Abstract
Background: Data on combined hormonal oral contraceptives' (OCs) effects on metabolic changes in women with polycystic ovary syndrome (PCOS) have been conflicting and were predominantly based on OCs with cyproterone acetate (unavailable in the United States) Most studies did not include normal women as controls. We compared metabolic changes before and after an OC commonly used in the United States between women with and without PCOS.
Methods: Ten PCOS and 20 control women took ethinyl estradiol 35 μg and norgestimate 0.18/0.215/0.25 mg. Fasting glucose and insulin, area-under-the-curve (AUC) glucose and insulin, insulin sensitivity (homeostatic model assessment of insulin sensitivity index [HOMA-ISI] and Matsuda index), insulinogenic index (Δinsulin0–30 minutes/Δglucose0–30 minutes), blood pressure, and lipids were evaluated at baseline and after three cycles of OC.
Results: At baseline, PCOS women had lower insulin sensitivity (Matsuda index p = 0.0093, HOMA-ISI p = 0.0397), higher fasting insulin (p = 0.0495), fasting glucose (p = 0.0393), AUC insulin (p = 0.0023), and triglycerides (p = 0.0044) versus controls. Baseline AUC glucose did not differ between PCOS women and controls. After 3 months of OC use, glucose tolerance worsened in PCOS women versus controls (p = 0.0468). Higher baseline androgens were predictive of worsened glucose tolerance, and a reduction of AUC insulin during OC use. The insulinogenic index significantly decreased in PCOS women (p < 0.01), while fasting insulin and insulin resistance significantly worsened in control women.
Conclusion: Women with PCOS exhibited worsened glucose tolerance (demonstrated by AUC glucose) after 3 months of a commonly used OC compared with control women. Larger studies with longer follow-up should confirm these findings.
Introduction
Polycystic ovary syndrome (PCOS) is a common endocrine disorder in reproductive-age women.1 It is associated with insulin resistance and a high prevalence of obesity, dyslipidemia, and the metabolic syndrome.2–5 A common treatment for cycle control and symptoms of androgen excess (i.e., hirsutism and acne) associated with PCOS are combined oral contraceptives (OCs), preferably those with low-androgenic or antiandrogenic properties.6,7 However, OCs may be associated with insulin resistance and glucose intolerance.8–10 Thus, there is concern that OCs may further exacerbate the underlying metabolic dysfunction and insulin resistance in women with PCOS.11 This is specifically concerning in obese women with PCOS, who have insulin resistance both due to PCOS and excess adiposity.12
Data on OC effect on carbohydrate metabolism in overweight/obese women with PCOS have been conflicting. Studies with OCs containing the antiandrogen cyproterone acetate have shown either no effect on insulin sensitivity13 or an aggravation in insulin resistance14,15 and decrease in glucose tolerance.16 Studies with low androgenic OC, desogestrel, have shown a decrease in glucose tolerance11 and insulin resistance.17 A recent meta-analysis of studies investigating the association between use of OCs and metabolic changes reported that OC use was not associated with a significant change in fasting glucose or insulin, and homeostasis model assessment of insulin resistance.18 However, OCs with varying types of progestins were included, and the studies showed significant heterogeneity, with body–mass index (BMI) being a contributory factor to OC effects on fasting glucose and insulin resistance.
Lack of a control group is a common limitation of most studies evaluating OC metabolic effects in women with PCOS. OCs may be associated with insulin resistance and glucose intolerance even in women without PCOS.8–10 Whether women with PCOS are more susceptible than normal women has only been evaluated in two studies. One study evaluated a norethindrone-containing OC in PCOS women compared to BMI-matched controls.19 After 3 months of this OC, a reduction in insulin sensitivity index, as measured by the hyperglycemic clamp, was observed in both groups. Whether these findings are translatable to low-androgenic OCs more commonly used in PCOS women is unknown. Another study evaluated a low-androgenic OC containing norgestimate and found no change in insulin sensitivity in lean PCOS and control women.20 A large number of women with PCOS in North America are overweight or obese, and unfortunately, results of this previous study are not generalizable to these PCOS women.
In this pilot study, we report the effects of a commonly used low-androgenic OC (35 μg ethinyl estradiol and 0.18/0.215/0.25 mg norgestimate) on carbohydrate and lipid metabolism in women with and without PCOS. The two groups of women were overweight or obese and BMI matched. This particular OC was studied because it is one of the most commonly used21,22 and because the safety profile of low-androgenic norgestimate has been established, with previous data suggesting no significant worsening of fasting insulin, glucose, or glycosylated hemoglobin.23
Materials and Methods
Participants
Participants were taken from two clinical studies each conducted with the OC ethinyl estradiol 35 μg and norgestimate 0.18/0.215/0.25 mg. One study evaluated PCOS women exclusively (NCT00682890)24 and compared the OC to metformin. The other study evaluated the OC in regular cycling women without PCOS exclusively (NCT00205504).9 Both the previous studies and the present study were approved by the Virginia Commonwealth University Institutional Review Board. All study participants provided informed consent. For this report, we included all PCOS women randomized to OC, but not metformin (from NCT00682890)24 and matched their BMI to normal women (from NCT00205504)9 using the nearest neighbor method. All subjects included in this report were overweight or obese.
