Abstract
Brain-derived neurotrophic factor (BDNF) regulates neuronal development and function. However, it has been difficult to discern its role in the adult brain in influencing complex behavior. Here, we use a recently developed inducible knockout system to show that deleting BDNF in broad forebrain regions of adult mice impairs hippocampal-dependent learning and long-term potentiation. We use the inducible nature of this system to show that the loss of BDNF during earlier stages of development causes hyperactivity and more pronounced hippocampal-dependent learning deficits. We also demonstrate that the loss of forebrain BDNF attenuates the actions of desipramine, an antidepressant, in the forced swim test, suggesting the involvement of BDNF in antidepressant efficacy. These results establish roles for BDNF in the adult, and demonstrate the strength of this inducible knockout system in studying gene function in the adult brain.
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family of growth factors (1, 2). It is widely expressed in the mammalian brain (3) and regulates many aspects of neuronal function (4–8). However, a role for BDNF in regulating complex behavior has been difficult to assess, because of the lack of specific pharmacological agents and the early postnatal lethality of BDNF null (-/-) mice (9). Studies examining heterozygous BDNF (+/-) mice, which display roughly half the normal levels of BDNF in brain, have reported increased feeding behavior, obesity, hyperactivity, and aggressiveness (10–12). However, these alterations may arise from developmental abnormalities and complicate the interpretation of the role of BDNF in the adult brain.
The recent generation of conditional BDNF knockout (KO) mice circumvents the problem of postnatal lethality and allows for some regional specificity of gene deletion. For example, conditional BDNF KO mice survive to adulthood and exhibit a range of deficits similar to those seen in the heterozygous null mice (13). However, such conditional KO mice still suffer from the fact that they delete a gene of interest during late-embryonic or early postnatal periods and thus do not preclude developmental abnormalities as the cause of behavioral impairments observed. This is of a particular concern in studying complex behavior because early developmental periods in humans are crucial for the manifestation of many complex neuropsychiatric illnesses. To truly investigate the influence of BDNF on complex behavior in adults, it is necessary to have a system in which BDNF is deleted in an inducible manner in specific brain regions. We have generated an inducible KO system in which BDNF can be deleted selectively in the brain of adult mice. Here we describe the phenotype of mice in which BDNF has been deleted in broad forebrain regions of adult mice. We use the inducibility of the system to examine the phenotype of mice in which BDNF has been deleted at earlier stages of development in the same brain regions. Our results demonstrate that the role of BDNF in the adult brain is different from in the embryonic brain.
Materials and Methods
Maintenance of Inducible KO Mice. The BDNF inducible KO mice were generated by crossing three lines of mice. The neuron-specific enolase–tetracycline transcriptional activator (NSE–tTA) line are on a BL6/SJL × ICR background, the TetOp-Cre are on an ICR background, and the floxed BDNF mice are on a BL6/sv129 background. The NSE–tTA mice (14) and the TetOp-Cre mice (15, 16) were maintained as homozygotes then crossed to generate the bigenic mice. The floxed LacZ reporter mice (17) or floxed BDNF mice (13) were then crossed with the bigenic NSE–tTA/TetOp-Cre mice to generate the inducible KO mice. All experiments were performed on littermates derived from this mating paradigm to ensure analysis by matched controls. Genomic DNA was isolated from tails for genotyping by PCR analysis at 3 weeks of age. Genotyping details can be found in Supporting Text, which is published as supporting information on the PNAS web site.
Adult KO Mice. Trigenic NSE–tTA × TetOp-Cre × floxed mice were bred on doxycycline (dox) (1 mg/ml) in their drinking water to suppress recombination. Mice were weaned at 21 days of age, at which time they were separated by gender with food and dox-treated water available. To induce recombination, the dox was removed from the drinking water of 12-week-old mice for 12 weeks, and then the mice were tested in behavioral paradigms or killed for analysis. Adult control (CTL) mice were trigenic (NSE–tTA/TetOp-Cre/fBDNF) mice maintained on dox throughout their lifetime. Mice containing only the NSE–tTA/TetOp-Cre constructs, which expressed similar levels of BDNF as wild-type mice, were indistinguishable from adult CTL mice in the analysis described in this study.
Early KO Mice. Trigenic NSE–tTA × TetOp-Cre × floxed mice were bred in the absence of dox. Mice were weaned at 21 days of age, at which time they were separated by gender with food and water available. Early CTL mice were littermates expressing NSE–tTA/TetOp-Cre with BDNF levels indistinguishable from wild-type mice.
