpiration rates than when ticks are not part of an aggregation [3,48]. This same theory of “power in numbers” also applies to the formation of aggregations to reduce individual risk of predation and physical injury. Additionally, when a host encounters a tick aggregation, clustering conspecific male and female ticks will attach to the host and begin to blood feed as a cohort. Since large tick densities enhance feeding and molting success, it may be advantageous for ticks to form large aggregations off the host to improve success on the host [64]. The aggregation of adult males and females also may increase the reproductive success of ticks by increasing successful feeding of both sexes on the same host, creating the opportunity for blood fed adults to subsequently mate. Additionally, Ixodes species that produce assembly pheromones can engage in mating without a blood meal and before host attachment. Further research is needed to better characterize the behavioral responses associated with the assembly pheromone in ticks that can mate prior to blood feeding and those that mate after blood feeding. It would also be interesting to determine if mites produce similar assembly pheromones.
5. Chemistry of reproductive (sex) pheromones
5.1. Mite immature female pheromone
Male mites must commonly compete to reproduce with conspecific females. Adult female mites have a short period of time for fertilization after eclosion, so the first males to mate are typically the most successful. Males increase fecundity by guarding quiescent deutonymph females and then mating with the emerging reproductives. Male mites are able to differentiate deutonymph females from protonymphs with a high degree of accuracy due to the mite immature female pheromone produced only by deutonymphs [65]. Table 1 lists the currently known components of the immature female pheromone, i.e., farnesol, nerolidol, geraniol and citronellol. Tetranychus urticae males were significantly attracted to crude extracts of the immature female pheromone and to its individual chemical components citronellol [66], farnesol [67], and nerolidol [68]. Similar behavior of males associating with deutonymph females has been observed in Hericia sp. [69], Macrocheles muscaedomesticae [70], Proctophyllodes stylifer, P.picae [71], and T. kanzawai [72], though there has been no identification of the pheromones involved. Male mite guarding of deutonymph females enhances male reproductive success by increasing the likelihood of guarding males mating first with emerging mature females. It would be interesting to evaluate immature female pheromones and the chemical components as potential attractant lures in novel control methods (discussed in more detail later).
5.2. Mite female sex pheromone
Fecundity of both male and female mites relies heavily on proper nutrition. Reproduction only occurs after feeding to ensure that adequate nutrients and energy are available for successful mating and egg development. Female mites release female sex pheromone after feeding to attract males for reproduction [73,74]. Currently female sex pheromone and its chemical components have been identified in 9 species of Argasid mites (Table 1): 2-hydroxy-6-methylbenzaldehyde in Acarus immobilis [73], Aleuroglyphus ovatus [74], Cosmoglyphus hughesi [75], and Dermatophagoides farinae [76]; undecane in C. rodriguezi Sams [77]; (2R, 3R)-epoxyneral in Caloglyphus sp. [78]; rosefuran in Caloglyphus sp. [79]; β-acaridial in Caloglyphus sp. [35]; α-acaridial in Rhizoglyphus robini [80]; S-isorobinal in R. setosus [81]; and Sisopiperitenone in Tyrophagus similis [82]. S-isorobinal has also been identified in seven other species of Astimata mites, though its role as a pheromone is unknown. Table 1 lists all the currently identified attractive chemical components of female sex pheromone.
The female sex pheromone is also not exclusively produced by females, and is often found in conspecific males in lower concentrations [81,83]. It is currently unknown why male mites would produce small amounts of female sex pheromone, though it may allow males to identify other conspecifics competing to reproduce with females. The presence of small amounts of female sex pheromone in males may also explain why mounting behavior is often observed between male mites [83]. Mounting behavior between males may be an act of ascendency with larger, stronger males asserting dominance over smaller, weaker conspecifics. This delineation of dominant and submissive male mites may also explain why dominant males typically mate with the larger, more reproductively successful females and submissive males with smaller, less reproductively successful females [84]. This mating hierarchy is a form of reproductive natural selection important for the survival of the species.
5.3. Tick 2,6-dichlorophenol sex pheromone
Ixodid adults become sexually mature shortly after initiating blood feeding. In adult female ticks, consumption of blood triggers production of the sex pheromone, 2,6-dichlorophenol (2,6-DCP) [85] (Table 2). Nearby feeding conspecific males detecting 2,6-DCP will stop feeding, detach, and begin searching for emitting females to begin courtship and mating [53]. Additional pheromones are involved in tick mating, though they are not currently known to be volatile attractants. The sex pheromone 2,6-DCP is currently known to be produced by seven genera and 12 species of ticks, listed in Table 2 [9,26]. There is currently no evidence of 2,6-DCP in the genus Ixodes. Females primarily utilize 2,6-DCP as a sex pheromone, though A. hebraeum and A. variegatum males will secrete 2,6-DCP to stimulate attraction and attachment of immature and mature males and females on the host [86]. The sex pheromone 2,6-DCP is extremely potent for adult male ticks. Plastic spherical baits impregnated with 50 ng of 2,6-DCP induced 100% of Anocentor (Dermacentor) nitens adult males to engage in mate-seeking and mounting behavior on the impregnated plastic baits [87]. Similar behavior was observed in A. cajennense when exposed to 500 ng of 2,6-DCP [88] and in A. americanum when exposed to 100 ng [89]. A. cajennense [90], D. andersoni, D. variabilis [85], and R. sanguineus [90] also exhibit attractive behavior when exposed to 2,6-DCP in olfactometer bioassays. Electrophysiological studies determined that sensilla in the anterior pit of the Haller's organ of A. cajennense [91], A. variegatum, D. variabilis, and R. appendiculatus [92] immature and mature males are responsible for detecting 2,6-DCP.
The sex pheromone 2,6-DCP plays a vital role in reproduction being responsible for alerting males to the presence of proximal, reproductive females. This only applies to adult ticks that must blood feed to become sexually mature and mate. The exception is ticks in the Ixodes genus. Ixodes sp. can mate prior to blood feeding, though the sex pheromones involved in courtship and reproduction are currently unknown. Ixodes sp. may utilize 2,6-DCP as a sex pheromone or some other uncharacterized compound. It is also possible that males secrete small amounts of 2,6-DCP to function as an aggregation pheromone increasing the feeding success of both immature and mature males and females. Similarly, although there is evidence of sex pheromones regulating courtship and mating in argasid ticks, nothing is known about their identity.
6. Chemistry of alarm pheromones
Neral and neryl formate (Table 1) have been identified in 16 species of astigmatid mites as alarm pheromones produced in response to bodily injury [5,93]; there also is recent evidence of an additional attractant function for these compounds. In olfactometer studies, Schwiebea elongata exhibited an attractive behavioral response walking towards and clustering near filter paper treated with 1 and 3 ng of neral [94] (Table 1). When S. elongata were presented with filter paper treated with 30 ng of neral, mites moved away from the neral source. D. farinae and D. pteronyssinus adults also exhibited attractive responses to low doses of neryl formate in olfactometer bioassays [95] (Table 1).
Attraction and clustering of mites in response to low concentrations of alarm pheromone may represent a novel group survival strategy with clustering mites forming a “safety net”. Once mites detect higher concentrations of alarm pheromone they may transition from performing group survival strategies to individual survival strategies, i.e., scattering and hiding. Group survival strategies could be implemented in response to sources of indirect injury, such as unfavorable environmental conditions. Individual survival strategies would be executed in response to direct physical injury. More research is needed to examine the role of alarm pheromone in mite self-preservation. It is also unknown if alarm pheromone and the associated behaviors are expressed in ticks. It would be interesting to determine if ticks have similar group and individual survival strategies as mites, and what compounds might regulate these behaviors. Group clustering in both mites and ticks could also be used as lures for their control.
7. Chemistry of host kairomones
7.1. Mite attractive host chemistry
Host seeking in parasitic mites is heavily dependent on the detection of host kairomones. Behavioral assays have demonstrated the attractive quality of a few chemicals against mites that parasitize insect and mammal hosts. Unfortunately, the information available is limited to a few select parasitic mite species. Chemical components of host kairomones that are attractive to parasitic mites are listed in Table 3. Macrocheles muscaedomesticae is a predatory mite of Musca domestica housefly eggs and first instar larvae. M. muscaedomesticae commonly engages in phoresy with adult M. domestica to gain access to offspring. N-phenyl-N-glucoside and N-phenyl-N-mannoside identified in whole body extracts of both male and female adult houseflies, 5–7 days after emergence, were documented as being significantly attractive to M. muscaedomesticae adults [96] (Table 3). These attractive compounds are also present in younger houseflies, though at lower concentrations which did not elicit strong attraction in M. muscaedomesticae [97]. Since it has been documented that the release of M. muscaedomesticae results in extremely high levels of house fly parasitism [98], one control strategy for houseflies could use applications of N-phenyl-N-glucoside and N-phenyl-N-mannoside with or without the release of M. muscaedomesticae.
Table 3.
Chemical components of host kairomones that elicit attractive behavioral responses in mites.
| Chemical component | Associated host | Mite speciesa | Reference | Formula | Structure |
|---|---|---|---|---|---|
| Methyl linolenate | Honeybee | V. destructor | [101,102] | C19H32O2 | 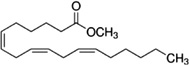 |
| Methyl palmitate | Honeybee | V. destructor | [101,102] | C17H34O2 | 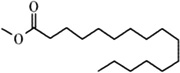 |
| N-Phenyl-N-glucoside | Housefly | M. muscaedomesticae | [96,97] | C12H16O6 | 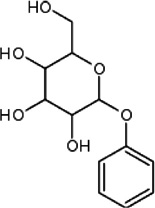 |
| N-Phenyl-N-mannoside | Housefly | M. muscaedomesticae | [96,97] | C12H16O6 | 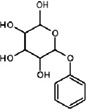 |
| Carbon dioxide | Poultry | D. gallinae | [103] | CO2 |
Dermanyssus gallinae, Macrocheles muscaedomesticae, Varroa destructor.
