Abstract
Thrombosis is a necessary physiological process to protect the body from uncontrolled bleeding. Pathological thrombus formation can lead to devastating clinical events including heart attack, stroke, deep vein thrombosis, pulmonary embolism, and disseminated intravascular coagulation. Numerous drugs have been developed to inhibit thrombosis. These have been targeted to coagulation factors along with proteins and receptors that activate platelets. While these drugs are effective at preventing blood clotting, their major side effect is inadvertent hemorrhage that can result in significant morbidity and mortality. There exists a need for anticoagulants that are not only effective at preventing thrombosis but can also be readily reversed. Aptamers offer a potential solution, representing a new class of drug agents that can be isolated to any protein and where antidote oligonucleotides can be designed based on the sequence of the aptamer. We present a summary of the anticoagulant and antithrombotic aptamers that have been identified and their stage of development and comment on the future of aptamer-based drug development to treat thrombosis.
Introduction
Thrombosis is ubiquitous in society and medicine. It can either be protective in the form of hemostasis or harmful when it manifests as acute myocardial infarction, ischemic stroke, deep vein thrombosis, pulmonary embolism, or disseminated intravascular coagulation that accompanies shock states. Our understanding of thrombosis has evolved from early observations in which coagulation was understood as a series of well-orchestrated biochemical steps, to a series of events that take place on tissue factor-bearing cells and platelets that integrates biochemistry, cellular and molecular biology. A more rich and detailed perspective of thrombosis introduces a myriad of potential targets that may successfully uncouple benefit and risk. Specifically, a drug strategy that attenuates or fully prevents thrombosis while not disturbing hemostasis or vascular integrity represents the “holy grail” in vascular medicine.
Antithrombotic aptamers were developed to take full advantage of several highly attractive intrinsic properties. First, they can be generated to specifically inhibit one or more protein or cellular targets participating in thrombosis. Second, the extent or degree of inhibition by aptamer agents can be carefully regulated by employing complementary “antidote” oligonucleotides, which bind and inhibit their complementary active agents. Third, the desired duration of aptamer-mediated inhibition could be determined either on the “front-end” of development and manufacturing, during the clinical implementation stage by selecting a specific route of administration or on the “back-end” by the availability of rapid and selective reversal agents.
Herein, we provide a summary of several discovery and developmental programs for antithrombotic aptamers for use in cardiovascular and related fields. We also describe several challenges that we faced during the 10-year journey and suggest new paths for development.
Properties and Distinct Characteristics of Aptamers
The characteristics of an aptameric system, notably high specificity and easy reversibility, are ideally suited to the development of antithrombotic therapies in which the dose limiting toxicity is bleeding [1,2].
Due to the small size of aptamers (in comparison is antibodies or other peptide inhibitors) and the vast libraries that can be generated using random sequences of oligonucleotides, aptamers are uniquely suited to the development of inhibitors of proteins in the coagulation cascade, which have been difficult to target by other routes, including upstream factors, which are present in only small quantities and whose activated states have been difficult to target with inhibitors [3].
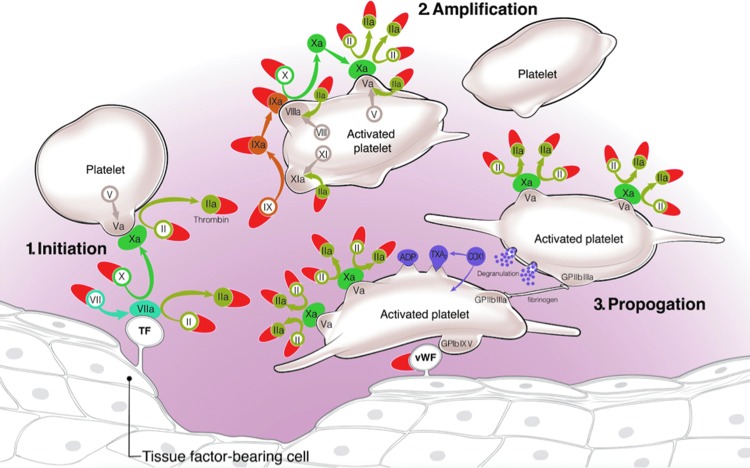
Our understanding of coagulation has undergone significant evolution from a traditional “waterfall” model to a “cell-based” model in which the interplay between coagulation proteases and cell surfaces and in particular platelet surfaces are highlighted (Fig. 1) [4–6]. In this model, a complex of tissue factor and factor VIIa initiates coagulation by binding to and activating factors IX and X. This leads to a small amount of thrombin generation, which, on the surface of platelets, leads to amplification or “priming” of the coagulation signal via direct stimulation of the platelet protease-activated receptors PAR1 and PAR4. Thrombin propagation continues via additional generation and stabilization of factor IXa on the platelet surface in combination with VIIIa, leading to activation of factor Xa. At this point, the activation of factor Xa on the platelet surface is more than 50-fold more efficient then direct activation by the tissue factor/VIIa complex in the plasma, where factor Xa is quickly inhibited by circulating tissue factor inhibitor and antithrombin III.
FIG. 1.
Cell-based model of coagulation. Red semilunar shapes represent aptamers that have been isolated to targets affecting coagulation and platelet aggregation.
Factor IX/IXa Aptamer and Antidote Development Program
In this model, the factor IXa/VIIIa complex is central to multiple steps of coagulation, and it is particularly important in the transition from initial activation to propagation phases of coagulation, likely due to the relatively inefficient way in which it is inhibited by circulating antithrombin III and other inhibitors.
In addition, factor IXa is an attractive target for therapeutic anticoagulation given a number of other properties and observations, including the following:
• Factor IXa is up to 7-fold more thrombogenic than factor Xa and up to 60-fold more thrombogenic than thrombin [7].
• Factor IX is the rate-limiting step in thrombin generation in models of thrombosis [8].
• Given it upstream role in both initiation and amplification portions of the cell-based theory of coagulation, its concentration is lower and less variable than downstream targets such as factor X or thrombin.
• The thrombogenicity of prothrombin concentrates is most closely associated with their factor IXa levels [9].
• Murine models demonstrate that in vivo FIXa activity is primarily deterministic of venous thrombosis in a model of saphenous vein thrombosis [10].
• Hemophilia B patients, with ∼50% reduction in FIX levels, have been shown in epidemiological studies to be at lower risk for cardiovascular events [11,12].