LacZ Staining. Mice were anesthetized with sodium pentobarbital and transcardially perfused with PBS followed by cold 4% paraformaldehyde. Brains were postfixed, and then transferred to a 30% sucrose solution in PBS and cryoprotected at 4°C overnight. Brains were sectioned (30-μm sections) on a freezing microtome, and sections were stored free-floating in PBS with 0.1% sodium azide. Sections were rinsed in PBS then placed in 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-gal) reaction buffer (50 mM PB/150 mM NaCl/1 mM MgCl2/0.01% sodium deoxycholate/0.02% Nonidet-P40/3.1 mM potassium ferrocyanide/3.1 mM potassium ferricyanide/0.2 mg/ml X-gal) and stained overnight at 37°C. Sections were rinsed in PBS, then mounted onto glass slides, dehydrated, and coverslipped by using 1,3-diethyl-8-phenylxanthine (DPX).
Immunohistochemistry. Mice were perfused, and brains were treated as described above. Sections were mounted onto glass slides and allowed to dry, then washed in PBS and blocked in 3% normal serum, 0.3% Triton X-100 in PBS for 1 h at room temperature. Sections were then incubated with goat anti-NeuN (1:50; Chemicon) and rabbit anti-β-galalactosidase (β-gal) (1:500; 5′ to 3′) or rabbit anti-GFAP (1:1,000; DAKO) and mouse anti-β-gal (1:500; Sigma) in 1% normal serum, 0.3% Triton X-100 in PBS at 4°C overnight. Sections were rinsed with PBS and then incubated 1:200 with the appropriate fluorescent secondary antibodies (1:200; Molecular Probes) for 2 h. Sections were rinsed in PBS, dehydrated, and coverslipped.
In Situ Hybridization. Animals were killed by rapid decapitation, and the brains were immediately placed on dry ice then stored at -80°C. The brains were sectioned (14 μm) on a cryostat and mounted on coated slides. An antisense probe was produced by in vitro transcription to the coding exon V of the BDNF gene. All other steps were essentially the same as described (18).
Ribonuclease Protection Assay. Animals were killed by rapid decapitation, and the hippocampus was dissected and frozen on dry ice. Total RNA was isolated by using TRIzol (GIBCO/BRL). Fifteen micrograms of total RNA was hybridized with a BDNF-specific probe from coding exon V of the BDNF gene for each reaction by using the Ribonuclease Protection Assay III kit (Ambion). Protected fragments were electrophoresed and visualized on a 5% acrylamide/8 M urea denaturing gel. Each sample was run with a β-actin control. Band intensity was quantitated by using a PhosphorImager (Molecular Devices).
Electrophysiology. Hippocampal slices including the CA1 region were prepared as coronal sections (400 μm) on a vibratome, then transferred to a chamber at room temperature in an oxygenated saline solution (artificial cerebrospinal fluid, ACSF). Slices were permitted to recover from cutting for 90 min before the recordings. Hippocampal slices were placed in a submerged type recording chamber. Excitatory postsynaptic field potentials (fEPSPs) were recorded in the stratum radiatum of the CA1 region while stimulating the Schaffer collaterals (input 1) and a second stimulating input on the opposite side of the same recorded pyramidal neurons (input 2). Stimuli (≈2.5 volts with a tungsten bipolar stimulating electrode, resistance ≈0.5 MW in ACSF) were delivered at intensities that evoked fEPSP slopes equal to 40% of the maximum in each slice. Test stimuli were delivered every minute (four times), and a baseline was recorded for 10–15 min before beginning the experiments to guarantee the stability of responses. Slices were stimulated by using the following paradigm: input 1 delivered a train of five stimuli at 100 Hz followed by a single stimulus from the second stimulating electrode (input 2) with a delay of 5 ms. Responses were recorded for 140 min after stimulation. The initial slope (10–50% of maximum) of the fEPSPs was measured over time, normalized to baseline (mean response before stimulation), and plotted.
Behavior. Locomotor activity was assessed by placing animals in a new home cage and measuring locomotor activity for 2 h by photobeams linked to computer data acquisition software (San Diego Instruments). For the resident intruder test, mice were isolated for 3 weeks before the test. The test was performed under dim lights for 10 min for 5 consecutive days. The intruder (C57 wild-type mouse) was introduced into the resident's (test mouse) cage, and the intensity of the aggressive and defensive behaviors were deduced from ethological analysis of behavioral sequences displayed by both animals. Aggressive behavior was defined as latency to first bite and number of bites (19). Fear conditioning was performed essentially as described (20). Further details on these behaviors can be found in Supporting Text.
Forced Swim Test. The forced swim test was conducted based on published protocols (21). Briefly, swim sessions were conducted by placing mice in glass cylinders for 6 min and recording their behavior with a video camera (Ethovision 3.0, Noldus). Immobility was measured during the last 4 min of the test. The automation parameters and optimal dose of desipramine (Sigma) in the assay were first validated by running a dose–response curve for the drug in our experimental mice (data not shown). Saline or desipramine (15–20 mg/kg) was administered subchronically by giving three injections over a 24-h period. Doses were spaced 24, 4, and 1 h before the swim test.
