Abstract
Adolescent idiopathic scoliosis (AIS) is a common disease. It is a multi-factorial (polygenic) disease controlled by genetic and environmental factors. Studies searching for genetic factors of AIS using linkage and association analyses have been conducted and several susceptibility genes have been reported. This paper reviews the recent progress in the genome-wide association study of AIS in Japan and comments on its future task.
Keywords: Genetics, Adolescent idiopathic scoliosis, GWAS, Japan, LBX1, GPR126, BNC2
Background
Scoliosis is a common spinal deformity. It is a lateral bending of the spine diagnosed by using the antero-posterior plane radiograph of the whole spine in the standing position; however the deformity (spinal curve) is actually 3-dimentional. The degree of spinal curve is evaluated by the Cobb angle. The Cobb angle of more than 10–15° is considered pathological (disease) since some degree of scoliosis is commonly seen in the general population.
Scoliosis is a mysterious disease. It can be congenital. A variety of spinal deformities including hemi-vertebra and block vertebra results in scoliosis. Scoliosis can be neuromuscular. A lot of conditions could lead to scoliosis, including cerebral palsy, spina bifida, polio, and muscular dystrophy. Scoliosis can also be syndrome-related. Connective tissue diseases like Marfan syndrome and Ehlers-Danlos syndrome, skeletal dysplasias, and neurofibromatosis present with scoliosis. However, the most common type of scoliosis is idiopathic. Specific cause is unknown in over 80 % of all scoliosis cases [1].
Idiopathic scoliosis is classified into three types, i.e., infantile, juvenile and adolescent scoliosis. Adolescent idiopathic scoliosis (AIS) is the most common and the most mysterious form of scoliosis. If the Cobb angle of ≧10° is a criterion, ~1 % of children are suffering from it in Japan [1]. Previously normal children suddenly come to suffer from progressive bending of the spine during their adolescence. There is also a strong female predominance.
GWAS
To solve the mystery of scoliosis, of AIS in particular, a group of expert scoliosis surgeons in Japan stood up and made a consortium, the Japan Scoliosis Clinical Research Group (JSCRG). This consortium was launched in January 31, 2009, supported by a research grant from Japanese Orthopedic Society. The leader is Prof. Morio Matsumoto of Keio University. Nine representative hospitals in Japan that are famous for their scoliosis clinics joined it and collected DNAs of scoliosis patients, together with detailed clinical information necessary for genetic research. The consortium collected far more than 3,000 AIS subjects (4,109, as of January 12, 2016). With a help of this huge resource, we started the genetic study of AIS using a genome-wide association study (GWAS) method.
We conducted the GWAS using Illumina platforms for the Japanese population consisting of 1,050 AIS patients and 1,474 controls [2]. Inclusion criteria for case subjects were patients who saw expert scoliosis surgeons and: 1) the age at diagnosis was between 10–18 years, 2) had a Cobb angle of ≧ 15°, and 3) female. The control subjects were Japanese females. More than half million SNPs were successfully genotyped. After quality controls, data of 455,121 SNPs for 1,033 cases and 1,473 controls were analyzed for their association with AIS.
1st locus
Three SNP on 10q24 showed genome-wide significance level in the GWAS [2]. The association of SNPs was replicated in independent Japanese female (326 cases and 9,823 controls) and male (94 cases and 1,849 controls) populations. The most significantly associated SNP was rs11190970. The P-value was 1.24 x 10-19 with odds ratio (OR) of 1.56 (95 % confidence interval (CI) = 1.41–1.71).
The association of rs11190970 was replicated in a Hong Kong Chinese population consisting of 300 cases and 788 controls (P = 9.10 x 10-10; OR (95 % CI) = 1.85 (1.52-2.25) [3]. The association was further replicated in Han Chinese in Nanjing [4] and Guangzhou [5]. We performed an international meta-analysis using Japanese, Han Chinese and Caucasian populations [6]. The meta-analysis using a total of 5,159 cases and 17,840 controls showed that rs11190870 is a global AIS susceptibility SNP (P = 1.22 x 10-45; OR = 1.60).
rs11190870 is in a 80 kb-LD block, which contains 2 genes (LBX1, FLJ41350). The two genes exist head to head on chromosome 10q, only 633 bp apart (database). rs11190870 is located 7.5 kb 3’ of LBX1 (lady bird late, Drosophila homolog of, 1/ lady bird-like homeobox). LBX1 is a homeobox gene, consisting of 2 exons and encodes a 280 amino-acid protein. It is specifically expressed during embryogenesis in mouse, with restricted expression to the developing central nervous system and muscles [7]. Lbx1 knock-out (KO) mice had extensive muscle loss at birth [8, 9]. The KO mice also had defects in heart looping and myocardinal hyperplasia [10]. Lbx1 is suggested to control the expression of genes that guide migrating muscle precursors and maintain their migratory potential. However, scoliosis was not found in the KO mice. On the other hand, neurons of the KO mice that arise in the dorsal spinal cord are eliminated by apoptosis [11]. Thus, LBX1 could be implicated in myogenic and neurogenic etiology of AIS.
