Abstract
Intraflagellar transport (IFT) is essential for assembly and maintenance of cilia and flagella as well as ciliary motility and signaling. IFT is mediated by multisubunit complexes, including IFT-A, IFT-B, and the BBSome, in concert with kinesin and dynein motors. Under high salt conditions, purified IFT-B complex dissociates into a core subcomplex composed of at least nine subunits and at least five peripherally associated proteins. Using the visible immunoprecipitation assay, which we recently developed as a convenient protein-protein interaction assay, we determined the overall architecture of the IFT-B complex, which can be divided into core and peripheral subcomplexes composed of 10 and 6 subunits, respectively. In particular, we identified TTC26/IFT56 and Cluap1/IFT38, neither of which was included with certainty in previous models of the IFT-B complex, as integral components of the core and peripheral subcomplexes, respectively. Consistent with this, a ciliogenesis defect of Cluap1-deficient mouse embryonic fibroblasts was rescued by exogenous expression of wild-type Cluap1 but not by mutant Cluap1 lacking the binding ability to other IFT-B components. The detailed interaction map as well as comparison of subcellular localization of IFT-B components between wild-type and Cluap1-deficient cells provides insights into the functional relevance of the architecture of the IFT-B complex.
Keywords: cell biology, cilia, intracellular trafficking, primary cilium, protein assembly, Cluap1, IFT-B complex, VIP assay
Introduction
Cilia and flagella are microtubule-based appendages on the surfaces of a wide variety of eukaryotic cells. Their assembly and maintenance by intraflagellar transport (IFT)3 were revealed in Chlamydomonas reinhardtii by the pioneering studies of Rosenbaum and colleagues (1). Subsequently, due to the critical roles for cilia and flagella in various physiological and developmental processes, including cell motility, signaling, and sensory reception, these structures have been studied intensively in metazoans (2–4). IFT, which moves various proteins bidirectionally between the base and tip of cilia/flagella along a microtubule-based structure called the axoneme, is mediated by the large IFT particles with the aid of the anterograde molecular motor kinesin and the retrograde motor dynein. Under high salt conditions, the IFT particle purified from Chlamydomonas flagella can be divided into two complexes, IFT-A and IFT-B. These complexes are composed of ∼6 and ∼14 subunits, respectively, and are thought to connect cargo proteins with molecular motors (4, 5). Mutational analyses in Chlamydomonas and other ciliated organisms suggested that the IFT-A and IFT-B complexes are primarily involved in retrograde and anterograde ciliary trafficking, respectively.
Biochemical studies revealed the approximate architecture of the Chlamydomonas IFT-A and IFT-B complexes (6–12), and subsequent studies by Lorentzen and colleagues (13–15) revealed the structural basis of the interactions among several IFT-B subunits. The Chlamydomonas IFT-B complex consists of the core subcomplex, including at least nine subunits (IFT88, -81, -74, -70, -52, -46, -27, -25, and -22) and at least five peripherally associated proteins (IFT172, -80, -57, -54, and -20) (reviewed in Refs. 4 and 5). Although the IFT-B subunits are evolutionarily conserved (2, 16), the architectures of the IFT-B complex in other ciliated organisms, including mammals, remain poorly understood. Furthermore, it is also unclear how the peripherally associated proteins are integrated into the full IFT-B complex.
Recently, we developed a novel technique, the visible immunoprecipitation (VIP) assay, as a method for studying protein-protein interactions and used it to determine the architectures of two multisubunit complexes, the BBSome and exocyst (17), both of which consist of eight subunits and have been implicated in protein trafficking to and/or within cilia. The VIP assay can visually detect binary protein interactions under a conventional fluorescence microscope without the necessity of electrophoresis and immunoblotting. Furthermore, the assay can determine interactions between more than two proteins at a time, including one-to-many and many-to-many protein interactions (17).
In this study, we applied the VIP assay to delineate the architecture of the mammalian IFT-B complex. The results revealed that the complex consists of 16 subunits and can be divided into core and peripheral subcomplexes containing 10 and 6 subunits, respectively. In particular, our data unequivocally showed that TTC26 and Cluap1, both of which have been referred to as IFT-B accessory proteins or candidate IFT-B subunits in previous review articles (2, 4, 18), are integral components of the core and peripheral subcomplexes, respectively. Furthermore, our findings reveal how the six peripheral subunits interact with one another to constitute the peripheral subcomplex.
