Abstract
Background: Medication utilization and costs increased over the last decade, but the effects of race/ethnicity have never been well studied in longitudinal data. We analyzed reports of prescription medication use to (1) identify trajectories of use and (2) determine predictors associated with a large increase in use. Specifically, variations in medication use by race/ethnicity were examined.
Methods: We analyzed the Study of Women's Health Across the Nation cohort with a median of 14 years of follow-up. Group-based trajectory models helped distinguish women with a low use of medications versus those with heavy use. Logistic regression was used to estimate the odds ratio (OR) for each racial/ethnic group associated with heavy use, controlling for potential baseline confounders.
Results: The 2,798 women sampled had a mean age of 46 years at baseline and the median number of medications at baseline was 2, increasing to 4 over the follow-up period. Trajectory models identified that 16% of participants demonstrated heavy use of medications, from a median of 5 at baseline to 10 medications at final follow-up. Regression models controlling for age, obesity, number of comorbid conditions, and pain found that Hispanic (OR = 0.085, 95% confidence interval [CI]: 0.037–0.20), Chinese (OR = 0.32, 95% CI: 0.16–0.63), Japanese (OR = 0.33, 95% CI: 0.17–0.64), and Black (OR = 0.79, 95% CI: 0.57–1.11) women had lower odds for heavy use compared with White women.
Conclusions: Longitudinal medication use among women in Study of Women's Health Across the Nation (SWAN) differed by race/ethnicity with non-White women having a lower odds of heavy use.
Introduction
Concurrent with an aging population, prescription medication use has grown in the United States. Data from the annual U.S. National Health and Nutrition Examination Survey (NHANES) demonstrate that use of one or more prescription drugs was reported by 48% of Americans in 2007–2008 versus 44% in 1999–2000.1 Individuals reporting the use of five or more drugs approximately doubled from 6% to 11% over the same period; and in respondents 60 years and over, 37% used five or more. The Rochester Epidemiology Project in Olmstead County, MN found that in 2009, 68% of the population received at least one prescription medication.2 Both of these studies include both men and women and report greater use by women, increasing use with age, and the NHANES found that White race was significantly associated with greater medication use compared to other races. One European study suggested that menopause may drive more medication use, but this has never been assessed directly.3 None of these surveys questioned the same individuals longitudinally. Little attention has been directed toward comparing medication use among people from different race/ethnicity groups; and the possible influence of the menopause transition on medication use has not been examined.
One might assume that clinical symptoms or diagnoses drive drug utilization, however, other factors amenable to intervention may predict use patterns, such as insurance coverage, health-seeking behaviors, cultural norms, and provider predisposition to prescribe. The annual NHANES found that having regular medical care, medical insurance, and drug insurance individually increase the frequency of prescription drug use.1 Based on differences in medical care by race and ethnicity in other areas of care (e.g., cardiovascular care), it is likely that there are also important differences by race and ethnicity with respect to medication use.4,5 Race and ethnicity differences in medication use may be driven by nonhealth-related issues. However, this has not been widely examined and never within the context of a study that collected medication data over an extended longitudinal follow-up.
While many medications are life saving or substantially improve quality of life, the value of other medications is less obvious.6 Moreover, the cost of medications in the United States has increased from ∼$90B in 1997 to $230B in 2007.7 To better understand longitudinal patterns of medication use and how they may differ across racial and ethnic groups, we conducted analyses on 14 years of prescription drug use data from a cohort of midlife women. We hypothesized that medication use would increase over time and patterns of use would vary by race/ethnicity.
Methods
Study cohort and design
We conducted the analyses in the setting of the Study of Women's Health Across the Nation (SWAN), a U.S.-based longitudinal cohort study of women recruited at seven sites between 1996 and 1997. Details of the design and methods for SWAN have been described in detail elsewhere,8 with relevant details included herein.
Briefly, SWAN is an NIH-funded cohort study of women passing through the menopause, assessing predictors and outcomes of this transition. Written informed consent is provided by participants for SWAN that includes secondary analyses, such as these. The research centers are located in Inkster, MI (University of Michigan), Boston, MA (Massachusetts General Hospital), Chicago, IL (Rush Presbyterian-St. Luke's Medical Center), Alameda and Contra Costa County, CA (University of California-Davis, and Kaiser Permanente), Los Angeles, CA (University of California-Los Angeles), Hackensack, NJ (Hackensack University Medical Center), and Pittsburgh, PA (University of Pittsburgh). SWAN participants represent five self-identified racial/ethnic groups—White, Black, Japanese, Chinese, and Hispanic—and a variety of cultural backgrounds. At the time of recruitment, women were 42–52 years of age and were premenopausal or early perimenopausal. Each site enrolled White women as well as women in one of four prespecified minority groups. Black women were enrolled in Boston, Chicago, Detroit, and Pittsburgh. Chinese, Japanese, and Hispanic women were enrolled in Oakland, Los Angeles, and Hudson County, respectively. The site that enrolled Hispanic women was closed for several years, producing a gap in data collection for this group.
