Abstract
Previously, we observed that developmental polychlorinated biphenyl (PCB) exposure resulted in an increase in audiogenic seizures (AGSs) in rats. However, the rats were exposed to loud noise in adulthood, and were not tested for AGS until after 1 year of age, either of which could have interacted with early PCB exposure to increase AGS susceptibility. This study assessed susceptibility to AGS in young adult rats following developmental PCB exposure alone (without loud noise exposure) and investigated whether there was a decrease in GABA inhibitory neurotransmission in the inferior colliculus (IC) that could potentially explain this effect. Female Long-Evans rats were dosed orally with 0 or 6 mg/kg/day of an environmentally relevant PCB mixture from 28 days prior to breeding until the pups were weaned at postnatal day 21. One male-female pair from each litter was retained for the AGS study whilst another was retained for Western blot analysis of glutamic acid decarboxylase (GAD) and GABAAα1 receptor in the IC, the site in the auditory midbrain where AGS are initiated. There was a significant increase in the number and severity of AGSs in the PCB groups, with females somewhat more affected than males. GAD65 was decreased but there was no change in GAD67 or GABAAα1 in the IC indicating decreased inhibitory regulation in the PCB group. These results confirm that developmental PCB exposure alone is sufficient to increase susceptibility to AGS, and provide the first evidence for a possible mechanism of action at the level of the IC.
Keywords: audiogenic seizures, IC, GAD, PCBs
Polychlorinated biphenyls (PCBs) were banned from production in North America several decades ago due to their toxicity but continue to cause human health concerns due to their chemical stability. Historically, the main source of human exposure has been via consumption of contaminated fish (Crinnion, 2011) but recently building materials including caulking, fluorescent light ballasts (Ampleman et al., 2015), and paint pigments (Anezaki et al., 2014; Hu and Hornbuckle, 2010) have been identified as other important sources of PCBs. PCBs accumulate in adipose tissue and can later be mobilized and transported across the placenta and into the breast milk in mammals (Goldey et al., 1995; Jacobson et al., 1984). Furthermore, PCBs have been shown to enter the human central nervous system (CNS) (Chu et al., 2003). Thus, PCB exposure could permanently alter the development and maturation of the nervous system.
Maternal exposure of rats to a commercial mixture of PCBs (Aroclor 1254) during the perinatal period drastically lowered circulating thyroid hormone levels (Goldey and Crofton, 1998) and resulted in low frequency hearing loss in adult offspring (Goldey et al., 1995; Herr et al., 1996). The same PCB exposure also caused a loss of outer hair cells (OHCs) within the cochlea (Crofton et al., 2000) and functional deficits of the OHCs, as indicated by distortion product otoacoustic emission (DPOAE) measures, with PCB exposure resulting in reduced DPOAE amplitudes and increased thresholds across a range of frequencies (Lasky et al. 2002). Similarly, in more recent studies of rats perinatally exposed to an environmentally relevant PCB mixture (Fox River PCB mixture; Kostyniak et al., 2005), we found increased DPOAE thresholds and decreased DPOAE amplitudes (Poon et al., 2011; Powers et al., 2006, 2009). We also found that PCB exposure increased auditory brainstem response (ABR) thresholds but did not affect ABR latencies or amplitudes, further indicating that PCBs act at the level of the OHCs in the cochlea to produce hearing loss (Herr et al., 1996; Powers et al., 2006).
Recently, we conducted a study to evaluate whether developmental PCB exposure would exacerbate noise-induced hearing loss in adulthood, and we unexpectedly observed audiogenic seizures (AGSs) in the PCB-exposed animals when they were exposed to noise. The same animals were later tested using a modified AGS procedure (Ross and Coleman, 1999), and PCB-exposed rats showed a dramatically increased incidence and severity of seizures and a decreased latency to onset of seizures (Poon et al., 2015).
Acoustically evoked convulsions or AGS are elicited by an intense high-frequency sound. The initiation and propagation of AGS activity results from hyperexcitability in the auditory brainstem and, in particular, the inferior colliculus (IC) (Coleman et al., 1999; Eells et al., 2004; Ishida et al., 1995; Ross and Coleman, 2000). In the IC, the key excitatory and inhibitory neurotransmitters are glutamate and GABA, respectively. Pathologies such as tinnitus, age-related hearing loss, and AGS have all been associated with abnormalities in GABA neurotransmission at the level of the IC (Bauer et al., 2000; Caspary et al., 1999; Faingold, 1999). In the mammalian brain, GABA is synthesized via the decarboxylation of glutamate by GAD (Petroff, 2002). Two isoforms of GAD, GAD65 and GAD67, exist in most GABA-producing neurons in the CNS and the amount of GAD present in a neuron is a direct indication of the amount of GABA that is produced (Petroff, 2002; Rowley et al., 2012).
The rats in our previous study were exposed to loud noise in adulthood and were not tested for AGS until after 1 year of age. Thus, noise exposure and/or advanced age could have interacted with early PCB exposure to increase AGS susceptibility. The purpose of the current experiment was to assess the effects of the environmentally relevant “Fox River PCB mixture” on AGS in young adult animals exposed to PCBs during early development but without any exposure to loud noise. GAD and GABAA receptor levels in the IC were measured to determine if there were changes in these proteins that could explain the increase in AGS. It was hypothesized that developmentally PCB-exposed animals would be more susceptible to AGS, and that GAD levels in the IC would be downregulated in these animals. It was also hypothesized that GABAA receptor protein expression in the IC would be decreased in PCB-exposed rats, reflecting the impaired inhibition that is often associated with seizure disorders. A classic AGS paradigm, where animals were exposed to a loud noise for a brief duration of time (eg, 2 min) and behavioral evidence of seizure activity was recorded and Western blot assays to quantify GAD65, GAD67, and GABAAα1 in the IC were used for these experiments.
MATERIALS AND METHODS
Animals
Primiparous female Long-Evans rats, approximately 8–10 weeks of age, were purchased from Harlan (Indianapolis, Indiana). They were individually housed in standard polycarbonate plastic shoebox cages with wood-chip bedding and were given access to rat chow (Harlan Teklad rodent diet (W) 8604) and water ad libitum. All rats were housed in a temperature- and humidity-controlled room, on a 12/12-h light cycle (lights on at 0700 h). The rats were maintained in facilities accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Illinois at Urbana-Champaign and were in accordance with the guidelines of the National Institutes of Health (2002) and National Research Council (2003).
