Abstract
We present a case of a 22-year-old asymptomatic female whose CT study (performed following trauma) incidentally discovered a posterior fossa mass. The lesion was further evaluated with a MRI study, and (following discussion with the patient and her family) elective surgical resection of the lesion was performed. On pathology, histological evaluation revealed a diagnosis of rosette-forming glioneuronal tumor of the fourth ventricle. RGNT of the fourth ventricle or posterior fossa should always be considered in the differential diagnosis of infratentorial lesions, especially in young adults.
Case report
A 22-year-old female presented to the ER following a motor vehicle accident. A CT study, obtained for trauma workup, revealed a posterior fossa mass lesion. No acute intracranial findings related to the trauma were seen.
On physical exam, there were no positive neurological findings. The patient had a normal motor and sensory exam, with no difficulty with coordination or balance, and had normal gait. No clinical signs of hydrocephalus were present.
The CT study of the head revealed a heterogeneously hypodense mass lesion involving the fourth ventricle and the cerebellar vermis, with some calcifications within (Figs. 1A, B).
Figure 1.
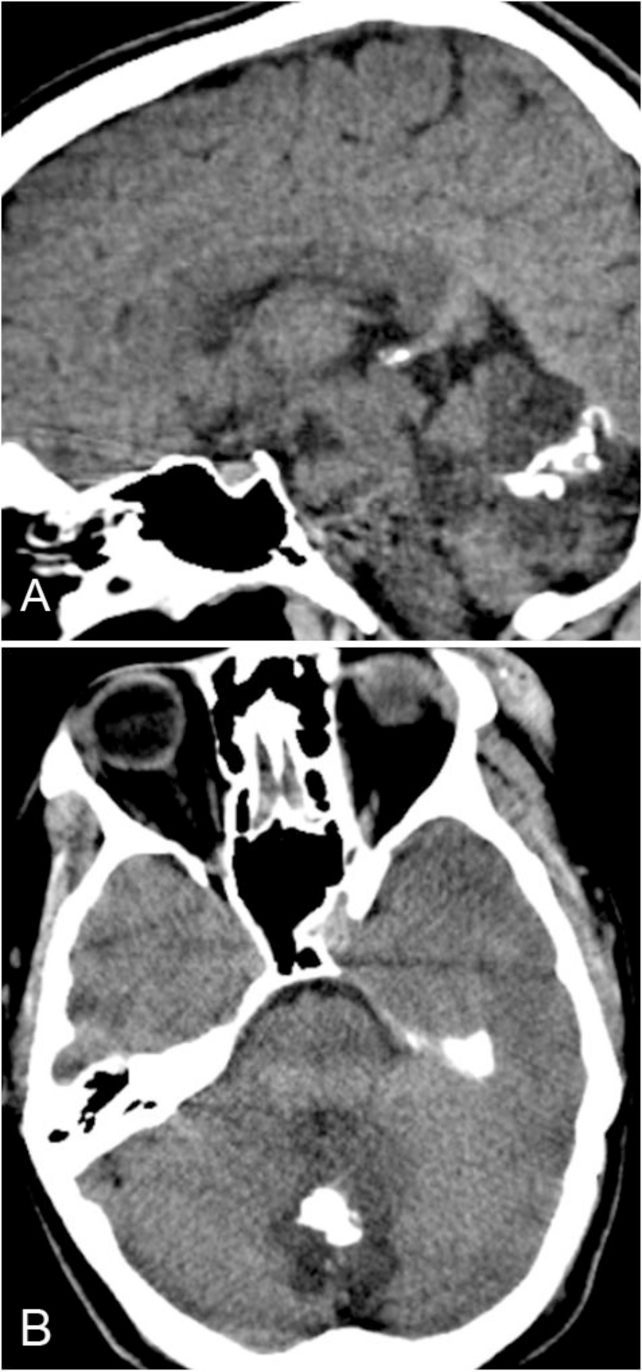
22-year-old female with rosette-forming glioneuronal tumor. Images reveal a hypodense mass lesion involving the midline posterior fossa, with predominant involvement of the fourth ventricle. Calcifications are seen within the lesion. A: Sagittal, noncontrast CT image. B: Axial, noncontrast CT image.