Participants underwent examinations approximately every 12–18 months that included anthropometric measurements, specimen collection, questionnaires, and measures of performance. Participants were asked to bring the original containers of all prescription medications that they used since their last study visit, including pills, creams, and liquids. If they did not bring containers (∼1/3 of visits), then medication lists were reviewed in person or by telephone. The 11th follow-up visit was not part of the original plan and data collection was sparse; data from this visit were not used for these analyses. Fourteen years of follow-up were potentially included for the current analyses. Because these analyses focused on longitudinal patterns of medication use, we excluded women who did not complete any of the medication use questionnaires (n = 126) or who had fewer than five total visits in SWAN (n = 378).
The Institutional Review Boards from each site have approved the SWAN cohort study protocol and all participants gave written, informed consent.
Medication utilization
At the study visits, trained interviewers reviewed all prescription medications brought by participants to determine which were currently being used; they also asked about other medication use. Names, dosages, and frequency of use were recorded, but many dosages were missing. Duration of use was not uniformly reported in SWAN. After error checking, these medication names were mapped to a standard drug index (the Iowa Drug Information Service [IDIS], Index), allowing assignment of standard medication categories. We used the IDIS categories when clinically relevant, but also created several customized categories noted in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/jwh).
Medication use was treated both as a general variable and by category of medication. Calcium and vitamin D supplements were included as medications, but other supplements were collected less systematically by the study sites and not included.
Patient characteristics
Baseline visit characteristics considered in this analysis included body mass index (BMI), sociodemographic variables (age, race/ethnicity, income, marital status, and education), lifestyle factors (tobacco use, alcohol intake), self-assessed health status, social support (4 items from the 20-item Medical Outcomes Study Social Support Survey),9 vasomotor symptoms, physical activity (modified version of the Baecke Physical Activity Questionnaire),10,11 depressive symptoms, pain, and self-reported comorbid conditions.
Comorbid conditions were self-reported on standardized forms and consisted of osteoporosis, thyroid disease, any cancer, diabetes mellitus, any cardiovascular disease, osteoarthritis, hypertension, migraine, and hyperlipidemia. Each condition was considered individually and as a summed count variable. Depressive symptoms were measured using the Center for Epidemiologic Studies Depression scale (CES-D). The CES-D consists of 20 items that ask about the frequency of being bothered by depressive symptoms during the previous week on a scale of 0 (rarely) to 3 (most or all of the time) for a total summed score ranging from 0–60.12 Pain was assessed with the bodily pain scale from the MOS 36-Item Short-Form Health Survey (SF-36). This scale assesses how much bodily pain a person has had during the past 4 weeks and how much her pain interfered with normal work. SF-36 bodily pain scale has a range of 1–100 points with higher scores denoting less pain.13
Menopause transition stage was assessed in SWAN based on bleeding criteria. Categories were as follows: premenopause (no decreased regularity in menstrual bleeding during the last year), early perimenopause (decreased menstrual regularity in the past year and menstrual bleeding in the past 3 months), late perimenopause (no menses for 3–11 months), and postmenopause (no menses for 12 or more months). Women reporting bilateral oophorectomy with or without hysterectomy were classified as surgically menopausal. Women who had not yet reached postmenopause, but used hormone therapy, were not classified. Menopause transition stage was updated at every study visit. Once a woman had advanced to a later transition stage, she could not be reclassified to an earlier transition stage. The final menstrual period (FMP) date was the month and year at which 12 months of amenorrhea commenced.
Statistical analyses
The characteristics for the total cohort and by race/ethnicity were compared using measures of central tendency (means, median, range) and proportions. Significance testing across race/ethnicities was performed using ANOVA, a Kruskal–Wallis test, or chi-square. Mixed-effects regression models with the random intercept and slope were used to test whether there was an increase in medication use during follow-up and between different race/ethnicities.14–16 Trends in use by medication category were also examined.
Group-based trajectory modeling was used to identify patterns and distribution of medication use over time. This method was used as it takes advantage of all of the data to discern different trends. We were interested in comparing two groups of women, those with relatively low use of medications to those with heavy use. Variables included in the trajectory models were age in years, race/ethnicity, study site, and total number of comorbid conditions at baseline. We utilized only baseline covariates because the goal of these analyses was to determine if trends in future medication use could be determined at the start of follow-up. Logistic regression was then used to evaluate baseline patient characteristics in relationship to membership in each of the groups determined in the trajectory modeling.17 Recruitment site was forced into all models and other variables significant in univariate analyses were considered for adjusted analyses based on p-values <0.10.