Materials and Methods
Plasmids
The full coding sequences of IFT-B proteins listed in supplemental Table S1 were cloned into various fluorescent protein vectors, as shown in supplemental Table S2.
Antibodies and Reagents
Preparation of polyclonal rabbit anti-Cluap1 antibody was described previously (19). The following antibodies were obtained from the indicated vendors: monoclonal mouse anti-acetylated α-tubulin (6-11B-1, Sigma-Aldrich) and anti-γ-tubulin (GTU-88, Sigma-Aldrich); monoclonal mouse anti-GFP (JL-8, BD Biosciences); polyclonal rabbit anti-TagRFP (tRFP) antibody (AB233, Evrogen); polyclonal rabbit anti-RFP antibody (PM005, MBL) (referred to as anti-mRFP under “Results” to distinguish it from anti-tRFP);polyclonal rabbit anti-IFT57 (11083-1-AP, Proteintech) and anti-IFT88 (13967-1-AP, Proteintech); monoclonal mouse anti-actin (C4, EMD Millipore); Alexa Fluor 555-conjugated goat anti-mouse IgG (A21424, Invitrogen); Alexa Fluor 488-conjugated goat anti-rabbit IgG (A11034, Invitrogen); Alexa Fluor 555-conjugated goat anti-mouse IgG1 (A21127, Invitrogen); Alexa Fluor 647-conjugated goat anti-mouse IgG2b (A21242, Invitrogen); and horseradish peroxidase-conjugated secondary antibodies (115-035-166 and 111-035-144, Jackson ImmunoResearch Laboratories). Hoechst33342, Mowiol, and Polyethylenimine Max were purchased from Invitrogen, Sigma-Aldrich, and Polysciences, respectively.
Preparation of GST-Anti-GFP and GST-Anti-mCherry Nanobody Beads
Construction of the expression vector for GST-anti-GFP nanobody and preparation of GST-anti-GFP nanobody beads were described previously (17). An expression vector for GST-anti-mCherry nanobody was constructed as follows. A DNA fragment encoding anti-mCherry nanobody (LaM-4), synthesized based on its amino acid sequence (20), was subcloned into pGEX-6P-1 (GE Healthcare). We deposited the plasmid encoding GST-anti-mCherry nanobody in Addgene (ID 70696). Escherichia coli BL21(DE3) cells transformed with the nanobody vector were treated with 0.1 mm isopropyl 1-thio-β-d-galactopyranoside for 4 h at 30 °C to induce protein expression and lysed, and the recombinant protein was purified using glutathione-Sepharose 4B beads (GE Healthcare). Purified GST-anti-GFP or anti-mCherry nanobody was adjusted to a concentration of ∼200 μg/ml for immunoprecipitation assays.
VIP Assays
VIP assays were performed as described previously (17). HEK293T cells (∼1.6 × 106 cells in 6-well plate) cultured in DMEM with high glucose supplemented with 5% fetal bovine serum (FBS) were transfected with EGFP and tRFP/mCherry fusion constructs (2 μg each) using Polyethylenimine Max (20 μg) and then cultured for 24 h. HEK293T cells were kindly provided by Hiroyuki Takatsu (Kyoto University). Before cell lysis, expression of fluorescent fusion proteins in transfected cells was confirmed under a fluorescence microscope. The cells were lysed in 250 μl of lysis buffer (20 mm HEPES-KOH (pH 7.4), 100 mm KCl, 5 mm NaCl, 3 mm MgCl2, 1 mm dithiothreitol, 10% glycerol, and 0.1% Triton X-100) containing EDTA-free protease inhibitor mixture (Nacalai Tesque). After 15 min on ice, the cell lysates were centrifuged at 16,100 × g for 15 min at 4 °C in a microcentrifuge. The supernatants (200 μl) were incubated with 5 μl of GST-tagged anti-GFP or anti-mCherry nanobody prebound to glutathione-Sepharose 4B beads in 0.2-ml 8-Tube Strips (Greiner) for 1 h at 4 °C. The tube strips were centrifuged at 2,000 × g for 30 s at room temperature. The precipitated beads were washed three times with 180 μl of lysis buffer and then transferred to a 96-well plate for observation. Precipitated beads bearing fluorescent fusion proteins were observed using an all-in-one-type fluorescence microscope (Biozero BZ-8000, Keyence) using a 20×/0.75 numeric aperture objective lens under constant conditions (sensitivity ISO 400, exposure to s (depending on the experiment) for green fluorescence and sensitivity ISO 800, exposure s for red fluorescence). Image acquisition was performed under constant conditions in the same series of experiments. When indicated, the materials bound to the beads were subjected to immunoblotting analysis using anti-GFP, anti-tRFP, or anti-mRFP antibody after image acquisition. For expression of combinations of EGFP, tRFP/mCherry, and TagBFP (tBFP) fusion constructs, ∼3.2 × 106 HEK293T cells grown on 6-cm dishes were transfected with expression vectors (8 μg total) using Polyethylenimine Max (40 μg) and then cultured for 24 h. Immunoprecipitation was performed as described above. The precipitated beads were observed with a confocal laser-scanning microscope (Nikon A1R-MP) equipped with a 20×/0.75 numeric aperture objective lens.