Exposure
The female rats were randomly assigned to two exposure groups and given treatments consisting of corn oil vehicle alone or PCBs in corn oil. Exposure began 28 days prior to breeding and continued until weaning of the pups on postnatal day (PND) 21. The PCB mixture (Fox River PCB mixture) was formulated to mimic the congener profile found in walleye fish from the Fox River in Northeast Wisconsin. The mixture consisted of 35% Aroclor 1242 (Monsanto lot KB 05-415; St Louis, Missouri), 35% Aroclor 1248 (AccuStandard lot F-110; New Haven, Connecticut), 15% Aroclor 1254 (Monsanto lot KB 05-612), and 15% Aroclor 1260 (AccuStandard lot 021-020) (Kostyniak et al., 2005). The dose (6 mg/kg/day) of the PCB mixture was selected based on previous dose-effect studies demonstrating ototoxicity (Poon et al., 2011; Powers et al. 2006) but no overt signs of clinical toxicity (Kostyniak et al., 2005) at this dose. The PCB mixture diluted in corn oil (Mazola) or the corn oil vehicle alone was pipetted onto one-half of a vanilla wafer cookie (Keebler Golden Vanilla Wafers). To arrive at a dose of 6 mg/kg, the PCB solution was mixed at a concentration of 15 mg/ml, and a volume of 0.4 ml/kg was administered via the cookies. The PCB and vehicle treated cookies were fed to the female rats daily with the amount of dosing solution applied to the cookies adjusted daily to account for weight gain.
Breeding, pregnancy, and weaning
After 28 days of PCB exposure, each female was paired with an unexposed male Long-Evans rat (Harlan) in a hanging wire cage for 8 consecutive days with food and water available ad libitum. The females were returned to their home cages each day for PCB dosing. The females were monitored for the presence of a sperm plug to establish gestational day 0.
On the first day after birth (PND 1), the pups were examined for abnormalities, sexed, and weighed. On PND 2, the litters were culled to 10 pups (five males and five females when possible), and litters with at least 7 pups had extra pups cross fostered into them from the same treatment group to bring the litters to 8–10 pups. Cross-fostered pups were ear marked and not used for the experiment. There were 19 successful litters (9 control and 10 PCB). Of the remaining dams, 9 were not pregnant and 4 had litters too small to be included in the study (< 7 pups). Overall the nonpregnant dams and dams with small litters were evenly distributed across the 2 treatment groups. Dosing continued until the pups were weaned on PND 21. Two males and 2 females were retained after weaning for these studies, 1 pair for the AGS study and the other pair for the Western blot assays.
Audiogenic seizures
At approximately PND 90 (young adulthood) 1 male and 1 female from each litter were tested in the AGS protocol. For testing, each rat was placed individually into a cylindrical plexiglass tube (12 in × 12 in) lined on the bottom with sound insulating material (Soundsoak, Armstrong World Industries, Lancaster, Pennsylvania). The chamber had a removable top lined with 4 equally spaced tweeter speakers (3″ OEM cone tweeter, MCM Electronics, Dayton, Ohio). Using a sound pressure level meter (RadioShack SPL-meter 33-4050), the noise level was calibrated to 100 105, or 110 dB when the chamber was closed. The tweeter speakers were connected to an amplifier (Techron 5507 power supply amplifier), which was connected to a music player (Sandisk Sansa Clip+ 4 GB MP3 player) loaded with the octave band noise with a center frequency of 8 kHz and played on a loop. The noise was created using MATLAB software (The MathWorks, Inc).
On the first AGS testing day, each rat was placed in the plexiglass chamber and exposed to 2 min of noise at 100 dB and monitored for any AGS behaviors. The stages of AGSs were observed as follows: 0-no abnormal behavior, 1-1st running fit, 2-2nd running fit, 3-clonus with full-body loss of posture, and 4-immobile postictal recovery (Ross and Coleman, 2000). Wild running is a characteristic phenotype of AGS and was defined as intense running mixed with high jumps. A rat was removed from the experiment and not tested further if it experienced clonus at any noise intensity. If a rat did not experience clonus at 100 dB, then it was retested 24–48 h later at 105 dB. If the rat still did not experience clonus, it was retested 24–48 h later at 110 dB. Animals that did not exhibit clonus at 110 dB were not tested further at louder noise intensities.
Western blot analysis for GAD65, GAD67, and GABAAα1
Littermates of the AGS tested animals were decapitated as adults (> PND 90). The brains were quickly removed and the IC was dissected from the rest of the brain (within 2 min post decapitation) and both the remaining brain tissue and the IC were stored at −80°C until processed. To collect tissue from control brain regions outside of the auditory midbrain (hippocampus; somatosensory cortex) each frozen brain was later positioned on the frozen stage (−20°C) of a microtome so that successive coronal slices could be taken in an anterior to posterior direction. Landmarks from the Paxinos and Watson (1998) brain atlas were used to guide cutting. Slices were removed until bregma −2.16 mm was reached, at which point 1.0 mm thick coronal slices were cut. The slice was placed on a glass slide with the posterior side facing down. Using a 1.0 mm circular tissue punch (Harris Uni-Core; Ted Pella, Inc) a single punch of hippocampus and somatosensory cortex was collected from each hemisphere with the punch directed perpendicular to the plane of the slide. All tissue punch specimens were immediately placed in closed vials and immersed in liquid nitrogen with subsequent storage at −80°C until the time of Western blot analysis.
For analysis of total protein, one 10 mg protease inhibitor (PI) tablet was dissolved in 10 ml tissue protein extraction reagent T-PER (ThermoFisher Scientific). The left and right IC from each animal were pooled and homogenized in 350 µl T-PER/PI (tissue protein extraction reagent with PI tablet) for IC and 160 µl for tissue punches from other structures. The homogenized sample was then centrifuged at 10 000 × g for 30 min at 4°C. The supernatant was used in the bicinchoninic acid assay (BCA) protein assay (Smith et al., 1985). The absorbance of the samples at 562 nm measured using a Multiskan Ascent microplate reader (Type 354; Thermo Scientific). Protein concentrations were calculated using Ascent Software (v. 2.6, Revision 3.1, December 2003; Thermo Scientific). To be able to compare protein expression between different gels, a protein standard was created by pooling IC tissue from nontreated animal and was loaded into one lane of every gel.
For each sample, a volume equal to a target amount of 5 μg protein was denatured while heating at 100°C for 15 min. Each gel contained samples of both male and female control and PCB-exposed rats. Once running buffer was added, gels were electrophoresed at 125 volts for 90 min. At the completion of gel electrophoresis, samples were transferred to a PVDF membrane via a transfer module and electrophoresed in a 4°C cold room at 30 volts for 90 min.
