Abstract
Objective: The use of noncultured autologous stromal vascular fraction or clinical grade adipose-derived regenerative cells (ADRCs) is a promising strategy to promote wound healing and tissue repair. Nevertheless, issues regarding the optimal mode of administration remain unclear. The purpose of this study was to compare the effects of local injection and topical spray delivery of ADRCs in a porcine model of thermal burns.
Approach: Full-thickness thermal burns were created on the dorsum of 10 Gottingen minipigs. Two days following injury, wounds underwent fascial excision and were randomized to receive control vehicle or freshly isolated autologous ADRCs delivered by either multiple injections into or surrounding the wound bed, or by spray onto the wound surface (0.25 × 106 viable cells/cm2). Healing was evaluated by planimetry, histopathology, and immunohistochemistry at day 7, 12, 16, 21, and 28 posttreatment.
Results: In vitro analysis demonstrated that there was no substantial loss of cell number or viability attributable to the spray procedure. Planimetric assessment revealed that delivery of ADRCs by either local injection or topical spray increased wound reepithelialization relative to control at day 14. No significant difference in wound reepithelialization was observed between both delivery approaches. In addition, on day 7 posttreatment, blood vessel density was greater in wounds receiving local or topical spray ADRCs than in the wounds treated with vehicle control. Histopathologic analysis suggests that ADRC treatment may modulate the inflammatory response by reducing neutrophil infiltration at day 7 and 12 posttreatment, irrespective of the route of administration.
Conclusions: These data demonstrate that local injection and spray delivery of ADRCs modulate inflammation and improve wound angiogenesis and epithelialization. Importantly, both delivery routes exhibited similar effects on wound healing. Given the greater ease-of-use associated with topical spray delivery, these data support the use of a spray system for autologous ADRC delivery.

Philippe Foubert, PhD
Introduction
Burn injury is a severe form of trauma responsible for substantial morbidity and mortality worldwide. While improvements in acute burn care and resuscitation have led to a notable decline in the burn-related mortality rate over recent decades, the healing of large burns remains a challenge.1,2 Development of novel therapeutic approaches is needed to improve burn care and wound repair in these patients.
Recent progress in regenerative medicine has demonstrated the potential of cellular therapy in improving the rate and quality of wound healing and tissue regeneration.3,4 Specifically, the heterogeneous stromal vascular fraction (SVF) of adipose tissue is an attractive option for autologous cell-based therapy.5–7 Indeed, SVF is an easily accessible and rich source of stem and regenerative cells, including endothelial cells, fibroblasts, smooth muscle cells, macrophages, and adipose-derived stromal cells.8,9 We have referred to clinical grade SVF as adipose-derived regenerative cells (ADRCs).10,11 Studies in renal and cardiovascular injury models have described a beneficial role of ADRCs during repair that appears to be mediated, at least in part, by modulation of the inflammatory response and/or promoting tissue vascularization and matrix deposition. This activity arises from the ability of ADRCs to secrete proangiogenic factors and/or differentiate toward lineage-committed cells.5 For instance, Hao et al. showed that ADRC therapy appears to promote vascularization by modulating local inflammation in a mouse model of hind limb ischemia.12 Similarly, our group has previously shown that ADRC treatment downregulates inflammatory-related gene expression (interleukin 6 [IL-6] and CXC chemokine ligand 12 [CXCL-12]) in a rat model of acute kidney injury.13 We have also reported that seeding ADRCs onto a widely used collagen dermal substitute increases wound angiogenesis, blood vessel maturation, and matrix remodeling.11 On the basis of studies such as these, the use of ADRCs as an adjunct to healing has advanced worldwide into multiple clinical trials (e.g., NCT0232696,14,15 NCT01813279,16 and NCT0205242717). Small case series applying this approach in chronic cutaneous wounds have similarly been published.18–20
Despite the advances in cell-based therapies, many challenges still remain regarding safety, efficacy, and robustness of various delivery methods. Several approaches (e.g., fibrin spray, systemic, local injection, topical delivery, and scaffold delivery) have been tested for delivery of stem/progenitor cells to acute and chronic wounds.3,21–23 In addition, from an efficacy and disease-impact perspective, it is important to define appropriate methods to successfully deliver cells. For instance, for applications wherein it is crucial to rapidly deliver cells to a large surface area, for example, for fragile patients with large burns, local injection may not be feasible or desirable as it is invasive and requires multiple injections around a large wound perimeter. To fully optimize cell therapy safety and efficacy, further research refining the most efficient delivery method and timing is required. Consequently, the aim of this study was to compare the effects of two modes of delivery of ADRCs (topical spray and multiple local injections) in a porcine model of full-thickness thermal burn injury.
Clinical Problem Addressed
ADRC therapy is an attractive option to promote wound healing in burn patients. Our data demonstrate that local injection and topical spray approaches of ADRC delivery after thermal burns are feasible and effective resulting in greater wound epithelialization and vascularization compared to the control condition. In addition, we were able to show that both delivery techniques reduced neutrophil infiltration within the burn wounds. Considering the greater ease-of-use and noninvasive character of topical spray delivery compared to multiple local invasive injections, these data support the use of a spray system for autologous ADRC delivery for future clinical use.
Materials and Methods
Animals
This study was conducted according to a research proposal approved by the Institutional Animal Care and Use Committee (IACUC). Ten female Gottingen minipigs (7–8 months; 12–16 kg) were purchased from Marshall BioResources. Animals were identified by ear tags, cage cards, and/or color identifiers in the animal's back.
Porcine model of thermal burn injury
Full-thickness burn and excision procedures were performed as previously described.11 Briefly, six full-thickness burns (∼10 cm2 each) were created on the dorsum of each animal using a custom-made burn induction device. Two days postinjury, wound sites were excised to the level of the underlying muscular fascia.