Results
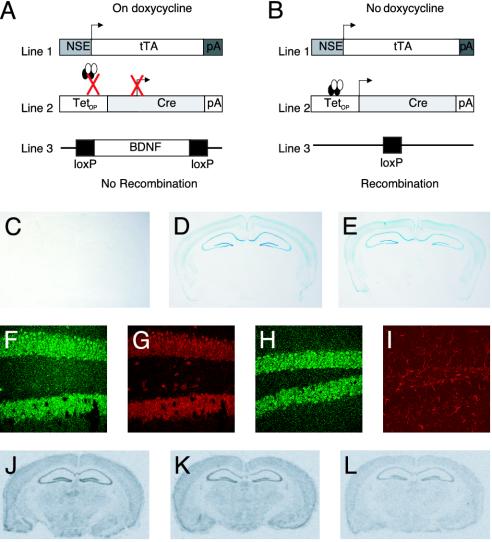
Characterization of the Inducible KO System. We have developed an inducible KO system by combining the tetracycline-inducible system (22, 23) with the bacteriophage P1-derived Cre/loxP recombination system (24) to delete BDNF in the brain in a regionally and temporally specific manner. This is a trigene system (Fig. 1 A and B). The first transgene consists of the NSE promoter driving the expression of the tTA, which is inhibited by tetracycline (14). These mice were crossed with mice that contain a transgene consisting of the TetOp promoter (which is activated by tTA) driving expression of Cre recombinase (15, 16). These bigenic NSE–tTA × TetOp-Cre mice were then crossed with mice containing a floxed BDNF locus (13) to generate the inducible KO mice (Fig. 1 A and B). This is an inducible KO because the tetracycline derivative, dox, completely represses the expression of Cre recombinase throughout development; the removal of dox induces Cre expression and subsequent recombination in the fully developed adult brain.
Fig. 1.
Inducible, cell-targeted KO system. (A and B) The NSE promoter drives expression of tTA. (A) In the presence of dox, tTA cannot activate the TetOp promoter. (B) In the absence of dox, tTA binds to TetOp and drives Cre expression. Cre then mediates recombination of a floxed (e.g., BDNF) gene locus. (C–E) Coronal sections from the brain of adult (6-month-old) male NSE–tTA × TetOp-Cre × Rosa26 trigenic mice stained with X-gal. (C) In mice maintained on dox, no recombination was observed. In contrast, comparable levels of recombination were observed in mice bred and maintained in the absence of dox (early KO; D) and in mice bred on dox and then removed at 12 weeks of age (adult KO; E). (F–I) Coronal hippocampal sections of adult trigenic mice subjected to immunofluorescence staining with antibodies against β-gal (F and H) and either NeuN (G) or GFAP (I). (J–L) In situ hybridization analysis of BDNF mRNA levels in the brains of NSE–tTA × TetOp-Cre × fBDNF trigenic mice. Images of coronal brain sections with a 35S-labeled BDNF cRNA probe in a wild-type animal (J); trigenic animal maintained on dox (adult CTL; K); and trigenic animal bred on dox and then removed at 12 weeks of age (adult KO; L).
To determine whether we could mediate recombination in the adult brain we initially bred the lacZ reporter mouse (Rosa26) into the NSE–tTA × TetOp-Cre bigenic mice (17). Rosa26 only expresses β-gal in cells upon recombination. X-gal staining revealed β-gal expression in the cortex, hippocampus, basolateral amygdala, and striatum of mice that were bred and raised off dox, but no detectable expression in animals bred and raised on dox (Fig. 1 C and D). To determine whether this system could mediate recombination in the adult brain, we bred these mice in the presence of dox and then removed the inhibitor when the mice were 3 months of age. Based on our previous experience with the tetracycline-inducible system (14), we allowed for a 3-month dox washout period to assess whether recombination had occurred. X-gal staining demonstrated equivalent levels of β-gal expression in the same brain regions (Fig. 1E) compared with mice bred off dox. No recombination was detected in peripheral tissues (data not shown).
We next examined whether the recombination observed took place in adult postmitotic neurons or glia by using double immunofluorescence staining with antibodies directed against β-gal and either NeuN (a neuronal marker) or glial fibrillary acidic protein (GFAP, an astroglial marker). We found that β-gal expression is colocalized exclusively with NeuN (Fig. 1 F and G) and not GFAP (Fig. 1 H and I). However, β-gal is not present in all neurons; rather, it is expressed in a subset of neurons in each affected region (Fig. 1 F and G). These observations are consistent with the fact that not all neurons in a given region express the NSE–tTA gene (14, 25) and indicate a mosaic pattern of recombination. Nevertheless, these data demonstrate the ability of our system to mediate recombination in adult postmitotic neurons.