PCOS was defined by the modified Rotterdam criteria, after excluding other endocrine disorders.25 In this study, all PCOS women had clinical or biochemical signs of hyperandrogenism, and oligo- or amenorrhea. The control groups consisted of regular cycling women.
The exclusion criteria for both studies were similar and included the following: pregnancy, contraindications to OCs (e.g., a history of thromoboembolism, blood pressure >140/90 mmHg, hepatic disease), diabetes, tobacco use, and the use of systemic hormonal contraceptives, insulin sensitizers, antiandrogens, glucocorticoids, antihypertensives, or antilipidemics within the last 3 months.
Study procedures
Study procedures were identical for the two studies. Measurements were taken during the follicular phase of the menstrual cycle, confirmed by a serum progesterone concentration of less than 2 ng/mL. For both studies, after a 12-hour fast, vital signs, height, weight, serum fasting lipids, insulin, and glucose were obtained. A 2-hour oral glucose tolerance test (OGTT) with a 75 g glucose load was administered, with glucose and insulin levels determined every 15 minutes. The participants began the OC (ethinyl estradiol 35 μg and norgestimate 0.18/0.215/0.25 mg) the next day. Subjects from both studies were prospectively followed for 3 months.
Laboratory analyses
Serum and plasma were stored at −80°C until assayed. Serum glucose was measured by the glucose oxidative method (YSI 2300 STAT Plus Glucose Analyzer; Yellow Springs Instruments). Serum insulin levels were measured by enzyme-linked immunosorbent assay (ELISA; ALPCO Diagnostics). Serum testosterone and sex hormone binding globulin were measured using ELISA (ALPCO Diagnostics). Serum-free testosterone was calculated using the method of Sodergard et al.26 For both studies, determinations were made in duplicate. All inter- and intra-assay coefficients of variation were <10%.
Statistical analysis
Fasting glucose and insulin, area-under-the-curve (AUC) glucose and insulin, incremental AUC glucose and insulin, insulin sensitivity (homeostatic model assessment of insulin sensitivity index [HOMA-ISI] and Matsuda index),27,28 early insulin response during OGTT (as estimated by the insulinogenic index, (Δinsulin0–30 minutes/(Δglucose0–30 minutes), blood pressure, and fasting lipid profile were evaluated at baseline and after three cycles of OC. AUC glucose and insulin upon OGTT were calculated by the trapezoidal rule, and incremental AUC glucose and insulin were calculated similarly after subtracting fasting values. As fasting values of insulin and glucose were already separately presented, incremental AUCs reflect changes in response to glycemic loads.29 Baseline comparisons between the control group (n = 20) and the PCOS group (n = 10) were performed using independent t-tests. Changes at 3 months from baseline within each group were analyzed using the paired t-test. Group differences were assessed by repeated measures analysis of variance (ANOVA), evaluating the interaction between time and group status (PCOS vs. normal women). Distribution of the data was assessed by normal quantile plots. Variables not in normal distribution were log-transformed for analyses and then back transformed into their original units for reporting. Data are presented as mean ± standard deviation, or geometric mean (95% confidence interval [CI]) for parameters that were transformed for analyses. p < 0.05 was considered statistically significant. Analyses were performed by JMP 11.0 (SAS Institute).
Sample size calculation
No current data exist regarding the effect of a norgestimate-containing OC in overweight/obese PCOS women compared to control women. For this exploratory pilot project, assumptions for sample size calculation were based on our previous data comparing the effects of the same norgestimate-containing OC on metabolic parameters between obese versus nonobese women.9 Similar to the current study, the obese and nonobese women in this previous study presented with between-group differences in baseline insulin sensitivity. In the previous study, the difference in the change in Matsuda index between obese and nonobese women was 3.1, with a common standard deviation of 1.5. In this study, we used similar assumptions but utilized a more conservative estimate for the standard deviation (common standard deviation of 2.0 instead of 1.5). Using these assumptions, eight women in each group would be needed to achieve a power of 80%, with α = 0.05.
Results
Baseline characteristics
At baseline, PCOS and control women were demographically similar and had comparable BMI (Table 1). As expected, women with PCOS were more insulin resistant at baseline, as assessed by the Matsuda index (p = 0.0093) and HOMA-ISI (p = 0.0397) (Table 2). The PCOS group also exhibited higher testosterone (p = 0.0021), fasting insulin (p = 0.0495) and glucose levels (p = 0.0393), higher AUC insulin (p = 0.0052), higher triglycerides (p = 0.0044), and waist-to-hip ratio (p = 0.0089) compared with control women.
Table 1.