ADRC isolation
Two days after burn injury (before excision of burn wounds), adipose tissue (14.3–27 g) was excised from the inguinal fat pad and isolated as previously described.11
ADRC yield and viability pre- and postspray delivery (in vitro)
Freshly isolated ADRCs were loaded into a 3-mL syringe connected to a spray atomization device (LMA MAD Nasal™; Teleflex), following the manufacturer's instructions. The cells were sprayed under manual pressure sufficient to generate a fine mist onto a 10-cm plastic culture Petri dish. Cell yield and viability of ADRC suspensions were determined on both prespray (baseline) and postspray samples, using the NucleoCounter® NC‐100™ (ChemoMetec).
Topical spray and local injection of ADRCs within the wound site
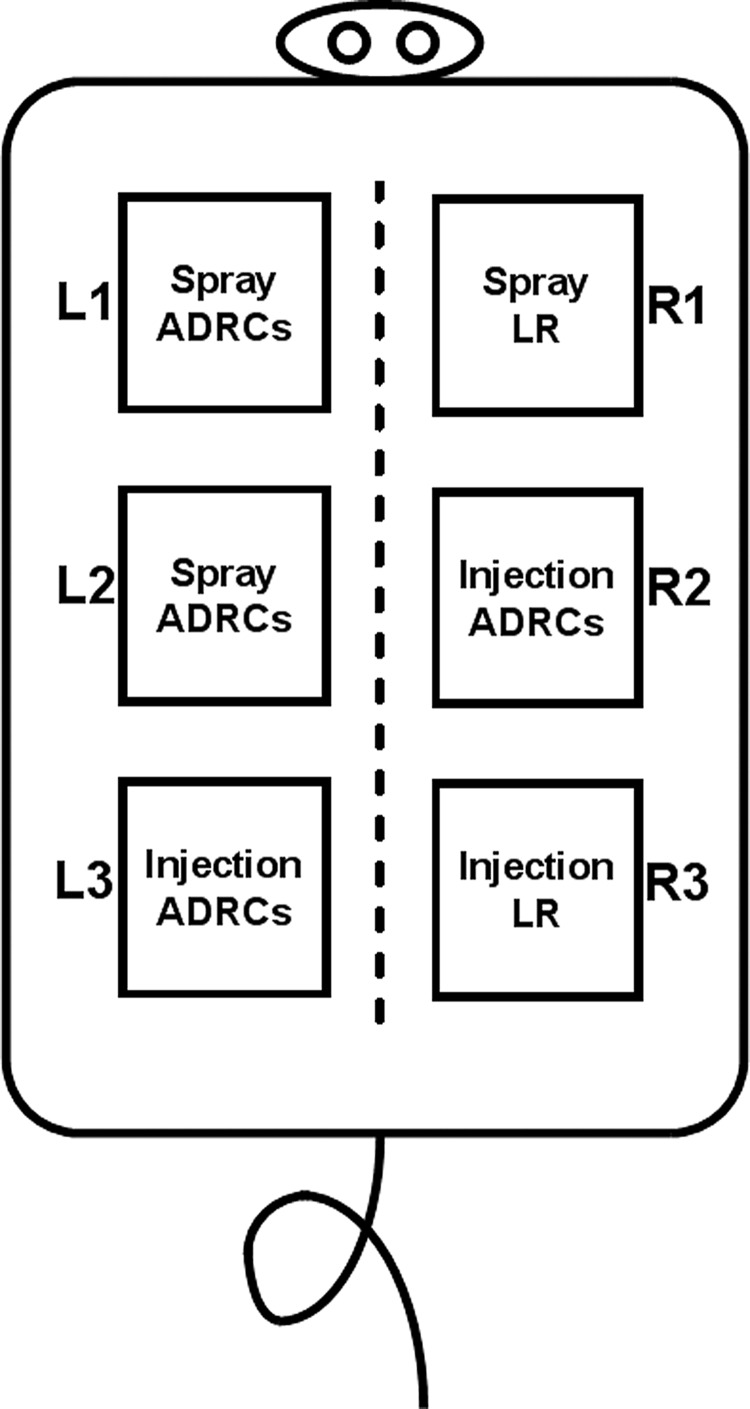
Following wound excision (day 2 postburn), freshly isolated ADRCs were locally injected subdermally and into the superficial fascia (total of 25 injections of 0.2 mL each) or topically sprayed (0.3 mL/wound) directly onto the wound bed to provide a dose of 0.25 × 106 ADRCs per cm2. Each of the three pairs of wounds was used to address a particular question: Pair 1: one wound received spray ADRCs and the contralateral wound received spray-lactated Ringer's (LR) (Control). Pair 2: one wound received spray ADRCs and the contralateral wound received local injection of ADRCs. Pair 3: one wound received local injection of ADRCs and the contralateral wound received local injection of LR (Control). This approach is illustrated in Fig. 1. To avoid potential cross-contamination of wounds with ADRCs as a result of accidental overspray, all wounds except the target were covered with an impermeable material during spraying. Following treatment, a multilayer dressing was used to protect the wound sites. Dressings were changed every 5–7 days.
Figure 1.
Treatment allocation to each burn wound site. This study consisted of 10 female Gottingen minipigs, each with three pairs of wounds. One pair in each animal compared adipose-derived regenerative cells (ADRCs) delivered by topical spray to a vehicle control delivered by the same spray mechanism (L1 vs. R1 wound); a second pair compared ADRCs delivered by spray to ADRCs delivered by local injection (L2 vs. R2 wound); and the third pair compared ADRCs delivered by local injection to a vehicle control delivered by local injection (L3 vs. R3 wounds).
Planimetry wound imaging
Digital imaging of wounds was conducted on day 0 (postburn), day 2 pre- and postexcision, day 9, 14, 18, 23, and 30 postinjury (i.e., day 7, 12, 18, and 28 posttreatment). All wounds were subjected to high-quality digital imaging using the SilhouetteStar Wound Camera (ARANZ Medical). For each time point, the entire burn size and wound area were traced and measured by planimetry using Silhouette Connect™ System (ARANZ Medical). Reepithelialized area was identified as pink/purple, fragile dry thin layer regions, whereas granulating open area was characterized as berry-like red moist area. Wound boundaries were manually performed by one investigator and then audited by another blinded to wound and treatment.