Despite this attractive role in the center of multiple steps of the coagulation cascade, inhibitors of factor IXa have not been tested in any meaningful way. In part, this is due to the difficulty of obtaining inhibitors to activated factor IX, as the binding site of factor IXa does not fully form until it is bound to factor VIIIa, leading to allosteric activation [13]. When bound to factor VIIIa, factor IXa remains effectively shielded from inactivation by a multitude of factors, including antithrombin III, nexin-2/amyloid beta-protein precursor, and protein Z-dependent protease inhibitor, and inhibition of Factor IXa activity is inefficient compared to that achieved for other coagulation factors such as Xa and thrombin [14].
While factor IXa inhibition has been tested and found to be effective in preclinical models [13,15–17], previously developed factor IXa inhibitors have either not been tested (the competitive antagonist IXai) or have not been effective in the clinical setting (TTP889) [13]. TTP889, an oral partial factor IXa inhibitor whose maximum reported achievable level of factor IXa inhibition is ∼90% [18], did not prevent venous thrombosis post-hip surgery. Biomarker studies suggested minimal efficacy at factor IXa inhibition and possibly inadequate dosing.
Oligonucleotide aptamers offer a unique opportunity to make a potent antithrombotic therapy targeting a central step in coagulation, effecting a high level of anticoagulation, with reversal available should the clinical need for anticoagulation change or bleeding ensue. Rusconi et al. were able to select a specific factor IXa inhibiting aptamer from over 1014 nucleic acid sequences using systematic evolution of ligands by exponential enrichment (SELEX), and subsequently design and identify a complementary RNA controlling agent, which was most efficacious at reversing its activity [19,20]. To increase the in vivo half-life and limit its volume of distribution, the initial aptamer (9.3t) was linked to a 40 kDa polyethylene glycol (PEG) moiety.
In vitro binding studies showed high affinity for factor IX and IXa, with little binding to factors VII, X, XI, or protein C [20,21]. In addition, the aptamer PEG-9.3t effectively inhibited factor IXa mediated activation of factor X and prolonged the activated partial thromboplastin time (aPTT) in human plasma, similar to what is observed in hemophilia B patients. Addition of the antidote 5.2C effected almost immediate, complete, and durable reversal of anticoagulant activity [20].
Based on these findings, the aptamers underwent further optimization to minimize degradation and clearance, and the aptamer pair RB006 (eventually renamed pegnivacogin) and its complementary controlling agent RB007 (anivamersen) were chosen for advanced testing and clinical application.
Preclinical evaluation
Aptamer RB006, developed as an inhibitor of human factor IXa, was demonstrated to cross-react with factor IX from a variety of species, allowing testing across a series of animal models [19]. Murine tail transection and arterial injury models were used to demonstrate that derivatives of aptamers 9.3t were able to cause bleeding at high doses, an event that was curtailed when the controlling agent was administered, while effectively preventing thrombosis in models of injury. Meanwhile porcine models were used to determine the effect of aptamer treatment on parameters of coagulation, demonstrating a dose related increase in the aPTT and whole blood clotting time. Amazingly these parameters were normalized within minutes of injection of the controlling agent, demonstrating that the theoretical concept of active control may in fact be feasible in vivo. The pharmacokinetics of factor IX inhibition and aptamer reversal were accurately predicted by in vitro modeling, and supported further development and clinical testing. Meanwhile porcine models were used to determine the effect of aptamer treatment on parameters of coagulation, demonstrating a dose-related increase in the aPTT and whole blood activated clotting time [22]. Finally, in a porcine model of cardiopulmonary byass, factor IXa inhibition maintained circuit patency for 60 min and upon administration of antidote-oligonucleotide, demonstrated durable reversal. In sample testing, the FIXa aptamer–antidote-treated pigs demonstrated lower thrombin generation and resulted in a much lower inflammatory response compared to heparin-protamine-treated animals [22].
Initial clinical testing
Clinical evaluation of the newly named REG1 anticoagulation system consisting of the anticoagulant RB006 and its controlling agent RB007, represented a clinical challenge in that a single therapeutic agent, the REG1 system, required testing and dosing optimization of disparate individual components. With this in mind, phase 1 testing commenced with dosing of the theoretical biologically inert component, RB007, followed by a cohort treated with RB006 to affect anticoagulation followed by reversal and finally a cohort treated with anticoagulant but no reversal [23]. After review of each treatment strata by the data safety monitoring board (DSMB), the process was repeated at the next dose of each of the anticoagulant and controlling agent.
Fixed doses of 15, 30, 60, and 90 mg of RB006 were administered, followed 3 h later by placebo or a 2:1 weight based dose of the controlling agent, which based on preclinical models was approximately fourfold above the minimal dose required to affect complete reversal of anticoagulant activity. Given the long half-life of the drug and the first-in-man nature of this study, a pharmacodynamics stopping rule was put in place such that if any subject demonstrated an increase in the aPTT >15% above 2.5-fold above the upper limit of normal (ULN), enrollment in that cohort would be terminated. In total, 85 subjects were randomized with 84 receiving study drug. This study [23] demonstrated the following:
• RB007 in the absence of RB006 resulted in no biologically measurable effect.
• Administration of RB006 resulted in a dose-dependent increase in the aPTT consistent with the predicted pharmacodynamics of the drug.
• In the absence of reversal, anticoagulation with RB006 appeared long lasting, with an estimated half-life of biological activity of ∼24 h.
• RB007 administration results in virtually immediate (1–5 min), complete, and durable (up to 7 days) reversal of RB006 anticoagulant effect.
• Dosing in the high dose cohort was curtailed after a single patient, a small female, exceeded the pharmacodynaimc stopping rule.
• Administration of RB007 resulted in immediate normalization of the aPTT, and no bleeding complications were noted.
These results were replicated in a phase 1B study enrolling patients with coronary disease on antiplatelet therapy, an important step in evaluating the safety of adding this potent anticoagulant to other therapies with potentially synergistic effects [24].