Mites are also the most successful parasites of honeybees [99]. Honeybee hives have an abundance of food and a constant regulation of temperature and humidity despite external conditions, which could be favorable for mite development. Varroa destructor, a parasitic honeybee mite, lays eggs in capped honeybee brood cells that have been stocked with nutrients. This makes the detection of honeybee colonies and brood cells essential for the reproduction and survival of V. destructor [100]. V. destructor are attracted to fatty acid esters including methyl palmitate and methyl linolenate found in the cuticle extracts of Apis mellifera workers and drone larvae, with no differentiation between these two honeybee castes [101,102] (Table 3). Lab exposure of V. destructor in a y-tube bioassay to methyl palmitate resulted in 50% of the tested mites arriving at the chemical source in 30 sec, and 70% after 400 sec [101]. Additionally, the parasitic mites D. farinae, D. pteronyssinus, L. konoi and T. putrescentiae are attracted to saturated and unsaturated fatty acids C16–C18 and methyl esters of C16–C18 [73].
Dermanyssus gallinae, the red poultry mite, is a parasitic mite that blood feeds on poultry. D. gallinae relies on the detection of heat, vibrations, and carbon dioxide levels higher than ambient air to detect and locate poultry (Table 3). Though, D. gallinae will cease locomotor activity and pause in response to large concentrations of carbon dioxide alone [103]. The latter appears to be a defense mechanism to prevent being eaten by the host; the host detects the mite, the mite is exposed to a high level of carbon dioxide from the breath of the host, and the mite decreases its chance of further detection by the cessation of movement. After a period of 30–40 sec, without further exposure to high concentrations of carbon dioxide, the mite will resume searching for the poultry host. Despite this defense mechanism, D. gallinae mites still exhibit attraction to lower concentrations of carbon dioxide [103]. D. gallinae also exhibits attractive responses to sebaceous gland secretions present on old feathers. When old feathers were tested in a y-tube bioassay against new feathers, 60–70% of tested mites exhibited attraction to the old feathers [104]. It is apparent that research investigating mite attractive host chemicals is sparse resulting in the identification of few chemical attractants. Mite attractive host chemicals have potential as putative lures in novel control methods, though this currently hindered by the limited information available and requires additional research.
7.2. Tick attractive host chemistry
The main determinant of tick host-seeking behavior can be attributed to the presence of host kairomones [105]. Ticks utilize host kairomones to detect host type and proximity. Behavioral assays have demonstrated the attractive quality of dermal pelage [106], host breath [105,107], gland secretions, and urine [105,108]. Acetone, ammonia, carbon dioxide, and 1-octen-3-ol (Table 4) are all attractive components of human and animal breath [36,109]. Carbon dioxide has long been recognized as an attractant for multiple species of ixodid ticks [107]. Ixodid ticks have both long-range and short-range sensilla in the Haller's organ that respond to small and large amounts of carbon dioxide, respectively. Sensilla cell responses to carbon dioxide have been recorded in A. americanum, A. maculatum, D. variabilis [110], and A. variegatum ticks [39]. Lab and field bioassays have also demonstrated the attractive quality of carbon dioxide to A. americanum [36], A. hebraeum [111], A. triguttatum [112], A. variegatum [109], D. andersoni [113], D. variabilis [36], I. dammini [113], I. ricinus [114], I. scapularis [115], R. microplus [28], and R. sanguineus [116]. Attractive behavioral responses to acetone and ammonia dissolved in water have been recorded for A. americanum [36] and to acetone alone in A. hebraeum [109]. Sensory cell responses to NH3 [39,117] and acetone [109] have also been recorded in A. variegatum.
Table 4.
Chemical components of host kairomones that elicit attractive behavioral responses in ticks.
| Chemical component |
Associated host | Tick speciesa | Reference | Formula | Structure |
|---|---|---|---|---|---|
| 1-Octen-3-ol | Cattle |
A. americanum A. cajennense A. hebraeum R. microplus I. ricinus |
[28,36,89,118,119] | C8H16O | 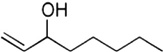 |
| 2-Ethylhexanoic acid | Cattle | R. microplus | [28] | C8H16O2 | 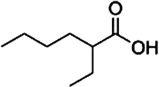 |
| 2-Nitrophenol | Cattle | R. microplus | [28] | C6H5NO3 | 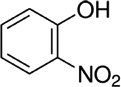 |
| Acetone | Cattle, Dogs, Humans, White-tailed deer |
A. americanum A. hebraeum |
[36,109] | C3H6O | 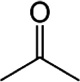 |
| Aliphatic aldehydes | Cattle Rabbit |
A. variegatum | [40] | N/A | Specific chemical structures are unknown |
| Ammonia | Cattle, Dogs, Humans, White-tailed deer |
A. americanum | [36] | NH4 | 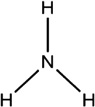 |
| Aromatic aldehydes | Cattle Rabbit |
A. variegatum | [39] | N/A | Specific chemical structures are unknown |
| Benzoic acid | Cattle | R. microplus | [28] | C7H6O2 | 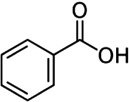 |
| Butyric acid | Cattle | R. microplus | [28] | C4H8O2 | 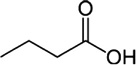 |
| Carbon dioxide | Cattle, Dogs, Humans, White-tailed deer |
A. americanum A. hebraeum A. maculatum A. triguttatum A. variegatum D. andersoni D. variabilis I. dammini I. ricinus I. scapularis R. microplus R. sanguineus |
[26,36,39,110,111,112,113,114,115,116] | CO2 | |
| Heptanoic acid | Cattle | R. microplus | [28] | C7H14O2 | 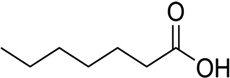 |
| Hexanal | Cattle | R. microplus | [28] | C6H12O | 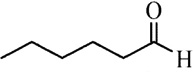 |
| Hexanoic acid | Cattle | R. microplus | [28] | C6H12O2 | 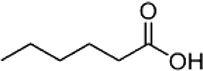 |
| Isobutyric acid | Cattle | R. microplus | [28] | C4H8O2 | 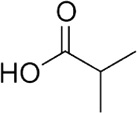 |
| Lactones | Cattle Rabbit |
I. ricinus | [126] | N/A | Specific chemical structures are unknown |
| Pentanoic acid | Cattle | R. microplus | [28] | C5H10O2 | 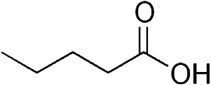 |
| Pyruvate | Cattle | R. microplus | [28] | C3H3O3 | 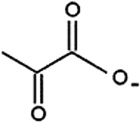 |
Amblyomma americanum, Amblyomma hebraeum, Amblyomma maculatum, Amblyomma triguttatum, Amblyomma variegatum, Dermacentor andersoni, Dermacentor variabilis, Ixodes dammini, Ixodes persulcatus, Ixodes ricinus, Ixodes scapularis, Rhipicephalus microplus, Rhipicephalus sanguineus.
1-Octen-3-ol (Table 4) is an important attractive component specifically associated with bovine breath and bovine odors [28]. 1-Octen-3-ol is attractive to A. hebraeum unfed adults [118] and R. microplus unfed nymphs [28,119] in field settings. Both A. hebraeum and R. microplus exclusively parasitize bovine hosts. 1-Octen-3-ol also elicited attractive behavioral responses from A. americanum in studies with an olfactometer [36] and electrophysiological responses in A. cajennense using tip-recording techniques [89].
Nitrogenous wastes, i.e., ammonia and ammonium hydroxide, are major components of mammal dermal pelage and urine. D. variabilis exhibits aggregative behavior in response to canine urine [120]. A. americanum and D. variabilis both exhibit aggregative behavior in response to domestic canine ear pelage and deer tarsal gland extracts [105,106]. Deer tarsal pelage and its odor are created by bacterial fermentation of urine that is retained in the gland after urination. Lipids secreted by sebaceous glands provide a habitable environment for microbial organisms [121,122]. Canine ear pelage consists of dog-ear wax, lipids from sebaceous glands, and a natural fauna of bacteria that live in the external ear canal [123]. D. variabilis does not parasitize white-tailed deer, but this tick has been reported to congregate around deer carcasses, increasing the likelihood of encountering canine scavengers, its primary host type. Its attraction to deer tarsal gland extracts reflects a means of feeding behavior, with tick aggregations in high host traffic areas identified by cervid odors [124]. D. variabilis, I. scapularis, and I. persulcatus also exhibit arrestant behavior in response to canine fur odors [120,125]. A. variegatum exhibits attractive behavior in response to rabbit and bovine dermal odors [40], I. ricinus to bovine and porcine dermal odors [28], and R. microplus to only bovine dermal odors [28]. Electrophysiological and bioassay studies paired with GC–MS analysis identified the attractive components of the rabbit and bovine dermal odors to be aliphatic and aromatic aldehydes, carboxylic acids, alcohols, phenols, as well as lactones [28,39,40,126]. Unfortunately, the majority of these compounds are currently unknown. Osterkamp et al. [28] identified 11 chemical compounds, mainly carboxylic acids, from bovine dermal odors that elicited questing activity in R. microplus larvae when presented at high doses; 2-ethylhexanoic acid, 2-nitrophenol, 1-octen-3-ol, benzoic acid, butyric acid, heptanoic acid, hexanal, hexanoic acid, isobutyric acid, pentanoic acid, and pyruvate (Table 4). Aliphatic aldehydes were detected by three different sensilla in the Haller's organ of A. variegatum adult ticks [40] and additional chemosensory cell responses to lactones recorded in the Haller's organ of I. ricinus adult ticks [126].