Characteristics of Study Participants
| Parameter | PCOS women (n = 10) | Control women (n = 20) | p |
|---|---|---|---|
| Age (years) | 24.3 ± 4.4 | 21.8 ± 3.8 | 0.1175 |
| BMI (kg/m2) | 32.6 ± 7.2 | 31.3 ± 8.8 | 0.7008 |
| Race | |||
| Caucasian | 4 (40%) | 12 (60%) | 0.2999 |
| Non-Caucasian | 6 (60%) | 8 (40%) | |
| Family history of diabetes | 2 (20%) | 4 (20%) | 1.0000 |
Values are mean ± SD, or numbers (%).
PCOS, polycystic ovary syndrome.
Table 2.
Effects of Combined Oral Contraceptive on Carbohydrate and Lipid Metabolism in Study Participants
| PCOS women (n = 10) | Control women (n = 20) | |||||
|---|---|---|---|---|---|---|
| Parameter | Baseline | 3 months | Baseline | 3 months | p-Value (baseline comparisons between groups)a | p-Value (comparisons of OC effects between groups)b |
| Total testosterone (ng/dL) | 57.6 ± 38.9 | 22.5 ± 19.9c | 25.5 ± 12.9 | 19.7 ± 9.4d | 0.0021 | 0.8769 |
| Free testosterone (ng/dL) | 8.1 ± 8.5 | 3.5 ± 4.4c | 4.4 ± 2.5 | 1.7 ± 0.8e | 0.0822 | 0.3785 |
| Fasting insulin (μU/L) | 7.9 (4.8–13.1) | 8.7 (6.3–12.1) | 4.7 (3.5–6.4) | 5.6 (4.3–7.4)c | 0.0495 | 0.6336 |
| Fasting glucose (mg/dL) | 88.7 ± 6.6 | 88.1 ± 7.0 | 84.1 ± 4.9 | 84.3 ± 6.0 | 0.0393 | 0.6543 |
| AUC insulin (μU/L·min) | 8,643 (5,655–13,210) | 7,864 (6,138–10,024) | 4,260 (3,207–5,661) | 4,675 (3,512–6,217) | 0.0052 | 0.32 |
| Incremental AUC insulin (μU/L·min) | 7,387 (4,947–11,031) | 6,682 (4,474–9,978) | 3,590 (2,704–4,767) | 3,837 (2,890–5,094) | 0.0021 | 0.1071 |
| AUC glucose (mg/dL) | 14,607 (11,631–18,345) | 23,153 (18,436–29,078) | 12,965 (11,036–15,231) | 14,109 (12,010–16,576)c | 0.0784 | 0.0468 |
| Incremental AUC glucose (mg/dL) | 4,250 (2,808–6,432) | 8,344 (3,024–23,017) | 2,671 (1,876–3,803) | 3,803 (2,758–5,245)c | 0.0365 | 0.0234 |
| OGTT glucose120minutes (mg/dL) | 119 ± 29.0 | 124 ± 27.9 | 111 ± 20.3 | 114 ± 27.7 | 0.3921 | 0.8144 |
| Matsuda index | 4.42 ± 2.63 | 4.13 ± 1.57 | 9.06 ± 4.88 | 7.45 ± 3.96f | 0.0093 | 0.1287 |
| HOMA-ISI | 0.73 ± 0.49 | 0.60 ± 0.35 | 1.21 ± 0.61 | 1.01 ± 0.58c | 0.0397 | 0.6234 |
| I (0–30)/G(0–30) (min) | 2.18 (1.72–2.79) | 1.49 (1.05–2.12)f | 1.51 (0.81–2.78) | 1.01(0.66–2.15) | 0.3827 | 0.6285 |
| Disposition index | 8.10 (3.99–16.42) | 5.76 (2.84–11.69) | 11.76 (4.13–19.39) | 7.88 (4.78–12.99) | 0.4352 | 0.8518 |
| Systolic blood pressure (mmHg) | 117 ± 10.5 | 117 ± 9.88 | 116 ± 14.8 | 116 ± 14.8 | 0.8655 | 0.8316 |
| Diastolic blood pressure (mmHg) | 69 ± 6.3 | 70 ± 6.1 | 72 ± 7.7 | 72 ± 7.9 | 0.2448 | 0.5006 |
| BMI (kg/m2) | 32.6 ± 7.2 | 32.5 ± 6.9 | 31.3 ± 8.8 | 31.3 ± 8.9 | 0.7008 | 0.7352 |
| Waist circumference (cm) | 97.4 ± 13.6 | 96.2 ± 16.1 | 87.8 ± 19.8 | 89.4 ± 18.6 | 0.1806 | 0.3056 |
| Waist-to-hip ratio | 0.84 (0.80–0.88) | 0.81 (0.75–0.87) | 0.75 (0.70–0.80) | 0.78 (0.75–0.81)f | 0.0198 | 0.0955 |
| Total cholesterol (mg/dL) | 168 ± 24.0 | 182 ± 25.4c | 161 ± 27.0 | 182 ± 24.6d | 0.452 | 0.2173 |
| LDL (mg/dL) | 100 ± 16.6 | 107 ± 19.7 | 90 ± 22.0 | 102 ± 25.6c | 0.2398 | 0.5785 |
| Triglycerides (mg/dL) | 116 (90.0–137.8) | 136 (93.3–198.5) | 66 (52.8–83.1) | 76 (59.4–98.0) | 0.0044 | 0.6599 |
| HDL (mg/dL) | 45 ± 8.9 | 48 ± 7.8 | 53 ± 11.9 | 61 ± 12.6d | 0.0947 | 0.0882 |
| Number of metabolic risk factors | 2.0 ± 1.2 | 1.6 ± 1.1 | 1.2 ± 1.2 | 0.9 ± 1.1 | 0.0719 | 0.6141 |
Values are indicated in mean ± SD or geometric mean (95% confidence interval).