Wound histology
Wound tissues from biopsies collected at day 9, 14, 23, and 30 postinjury were fixed in 10% neutral-buffered formalin, embedded in paraffin, sectioned (5 μm), and stained with hematoxylin and eosin (H&E) and Masson's trichrome stain. Entire slides were then digitally scanned using the Aperio ScanScope AT2 slide scanner (Aperio Technologies). Slides were viewed and analyzed using the ImageScope viewer (Aperio Technologies). The ImageScope analysis software package (version 12.1; Aperio Technologies) was applied to quantify histochemical staining.
Histopathologic evaluation
Slides stained with H&E and Masson's Trichrome were qualitatively evaluated by digital pathology by a board-certified veterinary pathologist blinded to the protocol and treatment allocation. Specifically, the presence and number of neutrophils, macrophages, and lymphocytes were semiquantitatively graded using a 5-point scale according to Mann et al., as follows: no inflammatory cells (score 0; within normal limit); 1–10 cells per high-power field (HPF) (score 1; minimal); 10–25 cells per HPF (score 2; mild); >25 cells per HPF (score 3; moderate); heavy infiltrate (score 4; marked); and packed lesions (score 5; severe).
Immunohistochemistry
Tissue specimens were fixed in 10% normal-buffered formalin and subsequently embedded in paraffin. Paraffin-embedded tissue sections (5 μm) were deparaffinized and rehydrated through alcohol to water. Sections were incubated with BLOXALL Solution (Vector Laboratories) for 10 min to inactivate an endogenous alkaline phosphatase (AP) activity. Tissue sections were subjected to an antigen retrieval step by boiling sodium citrate solution (pH 6; Vector Laboratories) for 15 min in a pressure cooker and then incubated with primary polyclonal rabbit CD31 (5 μg/mL; Abbiotec) or myeloperoxidase (MPO; 4 μg/mL; Dako) antibodies. AP-based detection of the primary antibody was performed using a Vectastain ABC-AP kit (Vector Laboratories) according to the manufacturer's instructions, followed by nuclear staining with Harris hematoxylin. As controls, tissue sections were stained as described above without adding the primary antibody. CD31 and MPO staining were digitally quantified using ImageScope analysis software package (Microvessel Analysis Algorithm and Positive Pixel Count; Aperio Technologies).
Statistical analysis
Results are expressed as means ± standard error of mean. Comparisons between two groups were performed using a paired t-test (Graph Pad Prism version 6.05). A value of p ≤ 0.05 was considered significant.
Results
ADRC yield and viability after spray delivery (in vitro)
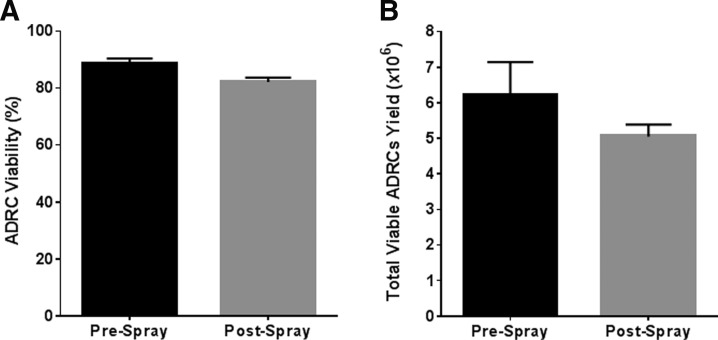
The process of spraying is associated with the potential for injury to the sprayed cells from shear force, particularly within the nozzle. To assess the effect of the spray on ADRC properties, both total cell yield and viability were evaluated before (baseline prespray) and after (postspray) cell delivery. Viable cell yield before and after spray was 6.2 × 106 ± 0.9 × 106 and 5.05 × 106 ± 0.3 × 106, respectively (Fig. 2A). In addition, the average percent of cell viability before and after spray was 88.4% ± 1.9% and 82.1% ± 1.5%, respectively (Fig. 2B), demonstrating that the loss in cell viability attributable to the spray device was <7%. Collectively, these data demonstrate that there is no substantial loss of cell number or viability attributable to the spraying application procedure.
Figure 2.
ADRC yield and viability using the atomization spray device. The total number of viable ADRCs (A) and percent of viability (B) were determined pre- and postspray. Results are presented as mean ± standard error of mean (SEM). n = 3.
Effects of topical spray and local injection of ADRCs on burn wound healing
The next step was to compare the efficacy profile of topical spray and local injection of ADRCs during the wound healing after full-thickness burn injury. We therefore evaluated the effects of both delivery approaches on wound epithelialization, inflammation, and angiogenesis.