The use of fixed dosing strategy in these initial studies afforded the opportunity to determine the relationship between weight-adjusted doses and predicted levels of factor IX inhibition. Utilizing mixing studies with plasma deficient in factor IX, a relationship between degree of aPTT prolongation and factor IX inhibition was defined and used to derive individual patient information [25]. Combination of phase 1A and 1B data demonstrated (1) a tight linear relationship between weight-based dosing and achieved plasma concentrations, suggesting a single compartment model for RB006 distribution; (2) a predicted EC50 of 15 μg/mL with a maximal predicted 2.4-fold increase in aPTT prolongation; (3) derivation of a model of both administered RB006 weight-based dose and concentration achieved with degree of factor IX inhibition, suggesting that a dose of 0.75 mg/kg results in reliable depletion of >99% of factor IX activity, resulting in prolongation of the aPTT to 2.5-fold above the ULN, which was consistent with prior modeling. Notably these results were consistent across the two studies, establishing that RB006 pharmacokinetics and pharmacodynamics are consistent across the populations enrolled [25].
These findings were used to select a weight-based dose of 0.75 mg/kg in a phase 1C study aimed to establish the pharmacodynamics of reversal. In this study, 39 patients were randomized to repetitive dosing with RB006 with or without intervening reversal with variable levels of RB007 [26]. This study established that (1) repeat dosing with RB006 resulted in consistent reproducible effect of RB006 on measured aPTT on repeat dosing; (2) a dose-dependent reversal of aPTT prolongation based on ratio of RB006:RB007 administered; and (3) effective reanticoagulation of subjects reversed with RB007 upon redosing with RB006. This last finding is notable and consistent with the known pharmacokinetics of these components of the REG1 system, in which administration of an excess of RB007 to patients anticoagulated with RB006 results in formation of a biologically inert and irreversibly bound RB007:RB006 complex, with rapid excretion of any excess RB007 [26]. While not specifically tested, these pharmacokinetics suggest that a patient could be anticoagulated, reversed, and reanticoagulated within minutes with additional RB006.
Testing the clinical utility of REG1
Phase 1 testing suggested that further testing of REG1 in indications in which the rapid reversibility inherent in this system might be maximally effective and attractive was warranted. A phase 2A study was performed to establish the feasibility of REG1-based anticoagulation in patients undergoing percutaneous coronary intervention (PCI), an indication in which there is a need for high level anticoagulation during the procedure, but where rapid reversal postprocedure or if bleeding is noted might be particularly attractive to mitigate bleeding risk [27]. A conservative stepwise strategy was employed in which two initial patients underwent PCI using RB006 with glycoprotein IIB/IIIA inhibition to afford a level of background antithrombotic therapy. After this initial experience using RB006 to affect anticoagulation during PCI, 20 patients underwent PCI utilizing RB006 as the sole anticoagulant. Given theoretical concerns that immediate reversal postprocedure might predispose patients to stent thrombosis, the first 10 patients underwent partial (50%) reversal immediately post-procedure, followed by 100% reversal 4 h later, while the second 10 patients underwent immediate full reversal post procedure. These dosing decisions speak to the complexity of fully evaluating an anticoagulation system in which one must establish the safety and efficacy of strategies for each component. The REVERSAL-PCI study demonstrated feasibility of either immediate partial, or full reversal, but full evaluation of the effectiveness of partial and full reversal strategies on bleeding and potentially ischemic events would require a much larger study [27].
The RADAR study was designed to answer three key clinical questions: (1) would a 1 mg/kg dose of pegnivacogin, the now renamed RB006, reliably provide near complete inhibition of factor IXa in an acute coronary syndrome population, who may be more thrombogenic than the stable patients studied to date; (2) to determine the dose of anivamersen (RB007) required to reverse anticoagulation post-PCI to allow safe femoral sheath removal postprocedure; and (3) to determine the feasibility and safety of using REG1 to provide sole anticoagulation during PCI by assessing the incidence of ischemic events in patients treated with REG1 compared with those treated with heparin [28].
RADAR was a partially blinded study in which patients were initially randomized 4:1 in an open label fashion to REG1 versus heparin. Those randomized to REG1 were also randomized to a blinded dose of reversal designed to provide 25%, 50%, 75%, or complete reversal of anticoagulant activity postprocedure, followed by immediate sheath removal. Each reversal strategy was carefully monitored, and the DSMB was empowered to suggest stoppage of low reversal arms for excess bleeding and high reversal arms for excess ischemic events [28]. The 25% reversal arm was in fact discontinued after the first DSMB review suggested high bleeding rates, and subjects who were to be randomized to this arm were distributed among the remaining reversal strategies.
RADAR ultimately met all its objectives. A pharmacodynamic substudy completed early during the course of enrollment definitively demonstrated that near complete factor IX inhibition was achieved after a 1 mg/kg dose of pegnivacogin, even in select patients who underwent catheterization and therefore PD assessment the day following dosing [29].
RADAR defined that a 50% reversal dose given immediately post catheterization was sufficient to allow safe immediate sheath removal with bleeding rates similar to heparin, while higher rates of reversal did appear to mitigate major bleeding, although the low number of events precludes a definitive conclusion in this regard [30].
Finally, REG1 appeared to act as a highly effective anticoagulant during PCI, as evidenced by acute ischemic event rates that were numerically lower than what was observed with heparin. All of these findings supported the further testing of REG1 in an adequately powered phase 3 trial [30].
RADAR was terminated due to the occurrence of three unexpected serious allergic events in females enrolled in Europe in a clustered fashion late in the course of enrollment. A thorough investigation by the sponsor concluded that study drug material did not undergo any significant degradation or aggregation during storage and that product obtained from the sites when tested as retrieved or after induced degradation, showed no ability to directly activate complement or precipitate any detectable immune or inflammatory response. No patients had a preformed antibody to the aptameric component of the drug, however, all three patients who had an allergy were found to have significant levels of antiPEG antibodies, suggesting that the reactions might be due to the PEG moiety of pegnivacogin [31]. Given the multitude of PEGylated products in use [32,33] and the rarity of allergic reactions that have been observed [34,35] it was felt most likely that the allergic reactions observed were a clustering of an otherwise rare event.
REGULATE-PCI
The REGULATE-PCI trial was designed to definitely test the efficacy and safety of the REG1 system to prevent ischemic complications in patients undergoing PCI for a broad range of indications. Based on the results of RADAR, an 80% reversal dose was chosen to mitigate bleeding risk while minimizing the risk of acute ischemic events.