Baseline comparisons between groups, performed by independent Student's t-tests.
Comparisons of OC effects between PCOS and control groups, performed by repeated measures analysis of variance (visit × PCOS status).
p < 0.05, fp < 0.01,dp < 0.005, ep < 0.001 for comparisons between baseline and 3 months within group, performed with paired t-tests.
AUC, area-under-the-curve; BMI, body–mass index; HDL, high-density lipoprotein; HOMA-ISI, homeostatic model assessment of insulin sensitivity index; LDL, low-density lipoprotein; OC, oral contraceptive; OGTT, oral glucose tolerance test.
Effects of OC within each group
Within the control group, after 3 months of OC use, there was a small but statistically significant increase in fasting insulin, AUC glucose, peak glucose, and a statistically significant decrease in insulin sensitivity compared to baseline (Table 2). PCOS women already had an adverse glucose metabolism profile at baseline compared to normal women (Table 2). Although the PCOS group had similar worsening in these parameters after 3 months of OC, the baseline-to-3-month changes in PCOS women did not reach significance. However, the PCOS group had a statistically significant decrease in the insulinogenic index after treatment with OC (Table 2).
Both PCOS and normal women had a significant increase in total cholesterol after 3 months of OC (Table 2). However, only the control women had a significant increase in high-density lipoprotein cholesterol. Control women also had a significant increase in low-density lipoprotein (LDL) cholesterol, but they had lower LDL cholesterol at baseline than PCOS women by 10 mg/dL. Even after a significant increase, their LDL cholesterol after OC treatment was still lower than that of PCOS women.
Effects of OC in PCOS women versus control women
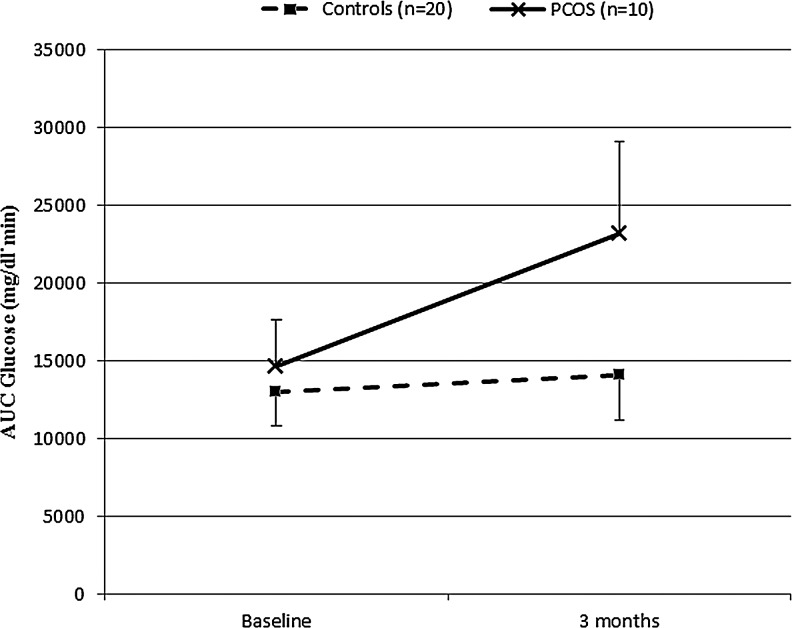
The use of OC for 3 months resulted in a divergent effect on AUC glucose and incremental AUC glucose between the PCOS and control groups (p = 0.0468 and p = 0.0234, respectively, for interaction between PCOS status and AUC glucose time trends, repeated measures ANOVA), as shown in Table 2 and Figure 1. In PCOS women, AUC glucose increased by more than 50%, from 14,603 (95% CI 12,370–16,751) mg/dL·min at baseline to 23,155 (95% CI 13,119–40,864) mg/dL·min at 3 months. In the control group, although within-group comparison by the paired t-test suggests a slight but significant increase in AUC glucose (Table 2), this increase in AUC glucose was less dramatic, from 12,965 (95% CI 12,049–13,951) mg/dL·min at baseline to 14,044 (12,848–15,491) mg/dL·min at 3 months (p = 0.0468 for between-group comparisons). Similar observations were made for incremental AUC glucose. There were no statistically significant differences between the PCOS and normal women in other glucose metabolism parameters during the 3 months of OC use (Table 2).