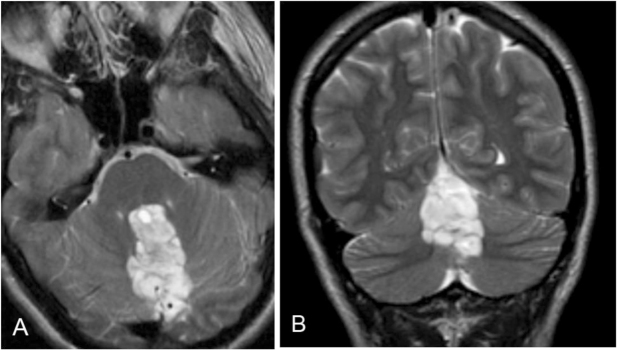
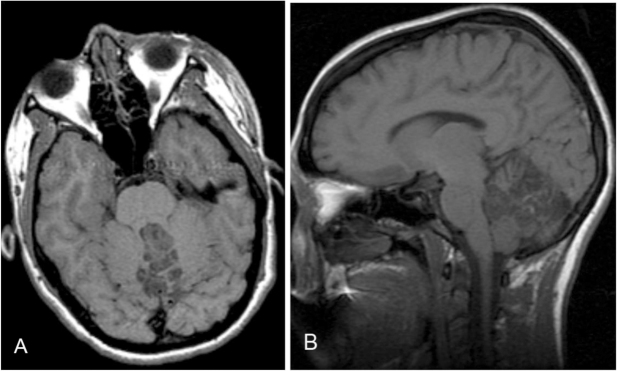
MRI study revealed a 5 × 4 × 2.5 cm, heterogeneously T2 hyperintense lesion, with multiple cystic areas, involving the fourth ventricle, which was expanded (Figs. 2A, B). The lesion was closely related to the midline vermis; no definite plane between the lesion and the vermis was seen. On the T1-weighted sequence, the lesion was predominantly hypointense and mildly heterogeneous (Figs. 3A, B). Areas of gradient susceptibility corresponding to the calcifications seen on the CT study were seen. Linear and serpiginous areas of enhancement were seen within the lesion (Figs. 4A, B). No significant dilatation of the third and lateral ventricles was seen.
Figure 2.
22-year-old female with rosette-forming glioneuronal tumor. T2-weighted MRI shows a heterogeneous, hyperintense lesion in the midline posterior fossa, with involvement of the fourth ventricle. A: Axial view. B: Coronal view.
Figure 3.
22-year-old female with rosette-forming glioneuronal tumor. Noncontrast, T1-weighted image shows a heterogeneous, hypointense lesion involving the midline posterior fossa, with predominant involvement of the fourth ventricle. A: Axial view. B: Sagittal view.
Figure 4.
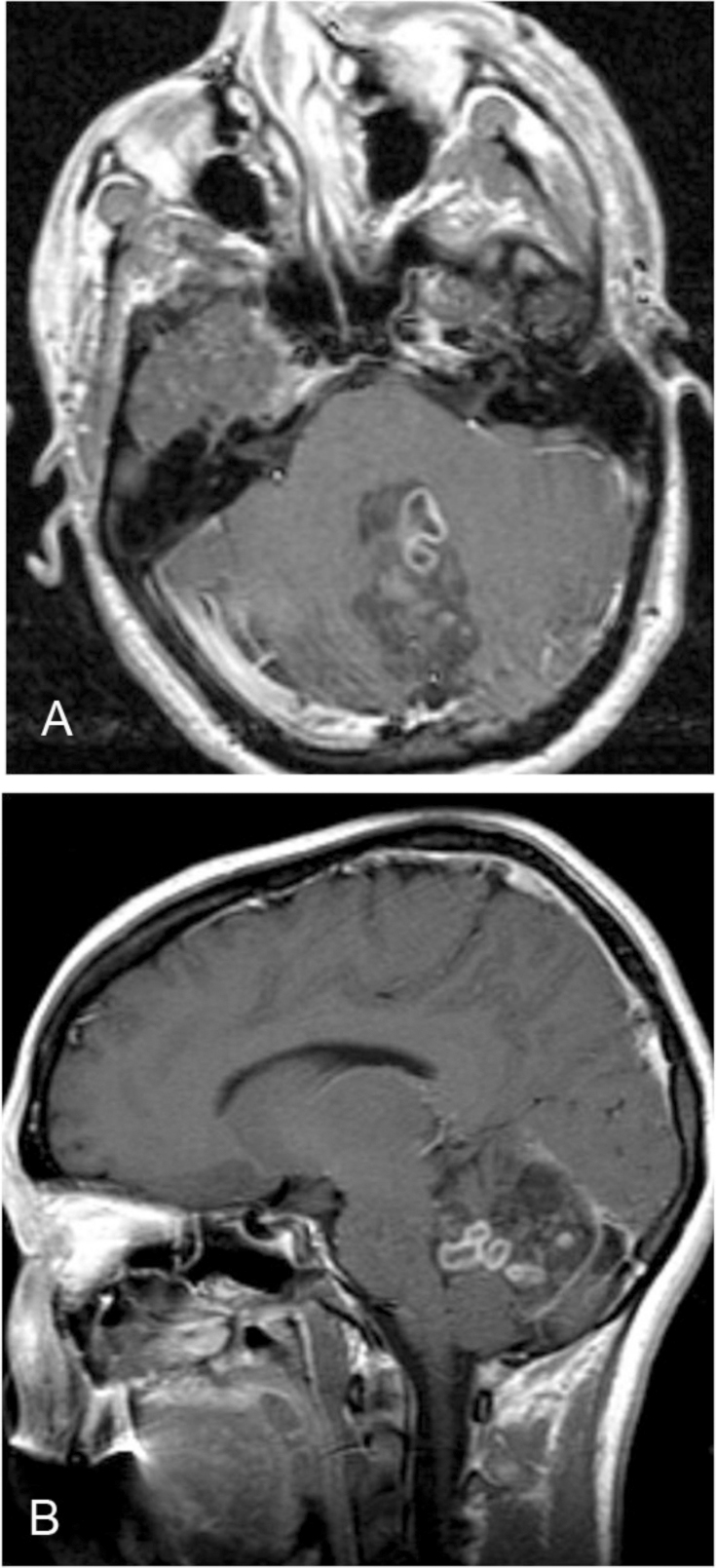
22-year-old female with rosette-forming glioneuronal tumor. Postcontrast T1-weighted image shows patchy areas of enhancement involving the midline posterior fossa lesion, with involvement of the fourth ventricle. A: Axial view. B: Sagittal view.
After discussion with the patient and her family, the lesion was surgically resected with a suboccipital midline craniotomy approach. A white and tan-red mass was totally resected.
On pathology, a low-grade neoplasm with bland histologic and cytologic features was seen with fragments of unremarkable cerebellum. The neoplasm had variable histology, with pilocytic astrocytoma-like areas, myxoid areas, and groups of oligodendoglial cells, neurocytic rosettes, and perivascular pseudorosettes. Microcalcifications were noted.