Following transfer of gel on to the PVDF membrane, the membrane was rinsed in Tris Buffered Saline and TWEEN 20 (T-TBS). T-TBS was used for all subsequent rinses. The membrane was blocked in 5% milk solution for 1 h and then incubated at 4°C with GAD65 antiserum (1:2500, Millipore), GAD67 antiserum (1:5000, Millipore), or GABAAα1 (1:2000 Millipore) wt/vol in 5% milk overnight. Incubation of secondary antibody (horseradish peroxidase, Santa Cruz Bio) at 1:2000 wt/vol in 1% milk was performed for 1 h at 23°C. The membrane was then treated with LumiGLO (Cell signaling technologies, Massachusetts) and chemiluminescence was captured by Proteinsimple FluoroChem R imaging system (San Jose, California). The membrane was subsequently stripped using a stripping buffer (Restore Western Blot Stripping Buffer, ThermoFisher Scientific) and then blocked again in 5% milk solution for 1 h. It was then incubated with anti-alpha tubulin primary antibody at 1:10 000 or anti-beta actin primary antibody (for GABAAα1) at 1:5000 wt/vol in 5% milk for 1 h at 23°C. Next, the membrane was incubated with goat anti-rabbit secondary antibody (Abcam) at 1:5000 or goat anti-mouse secondary antibody (Abcam) at 1:2000 wt/vol in 2% milk for 1 h. Then the membrane was treated with LumiGLO and chemiluminescence was captured using the same Proteinsimple FluoroChem R imaging system.
Densitometry of the bands was performed using ImageJ (version 1.46 r, http://imagej.nih.gov/ij). Briefly, images of the membrane were automatically saved on the Fluorochem R imaging system at 600 ppi in tagged image file format (TIFF) format at sequential time points following addition of LumiGLO to the membrane. The image with the best band representation and highest contrast was selected. The densities of samples for GAD and alpha tubulin or GABAAα1 and beta actin were determined and then standardized to the protein standard densities on each image to obtain relative densities for GAD and alpha tubulin or GABAAα1 and beta actin. Then adjusted densities for the samples were determined by dividing the relative density of each sample by the relative density of the standard.
Statistical analysis
All statistical analyses were conducted using SPSS for MS Windows (version 23.0; IBM SPSS Statistics with Exact Tests Module) with statistical significance set at P < .05. The incidence of AGSs was analyzed using a Pearsons Chi-square 2 × 2 ( χ2) test, for each sex separately and comparisons of the incidence of seizures between the control group, and the PCB group were conducted using a Fisher’s Exact Test with two sided exact P values. Seizure severity scores (ARS), latency to onset of wild running, and latency to clonus were analyzed using nonparametric statistics due to a lack of normal distribution of data (many rats reached the maximum value possible for each of these measures). Therefore, these scores were analyzed using Mann-Whitney tests to compare between control and PCB-exposed groups.
For the GAD and GABAAα1 analyses, adjusted densities (normalized to their internal loading control of either α-tubulin or β-actin and then standardized to the protein standard densities on each image) were determined using ImageJ and were then used in the statistical analyses. Males and females were analyzed separately with students t test, as not all litters were represented by both sexes. Data are reported as mean ± SEM.
RESULTS
There were no overt signs of clinical toxicity in the dams from either the control or PCB dose groups. In the PCB-exposed group, there was a significant effect (P < .01) of PCB exposure on pup body weight at PNDs 7, 14, and 21 days where PCB exposure reduced body weights compared with control animals. No other developmental abnormalities were noted.
AGS Incidences
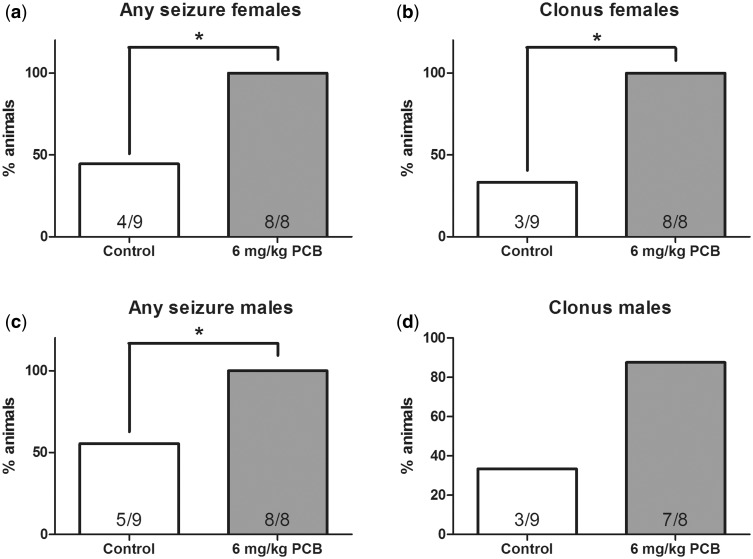
Analysis of the percentage of females that exhibited any seizure behavior (running fits or running fits progressing into clonus) at the lowest noise intensity of 100 dB revealed that there was a significant main effect of treatment (χ2 = 10.578, df = 1, P = .001), with 8/8 of the PCB-exposed females showing an incidence of seizure behavior relative to 2/9 of the controls (Figure 1A). Analysis of the number of female rats experiencing seizures that progressed to clonus (severest seizure form) at the lowest noise intensity (Figure 1B) also revealed a significant effect of treatment (χ2 = 10.578, df = 1, P = .001), where 8/8 of the PCB-exposed females had an incidence of clonic seizures compared with 2/9 of the control females.
FIG. 1.
A and C, Percentage of rats that showed any seizure behavior at 100 dB noise intensity for females and males (A and C). Percentage of rats that showed clonus seizures at 100 dB noise intensity for females and males (B and D). n = 9 for controls and 8 for polychlorinated biphenyl (PCB)-exposed group for both males and females. * indicates that the 6 mg/kg PCB exposure group significantly differed from the control group (P < .05).
The number of males that exhibited seizure behaviors is shown in Figure 1C. Analysis of the number of rats exhibiting any seizure behavior (running fit or running fits progressing to clonus) at the lowest noise intensity of 100 dB revealed that there was a significant effect of treatment (χ2 = 5.13, df = 1, P = .024) where 7/8 of the PCB-exposed males had an incidence of any seizure behavior compared with 4/9 of the control males. However, unlike the females, the analysis of male rats experiencing seizures that progressed to clonus at the lowest noise intensity did not reveal a significant difference between the treated and the control animals (5/8 and 2/9 of the animals, respectively; Figure 1D).