FLJ41350 (alias: LOC399806 and LBX1-AS1 (for LBX1 antisense RNA 1)) is a hypothetical (predicted) gene. We cloned FLJ41350 and determined its genomic structure. FLJ41350 is predicted to encode a 120 amino-acid protein; its nucleotide and amino-acid sequences are human specific. Its predicted protein has no known homology to other human proteins.
To gain an insight into the role of LBX1 and FLJ41350 in the etiology of AIS, we examined their function in vivo using zebrafish. Human LBX1 has three zebrafish homologues, namely, lbx1a, lbx1b and lbx2 (Table 1). Over-expression of the three lbx genes caused scoliosis, while FLJ41350 did not (Guo et al. manuscript under review). Knockdown of the three lbx genes by morpholino (MO) also caused early onset scoliosis in zebrafish. Further studies are necessary to clarify the role of LBX1 and FLJ41350 in AIS etiology and pathogenesis.
Table 1.
Amino-acid homology of human and zebrafish LBX genes
| Gene | Amino acids | Homology (%)a to | |
|---|---|---|---|
| LBX1 | LBX2 | ||
| zebrafish | |||
| lbx1a | 269 | 72.2 | 39.4 |
| lbx1b | 265 | 59.9 | 38.9 |
| lbx2 | 257 | 61.5 | 40.5 |
| human | |||
| LBX1 | 281 | - | 41.3 |
| LBX2 | 198 | 41.3 | - |
acalculated by Needleman-Wunsch Global Sequence Alignment Tool (NCBI)
2nd locus
To identify additional loci for AIS, we examined the association of top 30 SNPs in the GWAS. P-values of the SNPs were below 5.0 x 10-5. The replication study using 681 cases and 9,823 controls showed a significantly associated SNP, rs6570507 on chromosome 6q24.1 [12]. The combined P-value of the GWAS and the replication study was 6.96 × 10-10 (OR = 1.28). The association was replicated in Chinese and USA-Caucasian (non-Hispanic white) populations. The P-value in the meta-analysis was 1.27x 10-14 (OR = 1.27) [12].
The most significantly associated SNPs were in intron 2 of GPR126 (G protein-coupled receptor 126). GPR126 consists of 26 exons and encodes a 1,250-aa orphan receptor. In humans, GPR126 is highly expressed in cartilage. In mouse embryo, Gpr126 is highly expressed in the proliferating cartilage of the spine, suggesting its role in spinal development [12]. Gpr126 mRNA expression in early chondrogenic differentiation of ATDC5, a mouse model of chondrogenesis, showed that Gpr126 increases with cartilage differentiation [13]. Gpr126 over-expression increases expression of cartilage maker genes, Col2a1 (encoding type II collagen) and Acan (encoding aggrecan), while Gpr126 knock-down decreases their expression (Ikegawa et al. unpublished data). These data indicate that Gpr126 is a positive regulator of cartilage differentiation (Fig. 1).
Fig. 1.
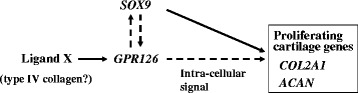
GPR126-related molecular network in cartilage. Solid and broken arrows indicate direct and indirect action
The association of GPR126 SNPs with AIS is replicated in another Chinese population [14]. rs6570507 also showed association with trunk length in a meta-analysis of GWAS in European populations [15]. Conditional loss of Gpr126 in chondrocyte lineages in mouse resulted in scoliosis and pectus excavatum [16]. Thus the scoliosis is post-natal onset and lacks malformations of vertebral units. The KO mouse would be a good model for AIS.
3rd locus
The associations of the two loci were highly significant and were replicated in multiple association studies using different ethnic populations [2–6, 12]. The candidate genes in the loci are highly likely from their functional analysis [2, 12]. However, they only explain ~1 % of the total genetic variance in AIS; heritabilities (calculated as the square of the correlation coefficient, r2) of LBX1 and GPR126 were 1.0 % and 0.2 %, respectively.