Characterization of BDNF Adult KO Mice. We next asked whether we could use this inducible system to delete BDNF in the adult brain. The floxed BDNF allele (13) was bred into the NSE–tTA × TetOp-Cre bigenic mice maintained on dox. The resulting progeny were removed from dox at 3 months of age and were used after a three-month washout period. Analysis of BDNF expression by in situ hybridization revealed a reduction in BDNF mRNA levels in cortex and hippocampus of the BDNF KO mice (adult KO) compared with controls of the same genotype maintained on dox (adult CTL) (Fig. 1 K and L). Ribonuclease protection assays showed a 70% reduction in BDNF mRNA levels in the hippocampus (Fig. 6A, which is published as supporting information on the PNAS web site). BDNF protein levels in the hippocampus were also reduced by ≈70% in the inducible KO mice (Fig. 6B). This partial reduction in BDNF expression is consistent with the β-gal reporter data showing that recombination is neuron-specific and does not occur in every neuron. The levels of BDNF mRNA in the adult CTL mice were indistinguishable from those seen in wild-type animals (Fig. 1J) and from littermates containing only the NSE–tTA × TetOp-Cre transgenes bred on dox and then either maintained on it or removed from it at 3 months of age (CTL-ON and CTL-OFF, respectively) (data not shown). Because the adult CTL mice were indistinguishable from the CTL-ON and CTL-OFF mice, we only show results from BDNF adult KO and adult CTL mice in subsequent analyses.
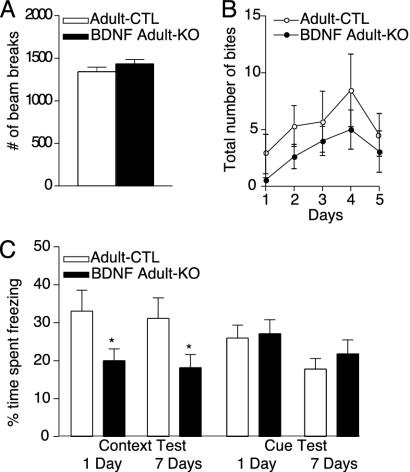
BDNF Adult KO Mice Show Deficits in Hippocampal-Dependent Learning. The BDNF adult KO mice were similar in body weight compared with adult CTL mice even up to 9 months of age (Fig. 6C). The BDNF adult KO mice were also similar in locomotor activity and levels of aggression compared with littermate controls (Fig. 2 A and B). Given the prominent loss of BDNF in the hippocampus of our mice and the implication of BDNF in the regulation of hippocampal function (26–28), we next examined the mice for context and cue-dependent fear conditioning. Contextual conditioning is a task that is sensitive to hippocampal and amygdala lesions. Cue conditioning is insensitive to hippocampal lesions but is dependent on an intact amygdala. The BDNF adult KO mice showed significantly less context-dependent memory than the adult CTL mice measured 24 h and 7 days after training (P < 0.05; Fig. 2C). In contrast, there was no difference in cue-dependent fear conditioning in BDNF adult KO compared with adult CTL mice. The threshold responses of the BDNF adult KO and adult CTL mice to foot shocks were indistinguishable (Fig. 6C), which indicates that the impairment in context conditioning represents a learning deficit and not a difference in pain sensitivity.
Fig. 2.
Behavioral effects of BDNF KO from adult brain. (A) Locomotor activity in the BDNF adult KO mice (n = 26) was indistinguishable from genotype controls maintained on dox (adult CTL; n = 21). Locomotor activity was assessed over a 10-min period, and the number of photocell beam breaks was recorded. (B and C) Adult KO mice (n = 16) do not display altered levels of aggression in the resident intruder paradigm compared with adult CTLs (n = 13). (D) Context-dependent fear conditioning is decreased in adult KO mice (n = 26) 24 h after training compared with adult CTL mice (*, P < 0.05; n = 22). The deficit in context-dependent fear conditioning persists on repeat testing 7 days after training (*, P < 0.05). No significant difference was observed in baseline freezing behavior. Cue-dependent fear conditioning was indistinguishable in inducible BDNF adult KO and adult CTL mice.
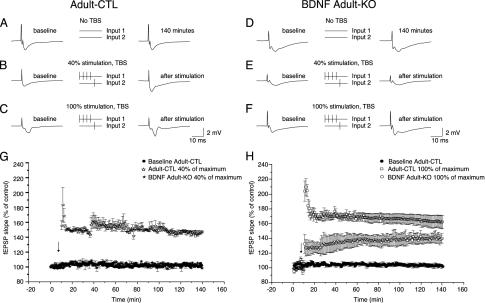
To determine whether the deficit in contextual conditioning is associated with changes in synaptic plasticity, we examined long-term potentiation (LTP) in the hippocampus of these mice. BDNF has been shown to facilitate NMDA receptor-dependent LTP at hippocampal synapses (7). To investigate the effect of the adult BDNF KO on LTP, we stimulated two independent Schaffer collateral inputs (determined by the absence of paired pulse facilitation between the inputs) to induce heterosynaptic, associative LTP as follows: input 1 received 4 stimuli at 20 Hz followed in 5 ms by a single stimulus applied to input 2 (Fig. 3 A–F). Stimulus was set at 40% of the amplitude needed to elicit a maximal fEPSP. LTP was induced in every case in the hippocampus of adult CTL mice, but the same protocol evoked no LTP in BDNF adult KO mice (Fig. 3G). LTP could be elicited in adult KO animals by using the same protocol, but with a stimulus amplitude of 100% of maximal; however, the magnitude of the enhancement of LTP was reduced compared with the controls (Fig. 3H). These findings demonstrate a dramatic modulatory action of BDNF on LTP induction and may be related to BDNF-dependent activation of dendritic calcium transients (8). Further experiments showed that the LTP deficits seen in the BDNF adult KO mice were not caused by significant changes in presynaptic function, because the input–output relationship between fEPSP slope and presynaptic fiber volley was unchanged in the KO (Fig. 7 A and B, which is published as supporting information on the PNAS web site). In addition, the paired pulse depression ratio, a measure of proportional neurotransmitter release probability, was unaffected by loss of BDNF (Fig. 7 C–F).