Effects on wound healing and reepithelialization
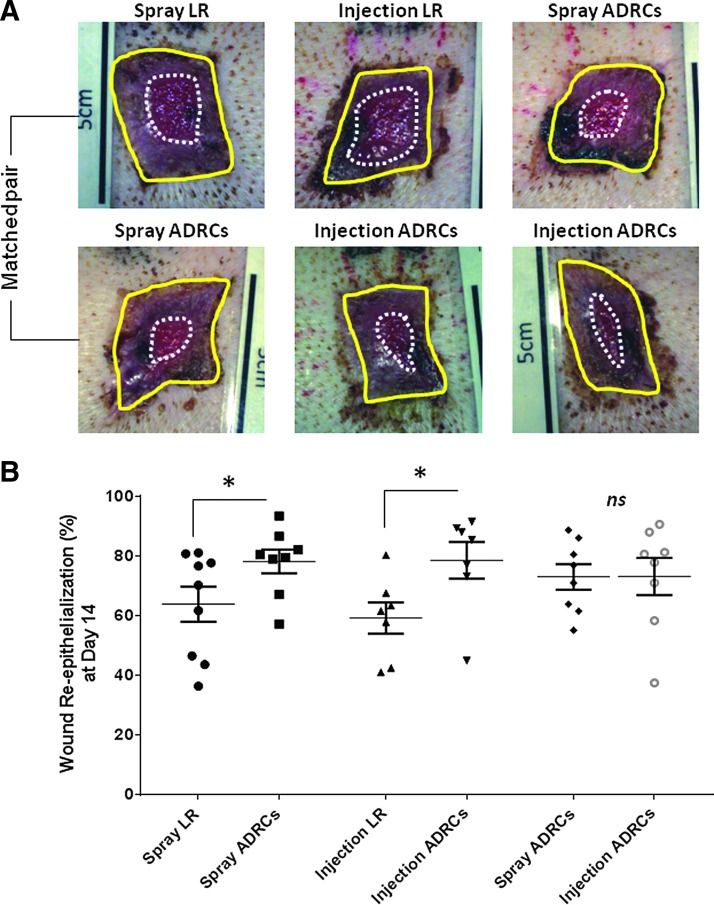
Planimetric assessment allowed quantification of wound contraction and epithelialization. There was no statistical difference between the levels of wound contraction observed in control LR, topical spray, and local injection of ADRCs over the course of the study (data not shown). By contrast, using a matched pair analysis, planimetry data showed that the percentage of the wound that was epithelialized at day 12 posttreatment was increased by 25% in wounds receiving local injection of ADRCs (p < 0.05) (Fig. 3). This was associated with 32% greater absolute epithelialized area in wounds treated with local injection of ADRCs than in those receiving control LR (5.8 ± 0.6 cm2 vs. 3.7 ± 0.5 cm2; p < 0.05, respectively) (data not shown). Similarly, topical spray delivery of ADRCs increased the percent of the wound that was epithelialized by 17% and led to a 28% increase in the absolute area of epithelialization (4.8 ± 0.4 cm2 vs. 3.7 ± 0.5 cm2; p < 0.05, respectively) compared to control spray LR at day 14 postinjury (p = 0.05) (Fig. 3). There was no significant difference in wound epithelialization between wounds treated by topical spray and those treated by local injection of ADRCs at day 14, as assessed by the percentage of wound that epithelialized (72.9% ± 4.4% vs. 73.1% ± 6.2%; p = 0.975, respectively) or by the absolute area of epithelialization (5.4 ± 0.6 cm2 vs. 4.9 ± 0.6 cm2; p = 0.253) (Fig. 3).
Figure 3.
Effect of ADRCs on wound reepithelialization. (A) Digital images of wound showing wound epithelialization on day 12 posttreatment. Entire wound size (solid yellow line) and open wound area (dotted white line) were manually traced and measured by planimetry using computer software to determine the mean percent of wound epithelialization. (B) Quantification of wound epithelialization. Results are presented as mean ± SEM. *p < 0.05 versus lactated Ringer's (LR) control vehicle.
Effects of ADRCs on the inflammatory response
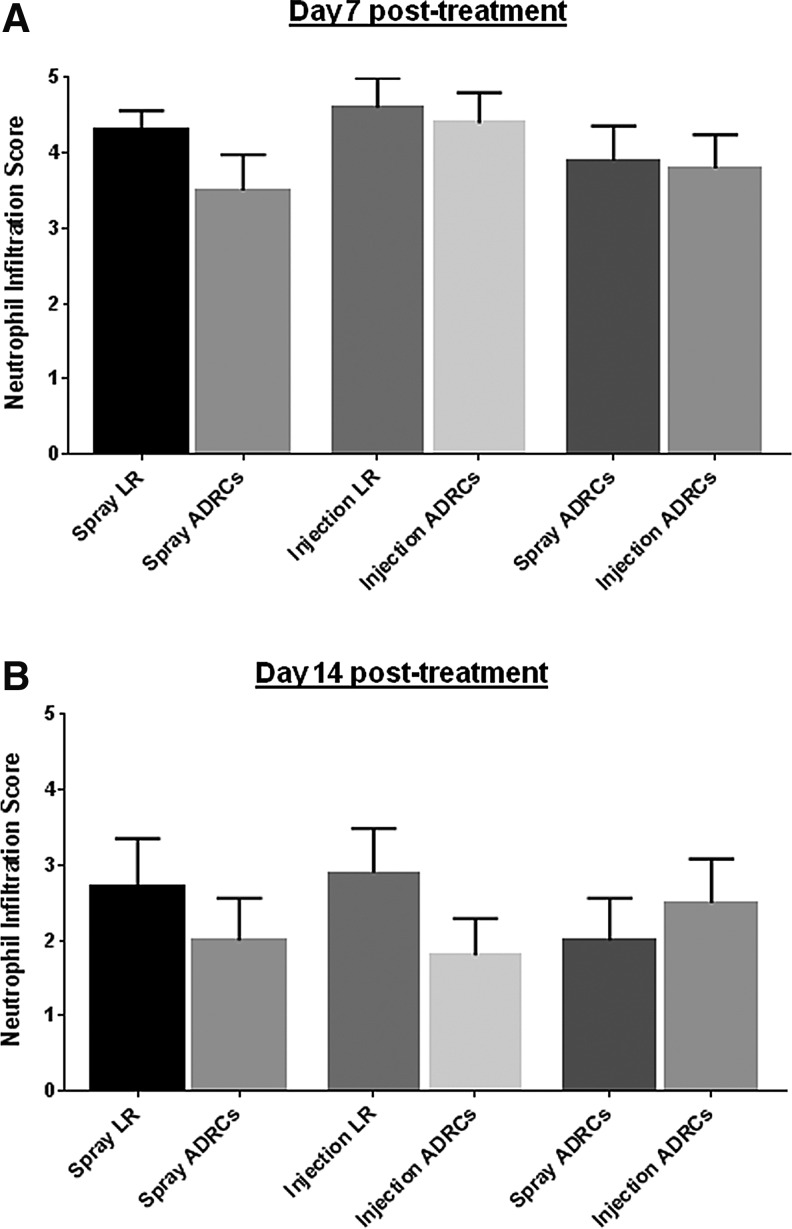
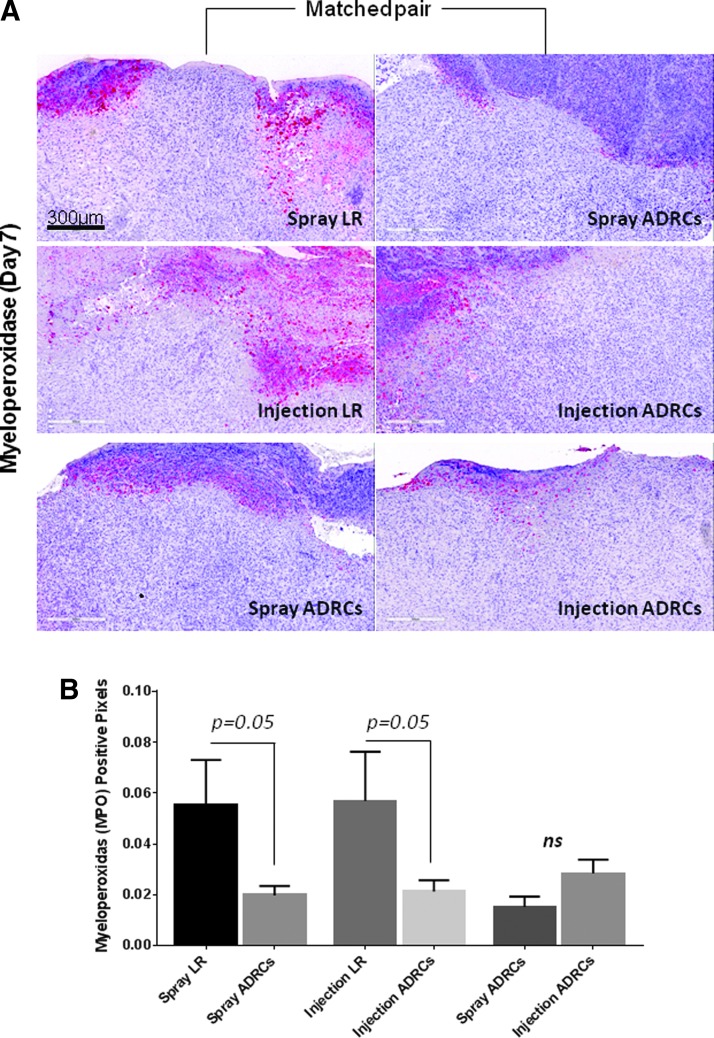