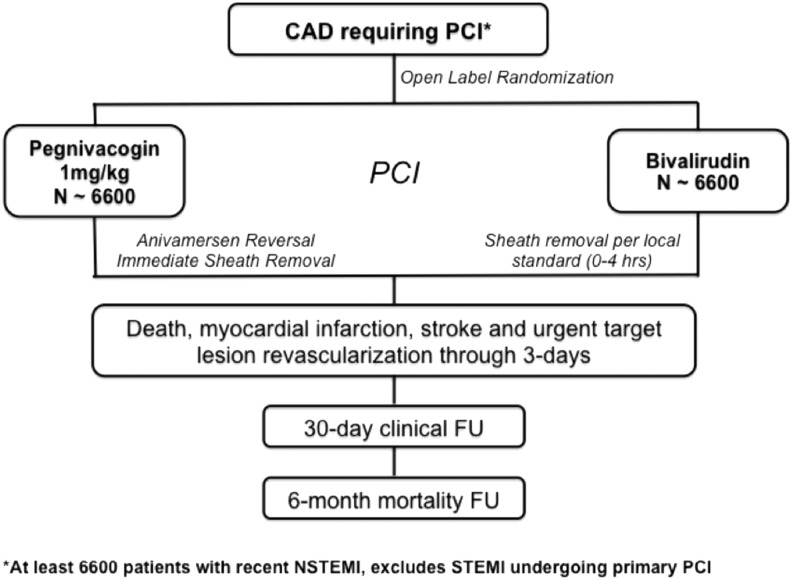
The design of REGULATE-PCI is shown in Fig. 2. The trial intended to enroll 13,200 patients randomized just before PCI to open-label REG1 versus bivalirudin. An open-label design was utilized to allow immediate sheath removal post procedure, reflecting the manner in which REG1 would be used in clinical practice. The primary endpoint was a composite of ischemic events including death, myocardial infarction, stroke, and urgent target lesion revascularization (stent thrombosis) through day 3 following PCI.
FIG. 2.
Design of the REGULATE-PCI trial. PCI, percutaneous coronary intervention.
Given the allergies observed in RADAR, a risk mitigation plan was established with FDA agreement, to both educate sites about the identification and treatment of allergic reactions, and to ensure collection of plasma samples obtained just before, and 1.5 and 20 h post PCI, to be used for mechanistic analyses if allergies were evident.
Ultimately REGULATE-PCI was curtailed after enrollment of only 3,232 of the planned 13,200 patients because of an excess of allergic responses in the REG1-treated patients, with 10 serious allergic events notes within 24 h of study drug dosing the REG1 patients versus only 1 in those treated with bivalirudin, without evidence of offsetting benefit [36]. While the sole serious allergy to bivalirudin manifested several hours postprocedure, 9 of 10 reactions in the REG1 arm were noted within minutes of study drug dosing and before administration of anivamersen, suggesting acute infusion reactions due to pegnivacogin. A full analysis of the mechanisms of these reactions is ongoing.
The primary results of REGULATE-PCI were somewhat disappointing. The use of REG1 during PCI failed to reduce the composite primary endpoint of death, myocardial infarction, or stroke [odds ratio (OR) 1.05, 95% confidence interval (95% CI) 0.80–1.39; P = 0.72] or change the rate of major bleeding (OR 3.49, 95% CI 0.73–16.82; P = 0.10), and it appeared to increase the incidence of major or minor bleeding (OR 1.64, 1.19–2.25; P = 0.002); however, several points are worth comment and consideration. First, as a prematurely discontinued trial, this study is not able to definitively answer whether or not reversible anticoagulation would fulfill its promise of greater efficacy without compromising safety (ie, bleeding); notably, the trial as terminated retained 70% conditional power if the underlying hypothesis is true. Second, the primary endpoint was driven almost exclusively by the incidence of peri-procedural myocardial infarctions, while other clinically important endpoints like stent thrombosis appeared to be mitigated by use of REG1. Finally, an 80% reversal dose was chosen partly due to the regulatory requirements to demonstrate superiority on ischemic events. Whether complete reversal might maintain ischemic efficacy while improving bleeding outcomes remains an intriguing avenue of investigation.
Aptamers Against Other Coagulation Proteins
In searching for the most effective anticoagulant, aptamers have been isolated to a number of different coagulation factors. Aptamers that have been developed against coagulation proteins have been summarized in Table 1.
Table 1.
Aptamers Isolated Against Coagulation Proteins
| Target | DNA/RNA | Binding (Kd) | In vitro activity | In vivo activity | Clinical studies | References |
|---|---|---|---|---|---|---|
| Prothrombin (FII) | RNA | 10 μM | Prolongs PT, aPTT, TCT Inhibits platelet activation | [38,58] | ||
| Thrombin (FIIa) | DNA | 25–200 nM | Prolongs PT | Prolongs PT and ACT in monkeys and canines | [40,41,39,59] | |
| RNA | 2.8 nM | Prolongs clotting time | [42] | |||
| Bivalent RNA | 1.3 nM | Prolongs PT and aPTT | [44] | |||
| FVII | RNA | 59–66 nM | Prolongs PT | [37] | ||
| FIX/FIXa | RNA | 0.6 nM | Prolongs PT and ACT | Increased bleeding in murine and porcine models with durable reversal with antidote. Patent circuit in porcine bypass | Phase 1A, 1B, 2A and 2B studies demonstrating increased aPTT with return to baseline following antidote administration; safety in cardiac catheterization and PCI; no significant bleeding with sheath removal | [19,20,23–26,28–30,60–62] |
| FX | RNA | 4.4 nM | Prolongs PT and aPTT | [37] | ||
| Bivalent RNA | 1.7 nM | Prolongs PT and aPTT | [44] | |||
| FXII | RNA | 8.9 nM | Prolongs aPTT and TCT | [44] |
ACT, activated clotting time; aPTT, activated partial thromboplastin time; PCI, percutaneous coronary intervention; PT, prothrombin time; TCT, thrombin clot time.
Coagulation factor II and IIa
Activation of prothrombin (FII) to thrombin (FIIa) is the critical step for clot formation. An aptamer targeting FII from a pool of protein [37] tightly bind to both FII and FIIa along with robust inhibitory activity in vitro and in vivo [38]. Moreover, a specific antidote-oligonucleotide demonstrates rapid reversal of aptamer function [38].