Although there has been research and development in the use of tick pheromones to attract ticks for the purpose of control, considerably less research is available on the use of host-associated attractants. One obvious disadvantage for the use of some of these host-associated attractants like carbon dioxide is the practical storage and long term delivery of dry ice or compressed gas at an economical cost; these are mostly engineering challenges. The use of dry ice to attract ticks, as one example, is a well-established research tool for tick collection and monitoring [111–115]. The use of other host-associated attractants in acarine control needs further consideration. Host-associated attractants could potentially have a wider range of activity for different acarines than a pheromone that is often species specific. Furthermore, research is needed to characterize both the bacteria associated with host-associated attractants and the volatiles that are produced from bacterial fermentation of mammal sebaceous gland secretions and urine. Since mites are attracted to similar sebaceous gland secretions, it is possible that they are also attracted to odors from bacterial fermentation in addition to host kairomones.
8. Chemistry of plant allomones
8.1. Mite attractive plant chemistry
Herbivore-induced plant volatiles (HIPVs) elicited by arthropod feeding, attract predatory mites that may help alleviate plant injury through predation. For example, the predatory mite Neoseiulus cucumeris is attracted to volatiles from arthropod infested cucumber plants, and is unresponsive to undamaged plants, plants with artificial damage, and volatiles from pest arthropods [127]. Similar results have been reported with N. cucumeris and tulip bulbs [128], N. womersleyi and tea plants [129], and Phytoseiulus macropilis and bean plants [130]. Table 5 lists four HIPVs that were detected from infestation damaged cucumber plants, i.e., linalool, methyl salicylate, α-ocimene and 4,8-dimethyl-1,3,7-nonatriene. One or more of these compounds may be responsible for attracting N. cucumeris to arthropod infested plants [127]. These four compounds are also involved in the attraction of the predatory mite P. persimilis to infested lima bean plants [131,132]. Additionally, Gols et al. [133] identified a fifth compound from the lima bean HIPVs, jasmonic acid (Table 5), to which P. persimilis exhibits attractive responses. Linalool and methyl salicylate have been shown to be successful attractants to N. californicus in field studies [134,135]. Slow release of methyl salicylate resulted in increased carnivorous predation of herbivorous pests over a 2-year period [42]. Predatory mites also show preference towards HIPVs that are produced by plants to which they have been previously exposed. N. womersleyi, previously associated with infested tea leaves, exhibited increased attraction to tea leaf volatiles, i.e., (E)-4,8-dimethyl-1,3,7-nonatriene, (E,E)-α-farnesene, and (E)-β-ocimene (Table 5) while remaining unresponsive to other HIPVs. Likewise, N. womersleyi previously associated with infested kidney bean plants exhibited 82% attraction to kidney bean plant volatiles, i.e., (E)-4,8-dimethyl-1,3,7-nonatriene, (E,E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene, β-caryophyllene, and methyl salicylate (Table 5), while remaining unresponsive to other HIPVs [129].
Table 5.
Plants and herbivore induced plant volatiles that elicit attractive behavioral responses in mites.
| Plant | Chemical components | Mite speciesa | Reference | Formula | Structure |
|---|---|---|---|---|---|
| Camellia tea plant | 4,8-Dimethyl-1,3,7-nonatriene | N. womersleyi | [129] | C11H18 | |
| α-Farnesene | N. womersleyi | [129] | C15H24 | ||
| β-Ocimene | N. womersleyi | [129] | C10H16 | 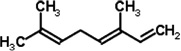 |
|
| Cucumber plant | 4,8-Dimethyl-1,3,7-nonatriene | N. cucumeris | [127] | C11H18 | |
| α-Ocimene | N. cucumeris | [127] | C10H16 | 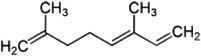 |
|
| Linalool |
N. californicus N. cucumeris |
[127,134,135] | C10H18O | ||
| Methyl salicylate |
N. californicus N. cucumeris |
[127,134,135] | C8H8O3 |  |
|
| Kidney bean plant | 4,8-Dimethyl-1,3,7-nonatriene | N. womersleyi | [129] | C11H18 | |
| 4,8,12-Trimethyl-1,3,7,11-tridecatetraene | N. womersleyi | [129] | C16H26 | 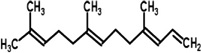 |
|
| β-Caryophyllene | N. womersleyi | [129] | C15H24 |  |
|
| Methyl salicylate | N. womersleyi | [129] | C8H8O3 | 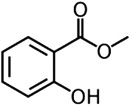 |
|
| Lima bean plant | 4,8-Dimethyl-1,3,7-nonatriene | P. persimilis | [131,132] | C11H18 | |
| Jasmonic acid | P. persimilis | [133] | C12H18O3 | 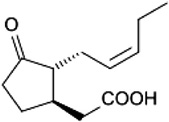 |
|
| α-Ocimene | P. persimilis | [131,132] | C10H16 | 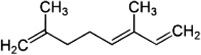 |
|
| Linalool | P. persimilis | [131,132] | C10H18O | ||
| Methyl salicylate | P. persimilis | [131,132] | C8H8O3 | 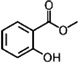 |
Neoseiulus cucumeris, Neoseiulus womersleyi, Neoseiulus californicus, Phytoseiulus persimilis.
Biotic stresses on plants have caused the evolution of tritrophic interactions between plants, herbivores, and herbivore enemies. HIPVs released by plants promote the effectiveness of natural enemies of herbivorous arthropods as a mechanism of indirect plant defense [136]. Interestingly, predatory mite species have been conditioned to associate certain HIPVs with the presence of herbivorous prey, becoming attracted to these volatile chemicals. Since HIPVs are produced in larger quantities than prey kairomones, they may be easier to detect and possibly become the primary chemical cue regulating hunting in predatory mites. The shift from utilizing prey kairomones to HIPVs would also result in changes in the olfactory sensory system, sensilla detecting prey kairomones losing function and sensilla detecting HIPVs becoming more active and robust. The phenomena of mite preference for HIPVs produced by plants to which they have been previously exposed can be explained by “imprinting”. Mites early in development associate specific plant HIPVs with prey and become dependent on that association for hunting throughout their life span. Though, it is still unclear how this preference for certain HIPVs over others is acquired and retained.
Tritrophic interactions between plants and predatory mites are interesting phenomena, although there is little information available on how these systems develop and function. Research has focused mostly on HIPVs, predatory mites, and their use in biological control. Tritrophic systems are unstable and continually changing as herbivorous pests evolve to evade detection by predators. The mechanisms by which these interactions are established could have significant value in development of biological based novel control strategies.
8.2. Tick attractive plant chemistry
A few plants have been found to be attractive to Rhipicephalus ticks. Freshly picked leaves from the Acalypha fruticosa plant were attractive to R. appendiculatus larvae [137], and oil extracts of dry leaves of Calpurnia aurea attractive to both R. appendiculatus and R. pulchellus adults (Table 6). A dose of 100 mg/ml of C. aurea extract attracted 52.2% of released R. pulchellus ticks and 44.4% of released R. appendiculatus ticks from a distance of 1 m in field settings [37]. The active ingredients in these plant extracts are currently unknown. It has been hypothesized that plant odors may mimic pheromones or represent safe habitat selection for ticks searching for protected locations to aggregate. Since Rhipicephalus ticks actively hunt or ambush hosts, they are more susceptible to environmental conditions than questing ticks, which remain on or near plants. Rhipicephalus ticks may need to identify specific plants in their environment as designated safe habitats to retreat back to during adverse weather conditions. Removing these plants could possibly force ticks to leave the area in search of new safe habitats or increase tick mortality since they no longer have protection from unsuitable environmental conditions. This phenomenon has obvious control implementations and warrants considerably more research emphasis. It at least suggests that ground landscaping could be used to create unviable habitats for ticks and reduce tick feeding on animals and humans. Tick questing behavior and plant preference would be of similar interest. Lastly, since mites exhibit similar aggregative behavior to that of ticks, the same study approaches might apply to mite control.
Table 6.
Plant volatiles that elicit attractive behavioral responses in ticks.
| Plant | Chemical components | Tick speciesa | Reference | Formula | Structure |
|---|---|---|---|---|---|
| Acalypha plant | Unknown | R. appendiculatus | [137] | Unknown | Unknown |
| Calpurnia plant | Unknown |
R. appendiculatus R. pulchellus |
[37] | Unknown | Unknown |
Rhipicephalus appendiculatus, Rhipicephalus pulchellus.
9. Chemistry of fungal allomones
Stored food mites are not ectoparasitic but exhibit attractive behavior in response to fungal allomones. Both cis- and trans-octa-1,5-dien-3-ol isolated from the fungi, Trichothecium roseum, have been determined to be attractive to T. putrescentiae mites [138] (Table 7). It has been suggested that in the absence of a traditional food source, stored food mites may utilize xerophilic fungi as a source of nutrients [139]. Because xerophilic fungi prefer dry environments, they are likely to thrive while traditional food sources for stored food mites may be limited or absent. This adaptation to obtain nutrients from nontraditional food sources, such as fungi, may have evolved in stored food mites to promote survival during long starvation periods. The ability of mites to obtain nutrients from nontraditional food sources during starvation periods should be of great interest. The availability of these nontraditional food sources under varying environmental conditions might be significant to the population dynamics of mites. Understanding these alternate food sources may be helpful in modifying current food preparation and storage methods for improved stored food mite control. This could also apply to other systems where mites are important, and therefore microbial-mite interactions will likely grow in importance in the future.
Table 7.
Chemical components of fungal allomones that elicit attractive behavioral responses in mites.
| Chemical component |
Associated host | Mite speciesa | Reference | Formula | Structure |
|---|---|---|---|---|---|
| cis-Octa-1,5-dien-3-ol | Fungi | T. putrescentiae | [138,139] | C8H14O | 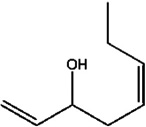 |
| trans-Octa-1, 5-dien-3-ol | Fungi | T. putrescentiae | [138,139] | C8H14O |
Tyrophagus putrescentiae.
10. Acarine control by trapping: attraction and kill
10.1. Tick lure and kill strategies
Lure and kill strategies combine attractants with an acaricide into a slow-release formulation or device. The attractive compound(s) lure the tick to some object that contains a contact acaricide [140]. Combining attractants and acaricides with slow release technology allows impregnated delivery devices, as one example, to remain attractive to ticks, and lethal for a period of up to 14weeks [141]. The delivery device provides a safer means of acaricide application both for the pest control operator and the environment since the amount of the pesticide applied is reduced with restricted bioavailability to the general landscape.