Immunohistochemically, GFAP and S-100 protein were positive in the glial areas, and synaptophysin was seen to highlight the neurocytic and perivascular rosettes. The Ki-67 proliferation index was 1%. EMA was negative. A trichome stain showed scattered hyalinized vessels. The neurofilament showed scattered axons focally in the neoplastic fragments.
One year after surgery, the patient has no complaints, is neurologically intact, and is doing well.
Discussion
Rosette-forming glioneuronal tumor (RGNT) was described as a distinct disease entity by Komori et al. in 2002, in a report of 11 cases (1). RGNTs were noted to be histologically distinct and separate from dysembryoplastic neuroepithelial tumor (DNET) of the cerebellum, a report of which was described in 1995 by Kuchelmeister et al (2). RGNT was recognized as a new category of grade 1 tumor, in the WHO classification of tumors of the central nervous system, in 2007 (3). It is a rare, slowly growing tumor of the posterior fossa, closely related to the fourth ventricle, and it predominantly affects young adults. Some studies have reported a female predominance (4). RGNT usually arises in the midline and primarily involves the wall and floor of the fourth ventricle and the cerebellum. It often occupies the fourth ventricle, may show parenchymal extension, and may extend to involve the aqueduct (3). Rarely, tumors involving the tectal/pineal and acqueductal regions have been described (1, 5, 6, 7), as well as multifocal lesions with mesencephalic and thalamic components (1, 5).
It has two separate histological components, glial and neurocytic, with the site of origin hypothesized to involve the periventricular germinal matrix.
The patient may remain asymptomatic and the lesion discovered incidentally, as in our case. It is often associated with hydrocephalus (8). If the patient is symptomatic, the clinical findings may be secondary to obstructive hydrocephalus, ataxia being the most common kind. Other clinical findings of nausea, vomiting, headaches, vertigo, visual disturbances, neck pain, and rigidity have been described (5).