FIG. 1.
AUC-Glucose in women with and without PCOS before and after 3 months of OC use. Values are geometric means (95% confidence interval). AUC, area-under-the-curve; OC, oral contraceptive; PCOS, polycystic ovary syndrome.
Relationship between baseline androgens and glucose metabolism
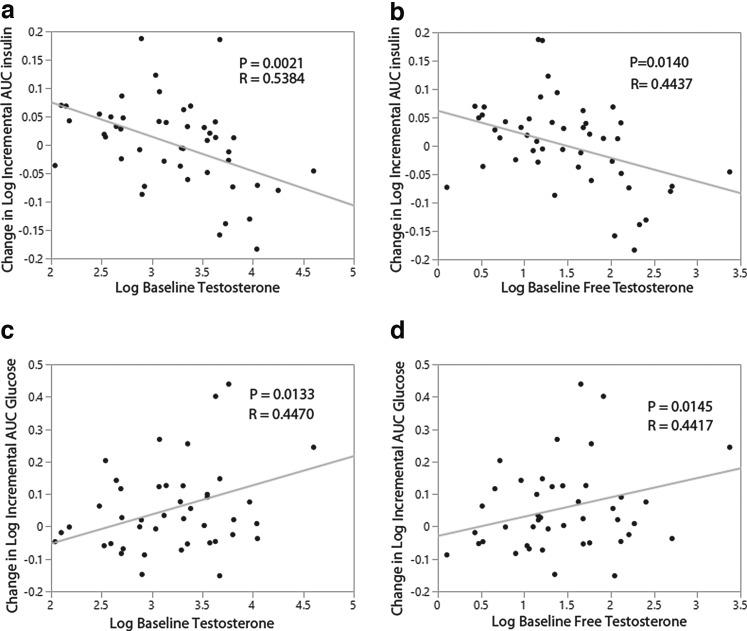
Both total testosterone (r = 0.442, p = 0.0144) and free testosterone (r = 0.470, p = 0.0088) were significantly associated with AUC insulin at baseline. Similarly, total testosterone (r = 0.407, p = 0.0258) and free testosterone (r = 0.608, p = 0.0004) were significantly associated with incremental AUC insulin at baseline. Higher androgens were associated with higher AUC and incremental AUC insulin. Relationships between androgens and other glucose/insulin parameters at baseline were not observed. As expected, treatment with OC significantly reduced total and free testosterone in both groups (Table 2). Baseline total testosterone and free testosterone were also predictive of changes in incremental AUC insulin (Fig. 2a, b) and incremental AUC glucose (Fig. 2c, d) during OC treatment. Higher baseline androgens were predictive of a decrease in incremental AUC insulin and an increase in incremental AUC glucose during OC use.
FIG. 2.
Relationship between baseline androgens and changes in incremental AUC-insulin and incremental AUC-glucose during 3 months of OC use. (a) Relationship between change in incremental AUC insulin and baseline total testosterone. (b) Relationship between change in incremental AUC insulin and baseline free testosterone. (c) Relationship between change in incremental AUC glucose and baseline total testosterone. (d) Relationship between change in incremental AUC glucose and baseline free testosterone.
Discussion
We set out to determine the metabolic effects of a norgestimate-containing OC in BMI-matched PCOS and normal women over 3 months. Women with PCOS were more insulin resistant, had higher fasting insulin and glucose levels, and higher AUC insulin at baseline. After 3 months of OC use, we found significant worsening of glucose tolerance (AUC glucose) in PCOS women compared to control women. There was no significant difference between the two groups in other metabolic parameters at the end of 3 months.
Worsened glucose tolerance in PCOS women with OC use has been previously reported.30 A randomized study by Morin-Papunen et al. compared the metabolic effects of an OC containing cyproterone acetate and metformin in obese women with PCOS.16 After 6 months, AUC glucose increased in the group receiving OC. However, no control women were included, so whether PCOS women developed glucose intolerance to a greater degree than women without PCOS could not be inferred.