Histologic scoring showed no significant difference in lymphocyte and macrophage infiltration between wounds treated with vehicle control, locally injected ADRCs, and topically sprayed ADRCs on day 7, 12, 21, and 28 posttreatment (data not shown). In contrast, as shown in Fig. 4, topical spray delivery of ADRCs tended to reduce neutrophil accumulation on day 7 and to a lesser extent on day 12 posttreatment, when compared to control. Local injection of ADRCs also slightly reduced neutrophil infiltration compared to local LR treatment only at day 12 posttreatment (Fig. 4B). When local and topical delivery approaches of ADRCs were compared, no significant difference in neutrophil infiltration was observed on day 7 and 12 posttreatment. To confirm these observations, biopsies were stained for MPO, a specific marker for neutrophils. Local injection and topical spray delivery of ADRCs both reduced neutrophil infiltration compared to control vehicle LR (Fig. 5). There was no apparent difference in neutrophil accumulation between the two ADRC delivery methods.
Figure 4.
(A) Effect of ADRCs on wound inflammation (histopathologic scoring). Biopsies collected on day 7 and 12 posttreatment were graded by an independent pathologist blinded to wound treatment. (B) Inflammatory infiltrate was graded according to the number of cells within the wound tissue in accordance with a 0–5 point scale as follows: 0 = no infiltrate; 1 = minimal infiltration (1–10 cells/field); 2 = mild infiltration (10–25 cells/field); 3 = moderate infiltration (>25 cells/field); 4 = marked infiltration (heavy infiltrate); and 5 = severe infiltration (packed).
Figure 5.
The influence of ADRCs on neutrophil infiltration. (A) Wound biopsies collected from animals receiving LR or ADRCs (topical spray or local injection) were stained for myeloperoxidase (MPO). Representative photomicrographs of sections stained with MPO for each wound pair at day 7 posttreatment are shown. (B) The total number of MPO-positive pixels was quantified using automated analysis of digitally scanned slides. Results are presented as mean ± SEM.