One hundred percent of female and nearly 100% of male PCB-exposed rats showed seizure activity at the lowest noise intensity of 100 dB but we also summed across all noise intensities to determine if the percentages of PCB exposed and control animals that experienced seizure behavior at any noise intensity was significantly different (Figs. 2A and C). This analysis across all noise intensities, 100, 105, and 110 dB revealed significant effects of treatment for females (χ2 = 7.2, df = 1, P = .007) and males (χ2 = 4.65, df = 1, P = .031). Even when the higher noise intensities were included, higher percentages of PCB-exposed animals, 8/8 for both males and females, experienced any seizure behavior compared with 4/9 of the female and 5/9 of the male controls. Similarly, the percentage of females that progressed to clonus (severest seizure form) across all noise intensities also revealed a significant effect (χ2 = 9.164, df = 1, P = .002), where 8/8 of the PCB-exposed females compared with 3/9 of the controls progressed to clonus (Figure 2B). However, for males, the percentage of PCB-exposed animals that progressed to clonus (7/8 animals) across all noise intensities tested did not differ significantly from controls (3/9 animals) (Figure 2D).
FIG. 2.
Percentage of rats that showed any seizure behavior summed across all noise intensities (100, 105, and 110 dB) for females and males (A and C). Running fits followed by clonus for females and males (B and D). Percentage of rats that showed clonus seizure at 100, 105, and 110 dB noise intensity. n = 9 for controls and 8 for PCB-exposed group for both males and females. F, females, M, males. * indicates that the 6 mg/kg PCB exposure group significantly differed from the control group (P < .05).
Because almost all animals, both male and female, in the PCB group experienced seizure behavior at the lowest noise intensity of 100 dB, the number of treated animals tested at 105 and 110 dB was very small. Therefore comparisons of latencies and ARS (as described later) were conducted using the data collected at 100 dB.
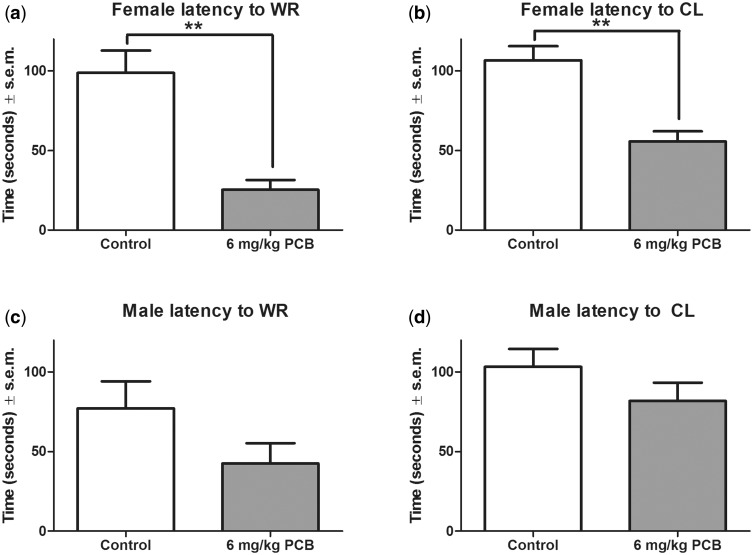
Latency to Onset of Wild Running and Clonus at 100 dB
Analysis of the mean latencies to the initiation of wild running (Figure 3A) and clonus (Figure 3B) revealed that in females there was a significant effect of treatment on the latency to both wild running [(2χ2(1, N = 17) = 6.5, P = .003> and clonus [(2χ2(1, N = 17) = 5.0, P = .002> where PCB-exposed females had shorter latencies to wild running and clonus compared with control females. In males, neither the analysis of latency to the initiation of wild running (Figure 3C) nor latency to the initiation of clonus (Figure 3D) revealed any significant difference between the treated and the control groups.
FIG. 3.
Latency to wild running (WR) (A) and clonus (CL) (B) in females. Latency to WR (C) and CL (D) in males. n = 9 for controls and 8 for PCB-exposed group for both males and females. * indicates that the 6 mg/kg PCB exposure group significantly differed from the control group (P < .001).
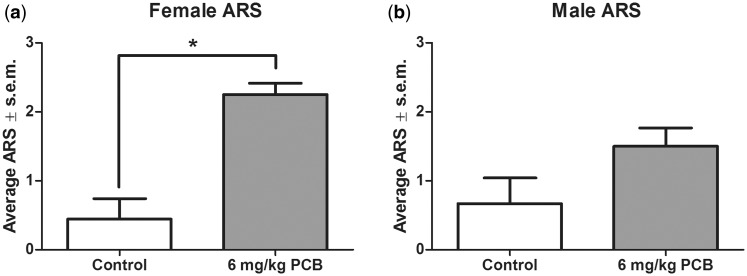
Audiogenic Seizure Response Score (ARS) at 100 dB
The mean ARS scores for each treatment group were calculated based on the behavioral manifestations of each animal during noise exposure. The mean ARS scores for females and males are shown in Figure 4. There was a significant effect of treatment on ARS for females, Figure 4A [χ2(1, N = 17) = 6, P = .002> where PCB-exposed females had a higher ARS score compared with control females. Males showed a marginally significant effect of treatment on ARS, Figure 4B [χ2(1, N = 17) = 18, P = .073>, with the PCB-exposed males having a higher ARS score compared with control males.
FIG. 4.
Audiogenic Seizure Response Score (ARS) for females (A) and males (B). ARS score 0: no observable behavioral changes, 1: Running fit only, 2: Two running fits followed by clonus, and 3: One running fit followed by clonus. n = 9 for controls and 8 for PCB-exposed group for both males and females. ** indicates that the 6 mg/kg PCB exposure group significantly differed from the control group (P < .001).