Whether PCOS women experience more pronounced glucose intolerance compared to normal women has seldom been studied. One previous study has compared the effects of OC on glucose metabolism between PCOS and control women. In a study by Korykowski et al., the metabolic effects of norethindrone-containing OC were examined in weight-matched PCOS women and controls.19 They found a significant decrease in the insulin sensitivity index (measured by the euglycemic-hyperinsulinemic clamp) in both PCOS and controls. However, the results of this study cannot be applied to current practice, given that norethindrone is an androgenic progestin10 and is seldom prescribed for PCOS women. Androgenic progestins have also been associated with more adverse effects on insulin sensitivity.30
While there have been studies on the metabolic effects of the low-androgenic progestin norgestimate in PCOS women, only lean PCOS women have been evaluated. Cibula et al. found no decrease in insulin sensitivity using the euglycemic hyperinsulinemic clamp in both lean PCOS women and controls who took norgestimate for 6 months.20 Similar to this study, we also did not find a difference in insulin sensitivity over the 3 months of OC use between overweight/obese PCOS and control women with norgestimate. The study by Cibula et al. did not measure glucose tolerance.20 In our study, we found a significant worsening of glucose tolerance in PCOS women during OC use. It is possible that obesity may have magnified OC adverse effects on glucose tolerance. In a cross-sectional study by Doar and Wynn, obese women taking OCs had increased AUC glucose compared to nonobese women.31
The worsened glucose tolerance in our PCOS women after OC use appears to be due primarily to a decrease in beta cell function and not due to worsened insulin resistance. This is not unexpected because at baseline, PCOS women were already profoundly more insulin resistant, and it was expected that OC may not further aggravate this insulin resistance. The insulinogenic index (beta cell function) significantly declined at 3 months in the PCOS group. The suggestion that beta cell function may be responsible for the worsened glucose tolerance in the PCOS women is also supported by our observation that women whose androgens were elevated at baseline (i.e., PCOS women) had a decrease in incremental AUC insulin during OC treatment. The insulin resistance already present at baseline, combined with decreased beta cell function during the OC treatment, may have led to greater glucose intolerance in PCOS women compared to normal women.
This impaired compensatory insulin secretion in PCOS women upon exposure to exogenous factors exacerbating insulin resistance has been previously reported by others.32 In a study by Ehrmann et al., BMI-matched obese PCOS women and obese controls were given dexamethasone to induce insulin resistance. Dexamethasone led to a greater deterioration in glucose tolerance during a 2-hour OGTT in PCOS versus controls. At the same time, an increase in AUC C-peptide to glucose ratio was less in PCOS women versus controls after dexamethasone use. Glucose intolerance in the PCOS group was thought to be due to a diminished beta-cell response in the face of increased demand placed on by factors exacerbating insulin resistance.
Insulin resistance did not significantly deteriorate in PCOS women during OC use in our study. Baseline obesity in the PCOS women may explain the lack of further worsening of insulin resistance during treatment. Chen et al. compared the effects of the OC containing cyproterone acetate in obese versus nonobese PCOS women. Insulin resistance did not change in obese PCOS women, but increased in nonobese PCOS women after 3 months of OC use.33 Here, the authors specifically cited BMI as the cause for the observed differences between the study groups. The effect of obesity is further supported by a meta-analysis evaluating the association between OCs and metabolic changes in women with PCOS.18 Meta-regression of included studies suggested that as mean BMI of the PCOS women in the studies increased, the association between HOMA-IR and OCs weakens.18
Hence, it appears that when insulin sensitivity is already profoundly reduced at baseline, as in the case of the combination of both PCOS and obesity, the effect of OCs in further reducing insulin sensitivity may not be evident. This may explain why only control women, but not PCOS women, experienced a significant increase in fasting insulin, and worsening of insulin sensitivity after 3 months of OC when compared to baseline. It is possible that given a much better insulin sensitivity profile at baseline compared with PCOS women, there is more room for OCs to induce a worsening of insulin sensitivity in normal women. In contrast, in obese PCOS women who were already profoundly insulin resistant at baseline, OCs may not further aggravate this insulin resistance. Even after this worsening of insulin sensitivity with OC, control women were still more insulin sensitive compared to PCOS women without OC. Nonetheless, pending confirmation with a bigger sample size, monitoring of metabolic parameters in obese women during OC use may be considered.
When comparing the lipid profile of the PCOS and control groups at baseline, the only significant difference found was higher triglycerides in the PCOS group (116 [95% CI 90.0–137.8] mg/dL in PCOS women versus 66 [95% CI 52.8–83.1] mg/dL in controls, p = 0.02). After three cycles of norgestimate, there were no significant differences in lipid parameters between the two groups, possibly due to the small number of subjects in our study.
PCOS subjects did not have significantly greater blood pressure than controls at baseline. There was also no detrimental effect of norgestimate on blood pressure in the PCOS and control groups at the end of three cycles. This supports earlier studies on norgestimate and other low-dose OCs demonstrating limited to no effects on blood pressure.34–36
Our study has several major limitations, notably a small sample size and only a 3-month follow-up. Because no data exist regarding the effects of a norgestimate-containing OC on metabolic parameters in obese PCOS and control women, we used data from a previous study in obese and nonobese women to serve as a starting point for sample size estimation for this pilot study. There may not have been sufficient power to observe significant changes in certain parameters. In addition, the 3-month follow-up time may have contributed to the inability to demonstrate significant changes in the lipid and metabolic profile of both PCOS and control groups. Because contraception methods are most likely used for much longer periods, findings of this pilot study will need to be confirmed by future studies with a much larger sample and a longer follow-up period, so that the long-term effects of OCs in obese women with and without PCOS can be ascertained. Finally, the lack of nonobese PCOS and control groups in this study prevents the direct comparison of OC effects among women of different obesity status with and without PCOS.