On imaging, T2-weighted MRI studies reveal a well-defined, hyperintense lesion, with limited mass effect and surrounding edema. In the study of Shah et al. (4), a majority of the lesions seemed to arise from the cerebellar vermis, without an obvious fourth ventricular element. In the case of Yasutaka et al. (9), the tumor was in the midline, abutting the fourth ventricle. T2-weighted images showed several high-intensity areas within the tumor, with T1 hypointensities and no significant postcontrast enhancement.
An association with type 1 neurofibromatosis (NF-1) has been demonstrated in some cases (10, 11). Our case did not have any physical stigmata or radiological findings to suggest NF-1.
On the CT study, the lesion in our patient was predominantly hypodense, with few areas of calcifications.
On pathology, the predominant feature of RGNT is presence of both neurocytic and astrocytic/glial components. The neurocytic component consists of small cells with round hyperchromatic nuclei in a background of mucinous matrix, forming rosettes around central aggregates of fibrillary material, with positive synaptophysin staining. The glial component consists of astrocytes with eosinophilic fibrillary processes, resembling pilocytic astrocytoma, with positive immunostaining for glial fibrillary acid protein (GFAP) and S-100 protein. The nuclei of tumor cells are round and small, with no mitosis or atypia (8). Shah et al. (4) reported two cases containing DNET-like “floating” neurons involving Purkinje cells in the adjacent cerebellar cortex. They also reported hyalinized small blood vessels, Rosenthal fibers, focal microvascular proliferation, and a less than 1% Ki-67 labeling index.
Differential diagnoses may include pilocytic astrocytoma, medulloblastoma, ependymoma, and metastases. The age of the patient, location of the tumor, and imaging features may help in the differential diagnosis, but histopathological evaluation is necessary for definite diagnosis (4, 9).
Pilocytic astrocytomas occur more often in children and young adults. They are more commonly seen in the posterior fossa. They are usually discrete lesions with cyst formation, often associated with an enhancing nodule (12). Calcification is seen in a few cases.
Medulloblastomas are posterior fossa midline lesions seen in children and young adults. They are generally T1 hypo- to isointense and iso- to mildly hyperintense on T2-weighted images. They show varying enhancement on postcontrast images. They may show areas of calcification and cystic changes (13).
Ependymomas are usually heterogeneous lesions that may involve the posterior fossa or supratentorium. Posterior fossa lesions are usually midline, are seen more often in children, and often extend through the foramina of Luschka and foramen of Magendie. They insinuate structures, often encasing blood vessels and lower cranial nerves. They are heterogeneously hypointense on T1, hyperintense on T2-weighted images, and show heterogeneous postcontrast enhancement. They may show cystic, hemorrhagic, and calcific components (14).
Posterior fossa metastases may have different radiological appearances. They generally show postcontrast enhancement and varying amount of associated edema. Hemorrhagic and cystic changes may be seen, depending on the primary tumor.
Few cases of DNET have been described in the posterior fossa, which is a predominantly a supratentorial lesion, with a majority of cases seen involving the temporal lobe. MRI findings may show a cystic lesion, which may show nodular components and calcifications, with varying enhancement (15, 16).
In view of the varying involvement of fourth ventricle and cerebellum, the term “RGNT of the posterior fossa” instead of “RGNT of the fourth ventricle” has been suggested by Shah et al. (4)
RGNTs may generally be treated with gross surgical resection with good results. Neurological complications like nerve palsies, diplopia, and cerebellar ataxia have been reported (1, 17). The surgical approach may vary depending on the predominant involvement of the cerebellar vermis versus the fourth ventricle.
In summary, RGNT needs to be considered in the differential diagnoses of posterior fossa tumors, especially in relation to the fourth ventricle and vermis. These are slow-growing tumors, with an indolent course, and they have good prognosis after gross surgical resection.