There are several aptamers that have been isolated against thrombin (FIIa), the first of which bound to an inhibited exocite I [39]. Further evaluation of this aptamer demonstrated activity in a canine model of cardiopulmonary bypass along with inhibition of thrombosis and sheep and monkey hemo-filtration models [2,40,41].
In an effort to find broader cross-reactivity of aptamer molecules, a toggle SELEX strategy isolated an aptamer to exocite II that demonstrated inhibitory activity in vitro [42]. Additional studies demonstrated that these aptamers inhibited thrombin synergistically, raising the possibility of multi-site inhibition of a particular target as a drug-development strategy [43].
In a novel approach to aptamer-based anticoagulant development, a bivalent aptamer was constructed from two monovalent aptamers—one that bound prothrombin/thrombin (FII/FIIa) and the second that bound coagulation factor X/Xa (FX/FXa). The bivalent aptamer bound to each target protein with high affinity. The bivalent aptamer prolonged clotting time in both aPTT and prothrombin time (PT) assays. An antidote-oligonucleotide designed against the bivalent aptamer completely reversed the anticoagulant activity of the aptamer in a dose-dependent manner [44].
Coagulation factor VII
Factor VII (FVII) activation and binding to tissue factor represents the initiating step in thrombin generation. There are two aptamers that have been isolated that target factor VII [37,45]. By using a novel strategy to direct aptamer identification against targets in relatively low concentration, Layzer and Sullenger identified a FVII aptamer with inhibitory activity in vitro [37].
Coagulation factor X/Xa
Factor Xa (FXa) represents an attractive target for inhibition and there are several drugs that have been approved by the FDA and are currently available clinically. Utilizing the same strategy that identified the FVII aptamer, seven aptamers were identified that bound to and inhibited factor X (FX) and Xa [37].
Coagulation factor XII
Factor XII (FXII) involved thrombin by directly activating FXI. It also activates plasma kallikrein to facilitate inflammation. An aptamer isolated against FXII binds both FXII and FXIIa. It prolonged clotting time and inhibited thrombin time in vitro. Moreover, it inhibited auto-activation of FXII and activation of FXIa. Interestingly, it did not inhibit inflammation [46].
Von Willebrand Factor Aptamer and Antidote Development Program
Antiplatelet agents remain our frontline agents for preventing both acute and chronic arterial ischemic complications, including myocardial infarction and ischemic stroke. Aspirin was first shown to be beneficial over 25 years ago [47]. In some cases, the use of aspirin led to an almost 50% reduction in risk of future myocardial infarction or death [48]. Not surprisingly, aspirin remains first-line therapy for treatment of ischemic events.
The possibility that more potent platelet inhibition would lead to further benefits in terms of ischemic events led to the development of inhibitors of the glycoprotein IIb/IIIa complex, first as intravenous antibody fragments and small molecules and subsequently as oral agents. While intravenous IIb/IIIa inhibition was shown to be a potent agent in the catheterization laboratory, a situation in which a mortality benefit was demonstrated [49], the benefit in other settings was less prominent or even not demonstrable [50] and appeared limited to patients undergoing PCI [51]. In addition, chronic oral therapy was found to be harmful [52].
While each of the aforementioned agents target metabolic pathways or mechanisms of aggregation of platelets, von Willebrand factor (vWF) plays a direct role in platelet attachment to vascular surfaces via the glycoprotein Ib-IX-V receptor. This pathway is independent of the metabolic and aggregation pathways targeted by other agents, and may be particularly important in conditions that involve pathological thrombosis, given its central role in initiation of thrombosis after endothelial disruption, the inciting injury leading to myocardial infarction and other thrombotic conditions. vWF is also activated by conditions of high shear stress, thus, its inhibition may be particularly effective at suppression of arterial events.
Deficiencies in vWF levels or function lead to von Willebrand's disease, which is associated with variable risk of bleeding, particularly at sites of vascular injury. While inhibition of vWF is a potent mechanism to prevent arterial thrombosis in animal models, it also leads to excessive bleeding.
The first aptamer isolated against vWF was ARC1172, a DNA oligonucleotide that bound to the A1-domain of vWF. The A1-domain on vWF binds to gpIb on platelets, which results in platelet adhesion, the first nonredundant step in platelet plug formation [53]. Subsequently, ARC1779, RNA/DNA-hybrid aptamer was developed that also inhibits vWF. Potent inhibition of vWF was associated with a large increase in the rate of embolic events as detected using transcranial Doppler in patients undergoing carotid endarterectomy, however, its use was also associated with bleeding risk [54]. ARC1779 was also tested in patients with Thrombotic Thrombocytopenic Purpura; however, the results were largely inconclusive [55]. Clinical development of this agent has been halted.
Aptamers are ideally suited as potentially ideal antithrombotics to target vWF, as rapid reversal might be used to mitigate bleeding risk associated with potent vWF inhibition when vascular hemostasis is again desired. With this principle in mind, an RNA aptamer was isolated against vWF that bound to the A1 domain—the specific portion of the protein that interacts with glycoprotein Ib, resulting in platelet adhesion to the endothelial wall of a blood vessel [56]. This aptamer demonstrated platelet inhibition in plasma platelet aggregometry and whole blood platelet inhibition. In vivo studies demonstrated increased bleeding in a murine hemorrhagic model. In the same model, an antidote-oligonucleotide demonstrated complete reversal of aptamer activity [57]. The aptamer was also tested in a clinically relevant murine carotid artery thrombosis model. Animals treated with the anti-vWF aptamer demonstrated persistent carotid artery patency for 60 min, when the experiment was electively concluded compared to control mice, where vessel occlusion occurred in ∼10 min [57].
Future Directions
The field of antithrombotic aptamers is at a relative state of infancy with less than two decades of experience. The highly attractive intrinsic properties remain a foundation for future investigation. Similarly, the knowledge gained through development of the factor IX/IXa aptamers will serve as a guiding light for testing new formulations. With each, the potential benefit of having a complementary antidote and reversal agent will be maintained in the development process. Last, the ability of aptamers to serve as deep probes and imaging molecules for understanding the complexities of human diseases, disorders, and conditions characterized by thrombosis will be leveraged and used to their utmost capacity.
Acknowledgment
The authors would like to acknowledge funding support from the National Institutes of Health National Heart, Lung, and Blood Institute (U54 grant).