Effects of ADRCs on wound vascularization
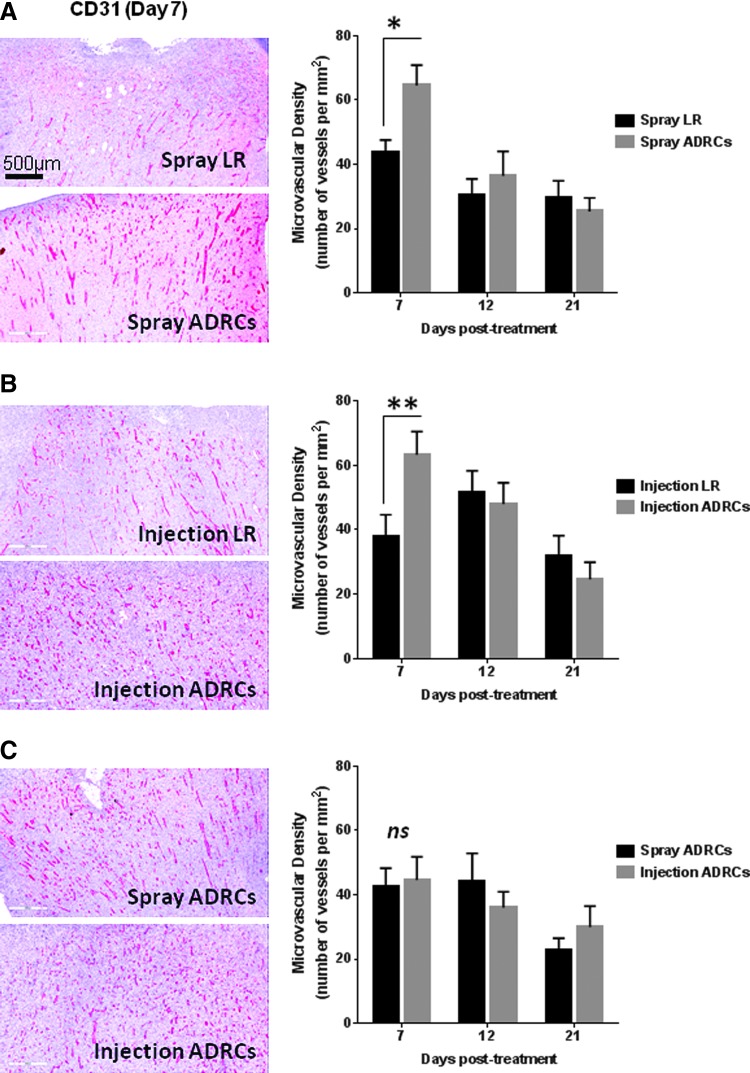
The formation of new blood vessels, or angiogenesis, is essential in driving wound healing and tissue repair.24,25 To determine the kinetics of new blood formation in the wound tissue, immunohistochemical analysis of tissue sections was performed on biopsies collected at day 7, 12, and 21 posttreatment. To localize neocapillaries, tissue samples from LR- and ADRC-treated animals were subjected to CD31 immunostaining. At day 7 posttreatment, digital analysis of CD31-positve blood vessels showed that the microvessel density (MVD) was significantly greater in wounds receiving local injection (1.7-fold) or topical spray of ADRCs (1.5-fold) compared to control LR treatment (p < 0.05) (Fig. 6A, B). No significant difference in MVD was observed between wounds treated with locally injected or topically sprayed ADRCs at any time point (Fig. 6C). Starting on day 12 posttreatment, a decrease in MVD was observed in wounds treated with locally injected or topically sprayed ADRCs (Fig. 6).
Figure 6.
Effect of ADRCs on wound vascularization after burn injury. (A–C, left) Wound biopsies collected from animals receiving LR or ADRCs (topical spray or local injection) were stained for CD31. Representative photomicrographs of sections stained with CD31 for each wound pair at day 7 posttreatment are shown. (A–C, right) Microvessel density (MVD) was quantified using automated analysis of digitally scanned slides. Results are presented as mean ± SEM. *p < 0.05; **p < 0.01 versus LR control vehicle.
Discussion
A substantial body of evidence supports the notion that delivery of ADRCs/SVF improves tissue repair in acute and chronic wounds.5,11,12,18,26–28 Nevertheless, a current problem in the development of such therapies is to optimize the delivery approaches so that the cell suspension efficiently reaches the wound beds. Currently, there are two basic delivery methods for acute and chronic wounds: local injections around the perimeter of the wounds and topical application.22 It is important to note that local injections may not be suitable as a large number of invasive injections will be required to treat patients with extensive burns. For such patients, topical spray delivery of ADRCs represents an attractive alternative to local injection as it has the potential to deliver cells to a large surface area much more quickly, easily, and conveniently and is much less invasive. This study compared topical spray and local injection of autologous ADRCs in a porcine model of full-thickness burns to assess the feasibility and efficacy of ADRC treatment through a topical spray delivery route. To our knowledge, this is the first study comparing two different modes of delivery for thermal burn injuries.
The present study demonstrated that while ADRC viability was not affected by the spray device, a trend for reduced cell numbers after spraying was observed. Similar findings were reported using the ReCell® spray kit device for which loss in cell numbers may be attributable to cell adhesion to the surfaces within the spray device.29 Furthermore, our data demonstrated that both local injection and topical spray delivery approaches effectively promote burn wound healing by modulating the inflammatory response and increasing wound angiogenesis and epithelialization. Importantly, local injection was equivalent to topically sprayed cells in terms of efficacy, suggesting that delivery through a spraying mechanism is a viable and favorable alternative to other, more cumbersome delivery routes.
Wound repair is a complex and dynamic process, which involves inflammation, angiogenesis, epithelialization, and tissue formation and remodeling.25,30 Using a matched pair analysis model, planimetry data showed that the percent of wound epithelialization and absolute epithelialized area were greater in wounds receiving topical spray and local injection of ADRCs compared to respective control.
Although the inflammatory process is essential for wound healing, excessive inflammation can hamper the repair process.25 In cutaneous wounds, neutrophils are the first immune cells to be recruited into the wound site where, among other activities, they protect against microbial infection. However, activated neutrophils release proteases and reactive oxygen species that may lead to significant tissue damage if they are not cleared from the tissue or persist in an activated state.31 Importantly, human burn wounds exhibit acute local inflammation characterized by the presence of inflammatory mediators (such as complement, CRP, neutrophils) up to 46 days postinjury.32 In our study, histopathologic and immunohistochemistry analyses revealed that local and topical spray delivery of ADRCs reduced neutrophil infiltration within the burn wounds at 7 days posttreatment. These data are consistent with other studies that suggest a role for ADRCs in reducing local inflammation.12,13 Blood vessel formation (angiogenesis) plays an important role during the wound healing process. In this study, both local injection and spray delivery of ADRCs equally increased microvessel density within the wound tissue after burn injury. Similarly, numerous preclinical and case study reports from our group and others have reported the angiogenic effect of ADRCs in several acute and chronic injury models.11,26,27,33 Taken together, these data suggest that ADRCs promote tissue repair through their effect on modulating the inflammatory response and promoting angiogenesis and epithelialization.