Footnotes
Published: February 19, 2013
References
- 1.Komori T, Scheithauer BW, Hirose T. A rosette forming glioneuronal tumor of the fourth ventricle: infratentorial form of dysembryoplastic neuroepithelial tumor? Am J Surg Pathol. May 2002;26(5):582–591. doi: 10.1097/00000478-200205000-00004. [PubMed] [DOI] [PubMed] [Google Scholar]
- 2.Kuchelmeister K, Demirel T, Schlorer E, Bergmann M, Gullotta F. Dysembryoplastic neuroepithelial tumor of the cerebellum. Acta Neuropathol. 1995;89(4):385–390. doi: 10.1007/BF00309634. [PubMed] [DOI] [PubMed] [Google Scholar]
- 3.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The WHO classification of tumors of the central nervous system. Acta Neuropathol. Aug 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah MN, Leonard JR, Perry A. Rosette forming glioneuronal tumors of the posterior fossa. J Neurosurg: Pediatrics. Jan 2010;5(1):98–103. doi: 10.3171/2009.7.PEDS09113. [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Rosenblum MK. The 2007 WHO Classification of nervous system tumors: newly recognized members of the mixed glioneuronal group. Brain Pathol. Jul 2007;17(3):308–313. doi: 10.1111/j.1750-3639.2007.00079.x. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacques TS, Eldridge C, Patel A, Saleem NM, Powell M, Kitchen ND, Thom M, Revesz T. Mixed glioneuronal tumor of the fourth ventricle with prominent rosette formation. Neuropathol Appl Neurobiol. Apr 2006;32(2):217–220. doi: 10.1111/j.1365-2990.2005.00692.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 7.Solis OE, Mehta RI, Lai A, Farchoukh LO, Green RM, Cheng JC, Natarajan S, Vinters HV, Cloughesy T, Yong WH. Rosette-forming glioneuronal tumor: a pineal region case with IDH1 and IDH2 mutation analyses and literature review of 43 cases. J Neurooncol. May 2011;102(3):477–484. doi: 10.1007/s11060-010-0335-1. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu C, Kwan G, Lau Q, Bhuta S. Rosette-forming glioneuronal tumor: Imaging features, histopathological correlation and a comprehensive review of literature. Br J Neurosurg. Apr 2012 doi: 10.3109/02688697.2012.655808. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Yasutaka YF, Akihiro AM, Hideaki HT, Kunihiro KA, Junko JH, Mitsunori MK, Kaori KT. Rosette forming glioneuronal tumor of the fourth ventricle with bilateral olivary degeneration. Jpn J Radiol. Jul 2011;29(6):445–448. doi: 10.1007/s11604-011-0566-x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Kemp S, Achan A, Ng T, Dexter MA. Rosette-forming glioneuronal tumor of the lateral ventricle in a patient with neurofibromatosis 1. J Clin Neurosci. Aug 2012;19(8):1180–1181. doi: 10.1016/j.jocn.2011.12.013. [PubMed] [DOI] [PubMed] [Google Scholar]
- 11.Alturkustani M, Ang LC. Rosette-forming glioneuronal tumor of the 4th ventricle in a NF1 patient. Can J Neurol Sci. Jan 2012;39(1):95–96. doi: 10.1017/s0317167100012786. [PubMed] [DOI] [PubMed] [Google Scholar]
- 12.Koeller KK, Rushing EJ. From the archives of the AFIP: pilocytic astrocytoma: Radiologic-Pathologic correlation. Radiographics. Nov 2004;24(6):1693–1708. doi: 10.1148/rg.246045146. [PubMed] [DOI] [PubMed] [Google Scholar]
- 13.Meyers SP, Kemp SS, Tarr RW. MR imaging features of medulloblastomas. AJR Am J Roentgenol. Apr 1992;158(4):859–865. doi: 10.2214/ajr.158.4.1546606. [PubMed] [DOI] [PubMed] [Google Scholar]
- 14.Yuh EL, Barkovich AJ, Gupta N. Imaging of ependymomas: MRI and CT. Childs Nerv Syst. October 2009;25(10):1203–1213. doi: 10.1007/s00381-009-0878-7. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koeller KK, Dillon WP. Dysembryoplastic neuroepithelial tumors: MR appearance. AJNR Am J Neuroradiol. Sept-Oct 1992;13(5):1319–1325. [PubMed] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujimoto K, Ohnishi H, Tsujimoto M, Hoshida T, Nakazato Y. Dysembryoplastic neuroepithelial tumor of the cerebellum and brainstem. Case report. J Neurosurg. Sept 2000;93(3):487–489. doi: 10.3171/jns.2000.93.3.0487. [PubMed] [DOI] [PubMed] [Google Scholar]
- 17.Adachi J, Nishikawa R, Hirose T, Matsutani M. Mixed neuro-glial tumor of the fourth ventricle and successful treatment of postoperative mutism with bromocriptine: case report. Surg Neurol. Apr 2005;63(4):375–379. doi: 10.1016/j.surneu.2004.05.039. [PubMed] [DOI] [PubMed] [Google Scholar]