To identify additional susceptibility gene(s) for AIS, we extended our GWAS by increasing the numbers of the subjects to more than 5 folds and conducting a whole genome imputation and a meta-analysis [17]. In total, we analyzed the association of 4,420,789 SNPs for 2,109 Japanese subjects with AIS and 11,140 control subjects. Three loci surpassed the genome-wide significance level of P < 5 × 10−8. Top two were the previously identified loci on 6q and 10q.
To confirm the association of the novel locus, we conducted a replication study with an independent Japanese cohort including 955 cases and 3,551 controls for 36 loci, yielding suggestive evidence of association (P < 1 × 10−5). In the replication study, we found evidence for association with one locus represented by a SNP, rs3904778 on chromosome 9p22.2. Combining the results of the GWAS and replication study, the P value of rs3904778 was 3.50 × 10−11 (OR = 1.21). We checked the association of rs3904778 in a Han Chinese population (1,268 cases and 1,173 controls). P value of the meta-analysis was 1.70 × 10−13 (OR = 1.21).
The most significantly associated SNPs were in intron 3 of BNC2 that encodes a zinc finger transcription factor, basonuclin 2. eQTL data on public databases suggested that the associated SNPs have the potential to regulate BNC2 transcription and the susceptibility alleles of the SNPs increase BNC2 expression. By a series of electrophoretic mobility shift assay and reporter assay, we identified a functional SNP in BCN2 (rs10738445), whose susceptibility allele had higher binding capacity to a transcription factor, YY1 (Yin yang 1) and higher BNC2 enhancer activity than the non-susceptibility allele in the co-transfection experiment of the BNC2 enhancer and YY1. Bnc2 over-expression in zebrafish embryo produced axial body curvature associated with abnormal somite formation. The in vitro and in vivo evidence strongly suggest that increased BNC2 expression predisposes to AIS.
Association with severity
The factors that influence the progression of scoliosis are clinically very important, as the AIS treatment depends on severity and progression. These factors are also reported to have genetic components [18, 19]: the meta-analysis of a Danish twin study reported that a significant correlation with curve severity was found in monozygous twins, but not in dizygous twins [20]. SNPs in ESR1, ESR2, MATN1, and IGF1 genes are reported to be associated with AIS severity [21–24].
Therefore, we performed a GWAS by using only severely affected AIS subjects (Cobb’s angle above 40°). Through a two-stage association study using a total of ~12,000 Japanese subjects, we identified six SNPs of genome-wide significance level association. Five of them were in the known loci of AIS susceptibility that we previously reported: three were close to LBX1 on chromosome 10q24.31 [2] and two on chromosome 6q24.1 in GPR126 [12]. We examined the correlation between risk allele frequency of SNPs on the LBX1 and GPR126 loci, but found that both loci had no correlation with AIS severity (Table 2). Therefore, we considered that these SNPs determine initiation (susceptibility), but not progression/severity.
Table 2.
Severity of AIS and risk allele frequency of previously identified associated SNPs
| Group | rs11190870 (LBX1) | rs6570507 (GPR126) | |||
|---|---|---|---|---|---|
| No. subject | RAF (%) | No. subject | RAF (%) | ||
| Patient | Cobb angle | ||||
| >50 | 407 | 67.1 | 491 | 50.5 | |
| 40 – 50 | 278 | 66.7 | 352 | 49.1 | |
| 30 – 40 | 270 | 66.9 | 440 | 46.6 | |
| 20 – 30 | 363 | 67.1 | 458 | 49.1 | |
| 15 – 20 | 41 | 67.1 | 7 | 45.5 | |
| General control | 11,294 | 56.5 | 25,939 | 42.9 | |
RAF, risk allele frequency
The remaining one, rs12946942 is on chromosome 17q24.3, and showed a significant association in the recessive model (P = 4.00 × 10-8, OR = 2.05). The association of the SNP was replicated in a Han Chinese population (meta-analysis combined P = 6.43 × 10-12, OR = 2.21). rs12946942 and the LD block containing it are in the gene desert region. The closest genes are SOX9 (MIM 608160) and KCNJ2 (MIM 600681). Both of them are ~1 Mb away from the SNP, but are excellent candidate genes.