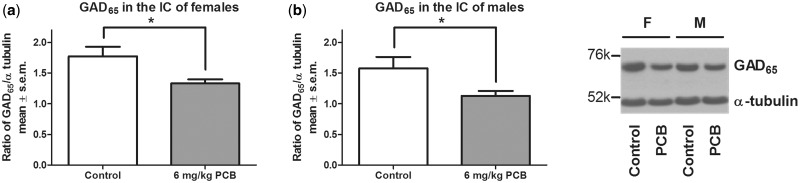
GAD and GABAAα1 Protein Expression in the IC
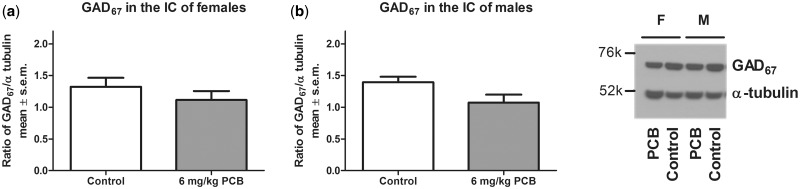
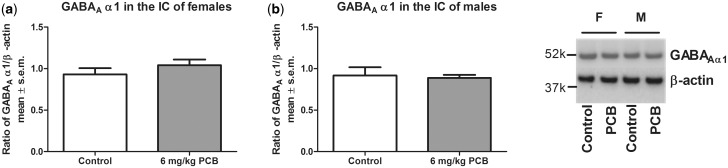
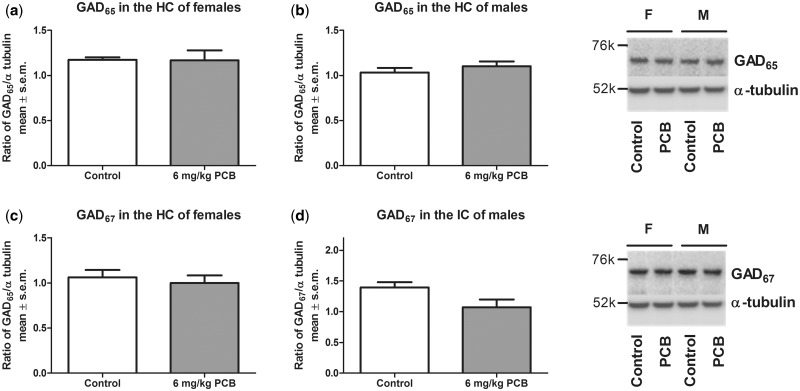
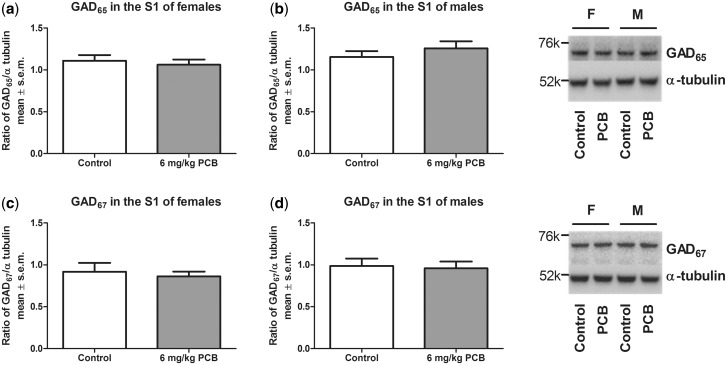
A significant effect of treatment was seen for females (P = .019) and for males (P = .046) where PCB-exposed animals had a lower concentration of GAD65 compared with control animals (Figure 5). For GAD67, there was no effect of PCB exposure on protein levels in the IC (Figure 6). Similarly, no PCB-related effect was observed for GABAAα1 in the IC (Figure 7). Analysis for a sex difference between control females and males and between PCB-exposed females and males revealed no significant effects of sex. Neither GAD65 nor GAD67 were significantly different between control and PCB-exposed animals in the control brain regions: hippocampus and somatosensory cortex (Figs. 8 and 9).
FIG. 5.
glutamic acid decarboxylase (GAD65) in the inferior colliculus (IC) of females (A) and males (B). Nine females for both control and PCB exposed and 7 males for both control and PCB exposed were used. * indicates that the 6 mg/kg PCB exposure group significantly differed from the control group (P < .05).
FIG. 6.
GAD67 in the IC of females (A) and males (B). Nine females for both control and PCB exposed and 7 males for both control and PCB exposed were used.
FIG. 7.
GABAAα1 in the IC of females (A) and males (B). Nine females for both control and PCB exposed and 7 males for both control and PCB exposed were used.
FIG. 8.
GAD65 in the hippocampus of females (A) and males (B). GAD67 in the hippocampus of females (C) and males (D). Nine females for both control and PCB exposed and 7 males for both control and PCB exposed were used.
FIG. 9.
GAD65 in the somatosensory cortex of females (A) and males (B). GAD67 in the somatosensory cortex of females (C) and males (D). Nine females for both control and PCB exposed and 7 males for both control and PCB exposed were used.
DISCUSSION
Audiogenic Seizures
This study confirms that developmental exposure to PCBs increases the susceptibility to AGS. Developmentally PCB-exposed female rats had increased susceptibility to seizures, shown by statistically significant effects for both any seizure behaviors and for clonus seizures at the lowest noise intensity of 100 dB. In males, any seizure behaviors reached statistical significance, but clonus at 100 dB noise intensity did not. Other indices of AGS, such as latency to the first wild run, latency to clonus and ARS scores, indicated that PCB-exposed animals were more susceptible to AGS than controls, with females generally showing a more robust effect of treatment than males.
These results are in accord with our previous study (Poon et al., 2015). However, in that study the rats were exposed to PCBs during early development and also to an extended period (5 days; 4 h per day) of loud noise in adulthood. Furthermore, they were not tested in the AGS paradigm until they were greater than 1 year of age. In our current study, we used young adult (90 days) rats that had not been exposed to noise in adulthood to eliminate prior noise exposure or advanced age as confounders, confirming that developmental PCB exposure alone is sufficient to increase AGS susceptibility in adulthood.
Although both male and female PCB-exposed rats showed increased susceptibility to AGS, the females appeared to be more sensitive than males in both the current and the previous AGS studies (Poon et al., 2015). In a genetically modified rat (GEPR), it has been shown that female rats are more susceptible to seizures than males (Mishra et al., 1988). In females, there is a positive correlation between seizure susceptibility and serum estradiol levels (Backstrom, 1976; Reddy and Rogawski, 2009). Ovariectomized female rats given testosterone had decreased AGS susceptibility whereas castrated males given estradiol had increased AGS susceptibility (Werboff and Corcoran, 1961).
Thyroid hormone plays a crucial role in the proper development and function of the cochlea (Bradley et al., 1994; Rusch et al., 2001; Uziel et al., 1983; Winter et al., 2006), and the increased AGS in developmentally PCB-exposed animals in this study may be due in part to PCB-induced disruption of thyroid hormone levels during development, given that neonatal hypothyroidism has been shown to increase AGS (Auso et al., 2004; Kato et al., 1996). Although no other investigators have studied the effects of PCB exposure on AGS, developmental PCB exposure has been shown to cause modest increases in other types of seizures in rodents. Lein et al. (2010) reported subtle increases of seizure susceptibility at a low dose of PCB 95 (a PCB congener that is active at the ryanodine receptor), including shorter latency to myoclonus and a trend toward tonic-clonic seizures when rats were exposed to the convulsant inhalant flurothyl. Similarly, perinatal exposure to PCB 95 or to Aroclor 1254 resulted in epileptic after-discharges in the evoked potentials of hippocampal slices when challenged with pictotoxin, a GABAA receptor antagonist (Kim and Pessah, 2011). These results suggest that PCB exposure may induce a global increase in seizure susceptibility that is not limited to the auditory system.