Arrestment pheromone, 2,6-DCP sex pheromone, and AAAP (discussed earlier) are examples of proven lures. Allan et al. [142] patented a device that incorporated I. scapularis arrestment pheromone (a mixture of guanine, xanthine and adenine) and permethrin acaricide into an oil formulation (Last Call™, IPM technologies, Portland OR) that was applied to ground cover and vegetation with a pump sprayer. The pheromone-acaricide mixture resulted in 95% I. scapularis mortality in treated areas over that of the untreated controls [142]. Two delivery devices have been evaluated incorporating 2,6-DCP and acaricides. The first involves a water emulsion of gelatin-microencapsulated 2,6-DCP combined with propoxur. The emulsion was applied to dogs infested with the American dog tick, D. variabilis, where the slow-release microcapsules continuously attracted ticks for multiple days. Tick mortality was observed, with a higher percentage of male ticks killed by the treatment than females. Mating disruption was also observed resulting in a 90% decrease in oviposition in females compared to untreated controls [143]. The second delivery device utilizing 2,6-DCP involved impregnating tick decoys with the pheromone-acaricide mixture. Tick decoys were plastic spherules made from polyvinyl chloride plastic. The decoys (5 mm × 5 mm) were impregnated with 2,6-DCP, cholesteryl oleate and propoxur and adhesively attached to rabbits infested with D. variabilis. Mortality of all the male ticks was observed, and there was no oviposition in females compared to untreated controls [144]. Similar results were observed when the impregnated tick decoys were placed onto tick-infested cattle [92] and camels [145]. AAAP was also effectively utilized as a chemical lure to control ticks. Plastic tags impregnated with AAAP and pyrethroids were placed on the tails of cattle infested with the ticks, A. hebraeum and A. variegatum. Tick control over a 3-month trial averaged 94.9% over that of the untreated controls [51,141].
Current tick control methods are heavily dependent on the use of acaricides. This approach can effectively control tick populations but typically requires multiple, large-scale applications [146,147]. Unfortunately, our dependency on traditional spraying promotes the application of large amounts of pesticides into the environment and fosters the development of pesticide resistance; these negative side effects could be limited by the use of lure and kill strategies focused not on the general pest populations but just on the resource needing protection. The current research on lure and kill technologies is limited and does not provide enough supporting evidence for their broad implementation as alternatives to chemical sprays.
10.2. Mite biological control and attractants
A different type of lure and kill strategy has been used for mite control in crop production using a biocontrol agent, i.e., predatory mites, as the kill mechanism. Attractive HIPVs, primarily methyl salicylate, have been shown to enhance natural enemies in laboratory experiments with a variety of plants [134,148,149]. Field-testing of methyl salicylate has been limited to grape vineyards [150], hops [42], cotton [151], and strawberry fields [150,152]. Methyl salicylate formulated for slow release dispensers (Predalure®, AgBio, Westminster, CO) have demonstrated the most effective predator attraction for the longest time period. Predalure dispensers repeatedly distributed in hops fields reduced infestations by the herbivorous mite T. koch 41–90% over a two year period over that of untreated controls [42]. Predalure dispensers placed in strawberry fields also consistently attracted predators from up to 10maway from the dispenser site [152]. It is apparent that chemically enhanced biocontrol is effective in reducing herbivory of a variety of crop plants. Though, research investigating HIPVs and the tritrophic interactions with predatory mites is still very limited and require additional study. There is also the potential for the development and implementation of lure and kill strategies (as previously described in ticks) to control parasitic mites of humans and animals using chemical attractants and miticides, though that also requires further study.
11. Summary and conclusions
The Acari are of significant economic importance in crop production and human and animal health, and chemical control an essential component for pest management. The acarines represent an ancient divergence in the Arthropoda and likely have unique strategies for the use of chemistry in their growth, development, and ecological interactions. However, the study of acarine attractants is limited as compared to that of insects. The acarines consists of two major groups, the mites that demonstrate a wide variety of life strategies, i.e., herbivory, predation, and endo- and ectoparasitism, and ticks which have evolved obligatory hemtophagy. Much of the research on attractants in mites versus ticks has occurred in relative isolation to each other. In addition, advances in the physiology and molecular biology of mite chemical communication have been limited by the small size of mites versus that of ticks. Although we now have genomes and transcriptomes to mites and ticks, which should rapidly advance our understanding of chemical communication, the larger size of ticks will still be important in correlating structure to function and be the driver for translational work with mites.
The important chemosensory organs in the acarines are the palps, chelicerae, and an organ on the tarsus on the front pair of legs called the Haller’s organ in ticks and the tarsal organ in mites. The relative importance of these different organs in their chemical ecology is poorly understood. Also the molecular mechanisms for attractant detection in these organs are a black box with no published papers. Data are presented in this review that suggests that the mechanism of olfaction in ticks is unlike that of insects. The tarsal organ of mites and the Haller's organ of ticks have a common evolutionary origin and have apparent similar functions; we recommend they be called the “foretarsal sensory organ” (FSO) in both mites and ticks. The FSO in acarines is not found in other arthropods and has multiple sensory functions, which are not clearly understood. New electrophysiology techniques are needed to map the function of individual sensilla in the FSO, chelicerae and palps. Transcriptomics coupled to genomics is needed along with new techniques like RNAseq, RNAi, and cell imaging to understand molecular mechanisms and the regulation of chemoreception; these studies are essentially lacking in the acarines, yet essential to any advancement for finding targets in these receptors for mite and tick control.
This review clearly demonstrates that over the past three to four decades, significant progress has been made in identifying chemical attractants and their associated behaviors in acarine species. However, there are still large gaps in the data currently available compared to insects and the application of this knowledge for control. The gaps are even greater for acarine pheromones with essentially all of the research on ticks as compared to mites. The importance of chemical communication on ecological positioning, aggregation, communication between conspecifics, courtship, and reproduction needs further attention. There appears to be a steady decline of research focusing on attractants and the role of attractants in development, reproduction, and control; research investigating acarine repellents for ticks has overshadowed the study of acarine attractants. Although repellents have a high commercial and military importance for protection against arthropod vectored diseases, the use of repellents for crop protection from mites has received essentially no consideration. Lure and kill and lure-enhanced biocontrol strategies have significant environmental advantages, which warrant further study. Also the use of pheromones and other compounds to disrupt chemical communication in insects has not been applied yet to acarines, even though proven technologies exist and are ready for commercial use.
Finally, mechanisms need to be established to encourage interactions between scientists working on mites and those with ticks. Certainly with the common evolutionary history of mites and ticks, even if they have evolved quite different life strategies, the acari must share many similar mechanisms in chemical communication. The tick model and employing techniques like organ specific transcriptomics, RNAi, and classical physiological techniques likely will be a driver for understanding mite function in the future and along with bioassay, chemical screening, and structure-activities studies will be the mechanism for the practical use of attractants for the control of tick and mite populations. There is also the need for translational research to examine chemical control approaches exploiting both attractants and repellents from ticks to mites and the reverse.
Acknowledgments
This work was funded by grants from NIH (1R21AI096268) and NSF (IOS-0949194) to RMR and the North Carolina Agricultural Experiment Station. ALC was supported in part by a Graduate Student Teaching Assistantship from the Department of Entomology at North Carolina State University and a Research Assistantship funded by the NIH grant mentioned earlier. We would also like to thank Dr. Daniel E. Sonenshine and Dr. Lewis B. Coons for their images of mite and tick sensory structures and for their review of the manuscript.