A secondary analysis was performed to examine whether or not FMP was an inflection point for number of medications by constructing a LOESS regression, a nonlinear regression method that creates smoothed plots. This subanalysis was restricted to the 1,548 women with a known FMP. Number of medications used was log transformed because this variable was not normally distributed.
All analyses were conducted using SAS, version 9.3, and STATA 10.
Results
After excluding the 504 women who were either missing medication data or had fewer than five follow-up study visits, data from 2,798 SWAN participants with a median follow-up of 14.0 years (mean follow-up of 12.9 years) were analyzed. Baseline characteristics of the women by race/ethnicity are shown in Table 1. Differences were noted for the excluded women (Supplementary Table S2). At baseline, participants were, on an average, 46 years old, 48.0% were White, 28.1% African American, 6.0% Hispanic, 8.4% Chinese, and 9.5% Japanese. Educational attainment of high school or greater was noted by 45% and current tobacco use by 15%. The mean CES-D depressive symptom score was 10 and the mean pain score was 70 (100 = least pain and 0 = most pain). On an average, women reported 1.3 comorbid conditions, including anemia, hyperlipidemia, hypertension, and arthritis, as the most common. Annual income was less than $20,000 in 13% of women. The baseline characteristics were similar across most race and ethnic groups; however, women of Hispanic ethnicity reported less educational attainment, worse CES-D depressive symptom scores, worse pain scores, lower annual household incomes, and lower social support.
Table 1.
Baseline Characteristics of Women in Study of Women's Health Across the Nation Reporting Medication Use by Race/Ethnicity
| Total cohort (n = 2,798) | White (n = 1,343) | African American (n = 785) | Chinese (n = 234) | Japanese (n = 267) | Hispanic (n = 169) | p-Value | |
|---|---|---|---|---|---|---|---|
| Age (years), mean ± SD | 46.4 ± 2.7 | 46.3 ± 2.7 | 46.3 ± 2.7 | 46.5 ± 2.5 | 46.7 ± 2.7 | 46.4 ± 2.8 | 0.1500 |
| Follow-up (years), mean ± SD | 12.9 ± 2.3 | 12.9 ± 2.3 | 12.6 ± 2.3 | 13.4 ± 2.3 | 13.4 ± 1.8 | 6.6 ± 0.8 | 0.0001 |
| BMI (kg/m2), mean ± SD | 28.1 ± 7.3 | 27.6 ± 6.9 | 31.8 ± 7.8 | 23.3 ± 3.9 | 23 ± 3.7 | 29.3 ± 6.5 | 0.0001 |
| Tobacco use, n (%) | 0.0001 | ||||||
| None | 1,633 (58.4%) | 710 (52.9%) | 425 (54.1%) | 220 (94.0%) | 168 (62.9%) | 110 (65.1%) | |
| Past | 713 (25.5%) | 442 (32.9%) | 166 (21.1%) | 11 (4.7%) | 63 (23.6%) | 31 (18.3%) | |
| Current | 432 (15.4%) | 187 (13.9%) | 181 (23.1%) | 3 (1.3%) | 34 (12.7%) | 27 (16.0%) | |
| Missing | 20 (0.7%) | 4 (0.3%) | 13 (1.7%) | 0 (0.0%) | 2 (0.7%) | 1 (0.6%) | |
| Symptom scales, mean ± SD | |||||||