Author Disclosure Statement
S.N., T.P., and R.B. have no competing financial interests. B.S. was a scientific founder of Regado Biosciences.
References
- 1.Becker RC, Povsic TJ, Cohen MG, Rusconi CP. and Sullenger BA. (2010). Nucleic acid aptamers as antithrombotic agents: opportunities in extracellular therapeutics. Thromb Haemost 103:586–595 [DOI] [PubMed] [Google Scholar]
- 2.Nimjee SM, Rusconi CP, Harrington RA. and Sullenger BA. (2005). The potential of aptamers as anticoagulants. Trends Cardiovasc Med 15:41–45 [DOI] [PubMed] [Google Scholar]
- 3.Becker RC, Oney S, Becker KCD. and Sullenger BA. (2009). Antidote-controlled antithrombotic therapy targeting factor IXa and von Willebrand factor. Ann N Y Acad Sci 1175:61–70 [DOI] [PubMed] [Google Scholar]
- 4.Monroe DM, Hoffman M. and Roberts HR. (1996). Transmission of a procoagulant signal from tissue factor-bearing cells to platelets. Blood Coagul Fibrinolysis 7:459–464 [DOI] [PubMed] [Google Scholar]
- 5.Monroe DM. (2002). Platelets and thrombin generation. Arterioscler Thromb Vasc Biol 47:3142–3148 [DOI] [PubMed] [Google Scholar]
- 6.Hoffman M, Monroe DM, Oliver JA. and Roberts HR. (1995). Factors IXa and Xa play distinct roles in tissue factor-dependent initiation of coagulation. Blood 86:1794–1801 [PubMed] [Google Scholar]
- 7.Gitel S, Stephenson R. and Wessler S. (1977). In vitro and in vivo correlation of clotting protease activity: effect of heparin. Proc Natl Acad Sci U S A 74:3028–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butenas S, Orfeo T, Gissel MT, Brummel KE. and Mann KG. (2004). The significance of circulating factor IXa in blood. J Biol Chem 279:22875–22882 [DOI] [PubMed] [Google Scholar]
- 9.Gray E, Tubbs J, Oates TSA, Bolsclair M, Kemball-Cook G. and Barrowcliffe T. (1995). Measurement of activated factor IX in factor IX concentrates: correlation with in vivo thrombogenicity. Thromb Haemost 73:675–679 [PubMed] [Google Scholar]
- 10.Buyue Y, Whinna H. and Sheehan J. (2008). The heparin-binding exosite of factor IXa is a critical regulator of plasma thrombin generation and venous thrombosis. Blood 112:3234–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sramek A, Kriek M. and Rosendaal F. (2003). Decreased mortality of ischaemic heart disease among carriers of haemophilia. Blood 108:52–66 [DOI] [PubMed] [Google Scholar]
- 12.Tuinenburg A, Mauser-Bunschoten E, Verhaar M, Biesma D. and Schutgens R. (2009). Cardiovascular disease in patients with hemophilia. J Thromb Haemost 7:247–254 [DOI] [PubMed] [Google Scholar]
- 13.Rothlein R, Shen JM, Naser N, Gohimukkula DR, Caligan TB, Andrews RC, Schmidt AM, Rose EA. and Mjalli AMM. (2005). TTP889, a novel orally active partial inhibitor of FIXa inhibits clotting in two A/V shunt models without prolonging bleeding times. Blood (ASH Annual Meeting Abstracts) 106:1886 [Google Scholar]
- 14.Howard EL, Becker KCD, Rusconi CP. and Becker RC. (2007). Factor IXa inhibitors as novel anticoagulants. Arterioscler Thromb Vasc Biol 27:722–727 [DOI] [PubMed] [Google Scholar]
- 15.Benedict C, Ryan J, Wolitzky B, Ramos R, Tijburg MP. and Stern D. (1991). Active site-blocked factor IXa prevents intravascular thrombus formation in the coronary vasculature without inhibiting extravascular coagulation in a canine thrombosis model. J Clin Invest 88:1760–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spanier T, Oz M, Madigan J, Rose E, Stern D, Nowygrod R. and Schmidt A. (1997). Selective anticoagulation with active site blocked factor IXa in synthetic patch vascular repair results in decreased blood loss and operative time. ASAIO J 43:M526–M530 [PubMed] [Google Scholar]
- 17.Wong A, Gunn A, Ku P, Hollenbach S. and Sinha U. (1997). Relative efficacy ofactive site-blocked factors IXa, Xa in models of rabbit venous and arterio-venous thrombosis. Thromb Haemost 77:1143–1147 [PubMed] [Google Scholar]
- 18.Eikelboom JW, Zelenkofske SL. and Rusconi CP. (2010). Coagulation factor IXa as a target for treatment and prophylaxis of venous thromboembolism. Arterioscler Thromb Vasc Biol 30:382–387 [DOI] [PubMed] [Google Scholar]
- 19.Rusconi CP, Roberts JD, Pitoc GA, Nimjee SM, White RR, Quick G, Jr., Scardino E, Fay WP. and Sullenger BA. (2004). Antidote-mediated control of an anticoagulant aptamer in vivo. Nat Biotechnol 22:1423–1428 [DOI] [PubMed] [Google Scholar]
- 20.Rusconi CP, Scardino E, Layzer J, Pitoc GA, Ortel TL, Monroe D. and Sullenger BA. (2002). RNA aptamers as reversible antagonists of coagulation factor IXa. Nature 419:90–94 [DOI] [PubMed] [Google Scholar]
- 21.Gopinath SCB, Shikamoto Y, Mizuno H. and Kumar PKR. (2006). A potent anti-coagulant RNA aptamer inhibits blood coagulation by specifically blocking the extrinsic clotting pathway. Thromb Haemost 95:767–771 [PubMed] [Google Scholar]
- 22.Nimjee SM, Keys JR, Pitoc GA, Quick G, Rusconi CP. and Sullenger BA. (2006). A novel antidote-controlled anticoagulant reduces thrombin generation and inflammation and improves cardiac function in cardiopulmonary bypass surgery. Mol Ther 14:408–415 [DOI] [PubMed] [Google Scholar]
- 23.Dyke CK, Steinhubl SR, Kleiman NS, Cannon RO, Aberle LG, Lin M, Myles SK, Melloni C, Harrington RA, et al. (2006). First-in-human experience of an antidote-controlled anticoagulant using RNA aptamer technology: a phase 1a pharmacodynamic evaluation of a drug-antidote pair for the controlled regulation of factor IXa activity. Circulation 114:2490–2497 [DOI] [PubMed] [Google Scholar]