References
- 1.Degenhardt J, Gershenzon J, Baldwin IT, Kessler A. Attracting friends on foes: engineering terpene emission to make crop plants more attractive to herbivore enemies. Curr. Opin. Biotechnol. 2003;14:169–176. doi: 10.1016/s0958-1669(03)00025-9. [DOI] [PubMed] [Google Scholar]
- 2.Jared S, Khan F, Ramirez-Fort M, Tying SK. Bites and mite: prevention and protection of vector-borne disease. Curr. Opin. Pediatr. 2013;25:488–491. doi: 10.1097/MOP.0b013e328362c4ab. [DOI] [PubMed] [Google Scholar]
- 3.Sonenshine DE, Roe RM. Biology of Ticks. Vol. 1. New York: Oxford University Press; 2014. [Google Scholar]
- 4.Balashov IS. An Atlas of the Ixodid Tick Ultrastructure. Leningrad: Nauka Press; 1983. [Google Scholar]
- 5.Kuwahara Y. Chemical Ecology in Astigmatid mites. In: Hidaka T, Matsumoto Y, Honda K, Honda H, Tatsuki S, editors. Environmental Entomology: Behavior, Physiology and Chemical Ecology. Tokyo: University of Tokyo Press; 1999. pp. 380–393. [Google Scholar]
- 6.Leonovich SA. Sensory Systems of Parasitic Ticks and Mites. St. Petersburg: Nauka Press; 2006. [Google Scholar]
- 7.Mulenga A. Molecular Biology and Physiology of Chemical Communication. In: Sonenshine DE, Roe RM, editors. Biology of Ticks. New York: Oxford University Press; 2014. pp. 368–397. [Google Scholar]
- 8.Šimo L, Sonenshine DE, Park Y, Žitňan D. Nervous and Sensory System, Function, Genomics and Proteomics. In: Sonenshine DE, Roe RM, editors. Biology of Ticks. New York: Oxford University Press; 2014. pp. 309–367. [Google Scholar]
- 9.Sonenshine DE. Tick pheromones and their use in tick control. Annu. Rev. Entomol. 2006;51:557–580. doi: 10.1146/annurev.ento.51.110104.151150. [DOI] [PubMed] [Google Scholar]
- 10.Bissinger BW, Roe RM. Tick repellents: past, present, and future. Pestic. Biochem. Physiol. 2010;96:63–79. [Google Scholar]
- 11.Bissinger BW, Roe RM. Tick Repellent Research, Methods and Development. In: Sonenshine DE, Roe RM, editors. Biology of Ticks. New York: Oxford University Press; 2014. pp. 382–402. [Google Scholar]
- 12.Kuwahara Y. Chemical ecology of astigmatid mites. Nippon Nogei Kaishi. 1990;11:1754–1757. [Google Scholar]
- 13.Peña JE, Mohyuddin AI, Wysoki M. A review on the pest management situation in mango agrosystems. Phytoparasitica. 1998;26:129–148. [Google Scholar]
- 14.Shipp JL, Boland GJ, Shaw LA. Integrated pest management of disease and arthropod pests of greenhouse vegetable crops in Ontario: current state and future possibilities. Can. J. Plant Sci. 1991;71:887–914. [Google Scholar]
- 15.Yamamoto I. Some chemical ecological approaches to the control of stored-product insects and mites. A.C.S. Symp. Ser. 1985;276:219–224. [Google Scholar]
- 16.Attia S, Grissa KL, Lognay G, Bitume E, Hance T, Mailleux AC. A review of the major biological approaches to control of the worldwide pest Tetranychus urticae (Acari: Tetranychidae) with special reference to natural pesticides. J. Pest. Sci. 2013;86:361–386. [Google Scholar]
- 17.Ridsdill-Smith TJ. Biology and control of Halotydeus destructor (Tucker) (Acarine: Penthaleidae): a review. Exp. Appl. Acarol. 1997;21:195–224. [Google Scholar]
- 18.Sparagano OAE, George DR, Harrington DW, Giangaspero A. Significance and control of the poultry red mite, Dermanyssus gallinae. Annu. Rev. Entomol. 2014;59:447–466. doi: 10.1146/annurev-ento-011613-162101. [DOI] [PubMed] [Google Scholar]
- 19.Strachecka A, Sawicki M, Borsuk G, Olszewski K, Paleolog J, Bajda M, Chobotow J. Use of acaricides for fighting Varroa destructor mites in bee colonies: efficiency and risk. Med. Weter. 2013;69:219–224. [Google Scholar]
- 20.Leonovich SA, Stanyukovich MK. Sensory organs of mesostimatic mites (Acarina, Mesostigmata) dwelling in body cavities of mammals and birds. Proc. Zool. Inst. Russ. Acad. Sci. 2011;315:263–273. [Google Scholar]
- 21.Leonovich SA. Tarsal sensory complex in the red chicken mite Dermanyssus gallinae (Gamasina) Parazitologiya. 2006;40:124–131. [PubMed] [Google Scholar]
- 22.Leonovich SA. Adaptation of sensory systems of gamasid mites (Parasitiformes, Gamasina) to dwelling in different environment. Parazitologiya. 2008;42:271–279. [PubMed] [Google Scholar]
- 23.Lees AD. The sensory physiology of the sheep tick, Ixodes ricinus L. J. Exp. Biol. 1948;25:145–207. [Google Scholar]
- 24.Gothe R, Beelitz P, Schol H. Morphology and structural organization of Haller's organ during postembryonic development of Argas (Persicargas) walkerae (Ixodoidea: Argasidae) Exp. Appl. Acarol. 1991;11:99–109. doi: 10.1007/BF01246083. [DOI] [PubMed] [Google Scholar]
- 25.Foelix RF, Axtell RC. Ultrastructure of Haller's organ in the tick Amblyomma americanum. Z. Zellforsch. Mikrosk. Anat. 1972;124:275–292. doi: 10.1007/BF00355031. [DOI] [PubMed] [Google Scholar]
- 26.Sonenshine DE. Biology of Ticks. Vol. 1. New York: Oxford University Press; 1991. [Google Scholar]
- 27.Waladde SM, Rice MJ. The Sensory Basis of Tick Feeding Behavior. In: Obenchain FD, Galun R, editors. Physiology of Ticks. Oxford: Pergamon Press; 1982. pp. 71–118. [Google Scholar]
- 28.Osterkamp J, Wahl U, Schmalfuss G, Haas W. Host odor recognition in two tick species coded in a blend of vertebrate volatiles. J. Comp. Physiol. 1999;185:59–67. doi: 10.1007/s003590050366. [DOI] [PubMed] [Google Scholar]
- 29.Leonovich SA, Dushbábek F. Pheromone Sensory Subsystem in Ticks: Correlation between Structure of Sensilla and Evolution of Behavior. In: Dushbábek F, Bukva V, editors. Modern Acarology. Vol. 1. Prague: Academia Press; 1991. pp. 53–58. [Google Scholar]
- 30.Grbić M, Van Leeuwen T, Clark RM, Rombauts S, Rouzé P, Grbić V, Osbourne EJ, Dermauw W, Ngoc PCT, Ortego F, Hernández-Crespo P, Diaz I, Martinez M, Navajas M, Sucena E, Magalhães S, Nagy L, Pace RM, Djuranović S, Smagghe G, Iga M, Christiaens O, Veenstra JA, Ewer J, Villalobos RM, et al. The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature. 2011;479:487–492. doi: 10.1038/nature10640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liggett SB. Phosphorylation barcoding as a mechanism of directing GPCR signaling. Sci. Signal. 2011;4:36. doi: 10.1126/scisignal.2002331. [DOI] [PubMed] [Google Scholar]
- 32.Rodgers ME, Jani MK, Vogt RG. An olfactory-specific glutathione-S-transferase in the sphinx moth Manduca sexta. J. Exp. Biol. 1999;202:1625–1637. doi: 10.1242/jeb.202.12.1625. [DOI] [PubMed] [Google Scholar]
- 33.Otieno DA, Hassanali A, Obenchain FD, Steinberg A, Galun R. Identification of guanine as an assembly pheromone of ticks. Insect Sci. Appl. 1985;6:667–670. [Google Scholar]
- 34.Arlian LG, Vyszenski-Moher DL. Response of Sarcoptes scabiei var. canis (Acari: Sarcoptidae) to lipid of mammalian skin. J. Med. Entomol. 1995;32:34–41. doi: 10.1093/jmedent/32.1.34. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu H, Mori N, Kuwahara H. Aggregation pheromone activity of the female sex pheromone, β-acaridial, in Caloglyphus polyphyllae (Acari: Acardidae) Biosci. Biotechnol. Biochem. 2001;65:1724–1728. [PubMed] [Google Scholar]
- 36.Carr AL, Roe RM, Arellano C, Sonenshine DE, Schal C, Apperson CS. Responses of Amblyomma americanum and Dermacentor variabilis to odorants that attract haematophagous insects. Med. Vet. Entomol. 2013;27:86–95. doi: 10.1111/j.1365-2915.2012.01024.x. [DOI] [PubMed] [Google Scholar]
- 37.Nana P, Maniania NK, Maranga RO, Kutima HL, Boga HI, Nchu F, Eloff JN. Attraction response of adult Rhipicephalus appendiculatus and Rhipicephalus pulchellus (Acari: Ixodidae) ticks to extracts from Calpurnia aurea (Fabaceae) Vet. Parasitol. 2010;174:124–130. doi: 10.1016/j.vetpar.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 38.Waladde SM. Tip-recording from ixodid tick olfactory sensilla: responses to tick related odours. J. Comp. Physiol. 1982;148:411–418. [Google Scholar]
- 39.Steullet P, Guerin PM. Perception of breath components by the tropical bont tick, Amblyomma variegatum Fabricius (Ixodidae) J. Comp. Physiol. 1992;170:665–676. doi: 10.1007/BF00198976. [DOI] [PubMed] [Google Scholar]
- 40.Steullet P, Guerin PM. Identification of vertebrate volatiles stimulating olfactory receptors on the tarsus I of the tick Ambylomma variegatum Fabricius (Ixodidae) J. Comp. Physiol. 1993;174:27–38. doi: 10.1007/BF00192003. [DOI] [PubMed] [Google Scholar]
- 41.Bissinger BW, Apperson CS, Watson DW, Arellano C, Sonenshine DE, Roe RM. Novel field assays and the comparative repellency of BioUD®, DEET and permethrin against Amblyomma americanum. Med. Vet. Entomol. 2011;25:217–226. doi: 10.1111/j.1365-2915.2010.00923.x. [DOI] [PubMed] [Google Scholar]