Fig. 3.
BDNF modulates LTP of the CA3 to CA1 inputs in adult hippocampus. (A–F) Traces show fEPSPs recorded in the stratum radiatum before (Left) and after (Right) two independent CA3 to CA1 inputs are stimulated according to the protocol indicated (Center). Stimulus intensities of 0% (A and D), 40% (B and E), or 100% (C and F) of the theta burst stimulation (TBS) intensity to induce a maximal fEPSP were used. Baseline amplitude of the slope of the fEPSP evoked by input 2 was established over 10 min; then the paired stimuli were applied and the fEPSP slope was monitored for 130 min (G and H). With a 40% stimulus intensity, the average control fEPSP was enhanced over the recording period by >150% in control (n = 9), but the BDNF KO (n = 9) slices showed no enhancement (G). At 100% stimulus intensity, control showed >170% enhancement and the BDNF KO slices showed an enhancement of ≈140% (H).
BDNF Early KO Mice Are Hyperactive and Show Deficits in Hippocampal-Dependent Learning. To compare the behavioral consequences of an adult KO of BDNF with the consequences of a KO that occurs earlier in development, we bred the inducible KO mice in the absence of dox. We have shown previously that the NSE–tTA transgene is expressed in late embryogenesis (14). The early KO mice were viable and survived into adulthood. The loss of BDNF in the early KO mice was found to be indistinguishable from the adult KO mice (Fig. 6A).
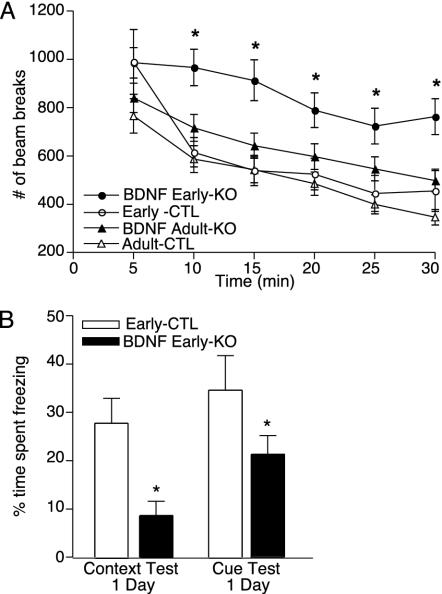
Early KO mice were similar in body weight compared with littermate controls (early CTL) up to 9 months of age (Fig. 8A, which is published as supporting information on the PNAS web site). Moreover, the early KO mice had a similar growth rate to the adult KO mice (data not shown). These findings indicate that the loss of BDNF in broad forebrain areas during development is not sufficient to mediate the obesity seen in heterozygous null mice and conditional KO mice (9, 13). The early KO mice also showed no difference in intermale aggressiveness compared with littermate controls as determined by the resident intruder paradigm (Fig. 8 B and C). In contrast, the early KO mice were hyperactive compared with their littermate controls (Fig. 4A). We tested the early KO and littermate control mice with the BDNF adult KO and adult CTL mice to determine whether this alteration in locomotor activity was caused by an increase in activity of the early KO mice or a difference in baseline activity of the two control groups. All mice were studied at ≈6 months of age. The early KO consistently displayed a higher level of activity compared with the three other groups, demonstrating that the early loss of BDNF causes hyperactivity.
Fig. 4.
Behavioral effects of BDNF KO from late embryonic brain. (A) Locomotor activity is increased in BDNF early KO mice compared with littermate controls (early CTLs), as well as with adult KO and adult CTL mice. Locomotor activity was assessed over a 2-h period, and the number of photocell beam breaks was recorded. (B) Selective deficit in context- and cue-dependent (*, P < 0.05) fear conditioning in BDNF early KO mice (n = 10) compared with littermate controls (n = 10) as assessed 24 h after testing. No significant difference was observed in baseline freezing behavior.