- 24.Chan MY, Cohen MG, Dyke CK, Myles SK, Aberle LG, Lin M, Walder J, Steinhubl SR, Gilchrist IC, et al. (2008). Phase 1b randomized study of antidote-controlled modulation of factor IXa activity in patients with stable coronary artery disease. Circulation 117:2865–2874 [DOI] [PubMed] [Google Scholar]
- 25.Povsic T, Cohen M, Chan M, Zelenkofske S, Wargin W, Harrington R, Alexander J, Rusconi C. and Becker R. (2011). Dose selection for a direct and selective factor IXa inhibitor and its complementary reversal agent: translating pharmacokinetic and pharmacodynamic properties of the REG1 system to clinical trial design. J Thromb Thrombolysis 32:21–31 [DOI] [PubMed] [Google Scholar]
- 26.Chan MY, Rusconi CP, Alexander JH, Tonkens RM, Harrington RA. and Becker RC. (2008). A randomized, repeat-dose, pharmacodynamic and safety study of an antidote-controlled factor IXa inhibitor. J Thromb Haemost 6:789–796 [DOI] [PubMed] [Google Scholar]
- 27.Cohen MG, Purdy DA, Rossi JS, Grinfeld LR, Myles SK, Aberle LG, Greenbaum AB, Fry E, Chan MY, et al. (2010). First clinical application of an actively reversible direct factor IXa inhibitor as an anticoagulation strategy in patients undergoing percutaneous intervention. Circulation 122:614–622 [DOI] [PubMed] [Google Scholar]
- 28.Povsic TJ, Cohen MG, Mehran R, Buller CE, Bode C, Cornel JH, Kasprzak JD, Montalescot G, Joseph D, et al. (2011). A randomized, partially-blinded, multicenter, active-controlled, dose-ranging study assessing the safety, efficacy, and pharmacodynamics of the REG1 anticoagulation system in patients with acute coronary syndromes: design and rationale of the RADAR phase IIb trial. Am Heart J 161:261–268 [DOI] [PubMed] [Google Scholar]
- 29.Povsic TJ, Wargin WA, Alexander JH, Krasnow J, Krolick M, Cohen MG, Mehran R, Buller CE, Bode C, et al. ; RADAR Investigators. (2011). Pegnivacogin results in near complete FIX inhibition in acute coronary syndrome patients: RADAR pharmacokinetic and pharmacodynamic substudy. Eur Heart J 32:2412–2419 [DOI] [PubMed] [Google Scholar]
- 30.Povsic TJ, Vavalle JP, Aberle LH, Kasprzak JD, Cohen MG, Mehran R, Bode C, Buller CE, Montalescot G, et al. (2013). A phase 2, randomized, partially blinded, active-controlled study assessing the efficacy and safety of variable anticoagulation reversal using the REG1 system in patients with acute coronary syndromes: results of the RADAR trial. Eur Heart J 34:2481–2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ganson NJ, Povsic TJ, Sullenger BA, Alexander JH, Zelenkofske SL, Sailstad JM, Rusconi CP. and Hershfield MS. (2015). Pre-existing anti-polyethylene glycol antibody linked to first-exposure allergic reactions to pegnivacogin, a PEGylated RNA aptamer. J Allergy Clin Immunol [Epub ahead of print]; DOI: 10.1016/j.jaci.2015.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schellekens H, Hennink W. and Brinks V. (2013). The immunogenicity of polyethylene glycol: facts and fiction. Pharm Res 30:1729–1734 [DOI] [PubMed] [Google Scholar]
- 33.Yang Q. and Lai SK. (2015). Anti-PEG immunity: emergence, characteristics, and unaddressed questions. Wiley Interdiscip Rev Nanomed Nanobiotechnol 7:655–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gabriel RS, Smyth YM, Menon V, Klein AL, Grimm RA, Thomas JD. and Sabik EM. (2008). Safety of ultrasound contrast agents in stress echocardiography. Am J Cardiol 102:1269–1272 [DOI] [PubMed] [Google Scholar]
- 35.Wei K, Mulvagh SL, Carson L, Davidoff R, Gabriel R, Grimm RA, Wilson S, Fane L, Herzog CA, et al. (2008). The safety of deFinity and Optison for ultrasound image enhancement: a retrospective analysis of 78,383 administered contrast doses. J Am Soc Echocardiogr 21:1202–1206 [DOI] [PubMed] [Google Scholar]
- 36.Lincoff AM, Mehran R, Povsic TJ, Zelenkofske SL, Huang Z, Armstrong PW, Steg PG, Bode C, Cohen MG, et al. (2016). Effect of the REG1 anticoagulation system versus bivalirudin on outcomes after percutaneous coronary intervention (REGULATE-PCI): a randomized clinical trial. Lancet 387 (10016):349–356 [DOI] [PubMed] [Google Scholar]
- 37.Layzer JM. and Sullenger BA. (2007). Simultaneous generation of aptamers to multiple gamma-carboxyglutamic acid proteins from a focused aptamer library using DeSELEX and convergent selection. Oligonucleotides 17:1–11 [DOI] [PubMed] [Google Scholar]
- 38.Bompiani KM, Monroe DM, Church FC. and Sullenger BA. (2012). A high affinity, antidote-controllable prothrombin and thrombin-binding RNA aptamer inhibits thrombin generation and thrombin activity. J Thromb Haemost 10:870–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bock LC, Griffin LC, Latham JA, Vermaas EH. and Toole JJ. (1992). Selection of single-stranded DNA molecules that bind and inhibit human thrombin. Nature 355:564–566 [DOI] [PubMed] [Google Scholar]
- 40.DeAnda A, Jr., Coutre SE, Moon MR, Vial CM, Griffin LC, Law VS, Komeda M, Leung LL. and Miller DC. (1994). Pilot study of the efficacy of a thrombin inhibitor for use during cardiopulmonary bypass. Ann Thorac Surg 58:344–350 [DOI] [PubMed] [Google Scholar]
- 41.Griffin LC, Tidmarsh GF, Bock LC, Toole JJ. and Leung LL. (1993). In vivo anticoagulant properties of a novel nucleotide-based thrombin inhibitor and demonstration of regional anticoagulation in extracorporeal circuits. Blood 81:3271–3276 [PubMed] [Google Scholar]