| Depression | 10.4 ± 9.5 | 10 ± 9.2 | 11.2 ± 10 | 8.4 ± 8.3 | 8.4 ± 7.9 | 15.5 ± 10.3 | 0.0001 |
| Pain | 69.6 ± 22.2 | 71.3 ± 19.9 | 66.9 ± 23.4 | 74.4 ± 23 | 75.6 ± 20 | 53.2 ± 25.5 | 0.0001 |
| Comorbid conditions | 1.3 ± 1.2 | 1.2 ± 1.1 | 1.6 ± 1.3 | 0.8 ± 0.9 | 0.9 ± 0.9 | 1.5 ± 1.2 | 0.0001 |
| Physical activity | 7.7 ± 1.8 | 8.1 ± 1.8 | 7.3 ± 1.7 | 7.3 ± 1.7 | 7.9 ± 1.6 | 6.8 ± 1.5 | 0.0001 |
| Hot flashes, n (%) | 1,074 (38.4%) | 481 (35.8%) | 360 (45.9%) | 70 (29.9%) | 86 (32.2%) | 77 (45.6%) | 0.0001 |
| Socioeconomic indicators | |||||||
| Educational attainment, n (%) | 0.0001 | ||||||
| Less than high school | 166 (5.9%) | 20 (1.5%) | 43 (5.5%) | 28 (12.0%) | 2 (0.7%) | 73 (43.2%) | |
| High school graduate | 459 (16.4%) | 178 (13.3%) | 157 (20.0%) | 38 (16.2%) | 43 (16.1%) | 43 (25.4%) | |
| Beyond high school | 900 (32.2%) | 410 (30.5%) | 312 (39.7%) | 51 (21.8%) | 94 (35.2%) | 33 (19.5%) | |
| College or beyond | 1,250 (44.7%) | 727 (54.1%) | 263 (33.5%) | 117 (50.0%) | 128 (47.9%) | 15 (8.9%) | |
| Missing | 23 (0.8%) | 8 (0.6%) | 10 (1.3%) | 0 (0.0%) | 0 (0.0%) | 5 (3.0%) | |
| Marital status, n (%) | |||||||
| Married | 1,858 (66.4%) | 961 (71.6%) | 368 (46.9%) | 187 (79.9%) | 216 (80.9%) | 126 (74.6%) | |
| Other | 903 (32.3%) | 360 (26.8%) | 412 (52.5%) | 45 (19.2%) | 50 (18.7%) | 36 (21.3%) | |
| Annual household income (US$), n (%) | |||||||
| <20,000 | 362 (12.9%) | 86 (6.4%) | 163 (20.8%) | 11 (4.7%) | 7 (2.6%) | 95 (56.2%) | |
| 20–50,000 | 912 (32.6%) | 414 (30.8%) | 312 (39.7%) | 80 (34.2%) | 55 (20.6%) | 51 (30.2%) | |
| 50–100,000 | 1,027 (36.7%) | 574 (42.7%) | 229 (29.2%) | 91 (38.9%) | 119 (44.6%) | 14 (8.3%) | |
| 100–150,000 | 296 (10.6%) | 180 (13.4%) | 32 (4.1%) | 36 (15.4%) | 45 (16.9%) | 3 (1.8%) | |
| >150,000 | 126 (4.5%) | 71 (5.3%) | 17 (2.2%) | 12 (5.1%) | 26 (9.7%) | 0 (0%) | |
| Social support, mean ± SD | 12.4 ± 3.2 | 12.7 ± 3.0 | 12.1 ± 3.4 | 11.8 ± 3.1 | 13.2 ± 2.5 | 10.9 ± 4.5 | |
Depression measured with the Center for Epidemiologic Studies Depression scale (higher score suggests greater likelihood of depression). Pain was assessed with the bodily pain scale from the MOS 36-Item Short-Form Health Survey (SF-36), with lower scores representing more pain. Physical activity was measured using a modified version of the Baecke Physical Activity Questionnaire (range 3–15). Comorbid conditions were self-reported and included osteoporosis, thyroid disease, any cancer, diabetes mellitus, cardiovascular disease, osteoarthritis, hypertension, migraine, and hyperlipidemia.
BMI, body mass index; SD, standard deviation.
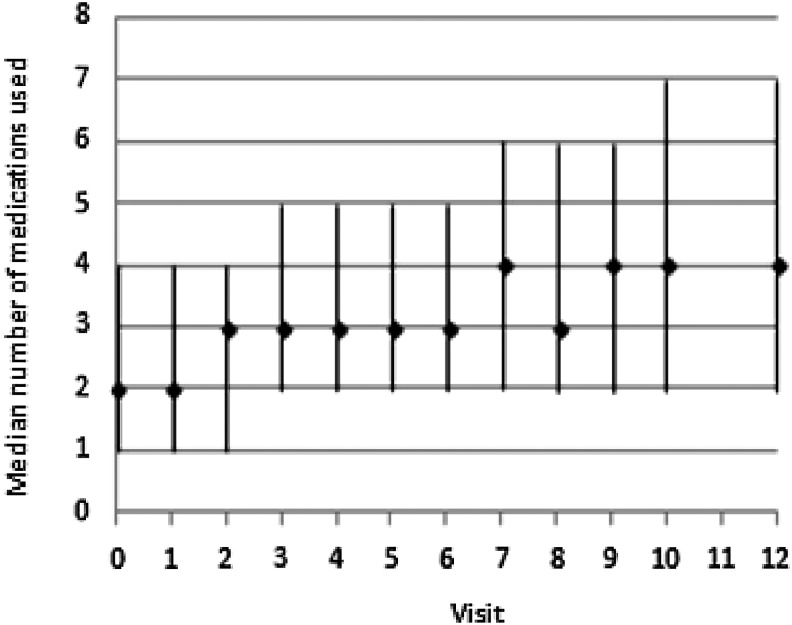
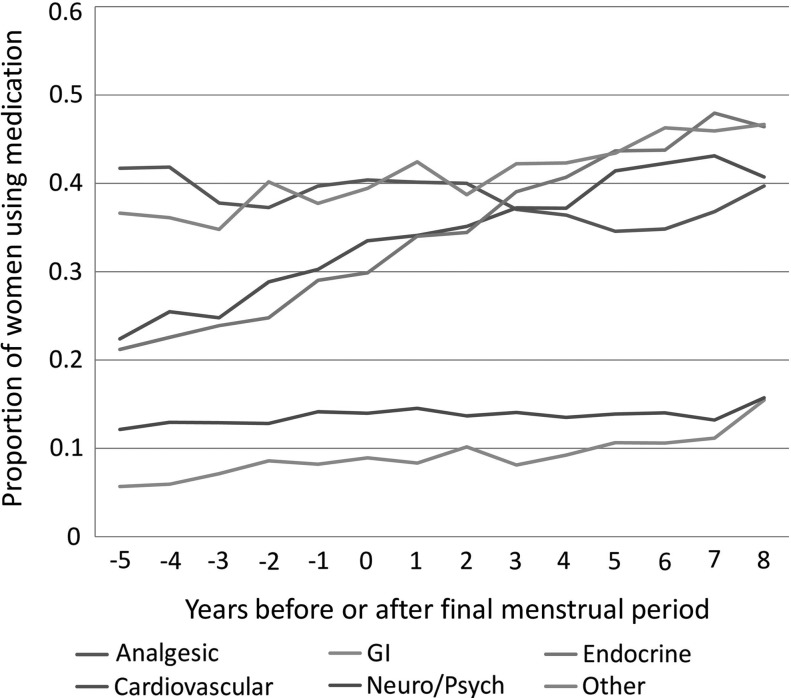