The current standard of care of full-thickness burns requires early wound excision and application of autologous split-thickness skin grafts (STSG).2,34 For large injuries, STSG must be often widely meshed due to limited donor sites and are often associated with scarring and functional cosmetic complications. Autologous ADRC therapy is an attractive option to optimize skin graft healing and functional outcome. The results presented herein clearly suggest that local injection or topical spray delivery of ADRCs may improve skin graft reepithelialization and healing outcome and support the use of ADRCs as an adjunct therapy to skin grafting in severe burn patients. Importantly, although cell spray delivery appears to be safe, standard, and blood-borne, pathogen precautions should be considered to avoid potential risks of exposure to the patient and surgical staff.
In summary, the present study shows that both local injection and topical spray approaches of ADRC delivery after thermal burns are feasible and effective. Both techniques resulted in similar effects on wound inflammation, angiogenesis, and epithelialization. Considering the greater ease-of-use and noninvasive character of topical spray delivery compared to multiple local invasive injections, these data support the use of a spray system for autologous ADRC delivery.
Innovation
ADRC therapy represents a novel strategy to promote wound healing in burn patients. Nevertheless, issues regarding the optimal mode of administration remain unclear. In this study, we compared two different modes of delivery for thermal burn injuries. Our data demonstrated that local injection and topical spray delivery of ADRCs exhibited similar effects on wound inflammation, angiogenesis, and epithelialization. Considering its ease-of use, topical spray delivery represents a safe and effective solution to administrate ADRCs and potentially optimize standard of care in fragile burn patients.
Key Findings.
• ADRC viability was not affected by the spray procedure.
• Local injections and topical spray delivery of ADRCs similarly increased wound epithelialization and angiogenesis after burn injury.
• Local injections and topical spray delivery of ADRCs similarly decreased neutrophil infiltration within the burn wound site.
Abbreviations and Acronyms
- ADRCs
adipose-derived regenerative cells
- AP
alkaline phosphatase
- H&E
hematoxylin and eosin
- LR
lactated Ringer's
- MPO
myeloperoxidase
- MVD
microvessel density
- SEM
standard error of mean
- STSG
split-thickness skin grafts
- SVF
stromal vascular fraction
Acknowledgments and Funding Sources
This work was supported by contract HHSO100201200008C from the Biomedical Advanced Research and Development Authority (BARDA), Department of Health and Human Services. S.T. was supported by a grant from the California Institute for Regenerative Medicine (CIRM; TB1-01175). The authors would also like to thank Dr. Kathy Mekjian for her valuable comments and suggestions to improve the article.
Author Disclosure and Ghostwriting
P.F., A.D.G., and J.K.F. are paid employees and stock holders of Cytori Therapeutics, Inc. M.T. receives consulting fees from Cytori Therapeutics, Inc. S.T., F.B., M.D.E., and K.Y. have no conflicts of interest with regard to this study.
All authors listed have contributed sufficiently to the project to be included as authors, and all those who are qualified to be authors are listed in the author byline. P.F. designed and coordinated the study, analyzed results, and wrote the article. A.D.G. carried out cell isolation, histological stains, and immunohistochemistry. S.T. performed in vitro analysis of topical spray delivery. M.D.E. directed the study at the testing facility and collected raw data. F.B. performed burn injury, lipectomy, wound excision, and biopsy collection. K.Y. performed histopathology scoring of digital slides. M.T. participated in study design and helped to draft and revised the article. J.K.F. conceived the study, participated in study design and coordination, and revised the draft of the article.
All authors read and approved the final article. No ghostwriters were used to write this article.
About the Authors
Philippe Foubert, PhD, is currently a scientist at Cytori Therapeutics. He is leading the preclinical activities related to wound healing and tissue repair. Dr Foubert has 10 years of biomedical research experience, including adult stem cell biology, vascular research, and tissue repair. Andreina D. Gonzalez is a research associate at Cytori. Stephan Teodosescu is a bioengineer intern at Cytori. Felipe Berard, DVM, is a Senior Clinical Veterinarian at Lovelace Respiratory Research Institute (LRRI). Melanie Doyle-Eisele, PhD, is an Associate Director at LRRI. Krishna Yekkala, BVSc, PhD, DACVP, is a veterinary pathologist at Toxikon, Inc. Mayer Tenenhaus, MD, FACS, is a plastic burn surgeon at University of California San Diego. John K. Fraser, PhD, is Chief Scientist at Cytori Therapeutics Inc. Dr. Fraser has more than 30 years of a broad experience and biomedical research experience working with adult stem cells in both academia and industry.