Conclusions
In summary, to the authors' knowledge, the current study is one of few reports comparing effects of a commonly used norgestimate-containing low-androgenic OC on glucose metabolism between PCOS and control women. Ours differ from previous reports in that we evaluated overweight/obese women. We observed significant worsening of glucose tolerance (AUC glucose) in PCOS women compared to control women. However, we did not find that the OC worsens insulin sensitivity in PCOS women. However, the OC may worsen insulin sensitivity in obese normal women compared to baseline. Further investigation should include a larger number of lean and obese PCOS and control women, and followed for longer periods
Acknowledgments
We thank nurses and staff at the Virginia Commonwealth University Clinical Research Services Unit for their invaluable assistance with this research. We also thank participants who have donated their time.
Funding
This work was supported, in part, by the National Institutes of Health Grant K23HD049454 (to K.I.C.) and R03HD047298 (to P.A.E.), Virginia Commonwealth University A.D. Williams Internal Research Grant (to K.I.C.), and Center for Clinical Translational Research UL1RR031990, National Institutes of Health. Sponsors and funding sources did not have involvement in the study design, collection, analysis, and interpretation of data, or in writing and submitting of this article.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Franks S. Polycystic ovary syndrome. N Engl J Med 1995;333:853–861 [DOI] [PubMed] [Google Scholar]
- 2.Apridonidze T, Essah PA, Iuorno MJ, Nestler JE. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2005;90:1929–1935 [DOI] [PubMed] [Google Scholar]
- 3.Diamanti-Kandarakis E, Kouli CR, Bergiele AT, et al. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: Hormonal and metabolic profile. J Clin Endocrinol Metab 1999;84:4006–4011 [DOI] [PubMed] [Google Scholar]
- 4.Holte J, Bergh T, Berne C, Berglund L, Lithell H. Enhanced early insulin response to glucose in relation to insulin resistance in women with polycystic ovary syndrome and normal glucose tolerance. J Clin Endocrinol Metab 1994;78:1052–1058 [DOI] [PubMed] [Google Scholar]
- 5.Lo JC, Feigenbaum SL, Yang J, Pressman AR, Selby JV, Go AS. Epidemiology and adverse cardiovascular risk profile of diagnosed polycystic ovary syndrome. J Clin Endocrinol Metab 2006;91:1357–1363 [DOI] [PubMed] [Google Scholar]
- 6.Azziz R. The evaluation and management of hirsutism. Obstet Gynecol 2003;101:995–1007 [DOI] [PubMed] [Google Scholar]
- 7.Yildiz B, Rizzi E, Dalla-Zuanna G. Oral contraceptives in polycystic ovary syndrome: Risk-benefit assessment. Semin Reprod 2008;26:111–120 [DOI] [PubMed] [Google Scholar]
- 8.Watanabe RM, Azen CG, Roy S, Perlman JA, Bergman R. Defects in carbohydrate metabolism in oral contraceptive uses without apparent metabolic risk factors. J Clin Endocrinol Metab 1994;79:1277–1283 [DOI] [PubMed] [Google Scholar]
- 9.Cheang KI, Essah PA, Sharma S, Wickham EP, Nestler JE. Divergent effects of a combined hormonal oral contraceptive on insulin sensitivity in lean versus obese women. Fertil Steril 2011;96:353–359.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodsland I, Crook D, Simpson R, et al. The effects of different formulations of oral contraceptive agents on lipid and carbohydrate metabolism. N Engl J Med 1990;323:1375–1381 [DOI] [PubMed] [Google Scholar]
- 11.Nader S, Riad-Gabriel MG, Saad MF. The effect of a desogestrel-containing oral contraceptive on glucose tolerance and leptin concentrations in hyperandrogenic women. J Clin Endocrinol Metab 1997;82:3074–3077 [DOI] [PubMed] [Google Scholar]
- 12.Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 1989;38:1165–1174 [DOI] [PubMed] [Google Scholar]
- 13.Harborne L, Fleming R, Lyall H, Sattar N, Norman J. Metformin or antiandrogen in the treatment of hirsutism in polycystic ovary syndrome. J Clin Endocrinol Metab 2003;88:4116–4123 [DOI] [PubMed] [Google Scholar]
- 14.Meyer CBTH. Effects of medical therapy on insulin resistance and the cardiovascular system in polycystic ovary syndrome. Diabetes Care 2007;30:471–478 [DOI] [PubMed] [Google Scholar]
- 15.Dahlgren E, Landin K, Krotkiewski M, Holm G, Janson PO. Effects of two antiandrogen treatments on hirsutism and insulin sensitivity in women with polycystic ovary syndrome. Hum Reprod 1998;13:2706–2711 [DOI] [PubMed] [Google Scholar]