SOX9 is the master transcription factor of cartilage [25]. SOX9 mutations cause campomelic dysplasia (MIM 114290), a skeletal dysplasia characterized by bowing of the long bones, small scapula, tracheobronchial narrowing, sex reversal, and kyphoscoliosis [26]. Very long-range cis-regulatory elements controlling tissue-specific SOX9 expression have been reported [27, 28]. The LD block containing rs12946942 has recently been defined as a susceptibility locus of prostate cancer [29]. The block contains six enhancer elements, of which the E1 enhancer forms a long-range chromatin loop to SOX9 in a prostate cancer cell line. Two SNPs within the E1 enhancer were shown to direct allele-specific gene expression. rs12946942 may likewise participate in scoliosis pathogenesis by controlling scoliosis-related tissue-specific SOX9 expression.
KCNJ2 encodes a potassium channel, a component of the inward rectifier current IK1 [30]. KCNJ2 mutations cause a cardiodysrhythmic type of periodic paralysis known as Andersen-Tawil syndrome (ATS; MIM 170390) [31]. The syndrome is characterized by ventricular arrhythmias, periodic paralysis, facial and skeletal dysmorphism including hypertelorism, small mandible, cleft palate, syndactyly, clinodactyly, and scoliosis [30, 31]. Furthermore, the 17q24.2-q24.3 micro-deletion syndrome whose deletion includes KCNJ2 and rs12946942 exhibited skeletal malformations similar to ATS, including progressive scoliosis [32]. However, a similar micro-deletion that includes KCNJ2, but not rs12946942, has no scoliosis phenotype [33]. Further studies are necessary to identify the causal gene in the locus.
Conclusions
International collaboration
I believe we need to identify more genes to clarify the whole picture of AIS. The total genetic variance in AIS explained by the three genes is still < 2 %. By increasing the number of samples, we can expect to increase the power of the association study. As in the other fields of GWASs, international collaboration for AIS genetic study is now in progress. An international consortium, ICSG (International Consortium for Scoliosis Genetics) has been established since 2012. Prof. Carol Wise (Texas Scottish Rite Hospital for Children) organized the consortium. Large-scale trans-ethnic association studies including GWASs will facilitate the identification of AIS susceptibility genes. We can see the good example of such collaboration in the GWAS of rheumatoid arthritis [34].
Association to function
Association does not mean causality. GWAS just shows a marker on genome, not a disease-causing sequence variation (causal variant). After all, a result of an association study is just a statistic. We must find a causal variant. We must clarify pathogenesis of AIS through functional studies. We must convert statistics to biology, and biology to medicine. Otherwise, we cannot reach to our ultimate goal, the treatment of AIS. Therefore, just like scoliosis, a long and winding road toward the truth of AIS is still before us.
Acknowledgements
The author thanks Ms. Naoko Atsumi for preparing the manuscript and Prof. Tohru Maruyama and Theodoros B. Grivas for giving the opportunity to the manuscript.
Footnotes
Competing interest
The author declares that he has no competing interests.
References
- 1.Ueno M, Takaso M, Nakazawa T, Imura T, Saito W, Shintani R, et al. A 5-year epidemiological study on the prevalence rate of idiopathic scoliosis in Tokyo: school screening of more than 250,000 children. J Orthop Sci. 2011;16(1):1–6. doi: 10.1007/s00776-010-0009-z. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi Y, Kou I, Takahashi A, Johnson TA, Kono K, Kawakami N, et al. A genome-wide association study identifies common variants near LBX1 associated with adolescent idiopathic scoliosis. Nat Genet. 2011;43(12):1237–40. doi: 10.1038/ng.974. [DOI] [PubMed] [Google Scholar]
- 3.Fan YH, Song YQ, Chan D, Takahashi Y, Ikegawa S, Matsumoto M, et al. SNP rs11190870 near LBX1 is associated with adolescent idiopathic scoliosis in southern Chinese. J Hum Genet. 2012;57(4):244–6. doi: 10.1038/jhg.2012.11. [DOI] [PubMed] [Google Scholar]