- 42.White R, Rusconi C, Scardino E, Wolberg A, Lawson J, Hoffman M. and Sullenger B. (2001). Generation of species cross-reactive aptamers using “toggle” SELEX. Mol Ther 4:567–573 [DOI] [PubMed] [Google Scholar]
- 43.Nimjee SM, Oney S, Volovyk Z, Bompiani KM, Long SB, Hoffman M. and Sullenger BA. (2009). Synergistic effect of aptamers that inhibit exosites 1 and 2 on thrombin. RNA 12:2105–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soule EE, Bompiani KM, Woodruff RS. and Sullenger BA. (2015). Targeting two coagulation cascade proteases with a bivalent aptamer yields a potent and antidote-controllable anticoagulant. Nucleic Acid Ther [Epub ahead of print]; DOI: 10.1089/nat.2015.0565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rusconi CP, Yeh A, Lyerly HK, Lawson JH. and Sullenger BA. (2000). Blocking the initiation of coagulation by RNA aptamers to factor VIIa. Thromb Haemost 84:841–848 [PubMed] [Google Scholar]
- 46.Woodruff RS, Xu Y, Layzer J, Wu W, Ogletree ML. and Sullenger BA. (2013). Inhibiting the intrinsic pathway of coagulation with a factor XII-targeting RNA aptamer. J Thromb Haemost 11:1364–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.No authors. (1988). Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17 187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 332:349–360 [PubMed] [Google Scholar]
- 48.Wallentin LC. (1991). Aspirin (75 mg/day) after an episode of unstable coronary artery disease: long-term effects on the risk for myocardial infarction, occurrence of severe angina and the need for revascularization. J Am Coll Cardiol 18:1587–1593 [DOI] [PubMed] [Google Scholar]
- 49.Kandzari DE, Hasselblad V, Tcheng JE, Stone GW, Califf RM, Kastrati A, Neumann FJ, Brener SJ, Montalescot G, Kong DF. and Harrington RA. (2004). Improved clinical outcomes with abciximab therapy in acute myocardial infarction: a systematic overview of randomized clinical trials. Am Heart J 147:457–462 [DOI] [PubMed] [Google Scholar]
- 50.Simoons ML; GUSTO IV-ACS Investigators. (2001). Effect of glycoprotein IIb/IIIa receptor blocker abciximab on outcome in patients with acute coronary syndromes without early coronary revascularisation: the GUSTO IV-ACS randomised trial. Lancet 357:1915–1924 [DOI] [PubMed] [Google Scholar]
- 51.Giugliano RP, White JA, Bode C, Armstrong PW, Montalescot G, Lewis BS, van ‘t Hof A, Berdan LG, Lee KL, et al. (2009). Early versus delayed, provisional eptifibatide in acute coronary syndromes. N Engl J Med 360:2176–2190 [DOI] [PubMed] [Google Scholar]
- 52.Boersma E, Harrington RA, Moliterno DJ, White H, Théroux P, Van de Werf F, de Torbal A, Armstrong PW, Wallentin LC, et al. (2002). Platelet glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: a meta-analysis of all major randomised clinical trials. Lancet 359:189–198 [DOI] [PubMed] [Google Scholar]
- 53.Huang RH, Fremont DH, Diener JL, Schaub RG. and Sadler JE. (2009). A structural explanation for the antithrombotic activity of ARC1172, a DNA aptamer that binds von Willebrand factor domain A1. Structure 17:1476–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Markus HS, McCollum C, Imray C, Goulder MA, Gilbert J. and King A. (2011). The von Willebrand inhibitor ARC1779 reduces cerebral embolization after carotid endarterectomy: a randomized trial. Stroke 42:2149–2153 [DOI] [PubMed] [Google Scholar]
- 55.Jilma-Stohlawtez P, Gilbert JC, Gorczyca M, Knobl P. and Jilma B. (2011). A dose ranging phase I/II trial of the von Willebrand factor inhibiting aptamer ARC1779 in patients with conge nital thrombotic thrombocytopenic purpura. Thromb Haemost 106:539–547 [DOI] [PubMed] [Google Scholar]
- 56.Oney S, Nimjee SM, Layzer J, Que-Gewirth N, Ginsburg D, Becker RC, Arepally G. and Sullenger BA. (2007). Antidote-controlled platelet inhibition targeting von Willebrand factor with aptamers. Oligonucleotides 17:265–274 [DOI] [PubMed] [Google Scholar]
- 57.Nimjee SM, Lohrmann JD, Wang H, Snyder DJ, Cummings TJ, Becker RC, Oney S. and Sullenger BA. (2012). Rapidly regulating platelet activity in vivo with an antidote controlled platelet inhibitor. Mol Ther 20:391–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bompiani KM, Woodruff RS, Becker RC, Nimjee SM. and Sullenger BA. (2012). Antidote control of aptamer therapeutics: the road to a safer class of drug agents. Curr Pharm Biotechnol 13:1924–1934 [DOI] [PubMed] [Google Scholar]
- 59.Griffin LC, Toole JJ. and Leung LL. (1993). The discovery and characterization of a novel nucleotide-based thrombin inhibitor. Gene 137:25–31 [DOI] [PubMed] [Google Scholar]
- 60.Nimjee SM, Keys JR, Pitoc GA, Quick G, Rusconi CP. and Sullenger BA. (2006). A novel antidote-controlled anticoagulant reduces thrombin generation and inflammation and improves cardiac function in cardiopulmonary bypass surgery. Mol Ther 14:408–415 [DOI] [PubMed] [Google Scholar]
- 61.Vavalle JP, Rusconi C, Zelenkofske S, Alexander JH. and Becker RC. (2011). Thrombin generation kinetics of the REG2 anticoagulation system: a first-in-human experience. Circulation 124:A13826. [DOI] [PubMed] [Google Scholar]
- 62.Vavalle JP, Rusconi CP, Zelenkofske S, Wargin WA, Alexander JH. and Becker RC. (2012). A phase 1 ascending dose study of a subcutaneously administered factor IXa inhibitor and its active control agent. J Thromb Haemost 10:1303–1311 [DOI] [PubMed] [Google Scholar]