The median number of medications reported at baseline was 2 (interquartile range [IQR], 1–4). As noted in Figure 1, the median number increased gradually over the next 14 years to a median of 4 (IQR 2–7). Medication use differed by drug category over the study period (Fig. 2). Analgesic use declined slightly, while the trends for all other medication categories increased (all p for trends <0.01 from mixed-effect regression). These patterns were similar by race and ethnicity for most drug categories (Supplementary Fig. S1A–F). However, Japanese and Chinese women showed no increase in neurologic or psychiatric drugs. We examined which specific medications drove the increase in both cardiovascular and endocrine drugs. The three most common cardiovascular drugs or drug categories at the last available visit were the following: aspirin (18.7% of cardiovascular medications), statin lipid-lowering drugs (19.0%), and blood pressure-lowering agents (40.4%). The top three endocrine drugs were the following: thyroid replacement (11.4%), diabetes drugs (16.1%), and calcium +/− vitamin D (51.4%).
FIG. 1.
The median number (and interquartile range) of medications as noted at each visit across the span of Study of Women's Health Across the Nation (SWAN), for all women. Visit 11 has missing medication data for all participants.
FIG. 2.
This plot illustrates the proportion of women in SWAN using medications by category of medication. The x-axis (time) is plotted as years before or after the final menstrual period (FMP), which is denoted by a zero.
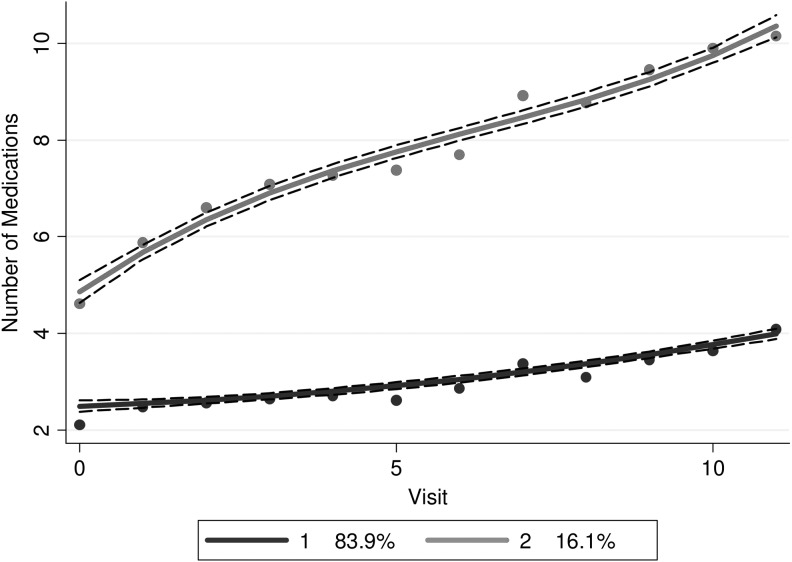
The group-based trajectory analysis of medication use revealed two dominant patterns (Fig. 3). The majority of SWAN participants (84%) demonstrated low use in medications during follow-up, from a median of 2 at baseline to 4 at the last follow-up and 16% of women showed heavy use in medications over time, from 5 at baseline to 10 at the last follow-up. We examined baseline patient characteristics associated with heavy use. The low use group differed from the heavy use group in several baseline patient characteristics (Supplementary Table S3). Table 2 provides baseline risk factors associated with medication trajectory group membership. The odds of being in the heavy use group were most strongly influenced by race/ethnicity. After controlling for age, obesity, number of comorbid conditions, site, physical activity, and pain, participants of Hispanic (odds ratio [OR] 0.085, 95% confidence interval [CI]: 0.037–0.20), Chinese (OR = 0.32, 95% CI: 0.16–0.63), and Japanese (OR = 0.33, 95% CI: 0.17–0.64) race/ethnicity had lower odds of being in the heavy use group compared with White women. Black women also had lower odds of being in the heavy use group, but the difference was not statistically significant (OR = 0.79, 95% CI: 0.57–1.11).