References
- 1.National Burn Repository. American Burn Association. http://www.ameriburn.org/2015NBRAnnualReport.pdf (last accessed July31, 2015)
- 2.Herndon D. Total Burn Care, 4th ed. Edinburgh: Saunders, 2012 [Google Scholar]
- 3.Kirby GT, Mills SJ, Cowin AJ, Smith LE. Stem cells for cutaneous wound healing. Biomed Res Int 2015;2015:285869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardien KL, Middelkoop E, Ulrich MM. Progress towards cell-based burn wound treatments. Regen Med 2014;9:201–218 [DOI] [PubMed] [Google Scholar]
- 5.Kokai LE, Marra K, Rubin JP. Adipose stem cells: biology and clinical applications for tissue repair and regeneration. Transl Res 2014;163:399–408 [DOI] [PubMed] [Google Scholar]
- 6.Gimble JM, Bunnell BA, Chiu ES, Guilak F. Concise review: adipose-derived stromal vascular fraction cells and stem cells: let's not get lost in translation. Stem Cells 2011;29:749–754 [DOI] [PubMed] [Google Scholar]
- 7.Gentile P, Orlandi A, Scioli MG, Di PC, Bocchini I, Cervelli V. Concise review: adipose-derived stromal vascular fraction cells and platelet-rich plasma: basic and clinical implications for tissue engineering therapies in regenerative surgery. Stem Cells Transl Med 2012;1:230–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimmerlin L, Donnenberg VS, Pfeifer ME, Meyer EM, Peault B, Rubin JP, et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry A 2010;77:22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013;15:641–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser JK, Hicok KC, Shanahan R, Zhu M, Miller S, Arm DM. The Celution system: automated processing of adipose-derived regenerative cells in a functionally closed system. Adv Wound Care (New Rochelle) 2014;3:38–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foubert P, Barillas S, Gonzalez AD, Alfonso Z, Zhao S, Hakim I, et al. Uncultured adipose-derived regenerative cells (ADRCs) seeded in collagen scaffold improves dermal regeneration, enhancing early vascularization and structural organization following thermal burns. Burns 2015. [Epub ahead of print]; DOI: 10.1016/j.burns.2015.05.004 [DOI] [PubMed] [Google Scholar]
- 12.Hao C, Shintani S, Shimizu Y, Kondo K, Ishii M, Wu H, et al. Therapeutic angiogenesis by autologous adipose-derived regenerative cells: comparison with bone marrow mononuclear cells. Am J Physiol Heart Circ Physiol 2014;307:H869–H879 [DOI] [PubMed] [Google Scholar]
- 13.Feng Z, Ting J, Alfonso Z, Strem BM, Fraser JK, Rutenberg J, et al. Fresh and cryopreserved, uncultured adipose tissue-derived stem and regenerative cells ameliorate ischemia-reperfusion-induced acute kidney injury. Nephrol Dial Transplant 2010;25:3874–3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinical Trial NCT02326961. Celution Prepared Adipose Derived Regenerative Cells in the Treatment of Osteoarthritis of the Knee (ACT-OA Knee). 2015
- 15.Magalon G, Daumas A, Sautereau N, Magalon J, Sabatier F, Granel B. Regenerative approach to scleroderma with fat grafting. Clin Plast Surg 2015;42:353–364 [DOI] [PubMed] [Google Scholar]
- 16.Clinical Trial NCT01813279. Assessment of the Subcutaneous Reinjection of Human Autologous Adipose-derived Stromal Vascular Fraction (Celution® System) in the Hands of Patients Suffering From Systemic Sclerosis. 2014
- 17.Clinical Trial NCT02052427. Safety & Efficacy of Adipose-Derived Regenerative Cells in the Treatment of Chronic Myocardial Ischemia (ATHENA II). 2014
- 18.Akita S, Yoshimoto H, Ohtsuru A, Hirano A, Yamashita S. Autologous adipose-derived regenerative cells are effective for chronic intractable radiation injuries. Radiat Prot Dosimetry 2012;151:656–660 [DOI] [PubMed] [Google Scholar]
- 19.Marino G, Moraci M, Armenia E, Orabona C, Sergio R, De SG, et al. Therapy with autologous adipose-derived regenerative cells for the care of chronic ulcer of lower limbs in patients with peripheral arterial disease. J Surg Res 2013;185:36–44 [DOI] [PubMed] [Google Scholar]
- 20.Cervelli V, De AB, Lucarini L, Spallone D, Balzani A, Palla L, et al. Tissue regeneration in loss of substance on the lower limbs through use of platelet-rich plasma, stem cells from adipose tissue, and hyaluronic acid. Adv Skin Wound Care 2010;23:262–272 [DOI] [PubMed] [Google Scholar]
- 21.Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng 2007;13:1299–1312 [DOI] [PubMed] [Google Scholar]
- 22.Sorrell JM, Caplan AI. Topical delivery of mesenchymal stem cells and their function in wounds. Stem Cell Res Ther 2010;1:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srijaya TC, Ramasamy TS, Kasim NH. Advancing stem cell therapy from bench to bedside: lessons from drug therapies. J Transl Med 2014;12:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mann PC, Vahle J, Keenan CM, Baker JF, Bradley AE, Goodman DG, et al. International harmonization of toxicologic pathology nomenclature: an overview and review of basic principles. Toxicol Pathol 2012;40(4 Suppl):7S-13S [DOI] [PubMed] [Google Scholar]
- 25.Johnson KE, Wilgus TA. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care (New Rochelle) 2014;3:647–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453:314–321 [DOI] [PubMed] [Google Scholar]
- 27.Kondo K, Shintani S, Shibata R, Murakami H, Murakami R, Imaizumi M, et al. Implantation of adipose-derived regenerative cells enhances ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol 2009;29:61–66 [DOI] [PubMed] [Google Scholar]
- 28.Murohara T, Shintani S, Kondo K. Autologous adipose-derived regenerative cells for therapeutic angiogenesis. Curr Pharm Des 2009;15:2784–2790 [DOI] [PubMed] [Google Scholar]
- 29.Atalay S, Coruh A, Deniz K. Stromal vascular fraction improves deep partial thickness burn wound healing. Burns 2014;40:1375–1383 [DOI] [PubMed] [Google Scholar]
- 30.Wood FM, Giles N, Stevenson A, Rea S, Fear M. Characterisation of the cell suspension harvested from the dermal epidermal junction using a ReCell(R) kit. Burns 2012;38:44–51 [DOI] [PubMed] [Google Scholar]
- 31.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738–746 [DOI] [PubMed] [Google Scholar]
- 32.Wilgus TA, Roy S, McDaniel JC. Neutrophils and wound repair: positive actions and negative reactions. Adv Wound Care (New Rochelle) 2013;2:379–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van de Goot F, Krijnen PA, Begieneman MP, Ulrich MM, Middelkoop E, Niessen HW. Acute inflammation is persistent locally in burn wounds: a pivotal role for complement and C-reactive protein. J Burn Care Res 2009;30:274–280 [DOI] [PubMed] [Google Scholar]
- 34.Ebrahimian TG, Pouzoulet F, Squiban C, Buard V, Andre M, Cousin B, et al. Cell therapy based on adipose tissue-derived stromal cells promotes physiological and pathological wound healing. Arterioscler Thromb Vasc Biol 2009;29:503–510 [DOI] [PubMed] [Google Scholar]
- 35.Orgill DP. Excision and skin grafting of thermal burns. N Engl J Med 2009;360:893–901 [DOI] [PubMed] [Google Scholar]