- 42.Woods JL, James DG, Lee JC, Gent DH. Evaluation of airborne methyl salicylate for improved conservation biological control of two-spotted spider mite and hop aphid in Oregon hop yards. Exp. Appl. Acarol. 2011;55:401–416. doi: 10.1007/s10493-011-9495-8. [DOI] [PubMed] [Google Scholar]
- 43.Carroll JF. Development of a bioassay for evaluating tick (Acari: Ixodidae) attractants in the field. Proc. Entomol. Soc. Wash. 2006;110:82–91. [Google Scholar]
- 44.Irvin AI, Hagler JR, Hoddie MS. Laboratory investigation of triple marking the parasitoid Gonatocerus ashmeadi with a fluorescent dye and two animal proteins. Entomol. Exp. Appl. 2012;143:1–12. [Google Scholar]
- 45.Hagler JR, Jones VP. A protein-based approach to mark arthropods for mark-capture type research. Entmol. Exp. Appl. 2010;2:177–192. [Google Scholar]
- 46.Kuwahara Y, Asami N, Morr M, Matsuyama S, Suzuki T. Chemical ecology of astigamtid mites. XXXVIII. Aggregation pheromone and kairomone activity of lardolure and its analogues against Lardoglyphus konoi and Carpoglyphus lactis. Appl. Entomol. Zool. 1994;29:253–257. [Google Scholar]
- 47.Maranga RO, Hassanali A, Kaaya GP, Mueke JM. Attraction of Amblyomma variegatum (ticks) to the attraction–aggregation–attachment pheromone with or without carbon dioxide. Exp. Appl. Acarol. 2003;29:121–130. doi: 10.1023/a:1024265529030. [DOI] [PubMed] [Google Scholar]
- 48.Bowman AS, Nuttal P. Ticks: Biology, Disease and Control. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- 49.Schoni R, Hess E, Blum W, Ramstein K. The aggregation–attachment pheromone of the tropical bont tick Amblyomma variegatum Fabricius (Acari: Ixodidae); isolation, identification and action of its components. J. Insect Physiol. 1984;30:613–668. [Google Scholar]
- 50.Norval RAI, Peter T, Yunker CE, Sonenshine DE, Burridge MJ. Responses of the ticks Amblyomma americanum and A. variegatum to known or potential components of the aggregation–attachment pheromone. I. Long-range attraction. Exp. Appl. Acarol. 1991;13:11–18. [Google Scholar]
- 51.Norval RAI, Peter T, Yunker CE, Sonenshine DE, Burridge MJ. Response of the ticks Amblyomma hebraeum and A. variegatum to known or potential components of the aggregation–attachment pheromones. III. Aggregation. Exp. Appl. Acarol. 1992;16:237–245. doi: 10.1007/BF01193806. [DOI] [PubMed] [Google Scholar]
- 52.Barré N, Garris GI, Lorvelec O. Field sampling of the tick Amblyomma variegatum (Acari: Ixodidae) on pastures in Guadeloupe: attraction of CO2 and/or tick pheromones and conditions of use. Exp. Appl. Acarol. 1997;21:95–108. doi: 10.1023/b:appa.0000031788.88306.77. [DOI] [PubMed] [Google Scholar]
- 53.Sonenshine DE. Pheromones and other semiochemicals of the Acari. Annu. Rev. Entomol. 1985;30:1–28. doi: 10.1146/annurev.en.30.010185.000245. [DOI] [PubMed] [Google Scholar]
- 54.Benoit JB, Lopez-Martinez G, Philips SA, Elnitsky MA, Yoder JA, Lee RE, Jr, Denlinger DL. The seabird tick, Ixodes uriae, uses uric acid in penguin guano as a kairomone and guanine in tick feces as an assembly pheromone on the Antarctic Peninsula. Polar Biol. 2008;31:1445–1451. [Google Scholar]
- 55.Treverrow NL, Stone BF, Cowie M. Aggregation pheromone in two Australian hard ticks, Ixodes holocyclus and Aponomma concolor. Experientia. 1977;33:680–682. doi: 10.1007/BF01946573. [DOI] [PubMed] [Google Scholar]
- 56.Dusbábek F, Šimek P, Jegorov A, Tříska J. Identification of xanthine and hypoxanthine as components of assembly pheromone in excreta of argasid ticks. Exp. Appl. Acarol. 1991;11:307–316. doi: 10.1007/BF01202877. [DOI] [PubMed] [Google Scholar]
- 57.Leahy MG, Hajkova Z, Bourchalova J. Two female pheromones in the metastriate tick, Hyalomma dromedarii (Acarina: Ixodidae) Acta Entomol. Bohemoslov. 1981;78:224–230. [Google Scholar]
- 58.Grenacher S, Kröber T, Guerin PM, Vlimant M. Behavioral and chemoreceptor cell response of the tick Ixodes ricinus to its own feces and faecal constituents. Exp. Appl. Acarol. 2001;25:641–660. doi: 10.1023/a:1016145805759. [DOI] [PubMed] [Google Scholar]
- 59.Allan SA, Sonenshine DE. Evidence of an assembly pheromone in the black-legged deer tick, Ixodes scapularis. J. Chem. Ecol. 2002;28:15–27. doi: 10.1023/a:1013554517148. [DOI] [PubMed] [Google Scholar]
- 60.Leahy MG, Sternberg S, Mango C, Galun RJ. Lack of specificity in assembly pheromones of soft ticks (Acari: Argasidae) Med. Entomol. 1975;31:413–414. doi: 10.1093/jmedent/12.4.413. [DOI] [PubMed] [Google Scholar]
- 61.Gothe R, Neitz AWH. Investigation into the participation of male pheromones of Rhipicephalus evertsi evertsi during infestation. Onderstepoort J. Vet. 1985;52:67–70. [PubMed] [Google Scholar]
- 62.Sonenshine DE, Adams T, Allan SA, McLaughlin J, Webster FX. Chemical composition of some components of the arrestment pheromone of the black-legged tick, Ixodes scapularis (Acari: Ixodidae) and their use in tick control. J. Med. Entomol. 2003;40:849–859. doi: 10.1603/0022-2585-40.6.849. [DOI] [PubMed] [Google Scholar]
- 63.McMahon C, Kröber T, Guerin PM. In vitro assay for repellents and deterrents for ticks: differing effects of products when tested with attractant or arrestment stimuli. Med. Vet. Entomol. 2003;17:370–378. doi: 10.1111/j.1365-2915.2003.00453.x. [DOI] [PubMed] [Google Scholar]
- 64.Brunner JL, Cheney L, Keesing F, Killilea M, Logiudice K, Previtali A, Ostfeld RS. Molting success of Ixodes scapularis varies among individual blood meal hosts and species. J. Med. Entomol. 2011;48:860–866. doi: 10.1603/me10256. [DOI] [PubMed] [Google Scholar]
- 65.Colloff M. Dust Mites. Melbourne: CSIRO Publishing; 2009. [Google Scholar]
- 66.Regev S, Cone WW. Evidence of farnesol as a male sex attractant of the two spotted spider mite, Tetranychus urticae Koch (Acarina: Tetranychidae) Environ. Entomol. 1975;4:307–311. [Google Scholar]
- 67.Regev S, Cone WW. Analyses of pharate female two spotted spider mites for nerolidol and geraniol: evaluation for sex attraction of males. Environ. Entomol. 1976;5:133–138. [Google Scholar]
- 68.Regev S, Cone WW. The monoterpene citronellol, as a male sex attractant of the two spotted spider mite, Tetranychus urticae (Acarina: Tetranychidae) Environ. Entomol. 1980;9:50–52. [Google Scholar]
- 69.Fashing NJ. Morphological adaptations associated with mate-guarding behavior in the genus Hericia (Acari: Algophagidae) In: Halliday RB, Walter DE, Proctor HC, Norton RA, Colloff MJ, editors. Acarology: Proceedings of the 10th International Congress. Melbourne: CSIRO Publishing; 2001. pp. 176–179. [Google Scholar]
- 70.Yasui I. The receptor sites of sex pheromone eliciting precopulatory mite guarding behavior of male predatory mite Macrocheles muscaedomesticae (Scopoli) J. Ethol. 1992;10:81–83. [Google Scholar]
- 71.Witalinski W, Dabert J, Walzl MG. Morphological adaptation for precopulatory guarding in astigmatic mites (Acari: Acarididae) Int. J. Acarol. 1992;18:49–54. [Google Scholar]
- 72.Oku K. Effects of density experience on mate guarding behavior by adult male Kanzawa spider mite. J. Ethol. 2009;27:279–283. [Google Scholar]
- 73.Sato M, Kuwahara Y, Matsuyama S. Chemical ecology of astigmatid mites XXXVII; fatty acids as food attractant of astigmatid mites, its scope and limitations. Appl. Entomol. Zool. 1993;28:565–569. [Google Scholar]
- 74.Kuwahara Y, Sato M, Koshii T, Suzuki T. Chemical ecology of astigmatid mites. XXXII. 2-Hydroxy-6-methyl-benzaldehyde, the sex pheromone of the brown-legged grain mite, Aleuroglyphus ovatus (Troupeau) (Acarina: Acaridae) Jpn. Soc. Appl. Entomol. Zool. 1992;27:253–260. [Google Scholar]
- 75.Ryono A, Mori N, Okabe K, Kuwahara Y. Chemical ecology of astigmatid mites, LVIII. 2-Hydroxy-6-methylbenzaldehyde: female sex pheromone of Cosmoglyphus hughesi Samsinak (Acari: Acaridae) Appl. Entomol. Zool. 2001;36:77–81. [Google Scholar]
- 76.Tatami K, Mori N, Nishida R, Kuwahara Y. 2-Hydroxy-6-methylbenzaldehyde: the female sex pheromone of the house dust mite Dermatophagoides farinae (Astigmata: Pyroglyphidae) Jpn. Soc. Med. Entomol. Zool. 2001;52:279–286. [Google Scholar]
- 77.Mori N, Kuwahara Y, Kurosa K, Nishida R, Fukushima T. Chemical ecology of astigmatid mites. XLI. Undecane: The sex pheromone of the acarid mite Caloglyphus rodriguezi Samsinak (Acarina: Acaridae) Appl. Entomol. Zool. 1995;30:415–423. [Google Scholar]
- 78.Mori N, Kuwahara Y, Kuroas K. Chemical ecology of astigamatid mites. XLV. (2R, 3R)-Epoxyneral: sex pheromone of the acarid mite Caloglyphus sp. (Acarina: Acaridae) Bioorg. Med. Chem. 1996;4:289–295. doi: 10.1016/0968-0896(96)00005-3. [DOI] [PubMed] [Google Scholar]