FIG. 3.
Trajectories of total medication utilization across the span of SWAN, for all women. The black line represents the low medication use trajectory and the grey line the heavy medication use trajectory.
Table 2.
Logistic Regression Model Predicting Rapid Increase in Medication Utilization According to the Group-Based Trajectory Analyses (n = 2,798)
| Baseline characteristics | Odds ratio (95% confidence interval) |
|---|---|
| Age | 1.08 (1.03–1.12) |
| Race/ethnicity | |
| White | 1.00 |
| Hispanic | 0.085 (0.04–0.20) |
| African American | 0.79 (0.57–1.11) |
| Japanese | 0.33 (0.17–0.64) |
| Chinese | 0.32 (0.16–0.63) |
| BMI | |
| Normal | 1.00 |
| Overweight | 1.24 (0.90–1.72) |
| Obese | 1.80 (1.27–2.56) |
| No. of comorbidities | 1.95 (1.76–2.16) |
| Pain, per 10-point increase | 0.82 (0.77–0.86) |
| Physical activity | 0.93 (0.86–0.99) |
Study site was also included in the model. Other variables noted in Table 1, but not included in the adjusted model were not statistically significant on univariate analysis. Model c-statistic = 0.81. Hosmer–Lemeshow χ2 = 4.97, p = 0.76. Pain is measured on a 0–100 scale with higher scores denoting less pain. BMI categories (Whites and Blacks): normal = 18.5–24.9; overweight = 25.0–29.9; obese ≥30.0. BMI categories (Japanese and Chinese): normal = <24.0; overweight = 24.0–26.9; obese ≥27.0. Physical activity is measured on a scale from 3 to 15 with higher scores being better. Age, number of comorbidities, and physical activity treated as continuous variables.
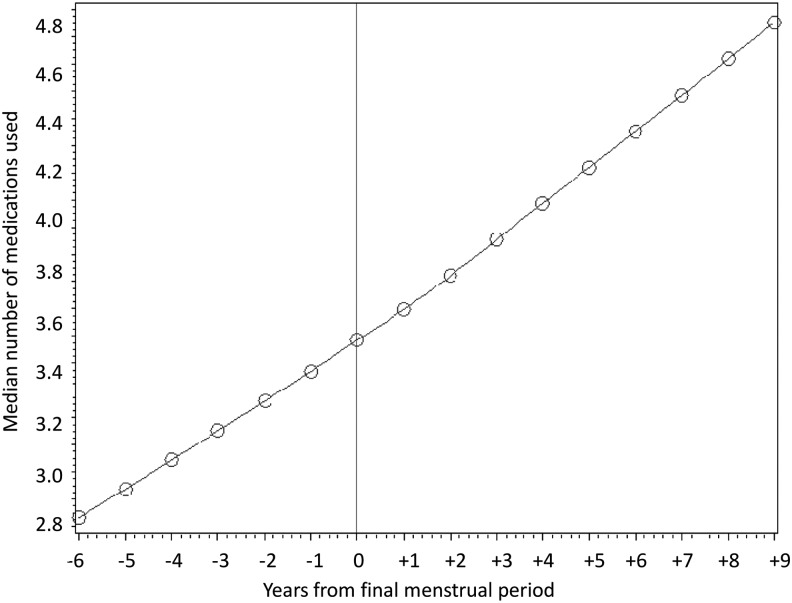
Finally, we constructed a regression plot to examine whether the slope for medication use changed around the FMP. Among the 1,548 women with known dates for the FMP, the slopes were continuous before and after the FMP, arguing against an effect on medication use by menopause (Fig. 4).
FIG. 4.
LOESS smoothed regression curve plotting the number of medications for women in SWAN during the 5 years before the FMP and the 10 years after.
Discussion
In this study, we analyzed 14 years of data on almost 2,800 women with a mean age of 46 years at baseline who represent the following race/ethnic groups: White, Black, Hispanic, Japanese, and Chinese. The results demonstrate a change in the overall medication use from a median of 2 medications to 4 over the study period. This pattern is observed for most participants. However, 16% of women reported heavy use in medications over the study period, starting with a median of 5 medications and ending with over 10 medications. While a number of baseline variables were correlated with this pattern, the most striking was race/ethnicity. For example, Hispanic women had 91% lower odds of being in the rapid increase group compared to White women. Finally, the menopause transition was not associated with change in medication use. This argues that increasing age (and possibly declining health) is a more important factor than hormonal factors associated with menopause.