- 16.Morin-Papunen LC, Vauhkonen I, Koivunen RM, Ruokonen A, Martikainen HK, Tapanainen JS. Endocrine and metabolic effects of metformin versus ethinyl estradiol-cyproterone acetate in obese women with polycystic ovary syndrome: A randomized study. J Clin Endocrinol Metab 2000;85:3161–3168 [DOI] [PubMed] [Google Scholar]
- 17.Kilic S, Yilmaz N, Zulfikaroglu E, Erdogan G, Aydin M, Batioglu S. Inflammatory-metabolic parameters in obese and nonobese normoandrogenemic polycystic ovary syndrome during metformin and oral contraceptive treatment. Gynecol Endocrinol 2011;27:622–629 [DOI] [PubMed] [Google Scholar]
- 18.Halperin IJ, Sujana Kumar S, Stroup DF, Laredo SE. The association between the combined oral contraceptive pill and insulin resistance, dysglycemia and dyslipidemia in women with polycystic ovary syndrome: A systematic review and meta-analysis of observational studies. Hum Reprod 2011;26:191–201 [DOI] [PubMed] [Google Scholar]
- 19.Korytkowski MT, Mokan M, Horwitz MJ, Berga S. Metabolic effects of oral contraceptives in women with polycystic ovary syndrome. J Clin Endocrinol Metab 1995;80:3327–3334 [DOI] [PubMed] [Google Scholar]
- 20.Cibula D, Sindelka G, Hill M, Fanta M, Skrha J, Zivny J. Insulin sensitivity in non-obese women with polycystic ovary syndrome during treatment with oral contraceptives containing low-androgenic progestin. Hum Reprod 2002;17:76–82 [DOI] [PubMed] [Google Scholar]
- 21.Top 200 drugs. www.drugs.com/top200.html 2010; accessed January3, 2015
- 22.Bartholow M. Top 200 drugs of 2012. Pharm Times 2013;79:42–44 [Google Scholar]
- 23.Burkman RT, Kafrissen ME, Olson W, Osterman J. Lipid and carbohydrate effects of a new triphasic oral contraceptive containing norgestimate. Acta Obstet Gynecol Scand Suppl 1992;156:5–8 [DOI] [PubMed] [Google Scholar]
- 24.Essah PA, Arrowood JA, Cheang KI, Adawadkar SS, Stovall DW, Nestler JE. Effect of combined metformin and oral contraceptive therapy on metabolic factors and endothelial function in overweight and obese women with polycystic ovary syndrome. Fertil Steril 2011;96:501–504.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azziz R, Carmina E, Dewailly D, et al. Positions statement: Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: An Androgen Excess Society guideline. J Clin Endocrinol Metab 2006;91:4237–4245 [DOI] [PubMed] [Google Scholar]
- 26.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem 1982;16:801–810 [DOI] [PubMed] [Google Scholar]
- 27.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 28.Matsuda M, Defronzo RA. Insulin Sensitivity Indices Obtained From Comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 29.Le Floch JP, Escuyer P, Baudin E, Baudon D, Perlemuter L. Blood glucose area under the curve. Methodological aspects. Diabetes Care 1990;13:172–175 [DOI] [PubMed] [Google Scholar]
- 30.Godsland IF. The influence of female sex steroids on glucose metabolism and insulin action. J Intern Med Suppl 1996;738:1–60 [PubMed] [Google Scholar]
- 31.Doar JW, Wynn V. Effects of obesity, glucocorticoid, and oral contraceptive therapy on plasma glucose and blood pyruvate levels. Br Med J 1970;1:149–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ehrmann DA, Breda E, Corcoran MC, et al. Impaired beta-cell compensation to dexamethasone-induced hyperglycemia in women with polycystic ovary syndrome. Am J Physiol Endocrinol Metab 2004;287:E241–E246 [DOI] [PubMed] [Google Scholar]
- 33.Chen M-J, Yang W-S, Chen H-F, et al. Increased follistatin levels after oral contraceptive treatment in obese and non-obese women with polycystic ovary syndrome. Hum Reprod 2010;25:779–785 [DOI] [PubMed] [Google Scholar]
- 34.Petersen KR, Christiansen E, Madsbad S, Skouby SO, Andersen LF, Jespersen J. Metabolic and fibrinolytic response to changed insulin sensitivity in users of oral contraceptives. Contraception 1999;60:337–344 [DOI] [PubMed] [Google Scholar]
- 35.Rautio K, Tapanainen JS, Ruokonen A, Morin-Papunen LC. Effects of metformin and ethinyl estradiol-cyproterone acetate on lipid levels in obese and non-obese women with polycystic ovary syndrome. Eur J Endocrinol 2005;152:269–275 [DOI] [PubMed] [Google Scholar]
- 36.Runnebaum B, Grunwald K, Rabe T. The efficacy and tolerability of norgestimate/ethinyl estradiol (250 μg of norgestimate/25 μg of ethinyl estradiol): Results of an open, multicenter study of 59,701 women. Am J Obstet Gynecol 1992:1963–1968 [DOI] [PubMed] [Google Scholar]