- 79.Mori N, Kuwahara Y, Kuroas K. Rosefuran: the sex pheromone of the acarid mite Caloglyphus sp. J. Chem. Ecol. 1998;24:1771–1779. [Google Scholar]
- 80.Mizoguchi A, Mori N, Nishida R, Kuwahara Y. α-Acaridial a female sex pheromone from an alarm pheromone emitting mite Rhizoglyphus robini. J. Chem. Ecol. 2003;29:1681–1690. doi: 10.1023/a:1024235100289. [DOI] [PubMed] [Google Scholar]
- 81.Mizoguchi A, Murakami K, Shimizu N, Mori N, Nishida R, Kuwahara Y. S-Isorobinal as the female sex pheromone from an alarm pheromone-emitting mite, Rhizoglyphus setosus. Exp. Appl. Acarol. 2005;36:107–117. doi: 10.1007/s10493-005-1666-z. [DOI] [PubMed] [Google Scholar]
- 82.Maruno G, Mori N, Kuwahara Y. Chemical ecology of the Astigmatid mites. LXXXVII. S-(+)-Isopiperitenone: re-identification of the alarm pheromone as the female sex pheromone in Tyrophagus similis (Acari: Acaridae) J. Chem. Ecol. 2012;38:36–41. doi: 10.1007/s10886-012-0059-0. [DOI] [PubMed] [Google Scholar]
- 83.Mori N, Kuwahara Y. Comparative studies of the ability of males to discriminate between sexes in Caloglyphus sp. J. Chem. Ecol. 2000;26:1299–1309. [Google Scholar]
- 84.Saito Y. Plant Mites and Sociality: Diversity and Evolution. Tokyo: Springer Publishing; 2010. [Google Scholar]
- 85.Sonenshine DE. 2,6-Dichlorophenol, the sex pheromone of the Rocky Mountain wood tick, Dermacentor andersoni (Stiles) and the American dog tick, Dermacentor variabilis (Say) J. Chem. Ecol. 1976;2:201–211. [Google Scholar]
- 86.Price T, Sonenshine DE, Norval RAI, Yunker CE, Burridge MJ. Pheromonal composition of two species of African Amblyomma ticks: similarities, difference and possible species specific components. Exp. Appl. Acarol. 1994;18:37–50. doi: 10.1007/BF00051401. [DOI] [PubMed] [Google Scholar]
- 87.Borges L, Eiras AE, Ferri PH, Lobo ACC. The role of 2,6-dichlorophenol as a sex pheromone of the tropical horse tick Anocentor nitens (Acari: Ixodidae) Exp. Appl. Acarol. 2002;27:223–230. doi: 10.1023/a:1021620215416. [DOI] [PubMed] [Google Scholar]
- 88.Gachoka KK, Ferreira LL, Louly CCB, Borges LMF. Induction of complete courtship ritual in Amblyomma cajennense using 2,6-dichlorophenol at female-equivalent quantities. Rev. Bras. Parasitol. Vet. 2011;20:347–350. doi: 10.1590/s1984-29612011000400017. [DOI] [PubMed] [Google Scholar]
- 89.Berger RS. 2,6-dichlorophenol, sex pheromone of the lone star tick. Science. 1972;177:704–705. doi: 10.1126/science.177.4050.704. [DOI] [PubMed] [Google Scholar]
- 90.Louly CCB, Silveira DD, Soares SF, Ferri PH, de Melo ACC, Borges LMF. More about the role of 2,6-dichlorophenol in tick courtship: identification and olfactometer bioassay in Amblyomma cajennense and Rhipicephalus sanguineus. Mem. Inst. Oswaldo Cruz. 2008;103:60–65. doi: 10.1590/s0074-02762008000100009. [DOI] [PubMed] [Google Scholar]
- 91.Soares SF, Borges MLF. Electrophysiological responses of the olfactory receptors of the tick Amblyomma cajennense (Acari: Ixodidae) to host-related and tick pheromone-related synthetic compounds. Acta Trop. 2012;124:192–198. doi: 10.1016/j.actatropica.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 92.Sonenshine DE, Hamilton JG, Lusby WR. Use of cholesteryl esters as mounting sex pheromone in combination with 2,6-dichlorophenol and pesticides to control populations of hard ticks. 5149526. United States Patent. 1992
- 93.Kuwahara Y. How Astigmatic Mites Control the Emission of Two or even Three Types of Pheromones from the Same Gland. In: Sabelis MW, Bruin J, editors. Trends in Acarology: Proceedings on the 12th International Conference. London: Springer Publishing; 2010. pp. 241–246. [Google Scholar]
- 94.Nishimura K, Shimizu N, Mori N, Kuwahara Y. Chemical ecology of astigmatid mites. LXIV. The alarm pheromone neral functions as an attractant in Schwiebea elongata (Banks) (Acari: Acaridae) Appl. Entomol. Zool. 2002;37:13–18. [Google Scholar]
- 95.Skelton AC, Cameron MM, Pickett JA, Birkett MA. Identification of neryl formate as the airborne aggregation pheromone for the American house dust mite and the European house dust mire (Acari: Epidermoptidae) Med. Vet. Entomol. 2010;47:798–804. doi: 10.1603/me09295. [DOI] [PubMed] [Google Scholar]
- 96.Achiano KA, Giliomee JH. Feeding behavior of the potential predators of the house flies Musca domestica L. and Fannia canicularis (L.) (Diptera: Muscidae) Afr. Entomol. 2006;14:69–75. [Google Scholar]
- 97.Wicht MC, Rodriguez JG, Smith WT, Jr, Jalil M. Attractant to Macrocheles muscaedomesticae (Acarina) present in the housefly, Musca domestica. J. Insect Physiol. 1971;17:63–67. [Google Scholar]
- 98.Kaufman PE, Burgess M, Rutz DA, Glenister C. Population dynamics of manure inhabiting arthropods under an integrated pest management (IPM) program in New York poultry facilities - 3 case studies. J. Appl. Poult. Res. 2002;11:90–103. [Google Scholar]
- 99.Tarpy DR, Summers J. North Carolina Cooperative Extension Service. Raleigh: N.C. State University; 2014. Managing Varroa Mites in Honeybee Colonies. [Google Scholar]
- 100.Sammataro D, Gerson U, Needham G. Parasitic mites of honeybees: life history, implications and impacts. Annu. Rev. Entomol. 2000;45:519–548. doi: 10.1146/annurev.ento.45.1.519. [DOI] [PubMed] [Google Scholar]
- 101.Le Conte Y, Arnold G, Trouiller J, Masson C, Chappe B, Ourisson G. Attraction of the parasitic mite Varroa jacobsoni to the drone larvae of honeybees by simple aliphatic esters. Science PRS. 1989:638–639. doi: 10.1126/science.245.4918.638. [DOI] [PubMed] [Google Scholar]
- 102.Calderone NW, Lin S. Behavioral responses of Varroa destructor (Acari: Varroidae) to extracts of larvae, cocoons, and food brood of worker and drone honey bees, Apis melifera (Hymenoptera: Apidae) Physiol. Entomol. 2001;26:341–350. [Google Scholar]
- 103.Kilpinen O. How to obtain a bloodmeal without being eaten by a host: the case of poultry red mite, Dermanyssus gallinae. Physiol. Entomol. 2005;30:232–240. [Google Scholar]
- 104.Koenraadt CJM, Dick M. The role of volatiles in aggregation and host-seeking of the haematophagous poultry red mite Dermanyssus gallinae (Acari: Dermanyssidae) Exp. Appl. Acarol. 2010;50:191–199. doi: 10.1007/s10493-009-9305-8. [DOI] [PubMed] [Google Scholar]
- 105.Carroll JF. Notes on the responses of host-seeking nymphs and adults of the ticks Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) to canine, avian and deer-produced substances. Proc. Entomol. Soc. Wash. 2002;104:73–78. [Google Scholar]
- 106.Carroll JF. Responses of three species of adult ticks (Acari: Ixodidae) to chemicals in the coats of principal and minor hosts. J. Med. Entomol. 1999;36:238–241. doi: 10.1093/jmedent/36.3.238. [DOI] [PubMed] [Google Scholar]
- 107.Garcia R. Carbon dioxide as an attractant for certain ticks (Acarina: Argasidae and Ixodidae) Ann. Entomol. Soc. Am. 1962;55:605–606. [Google Scholar]
- 108.Carroll JF. Interdigital gland substances of white-tailed deer and the response of host-seeking ticks (Acari: Ixodidae) J. Med. Entomol. 2001;38:114–117. doi: 10.1603/0022-2585-38.1.114. [DOI] [PubMed] [Google Scholar]
- 109.McMahon C, Guerin PM. Attraction of the tropical bont tick, Amblyomma variegatum to human breath and to the breath components acetone. NO, and CO2 . Naturwissenschaften. 2002;89:311–315. doi: 10.1007/s00114-002-0317-z. [DOI] [PubMed] [Google Scholar]
- 110.Holscher KH, Gearhart HL, Barker RW. Electrophysiological response of three tick species to carbon dioxide in the laboratory and field. Ann. Entomol. Soc. Am. 1980;73:288–292. [Google Scholar]
- 111.Anderson RB, Scrimgeour GJ, Kaufman WR. Responses of the tick, Amblyomma hebraeum (Acari: Ixodidae) to carbon dioxide. Exp. Appl. Acarol. 1998;22:667–681. [Google Scholar]
- 112.Gugliemone AA, Morehouse ED, Wolf G. Attraction to carbon dioxide of unfed stages of Ambyomma trigutatum under field conditions. Acarologia. 1985;26:123–129. [Google Scholar]
- 113.Falco RC, Fish D. Horizontal movement of adult Ixodes dammini (Acari: Ixodidae) attracted to CO2 baited traps. J. Med. Entomol. 1991;28:726–729. doi: 10.1093/jmedent/28.5.726. [DOI] [PubMed] [Google Scholar]
- 114.Gherman CM, Mihalca AD, Dumitrache MO, Gyorke A, Oroian I, Sandor M, Cozma V. CO2 flagging, an improved method for collection of questing ticks. Parasit. Vectors. 2012;5:125. doi: 10.1186/1756-3305-5-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mackay A, Foil L. Seasonal distribution of adult Ixodes scapularis Say (Acari: Ixodidae) in Louisiana. J. Vector. Ecol. 2005;30:168–170. [PubMed] [Google Scholar]