We next examined the early KO mice in the fear-conditioning paradigm to assess whether they have similar learning impairments as the adult KO mice. The early KO mice show a dramatic, virtually complete absence of context-dependent fear conditioning compared with littermate controls (P < 0.05; Fig. 4B). The magnitude of this impairment is more pronounced in the early KO mice than in the adult KO mice, suggesting that the loss of BDNF during development causes a more severe learning phenotype than such a loss in the adult. We also detected an impairment in cue-dependent fear conditioning in the early KO mice that was not observed in the adult KO mice (P < 0.05; Fig. 4B). Because the spatial pattern of BDNF KO at 6 months of age is indistinguishable between these two groups, these results suggest that impairment in cue-dependent fear conditioning results from a developmental effect of BDNF.
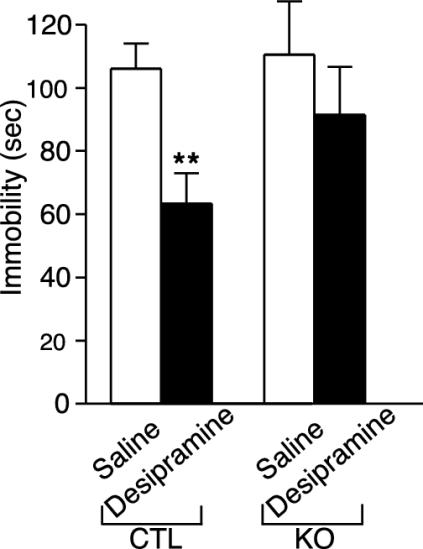
BDNF KO Mice Show Altered Response to Desipramine. Loss of BDNF expression in the hippocampus has been suggested to mediate “depressive-related” behaviors, whereas up-regulation of BDNF in the hippocampus has been suggested to mediate the therapeutic effect of antidepressants (29). To investigate the role of BDNF in “depression-like” behavior and antidepressant efficacy, we compared BDNF KO mice with littermate controls in the forced swim test. In this paradigm, the more time an animal spends inactive vs. active after placement in a beaker of water is interpreted as a measure of depressive-like behavior (21). This test reliably predicts antidepressant efficacy as measured by decreased immobility time of an animal in this test (30). In the absence of antidepressants, BDNF KO mice were indistinguishable from control mice in the amount of immobility in the forced swim test (Fig. 5). In contrast, after administration of the antidepressant desipramine, the control animals displayed the expected significant reduction in immobility (P < 0.05), whereas no significant decrease in immobility was observed in the KO mice (Fig. 5). This behavioral effect is not caused by changes in the level of the norepinephrine transporter, the site of action of desipramine, in our KO mice (data not shown).
Fig. 5.
Behavioral response of CTL and BDNF KO mice in the forced swim test to subchronic antidepressant treatment. Animals were administered saline or desipramine 24, 4, and 1 h before the swim test (10 mg/kg i.p., 10 mg/kg i.p., and 20 mg/kg s.c., respectively). Saline-treated control (n = 10) and BDNF KO (n = 10) mice exhibited similar immobility times. Subchronic desipramine treatment significantly reduced immobility time in the control mice (n = 10) but not in the BDNF KO mice (n = 10). Results are presented as mean immobility (sec) ± SEM; ANOVA and post hoc Tukey's test revealed a significance of P < 0.05 (asterisks) for saline control versus desipramine control [F(1, 57) = 8.51; P < 0.01].
Discussion
Here, we demonstrated that the loss of BDNF selectively in the brain of adult mice resulted in impaired hippocampal function, whereas the loss of BDNF at earlier stages of development contributed to hyperactivity as well as more severe impairments in hippocampal-dependent learning. Our data show that loss of BDNF in early development may produce more dramatic phenotypes than loss of BDNF in the adult. These findings suggest that the role of BDNF in the adult brain may be different from that in the developing brain.
The finding that the loss of BDNF in broad forebrain regions of 3- to 6-month-old mice does not alter body weight, aggression, or activity levels is interesting because abnormalities in each of these domains have been observed in BDNF heterozygous null mice and conditional BDNF KO mice (9, 13). Although the loss of BDNF in embryonic mice (early KO) increased locomotor activity, it did not produce alterations in weight gain or aggression. Importantly, our early KO mice showed an equivalent recombination pattern compared with our adult KO mice. This is in contrast to a recent report suggesting that different patterns of recombination may occur depending on the timing of dox treatment in the tetracycline-on system (31). This could reflect a difference between the tetracycline-off system used here and the tetracycline-on system used in the other study. Regardless, any behavioral differences observed between our early and adult KO mice cannot be attributed to different patterns of recombination. Thus, early loss of forebrain BDNF is sufficient to cause a hyperlocomotor phenotype, but not the weight gain and aggressiveness phenotypes, which are presumably mediated by the loss of BDNF from brain regions not affected in our system.