GAD and GABAAα1 in the IC
It is well documented that GABA is integral for maintaining the inhibitory balance in the IC, a relay nucleus of the midbrain that plays a crucial role in the initiation of AGS (reviewed by Faingold 2002). Bilateral microinfusions of muscimol, a GABAA agonist, into the IC at very low concentrations abolish the tonic-clonic components of AGS in GEPR-9 s rats (Browning et al., 1989), a strain that is documented to have both increased susceptibility to AGS and low concentrations of GABA in the IC (Lasley, 1991). Furthermore, in a study by Faingold et al. (1994) microinjections of baclofen, a GABAB receptor agonist, into the IC of GEPR-9 s protected against AGS, and blockade of the breakdown of endogenous GABA by gabaculine, a GABA transaminase inhibitor, increased GABA levels and decreased AGS susceptibility in these rats. Together, these studies indicate the importance of the GABA neurotransmitter system in preventing the initiation of AGS at the level of the IC.
GABA is synthesized by the decarboxylation of glutamate by GAD. The two isoforms of GAD, GAD65, and GAD67, exist in most GABA-producing neurons in the CNS. In this study, we measured GAD protein concentrations in the IC of developmentally PCB-exposed rats and saw a significant reduction in the level of GAD65 protein in the IC of both female and male PCB-exposed animals but no reductions in GAD67 or the post synaptic GABAAα1 receptor in either male or female PCB-exposed animals. Analysis of GAD levels between control or PCB-exposed males versus females revealed that there was no sex difference in GAD protein expression levels. Although we do see a sex difference in the behavioral evidence for seizures in our AGS model, this increase in sensitivity by sex is not explained by the GAD expression levels in the IC. There were no changes in either GAD65 or GAD67 in the hippocampus or the somatosensory cortex, which were analyzed as control regions outside of the auditory brainstem.
Interestingly, in GAD65 knockout mice, normal levels of GABA are found, indicating that GAD67 can compensate for the loss of GAD65 (Asada et al., 1996). However, when the otherwise behaviorally normal GAD65 knockout mice were challenged with picrotoxin or pentelenetetrazol, abnormalities surfaced. The latency to seizures induced by picrotoxin was shorter and the dose of pentylenetetrazol required for induction of seizures was lower (Asada et al., 1996). Therefore, it can be postulated that in developmentally PCB-exposed animals the reduction in GAD65 may be compensated for by the function of GAD67 under normal conditions. However, in situations of high inhibitory demand on the auditory brainstem, such as acoustic overstimulation, the reduction of synaptic GABA resulting from reduced GAD65 may lead to AGS. Perfusion of hippocampal slices with picrotoxin (to block GABA neurotransmission) led to increased field excitatory post synaptic potentials and epileptiform after-potential discharges in the CA1 region of hippocampal slices exposed to PCBs compared with control non-PCB perfused hippocampal slices (Kim et al., 2009). Together, these studies indicate that when given a neural stressor, PCB-exposure results in a pathological increase in excitability of neural activity compared with controls.
As mentioned before, developmental exposure to the environmentally relevant “Fox River PCB mixture” results in altered DPOAE in rats (Poon et al., 2011; Powers et al., 2006, 2009). The OHCs within the cochlea are responsible for frequency selectivity and for increasing the sensitivity to sounds (Dallos, 1992). Our prior DPOAE results suggest that developmental exposure to PCBs may disrupt OHC function, resulting in attenuated neural signaling at the level of the auditory brainstem. Similarly, Milbrandt et al. (2000) found that noise-induced cochlea hair cell damage results in a decrease in GAD65 expression in the IC. Therefore, attenuated neural signaling during development may result in a permanent down regulation of GAD65. Immunohistochemical and nonradioactive in situ hybridization for GAD65 and GAD67 have shown that GAD65 is more highly concentrated in axon terminals rather than in the somata (Esclapez et al., 1994) and in phasically active neurons which respond more rapidly in response to external stimuli (Feldblum et al., 1993). It is thought that GAD65 and GAD67 provide flexibility for regulating GABA synthesis. In support of this hypothesis, we observed down regulation of GAD65 protein levels in the IC and no changes in GAD67 or the post synaptic GABAA receptor in developmentally PCB-exposed animals. These animals, when subject to acoustic overstimulation, may lack sufficient synaptic GABA produced by GAD65 culminating in AGS.
CONCLUSIONS
In summary, we have shown that developmental exposure to PCBs during the critical period of cochlear development resulted in an increased susceptibility to AGS in adulthood. Similar to what we have observed before, AGS effects were more robust in females than in males. To understand the biological substrate for increased seizure susceptibility in PCB-exposed animals, we measured GAD levels in the IC and found that there was a significant decrease in GAD65 in both sexes of PCB-exposed animals. This indicates that PCB exposure was an important factor in priming the animals to be more susceptible to AGS via down regulation of inhibitory GABA activity in the IC. Similar decreases in GAD65 were not observed in brain regions outside the auditory pathway (hippocampus; somatosensory cortex) but our findings do not preclude possible deficits elsewhere in the midbrain AGS circuit in PCB-exposed animals, nor do they dismiss the possibility of changes in sensorimotor gating systems. Additional research will be needed to determine if this phenomenon of hyperexcitability is specific to the auditory brainstem or if developmental PCB exposure may cause a more generalized change in GABA function and seizure activity throughout the brain.
ACKNOWLEDGMENTS
The authors thank Dr Larry G. Hansen and Dr Paul J. Kostyniak for their help in formulating the PCB mixture and Dr Donald M. Caspary for his insight about IC GABA.
FUNDING
This work was supported by a grant from the National Institute of Environmental Health Sciences (NIEHS) (R01 ES015687 to S.L.S.).