- 116.Dykstra EA, Teel PD. Influence of daily activity of adult Rhipicephalus sanguineus (Acari: Ixodidae) on CO2-trap sampled in acaricide efficacy trials. Southwest. Entomol. 1994;19:119–128. [Google Scholar]
- 117.Steullet P, Guerin PM. Identification of vertebrate volatiles stimulating olfactory receptors on tarsus I of the tick Amblyomma variegatum Fabricius (Ixodidae) II, Receptors outside the Haller's organ capsule. J. Comp. Physiol. 1994;174:39–47. doi: 10.1007/BF00192003. [DOI] [PubMed] [Google Scholar]
- 118.Norval RAI. Field sampling of unfed adults of Amblyomma hebraeum Koch. Exp. Appl. Acarol. 1987;3:213–217. doi: 10.1007/BF01270457. [DOI] [PubMed] [Google Scholar]
- 119.Norval RAI, Yunker CE, Gibson JD, Deem SLD. Field sampling of unfed nymphs of Amblyomma hebraeum. Exp. Appl. Acarol. 1988;4:173–177. doi: 10.1007/BF01193875. [DOI] [PubMed] [Google Scholar]
- 120.Smith CN, Cole MM, Gouk HK. Biology and control of the American dog tick. USDA Tech. Bull. 1946;905:74. [Google Scholar]
- 121.Osborn DA, Miller KV, Hoffman DM, Dickerson WH, Gasset JW, Quist CF. Morphology of the white-tailed deer tarsal gland. Acta Theriol. 2000;45:117–122. [Google Scholar]
- 122.Alexy KJ, Gasset JW, Osburn DA, Miller KV, Russell SM. Bacterial fauna of the tarsal tufts of White-tailed deer (Odocoileus virginianus) Am. Midl. Nat. 2003;149:237–240. [Google Scholar]
- 123.Harvey RG, Harari J, Delauche AJ. Ear Diseases of the Dog and Cat. London: Manson Publishing; 2001. [Google Scholar]
- 124.Carroll JF, Grasela JJ. Occurrence of adult American dog ticks, Dermacentor variabilis (Say) around small animal traps and vertebrate carcasses. Proc. Entomol. Soc. Wash. 1998;88:253–256. [Google Scholar]
- 125.Dobrotvorsky AK, Tkachev AV, Naumov RI, Carroll CF. Study of host odour determinants of questing behavior in Ixodes ticks. In: Kazimírová M, Labuda M, Nuttall PA, editors. Proceedings of the 3rd International Conference on Tick and Tick Borne Diseases. Slovakia: High Tatra Mountains Press; 2000. pp. 229–233. [Google Scholar]
- 126.Leonovich SA. Phenol and lactone receptors in the distal sensilla of the Haller's organ in Ixodes ricinus ticks and their possible role in host perception. Exp. Appl. Acarol. 2004;32:89–102. doi: 10.1023/b:appa.0000018200.24760.78. [DOI] [PubMed] [Google Scholar]
- 127.Tatemoto S, Shimoda T. Olfactory responses of the predatory mites (Neoseiulus cucumeris) and insects (Orius strigicollis) to two different plant species infested with onion thrips (Thrips tabaci) J. Chem. Ecol. 2008;34:605–613. doi: 10.1007/s10886-008-9469-4. [DOI] [PubMed] [Google Scholar]
- 128.Aratchige NS, Lesna I, Sabelis MW. Below ground plant parts emit herbivore-induced volatiles: olfactory responses of a predatory mite to tulip bulbs infested by rust mites. Exp. Appl. Acarol. 2004;33:21–30. doi: 10.1023/b:appa.0000030011.66371.3f. [DOI] [PubMed] [Google Scholar]
- 129.Ishiwari H, Suzuki T, Maeda T. Essential compounds in herbivore-induced plant volatiles that attract the predatory mite Neoseiulus womersleyi. J. Chem. Ecol. 2007;33:1670–1681. doi: 10.1007/s10886-007-9344-8. [DOI] [PubMed] [Google Scholar]
- 130.Amin MM, Mizell RF, III, Flowers RW. Response of the predatory mite Phytoseiulus macropilis (Acari: Phytoseiidae) to pesticides and kairomones of three spider mite species (Acari: Tetranychidae) and non-prey food. Fla. Entomol. 2009;92:554–562. [Google Scholar]
- 131.Dicke M, Van Beek TA, Posthumus MA, Ben Don N, Van Bokhoven H, De Groot AE. Isolation and identification of volatile kairomone that affects acarine predator–prey interactions: involvement of host plant in its production. J. Chem. Ecol. 1990;16:381–396. doi: 10.1007/BF01021772. [DOI] [PubMed] [Google Scholar]
- 132.De Boer JG, Posthumus MA, Dicke M. Identification of volatiles that are used in discrimination between plants infested with prey or non–prey herbivores by a predatory mite. J. Chem. Ecol. 2004;30:2215–2230. doi: 10.1023/b:joec.0000048784.79031.5e. [DOI] [PubMed] [Google Scholar]
- 133.Gols R, Roosjen M, Dijman H, Dicke M. Induction of direct and indirect plant response by jasmonic acid, low spider mite densities, or a combination of jasmonic acid treatment and spider mite infestation. J. Chem. Ecol. 2003;29:2651–2666. doi: 10.1023/b:joec.0000008010.40606.b0. [DOI] [PubMed] [Google Scholar]
- 134.Shimoda T. A key volatile infochemical that elicits a strong olfactory response of the predatory mite Neoseiulus californicus, an important natural enemy of the two-spotted spider mite Tetranychus urticae. Exp. Appl. Acarol. 2009;50:9–22. doi: 10.1007/s10493-009-9275-x. [DOI] [PubMed] [Google Scholar]
- 135.Shimoda T, Ozawa R, Sano K, Yano E, Takabayashi J. The involvement of volatile infochemicals from spider mites and from food-plants in prey location of the generalist predatory mite, Neoseiulus californicus. J. Chem. Ecol. 2005;31:2019–2032. doi: 10.1007/s10886-005-6075-6. [DOI] [PubMed] [Google Scholar]
- 136.Sabelis MW, Janssen A, Takabayashi J. Can plants evolve stable alliances with the enemies of enemies? J. Plant Interact. 2011;6:71–75. [Google Scholar]
- 137.Hassan SM, Dipeolu OO, Malonza MM. Natural attraction of livestock ticks by the leaves of a shrub. Trop. Anim. Health Prod. 1994;26:87–91. doi: 10.1007/BF02239905. [DOI] [PubMed] [Google Scholar]
- 138.Vanhaelen M, Vanhaelen-Fastré R, Geeraets J, Wirthlin T. Cis- and trans-octa-1, 5-dien-3-ol; new attractants to the cheese mite Tyrophagus putrescentiae (Schrank) (Acaridae) identified in Trichothecium roseum (Fungi imperfecti) Microbios. 1979;23:199–212. [PubMed] [Google Scholar]
- 139.Vanhaelen M, Vanhaelen-Fastré R, Geeraets J. Occurrence in mushrooms (Homobasidiomycetes) of cis- and trans-octa-1, 5-dien-3-ol: attractants to the cheese mite Tyrophagus putrescentiae (Schrank) (Acaridae) Experientia. 1980;36:406–407. [Google Scholar]
- 140.Allan SA, Barré N, Sonenshine DE, Burridge MJ. Efficiency of tags impregnated with pheromone and acaricide for control of Amblyomma variegatum. Med. Vet. Entomol. 1998;12:141–150. doi: 10.1046/j.1365-2915.1998.00080.x. [DOI] [PubMed] [Google Scholar]
- 141.Norval RAI, Sonenshine DE, Meltzer MI, Burridge MJ. Attractant decoy for controlling bont ticks. 5,296,277. United States Patent Office. 1994
- 142.Allan SA, Sonenshine DE, Burridge MJ. Tick pheromones and uses thereof. 6331297. United States Patent Office. 2002
- 143.Sonenshine DE, Taylor D, Corrigan G. Studies to evaluate the effectiveness of sex pheromone impregnated formulations for control of populations of the American dog tick Dermacentor variabilis (Say) (Acari: Ixodidae) Exp. Appl. Acarol. 1985;1:23–24. doi: 10.1007/BF01262197. [DOI] [PubMed] [Google Scholar]
- 144.Hamilton JG, Sonenshine DE. Methods and apparatus for controlling arthropods populations. 4884361. United States Patent Office. 1989
- 145.Abdel-Rahman MS, Fahmy MM, Aggour MG. Trials for control of ixodid ticks using pheromone-acaricide tick decoys. J. Egypt. Soc. Parasitol. 1998;28:551–557. [PubMed] [Google Scholar]
- 146.George JE. Present and future technologies for tick control. Ann. N.Y. Acad. Sci. 2000;916:583–588. doi: 10.1111/j.1749-6632.2000.tb05340.x. [DOI] [PubMed] [Google Scholar]
- 147.Ginsberg HS. Tick Control: Trapping, Biocontrol, Host Management, and other Alternative Strategies. In: Sonenshine DE, Roe RM, editors. Biology of Ticks. New York: Oxford University Press; 2014. pp. 409–434. [Google Scholar]
- 148.Drukker B, Bruin J, Sabelis MW. Anthocorid predators learn to associate herbivore induced plant volatiles with presence or absence of prey. Physiol. Entomol. 2000;25:260–265. [Google Scholar]
- 149.Pickett JA, Bruce TJA, Chamerbalin K, Hassanali A, Khan ZR, Matthes MC, Napier JA, Smart LE, Wadhams LJ, Woodcock CM. Plant Volatiles Yielding New Ways to Exploit Plant Defense. In: Dicke M, Tekken W, editors. Chemical Ecology: From Gene to Ecosystem. The Netherlands: Springer Press; 2006. pp. 161–173. [Google Scholar]
- 150.Khan ZR, James DG, Midega CAO, Pickett JA. Chemical ecology and conservation biological control. Biol. Control. 2008;45:210–224. [Google Scholar]
- 151.Yu H, Zang Y, Wu K, Gao XW, Guo YY. Field-testing of synthetic herbivore-induced plant volatiles as attractants for beneficial insects. Environ. Entomol. 2008;37:1410–1415. doi: 10.1603/0046-225X-37.6.1410. [DOI] [PubMed] [Google Scholar]
- 152.Lee JC. Methyl salicylate as a natural enemy attractant in strawberry fields. Environ. Entomol. 2010;39:653–660. doi: 10.1603/EN09279. [DOI] [PubMed] [Google Scholar]