Although our inducible system suggests developmental roles for BDNF in mediating hyperactivity, it also supports previous data suggesting a nondevelopmental role for BDNF in eliciting LTP in the hippocampus (27, 32). We found that the threshold induction of LTP is increased in BDNF adult KO compared with wild-type mice. This finding is in agreement with previous studies showing that overexpression of BDNF can rescue hippocampal LTP in constitutive BDNF KO mice (27, 32) and strengthens the utility of our inducible KO system.
One leading hypothesis concerning the role of BDNF in mediating complex behavior, the neurotrophic hypothesis of depression, suggests that the loss of BDNF from hippocampus may contribute to alterations that underlie aspects of depression, whereas antidepressants may mediate some of their therapeutic effects by increasing BDNF levels in this brain region (29). We tested this hypothesis with our BDNF KO mice by using the forced swim test, a paradigm that reliably predicts antidepressant efficacy and by analogy is used to predict depression-related behavior (21, 30). In the absence of antidepressants, BDNF KO mice were indistinguishable from control mice in the amount of immobility in the forced swim test. In contrast, after administration of the antidepressant desipramine, the control animals displayed the expected significant reduction in immobility, whereas no significant decrease in immobility was observed in the KO mice. These data suggest that the loss of forebrain BDNF per se is not sufficient to mediate depression-like behavior, but rather may be essential for mediating aspects of antidepressant efficacy. It may be that loss of BDNF would make the mice more vulnerable to particular chronic perturbations, which can now be investigated. In any event, this is a direct demonstration in genetic models that BDNF is important in antidepressant action.
It is important to note that, although our inducible KO mice have a dramatic reduction in BDNF in cortical and hippocampal regions, there is not a complete ablation of BDNF. The inducible KO mice, similar to conditional KO mice, do not mediate recombination in all cells in a particular brain region, but rather in a subset of cells. This may well be due to the large heterogeneity of cell types within the brain. Regardless of the mechanism, this observation highlights an important concern when using inducible and conditional KO mice in the brain.
We have demonstrated the ability to knock out a gene selectively in the brain of fully developed adult mice. This inducible system has the additional strength of testing the consequences of a gene KO at different times during development. Indeed, our data show that loss of BDNF in early development may produce more extreme phenotypes than loss of BDNF in the adult. These findings, therefore, highlight the caution that must be used in interpreting conventional and even conditional KO mice. Although they often circumvent the problem of early lethality, conditional KO mice do not specifically test the role of a gene in the adult, because the KO still occurs relatively early in development, typically in late embryonic or early postnatal periods. Further studies of the inducible BDNF mice will enable a more complete understanding of the role played by this neurotrophin in the adult brain. Moreover, this inducible gene KO system can be applied more broadly to investigate the influence of other genes on adult brain function.
Supplementary Material
Acknowledgments
We thank C. Steffen, N. Sanchez, and T. Sasaki for excellent technical assistance and E. Kavalali for comments on the manuscript. This work was supported by grants from the National Institute of Mental Health and National Institute of Drug Abuse (to E.J.N.), National Alliance for Research on Schizophrenia and Depression (to L.M.M.), and National Alliance for Autism Research (to L.M.M.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: KO, knockout; dox, doxycycline; BDNF, brain-derived neurotrophic factor; NSE, neuron-specific enolase; tTA, tetracycline transcriptional activator; CTL, control; X-gal, 5-bromo-4-chloro-3-indolyl β-D-galactoside; β-gal, β-galalactosidase; fEPSP, excitatory postsynaptic field potentials.