Race and ethnicity were strong predictors of medication use patterns. While this is not a new finding,18 it has not been adequately explored in prior research. Even after considering markers of income, education, and social support in adjusted models, this finding persisted. Differential access to care, prevailing cultural norms, and divergent disease patterns are all possible explanations that require further study.19,20 Some of these issues can be further explored in SWAN, but information about patient preferences and disease severity were not collected as part of the study.
The cost implications of drug use are potentially substantial. When used wisely, medications can be beneficial at reasonable costs. However, some medications are known to be overused with large associated costs. For example, one of the most commonly used cardiovascular drug categories in our study was statin lipid-lowering drugs, which typically cost from $1 to $3 per day. While treatment recommendations from the medical profession for statins are controversial,20,21 most would agree that the health benefits of statins for primary prevention in typical women in their mid-50s would be questionable. Reducing use of such high-cost medications (e.g., statins and other lipid-lowering drugs, antidepressants) in populations without clear indications would likely be a useful cost-containment strategy.
Some of the factors associated with heavy use in medications may be modifiable, resulting in less long-term drug use. As noted in a prior study, BMI was strongly associated with a more rapid increase and is likely related to use of medications for diabetes or other weight-related conditions.18 Better solutions for obesity will likely lead to decreases in medication use.22
The majority of women studied reported a relatively stable medication use with a small rise in use over time, from 2 medications at baseline to 4 medications 14-years later. This pattern stands in stark contrast to the 16% who reported heavy use of prescription medications, from 5 medications at baseline to 10. These divergent trends suggest that aging is not the driving force for a rapid increasing medication use in this cohort.
The purposeful sampling of women from different races and ethnicities gave us the opportunity to compare these groups. Furthermore, the participants were all pre- or early perimenopausal at study entry, allowing us to examine the effects of menopause on drug use in a more precise manner than prior studies.2,3 Another important strength of this analysis is its longitudinal nature. To our knowledge, all other publications on medication use trends have used cross-sectional methods and compared different study samples over time. Finally, we considered a robust set of covariates, including demographics, symptoms, comorbid conditions, and BMI.
Limitations of this study pertain to the possibility that women participating in SWAN are not representative of the general U.S. population since recruitment was restricted to seven sites and not all race/ethnicities were included. However, at each of the seven sites, general population sampling was conducted to obtain a representative cohort. As noted, the sample includes women of various race/ethnic groups; thus, the comparisons by race/ethnicity and across trajectories would be internally valid. However, the site recruiting Hispanic women was closed for several years during follow-up for operational issues. Medications were collected at all study visits, and thus, we may have missed utilization that started and stopped between study visits such as for medications that would have been used for acute indications. Comorbidities were self-reported and may not always be accurate. Sparse information was available about insurance status for medications and the presence or absence of insurance may impact drug utilization, possibly underpinning increased use by White women. Race and ethnicity were self-designated and did not follow the census procedures that allow individuals to self-report both race and ethnicity. No information on folk remedies was collected. It is possible that the exclusion of women with less than five visits may have introduced selection bias. Finally, the indications for medication utilization (i.e., reasons for use) are not clearly linked for each of the drugs reported, and the patterns and predictors of utilization may not have fully captured the reasons why SWAN participants used specific medications.
We chose to focus on baseline patient characteristics as potential correlates of medication use. While it is true that health conditions and lifestyles may have changed over time, examining baseline variables allows one to determine if medication use can be correlated with variables collected at cohort inception. Future analyses will examine whether changes in participant characteristics correlate with medication utilization.
In conclusion, we found important differences across race and ethnicity in trajectories of medication utilization among women transitioning through the menopause. While the majority of women had low medication use, 16% started the study period using more medications and experienced a large increase; the strongest predictor of trajectory was a woman's race and ethnicity. While this analysis does not fully capture the reasons for these increasing patterns of utilization, it gives us important longitudinal experience among a cohort of racially and ethnically diverse women transitioning through the menopause. Further work may help understand factors that could be modified to reduce overall medication utilization or increase use where undertreatment is likely.
Supplementary Material
Acknowledgments
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR), and the NIH Office of Research on Women's Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The funding agencies had no direct role in the design or conduct of the study; the collection, management, analyses, and interpretation of the data; or preparation or approval of the article.
Clinical centers
University of Michigan, Ann Arbor–Siobán Harlow, PI 2011–present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA–Joel Finkelstein, PI 1999–present; Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL–Howard Kravitz, PI 2009–present; Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser–Ellen Gold, PI; University of California, Los Angeles–Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY–Carol Derby, PI 2011–present, Rachel Wildman, PI 2010–2011; Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry–New Jersey Medical School, Newark–Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, PA–Karen Matthews, PI.