- 4.Jiang H1, Qiu X, Dai J, Yan H, Zhu Z, Qian B, et al. Association of rs11190870 near LBX1 with adolescent idiopathic scoliosis susceptibility in a Han Chinese population. Eur Spine J. 2013;22(2):282–6. doi: 10.1007/s00586-012-2532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao W, Peng Y, Liang G, Liang A, Ye W, Zhang L, et al. Association between common variants near LBX1 and adolescent idiopathic scoliosis replicated in the Chinese Han population. PLoS One. 2013;8(1) doi: 10.1371/journal.pone.0053234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Londono D, Kou I, Johnson TA, Sharma S, Ogura Y, Tsunoda T, et al. A meta-analysis identifies adolescent idiopathic scoliosis association with LBX1 locus in multiple ethnic groups. J Med Genet. 2014;51(6):401–6. doi: 10.1136/jmedgenet-2013-102067. [DOI] [PubMed] [Google Scholar]
- 7.Jagla K, Dollé P, Mattei MG, Jagla T, Schuhbaur B, Dretzen G, et al. Mouse Lbx1 and human LBX1 define a novel mammalian homeobox gene family related to the Drosophila lady bird genes. Mech Dev. 1995;53(3):345–56. doi: 10.1016/0925-4773(95)00450-5. [DOI] [PubMed] [Google Scholar]
- 8.Brohmann H, Jagla K, Birchmeier C. The role of Lbx1 in migration of muscle precursor cells. Development. 2000;127(2):437–45. doi: 10.1242/dev.127.2.437. [DOI] [PubMed] [Google Scholar]
- 9.Gross MK1, Moran-Rivard L, Velasquez T, Nakatsu MN, Jagla K, Goulding M. Lbx1 is required for muscle precursor migration along a lateral pathway into the limb. Development. 2000;127(2):413–24. doi: 10.1242/dev.127.2.413. [DOI] [PubMed] [Google Scholar]
- 10.Schäfer K, Neuhaus P, Kruse J, Braun T. The homeobox gene Lbx1 specifies a subpopulation of cardiac neural crest necessary for normal heart development. Circ Res. 2003;92(1):73–80. doi: 10.1161/01.RES.0000050587.76563.A5. [DOI] [PubMed] [Google Scholar]
- 11.Gross MK, Dottori M, Goulding M. Lbx1 specifies somatosensory association interneurons in the dorsal spinal cord. Neuron. 2012;34(4):535–49. doi: 10.1016/S0896-6273(02)00690-6. [DOI] [PubMed] [Google Scholar]
- 12.Kou I, Takahashi Y, Johnson TA, Takahashi A, Guo L, Dai J, et al. Genetic variants in GPR126 are associated with adolescent idiopathic scoliosis. Nat Genet. 2013;45(6):676–9. doi: 10.1038/ng.2639. [DOI] [PubMed] [Google Scholar]
- 13.Shukunami C, Shigeno C, Atsumi T, Ishizeki K, Suzuki F, Hiraki Y. Chondrogenic differentiation of clonal mouse embryonic cell line ATDC5 in vitro: differentiation-dependent gene expression of parathyroid hormone (PTH)/PTH-related peptide receptor. J Cell Biol. 1996;133(2):457–68. doi: 10.1083/jcb.133.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu JF, Yang GH, Pan XH, Zhang SJ, Zhao C, Qiu BS, et al. Association of GPR126 gene polymorphism with adolescent idiopathic scoliosis in Chinese populations. Genomics. 2015;105(2):101–7. doi: 10.1016/j.ygeno.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Soranzo N, Rivadeneira F, Chinappen-Horsley U, Malkina I, Richards JB, Hammond N, et al. Meta-Analysis of Genome-Wide Scans for Human Adult Stature Identifies Novel Loci and Associations with Measures of Skeletal Frame Size. PLoS Genet. 2009;5(4):e1000445. doi: 10.1371/journal.pgen.1000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karner CM, Long F, Solnica-Krezel L, Monk KR, Gray RS. Gpr126/Adgrg6 deletion in cartilage models idiopathic scoliosis and pectus excavatum in mice. Hum Mol Genet. 2015;24(15):4365–73. doi: 10.1093/hmg/ddv170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogura Y, Kou I, Miura S, Takahashi A, Xu L, Takeda K, et al. A Functional SNP in BNC2 is associated with adolescent idiopathic scoliosis. Am J Hum Genet. 2015;97(2):337–42. doi: 10.1016/j.ajhg.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinstein SL, Dolan LA, Cheng JC, Danielsson A, Morcuende JA. Adolescent idiopathic scoliosis. Lancet. 2008;371(9623):1527–37. doi: 10.1016/S0140-6736(08)60658-3. [DOI] [PubMed] [Google Scholar]