References
- 1.Thoenen, H. (2000) Prog. Brain Res. 128, 183-191. [DOI] [PubMed] [Google Scholar]
- 2.Huang, E. J. & Reichardt, L. F. (2003) Annu. Rev. Biochem. 72, 609-642. [DOI] [PubMed] [Google Scholar]
- 3.Murer, M. G., Yan, Q. & Raisman-Vozari, R. (2001) Prog. Neurobiol. 63, 71-124. [DOI] [PubMed] [Google Scholar]
- 4.Levine, E. S., Dreyfus, C. F., Black, I. B. & Plummer, M. R. (1995) Proc. Natl. Acad. Sci. USA 92, 8074-8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McAllister, A. K., Katz, L. C. & Lo, D. C. (1997) Neuron 18, 767-778. [DOI] [PubMed] [Google Scholar]
- 6.Poo, M. M. (2001) Nat. Rev. Neurosci. 2, 24-32. [DOI] [PubMed] [Google Scholar]
- 7.Kovalchuk, Y., Hanse, E., Kafitz, K. W. & Konnerth, A. (2002) Science 295, 1729-1734. [DOI] [PubMed] [Google Scholar]
- 8.Blum, R., Kafitz, K. W. & Konnerth, A. (2002) Nature 419, 687-693. [DOI] [PubMed] [Google Scholar]
- 9.Ernfors, P., Lee, K. F. & Jaenisch, R. (1994) Nature 368, 147-150. [DOI] [PubMed] [Google Scholar]
- 10.Linnarsson, S., Bjorklund, A. & Ernfors, P. (1997) Eur. J. Neurosci. 9, 2581-2587. [DOI] [PubMed] [Google Scholar]
- 11.Montkowski, A. & Holsboer, F. (1997) NeuroReport 8, 779-782. [DOI] [PubMed] [Google Scholar]
- 12.Lyons, W. E., Mamounas, L. A., Ricaurte, G. A., Coppola, V., Reid, S. W., Bora, S. H., Wihler, C., Koliatsos, V. E. & Tessarollo, L. (1999) Proc. Natl. Acad. Sci. USA 96, 15239-15244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rios, M., Fan, G., Fekete, C., Kelly, J., Bates, B., Kuehn, R., Lechan, R. M. & Jaenisch, R. (2001) Mol. Endocrinol. 15, 1748-1757. [DOI] [PubMed] [Google Scholar]
- 14.Chen, J., Kelz, M. B., Zeng, G., Sakai, N., Steffen, C., Shockett, P. E., Picciotto, M. R., Duman, R. S. & Nestler, E. J. (1998) Mol. Pharmacol. 54, 495-503. [DOI] [PubMed] [Google Scholar]
- 15.Perl, A. K., Wert, S. E., Nagy, A., Lobe, C. G. & Whitsett, J. A. (2002) Proc. Natl. Acad. Sci. USA 99, 10482-10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radomska, H. S., Gonzalez, D. A., Okuno, Y., Iwasaki, H., Nagy, A., Akashi, K., Tenen, D. G. & Huettner, C. S. (2002) Blood 100, 4410-4419. [DOI] [PubMed] [Google Scholar]
- 17.Soriano, P. (1999) Nat. Genet. 21, 70-71. [DOI] [PubMed] [Google Scholar]
- 18.Monteggia, L. M., Eisch, A. J., Tang, M. D., Kaczmarek, L. K. & Nestler, E. J. (2000) Brain Res. Mol. Brain Res. 81, 129-139. [DOI] [PubMed] [Google Scholar]
- 19.Miczek, K. A., Maxson, S. C., Fish, E. W. & Faccidomo, S. (2001) Behav. Brain Res. 125, 167-181. [DOI] [PubMed] [Google Scholar]
- 20.Powell, C. M., Schoch, S., Monteggia, L., Barrot, M., Matos, M. F., Feldmann, N., Sudhof, T. C. & Nestler, E. J. (2004) Neuron 42, 143-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porsolt, R. D., Bertin, A. & Jalfre, M. (1977) Arch. Int. Pharmacodyn. Ther. 229, 327-336. [PubMed] [Google Scholar]
- 22.Gossen, M. & Bujard, H. (1992) Proc. Natl. Acad. Sci. USA 89, 5547-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajewsky, K., Gu, H., Kuhn, R., Betz, U. A., Muller, W., Roes, J. & Schwenk, F. (1996) J. Clin. Invest. 98, 600-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sauer, B. & Henderson, N. (1989) Nucleic Acids Res. 17, 147-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelz, M. B., Chen, J., Carlezon, W. A., Jr., Whisler, K., Gilden, L., Beckmann, A. M., Steffen, C., Zhang, Y. J., Marotti, L., Self, D. W., et al. (1999) Nature 401, 272-276. [DOI] [PubMed] [Google Scholar]
- 26.Kang, H. & Schuman, E. M. (1995) Science 267, 1658-1662. [DOI] [PubMed] [Google Scholar]
- 27.Patterson, S. L., Abel, T., Deuel, T. A., Martin, K. C., Rose, J. C. & Kandel, E. R. (1996) Neuron 16, 1137-1145. [DOI] [PubMed] [Google Scholar]
- 28.Minichiello, L., Korte, M., Wolfer, D., Kuhn, R., Unsicker, K., Cestari, V., Rossi-Arnaud, C., Lipp, H. P., Bonhoeffer, T. & Klein, R. (1999) Neuron 24, 401-414. [DOI] [PubMed] [Google Scholar]
- 29.Duman, R. S., Heninger, G. R. & Nestler, E. J. (1997) Arch. Gen. Psychiatry 54, 597-606. [DOI] [PubMed] [Google Scholar]
- 30.Dalvi, A. & Lucki, I. (1999) Psychopharmacology 147, 14-16. [DOI] [PubMed] [Google Scholar]
- 31.Lindeberg, J., Mattsson, R. & Ebendal, T. (2002) J. Neurosci. Res. 68, 248-253. [DOI] [PubMed] [Google Scholar]
- 32.Korte, M., Griesbeck, O., Gravel, C., Carroll, P., Staiger, V., Thoenen, H. & Bonhoeffer, T. (1996) Proc. Natl. Acad. Sci. USA 93, 12547-12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.