NIH program office
National Institute on Aging, Bethesda, MD–Winifred Rossi 2012–present; Sherry Sherman 1994–2012; Marcia Ory 1994–2001; and National Institute of Nursing Research, Bethesda, MD–Program Officers.
Authors' Contributions
Daniel H. Solomon developed the analysis plan, interpreted analyses, drafted the article, and approved it. He had access to the data and vouches for its accuracy. Kristine Ruppert helped develop the analysis plan, supervised and interpreted analyses, drafted parts of the article, and approved it. YinJuan Lian ran analyses and approved the article. Faith Selzer revised and approved the article. Gail A. Greendale interpreted the analyses, revised the article, and approved it. Joel S. Finkelstein interpreted the analyses, revised the article, and approved it.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Gu Q, Dillon CF, Burt VL. Prescription drug use continues to increase: U.S. prescription drug data for 2007–2008. NCHS Data Brief 2010;42;1–8 [PubMed] [Google Scholar]
- 2.Zhong W, Maradit-Kremers H, St. Sauver JL, et al. Age and sex patterns of drug prescribing in a defined American population. Mayo Clin Proc 2013;88:697–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardel A, Wallander MA, Svardsudd K. Factors associated with adherence to drug therapy: A population-based study. Eur J Clin Pharmacol 2007;63:307–314 [DOI] [PubMed] [Google Scholar]
- 4.Sequist TD, Fitzmaurice GM, Marshall R, Shaykevich S, Safran DG, Ayanian JZ. Physician performance and racial disparities in diabetes mellitus care. Arch Intern Med 2008;168:1145–1151 [DOI] [PubMed] [Google Scholar]
- 5.Ayanian JZ, Udvarhelyi IS, Gatsonis CA, Pashos CL, Epstein AM. Racial differences in the use of revascularization procedures after coronary angiography. JAMA 1993;269:2642–2646 [PubMed] [Google Scholar]
- 6.Avorn J. Balancing the cost and value of medications: The dilemma facing clinicians. Pharmacoeconomics 2002;20 Suppl 3:67–72 [DOI] [PubMed] [Google Scholar]
- 7.Aitken M, Berndt ER, Cutler DM. Prescription drug spending trends in the United States: Looking beyond the turning point. Health Aff (Millwood) 2009;28:w151–w160 [DOI] [PubMed] [Google Scholar]
- 8.Sowers MF, Crawford S, Sternfeld B, et al. Design, survey, sampling and recruitment methods of SWAN: A multi-center, multi-ethnic, community based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, eds. Menopause: biology and pathobiology. San Diego, CA: Academic Press, 2000:175–188 [Google Scholar]
- 9.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med 1991;32:705–714 [DOI] [PubMed] [Google Scholar]
- 10.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 1982;36:936–942 [DOI] [PubMed] [Google Scholar]
- 11.Sternfeld B, Ainsworth BE, Quesenberry CP. Physical activity patterns in a diverse population of women. Prev Med 1999;28:313–323 [DOI] [PubMed] [Google Scholar]
- 12.Farmer ME, Locke BZ, Moscicki EK, Dannenberg AL, Larson DB, Radloff LS. Physical activity and depressive symptoms: The NHANES I epidemiologic follow-up study. Am J Epidemiol 1988;128:1340–1351 [DOI] [PubMed] [Google Scholar]
- 13.Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–483 [PubMed] [Google Scholar]
- 14.SAS Education. Longitudinal data analysis with discrete and continuous responses course notes. Cary: SAS Institute, Inc., 2008 [Google Scholar]
- 15.Brown H, Prescott R. Applied mixed models in medicine, 2nd ed. West Sussex: John Wiley & Sons, Ltd., 2006 [Google Scholar]
- 16.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for mixed models, 2nd ed. Cary: SAS Institute, Inc., 2006 [Google Scholar]
- 17.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol 2010;6:109–138 [DOI] [PubMed] [Google Scholar]
- 18.Qato DM, Alexander GC, Conti RM, Johnson M, Schumm P, Lindau ST. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA 2008;300:2867–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: Part II: Variations in cardiovascular disease by specific ethnic groups and geographic regions and prevention strategies. Circulation 2001;104:2855–2864 [DOI] [PubMed] [Google Scholar]
- 20.Miranda J, McGuire TG, Williams DR, Wang P. Mental health in the context of health disparities. Am J Psychiatry 2008;165:1102–1108 [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM, Cook NR. Statins: New American guidelines for prevention of cardiovascular disease. Lancet 2013;382:1762–1765 [DOI] [PubMed] [Google Scholar]
- 22.Segal JB, Clark JM, Shore AD, et al. Prompt reduction in use of medications for comorbid conditions after bariatric surgery. Obes Surg 2009;19:1646–1656 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.