- 19.Lonstein JE, Carlson JM. The prediction of curve progression in untreated adolescent idiopathic scoliosis during growth. J Bone Joint Surg Am. 1984;66(7):1061–71. [PubMed] [Google Scholar]
- 20.Kesling KL, Reinker KA. Scoliosis in twins. A meta-analysis of the literature and report of six cases. Spine (Phila Pa 1976) 1997;22(17):2009–14. doi: 10.1097/00007632-199709010-00014. [DOI] [PubMed] [Google Scholar]
- 21.Wu J, Qiu Y, Zhang L, Sun Q, Qiu X, He Y. Association of estrogen receptor gene polymorphisms with susceptibility to adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2006;31(10):1131–6. doi: 10.1097/01.brs.0000216603.91330.6f. [DOI] [PubMed] [Google Scholar]
- 22.Zhang HQ, Lu SJ, Tang MX, Chen LQ, Liu SH, Guo CF, et al. Association of estrogen receptor beta gene polymorphisms with susceptibility to adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2009;34(8):760–4. doi: 10.1097/BRS.0b013e31818ad5ac. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z, Tang NL, Cao X, Qiao D, Yi L, Cheng JC, et al. Promoter polymorphism of matrilin-1 gene predisposes to adolescent idiopathic scoliosis in a Chinese population. Eur J Hum Genet. 2009;17(4):525–32. doi: 10.1038/ejhg.2008.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeung HY, Tang NL, Lee KM, Ng BK, Hung VW, Kwok R, et al. Genetic association study of insulin-like growth factor-I (IGF- I) gene with curve severity and osteopenia in adolescent idiopathic scoliosis. Stud Health Technol Inform. 2006;123:18–24. [PubMed] [Google Scholar]
- 25.Dy P, Wang W, Bhattaram P, Wang Q, Wang L, Ballock RT, et al. Sox9 directs hypertrophic maturation and blocks osteoblast differentiation of growth plate chondrocytes. Dev Cell. 2012;22(3):597–609. doi: 10.1016/j.devcel.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lekovic GP, Rekate HL, Dickman CA, Pearson M. Congenital cervical instability in a patient with camptomelic dysplasia. Childs Nerv Syst. 2006;22(9):1212–4. doi: 10.1007/s00381-006-0071-1. [DOI] [PubMed] [Google Scholar]
- 27.Wunderle VM, Critcher R, Hastie N, Goodfellow PN, Schedl A. Deletion of long-range regulatory elements upstream of SOX9 causes campomelic dysplasia. Proc Natl Acad Sci U S A. 1998;95(18):10649–54. doi: 10.1073/pnas.95.18.10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon CT, Tan TY, Benko S, Fitzpatrick D, Lyonnet S, Farlie PG. Long-range regulation at the SOX9 locus in development and disease. J Med Genet. 2009;46(10):649–56. doi: 10.1136/jmg.2009.068361. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Cowper-Sal Lari R, Bailey SD, Moore JH, Lupien M. Integrative functional genomics identifies an enhancer looping to the SOX9 gene disrupted by the 17q24.3 prostate cancer risk locus. Genome Res. 2012;22(8):1437–46. doi: 10.1101/gr.135665.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tristani-Firouzi M, Etheridge SP. Kir 2.1 channelopathies: the Andersen-Tawil syndrome. Pflugers Arch. 2010;460(2):289–94. doi: 10.1007/s00424-010-0820-6. [DOI] [PubMed] [Google Scholar]
- 31.Plaster NM, Tawil R, Tristani-Firouzi M, Canún S, Bendahhou S, Tsunoda A, et al. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen's syndrome. Cell. 2001;105(4):511–9. doi: 10.1016/S0092-8674(01)00342-7. [DOI] [PubMed] [Google Scholar]
- 32.Lestner JM, Ellis R, Canham N. Delineating the 17q24.2-q24.3 microdeletion syndrome phenotype. Eur J Med Genet. 2012;55(12):700–4. doi: 10.1016/j.ejmg.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Blyth M, Huang S, Maloney V, Crolla JA, Karen Temple I. A 2.3 Mb deletion of 17q24.2-q24.3 associated with 'Carney Complex plus'. Eur J Med Genet. 2008;51(6):672–8. doi: 10.1016/j.ejmg.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Okada Y, Wu D, Trynka G, Raj T, Terao C, Ikari K, et al. Genetics of rheumatoid arthritiscontributes to biology and drug discovery. Nature. 2014;506(7488):376–81. doi: 10.1038/nature12873. [DOI] [PMC free article] [PubMed] [Google Scholar]