REFERENCES
- Ampleman M. D., Martinez A., DeWall J., Rawn D. F., Hornbuckle K. C., Thorne P. S. (2015). Inhalation and dietary exposure to PCBs in urban and rural cohorts via congener-specific measurements. Environ. Sci. Technol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anezaki K., Kannan N., Nakano T. (2014). Polychlorinated biphenyl contamination of paints containing polycyclic- and naphthol AS-type pigments. Environ. Sci. Pollut. Res. Int. 22, 14478–14488. [DOI] [PubMed] [Google Scholar]
- Asada H., Kawamura Y., Maruyama K., Kume H., Ding R., Ji F. Y., Kanbura N., Kuzume H., Sanbo M., Yagi T., et al. (1996). Mice lacking the 65 kDa isoform of glutamic acid decarboxylase (GAD65) maintain normal levels of GAD67 and GABA in their brains but are susceptible to seizures. Biochem. Biophys. Res. Commun. 229, 891–895. [DOI] [PubMed] [Google Scholar]
- Auso E., Lavado-Autric R., Cuevas E., Del Rey F. E., Morreale De Escobar G., Berbel P. (2004). A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology 145, 4037–4047. [DOI] [PubMed] [Google Scholar]
- Backstrom T. (1976). Epileptic seizures in women related to plasma estrogen and progesterone during the menstrual cycle. Acta Neurol. Scand. 54, 321–347. [DOI] [PubMed] [Google Scholar]
- Bauer C. A., Brozoski T. J., Holder T. M., Caspary D. M. (2000). Effects of chronic salicylate on GABAergic activity in rat inferior colliculus. Hear. Res. 147, 175–182. [DOI] [PubMed] [Google Scholar]
- Bradley D. J., Towle H. C., Young W. S., III (1994). Alpha and beta thyroid hormone receptor (TR) gene expression during auditory neurogenesis: Evidence for TR isoform-specific transcriptional regulation in vivo. Proc. Natl. Acad. Sci. U.S.A. 91, 439–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning R. A., Lanker M. L., Faingold C. L. (1989). Injections of noradrenergic and GABAergic agonists into the inferior colliculus: Effects on audiogenic seizures in genetically epilepsy-prone rats. Epilepsy Res. 4, 119–125. [DOI] [PubMed] [Google Scholar]
- Caspary D. M., Holder T. M., Hughes L. F., Milbrandt J. C., McKernan R. M., Naritoku D. K. (1999). Age-related changes in GABA(A) receptor subunit composition and function in rat auditory system. Neuroscience 93, 307–312. [DOI] [PubMed] [Google Scholar]
- Chu S., Covaci A., Schepens P. (2003). Levels and chiral signatures of persistent organochlorine pollutants in human tissues from Belgium. Environ. Res. 93, 167–176. [DOI] [PubMed] [Google Scholar]
- Coleman J. R., Ross K. C., Mullaney M. M., Cooper W. A. (1999). Latency alterations of the auditory brainstem response in audiogenic seizure-prone Long-Evans rats. Epilepsy Res. 33, 31–38. [DOI] [PubMed] [Google Scholar]
- Crinnion W. J. (2011). Polychlorinated biphenyls: Persistent pollutants with immunological, neurological, and endocrinological consequences. Altern. Med. Rev. 16, 5–13. [PubMed] [Google Scholar]
- Crofton K. M., Kodavanti P. R., Derr-Yellin E. C., Casey A. C., Kehn L. S. (2000). PCBs, thyroid hormones, and ototoxicity in rats: Cross-fostering experiments demonstrate the impact of postnatal lactation exposure. Toxicol. Sci. 57, 131–140. [DOI] [PubMed] [Google Scholar]
- Dallos P. (1992). The active cochlea. J. Neurosci. 12, 4575–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eells J. B., Clough R. W., Browning R. A., Jobe P. C. (2004). Comparative fos immunoreactivity in the brain after forebrain, brainstem, or combined seizures induced by electroshock, pentylenetetrazol, focally induced and audiogenic seizures in rats. Neuroscience 123, 279–292. [DOI] [PubMed] [Google Scholar]
- Esclapez M., Tillakaratne N. J., Kaufman D. L., Tobin A. J., Houser C. R. (1994). Comparative localization of two forms of glutamic acid decarboxylase and their mRNAs in rat brain supports the concept of functional differences between the forms. J. Neurosci. 14(3 Pt 2), 1834–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faingold C. L. (1999). Neuronal networks in the genetically epilepsy-prone rat. Adv. Neurol. 79, 311–321. [PubMed] [Google Scholar]
- Faingold C. L. (2002). Role of GABA abnormalities in the inferior colliculus pathophysiology - audiogenic seizures. Hear. Res. 168, 223–237. [DOI] [PubMed] [Google Scholar]
- Faingold C. L., Marcinczyk M. J., Casebeer D. J., Randall M. E., Arneric S. P., Browning R. A. (1994). GABA in the inferior colliculus plays a critical role in control of audiogenic seizures. Brain Res. 640, 40–47. [DOI] [PubMed] [Google Scholar]
- Feldblum S., Erlander M. G., Tobin A. J. (1993). Different distributions of GAD65 and GAD67 mRNAs suggest that the two glutamate decarboxylases play distinctive functional roles. J. Neurosci. Res. 34, 689–706. [DOI] [PubMed] [Google Scholar]
- Goldey E. S., Crofton K. M. (1998). Thyroxine replacement attenuates hypothyroxinemia, hearing loss, and motor deficits following developmental exposure to aroclor 1254 in rats. Toxicol. Sci. 45, 94–105. [DOI] [PubMed] [Google Scholar]
- Goldey E. S., Kehn L. S., Lau C., Rehnberg G. L., Crofton K. M. (1995). Developmental exposure to polychlorinated biphenyls (aroclor 1254) reduces circulating thyroid hormone concentrations and causes hearing deficits in rats. Toxicol. Appl. Pharmacol. 135, 77–88. [DOI] [PubMed] [Google Scholar]
- Herr D. W., Goldey E. S., Crofton K. M. (1996). Developmental exposure to aroclor 1254 produces low-frequency alterations in adult rat brainstem auditory evoked responses. Fundam. Appl. Toxicol. 33, 120–128. [DOI] [PubMed] [Google Scholar]
- Hu D., Hornbuckle K. C. (2010). Inadvertent polychlorinated biphenyls in commercial paint pigments. Environ. Sci. Technol. 44, 2822–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida N., Kato N., Kanai H., Watanabe Y., Kuroda Y., McEwen B. S. (1995). Audiogenic seizure induces c-fos mRNA expression in the inferior colliculus and not in the hippocampus. Psychiatry Clin. Neurosci. 49, S280–2. [DOI] [PubMed] [Google Scholar]
- Jacobson J. L., Fein G. G., Jacobson S. W., Schwartz P. M., Dowler J. K. (1984). The transfer of polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs) across the human placenta and into maternal milk. Am. J. Public Health 74, 378–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Ishida N., Kanai H., Watanabe Y., Kuroda Y., McEwen B. S. (1996). Expression of c-fos mRNA after audiogenic seizure in adult rats with neonatal hypothyroidism. Brain Res. Mol. Brain Res. 38, 85–90. [DOI] [PubMed] [Google Scholar]
- Kim K. H., Inan S. Y., Berman R. F., Pessah I. N. (2009). Excitatory and inhibitory synaptic transmission is differentially influenced by two ortho-substituted polychlorinated biphenyls in the hippocampal slice preparation. Toxicol. Appl. Pharmacol. 237, 168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. H., Pessah I. N. (2011). Perinatal exposure to environmental polychlorinated biphenyls sensitizes hippocampus to excitotoxicity ex vivo. Neurotoxicology 32, 981–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyniak P. J., Hansen L. G., Widholm J. J., Fitzpatrick R. D., Olson J. R., Helferich J. L., Kim K. H., Sable H. J., Seegal R. F., Pessah I. N., et al. (2005). Formulation and characterization of an experimental PCB mixture designed to mimic human exposure from contaminated fish. Toxicol. Sci. 88, 400–411. [DOI] [PubMed] [Google Scholar]
- Lasky R. E., Widholm J. J., Crofton K. M., Schantz S. L. (2002). Perinatal exposure to aroclor 1254 impairs distortion product otoacoustic emissions (DPOAEs) in rats. Toxicol. Sci. 68, 458–464. [DOI] [PubMed] [Google Scholar]
- Lasley S. M. (1991). Roles of neurotransmitter amino acids in seizure severity and experience in the genetically epilepsy-prone rat. Brain Res. 560, 63–70. [DOI] [PubMed] [Google Scholar]
- Lein P. J., Kim K. H., Berman R. F., Pessah I. N. (2010). Exposure of the developing brain to polychlorinated biphenyls influences the suceptibility of the adult brain to stress. In Developmental Neurotoxicolgy Research: Principles, Models, Techniques, Strategies and Mechanisms (Wang C., Slikker W., Eds.), pp 211–229. John Wiley & Sons, Inc., Hoboken, NJ, USA. [Google Scholar]
- Milbrandt J. C., Holder T. M., Wilson M. C., Salvi R. J., Caspary D. M. (2000). GAD levels and muscimol binding in rat inferior colliculus following acoustic trauma. Hear. Res. 147, 251–260. [DOI] [PubMed] [Google Scholar]
- Mishra P. K., Dailey J. W., Reigel C. E., Tomsic M. L., Jobe P. C. (1988). Sex-specific distinctions in audiogenic convulsions exhibited by severe seizure genetically epilepsy-prone rats (GEPR-9s). Epilepsy Res. 2, 309–316. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. (2002). Public Health Service Policy on Humane Care and Use of Laboratory Animals. NIH/Office of Laboratory Animal Welfare, Rockville, MD. [Google Scholar]
- National Research Council. (2003). Institute for Laboratory Animal Research. Guidelines for the Care of Use of Mammals in Neuroscience and Behavioral Research . National Academy Press, Washington, DC. [Google Scholar]
- Paxinos G., Watson C. (1998). The Rat Brain in Stereotaxic Coordinates, Fourth Edition. Academic Press, New York. [Google Scholar]
- Petroff O. A. (2002). GABA and glutamate in the human brain. Neuroscientist 8, 562–573. [DOI] [PubMed] [Google Scholar]
- Poon E., Bandara S. B., Allen J. B., Sadowski R. N., Schantz S. L. (2015). Developmental PCB exposure increases susceptibility to audiogenic seizures inadulthood. Neurotoxicology 46, 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon E., Powers B. E., McAlonan R. M., Ferguson D. C., Schantz S. L. (2011). Effects of developmental exposure to polychlorinated biphenyls and/or polybrominated diphenyl ethers on cochlear function. Toxicol. Sci. 124, 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers B. E., Poon E., Sable H. J., Schantz S. L. (2009). Developmental exposure to PCBs, MeHg, or both: Long-term effects on auditory function. Environ. Health Perspect. 117, 1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers B. E., Widholm J. J., Lasky R. E., Schantz S. L. (2006). Auditory deficits in rats exposed to an environmental PCB mixture during development. Toxicol. Sci. 89, 415–422. [DOI] [PubMed] [Google Scholar]
- Reddy D. S., Rogawski M. A. (2009). Neurosteroid replacement therapy for catamenial epilepsy. Neurotherapeutics 6, 392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross K. C., Coleman J. R. (1999). Audiogenic seizures in the developmentally primed Long-Evans rat. Dev. Psychobiol. 34, 303–313. [DOI] [PubMed] [Google Scholar]
- Ross K. C., Coleman J. R. (2000). Developmental and genetic audiogenic seizure models: Behavior and biological substrates. Neurosci. Biobehav. Rev. 24, 639–653. [DOI] [PubMed] [Google Scholar]
- Rowley N. M., Madsen K. K., Schousboe A., Steve White H. (2012). Glutamate and GABA synthesis, release, transport and metabolism as targets for seizure control. Neurochem. Int. 61, 546–558. [DOI] [PubMed] [Google Scholar]
- Rusch A., Ng L., Goodyear R., Oliver D., Lisoukov I., Vennstrom B., Richardson G., Kelley M. W., Forrest D. (2001). Retardation of cochlear maturation and impaired hair cell function caused by deletion of all known thyroid hormone receptors. J. Neurosci. 21, 9792–9800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. (1985). Measurement of protein using bicinchoninic acid. Anal. Biochem. 150, 76–85. [DOI] [PubMed] [Google Scholar]
- Uziel A., Pujol R., Legrand C., Legrand J. (1983). Cochlear synaptogenesis in the hypothyroid rat. Brain Res. 283, 295–301. [DOI] [PubMed] [Google Scholar]
- Werboff J., Corcoran J. B. (1961). Effects of sex hormone manipulation on audiogenic seizures. Am. J. Physiol. 201, 830–832. [DOI] [PubMed] [Google Scholar]
- Winter H., Brain C., Zimmermann U., Geisler H., Franzer J., Weber T., Ley M., Engel J., Knirsch M., Bauer K., et al. (2006). Thyroid hormone receptors TRalpha1 and TRbeata differentially regulate gene expression of Kcnq4 and prestin during final differentiation of outer hair cells. J. Cell Sci. 119, 2975–2984. [DOI] [PubMed] [Google Scholar]