Culture of MEFs, DNA Transfection, and Immunofluorescence Microscopy
MEFs were derived from control or Cluap1−/− embryonic day 8.75 embryos as described previously (19). For immunofluorescence analysis, MEFs (∼9.0 × 104 cells on a coverslip in a 24-well plate) were cultured for 24 h in DMEM with high glucose supplemented with 10% FBS and starved for 24 h in DMEM with high glucose-containing 0.5% FBS. For immunostaining with anti-IFT57 or anti-IFT88 antibody, the MEFs were fixed and permeabilized with methanol at −20 °C for 5 min, and for immunostaining with anti-Cluap1 antibody, the MEFs were fixed with 3% paraformaldehyde in PBS at room temperature for 5 min and then fixed and permeabilized with methanol at −20 °C for 5 min. The fixed and permeabilized cells were washed twice with PBS and blocked in PBS containing 10% FBS at room temperature for 30 min. The cells were triply stained with any of anti-Cluap1 (3,000-fold dilution), anti-IFT57 (200-fold dilution) or anti-IFT88 (200-fold dilution), and anti-γ-tubulin (1,000-fold dilution) and anti-acetylated α-tubulin (500-fold dilution), followed by Alexa Fluor 488-conjugated anti-rabbit IgG (500-fold dilution), Alexa Fluor 555-conjugated anti-mouse IgG1 (1,000-fold dilution), and Alexa Fluor 647-conjugated anti-mouse IgG2b (1,000-fold dilution). In rescue experiments of Cluap1−/− MEFs, MEFs (∼8.0 × 104 cells on a coverslip in a 24-well plate) were cultured for 24 h in DMEM with high glucose supplemented with 10% FBS, transfected with an EGFP-fused mCluap1 construct (800 ng) using Lipofectamine 2000 (Invitrogen), and cultured for an additional 24 h. The MEFs were then cultured under starvation conditions (Opti-MEM (Invitrogen) with 0.2% BSA) for 48 h. Immunofluorescence analysis was performed as described previously (17, 21). The cells were fixed with 3% paraformaldehyde in PBS at 4 °C for 15 min, permeabilized with 0.1% Triton X-100 in PBS at room temperature for 5 min, and incubated in PBS containing 10% FBS at room temperature for 30 min. The cells were then incubated with anti-acetylated α-tubulin at room temperature for 1 h, washed three times with PBS containing 0.1% Triton X-100, and incubated with Alexa Fluor 555-conjugated anti-mouse IgG and Hoechst 33342 at room temperature for 1 h. The coverslip was placed onto Mowiol, and the cells were observed using an Axiovert 200M microscope (Carl Zeiss).
Immunoblotting
Proteins in cell lysates prepared as described above or on beads after VIP assays were separated by SDS-PAGE and electroblotted onto an Immobilon-P transfer membrane (EMD Millipore). The membrane was blocked in 5% skim milk and incubated sequentially with primary antibody (anti-GFP, anti-mRFP, anti-tRFP, anti-Cluap1, anti-IFT88, or anti-IFT57, diluted 1,000-fold, or anti-actin, diluted 2,000-fold) and horseradish peroxidase-conjugated secondary antibodies (diluted 3,000-fold). Detection was carried out using a Chemi-Lumi One L kit (Nacalai Tesque).
Results
All-by-all VIP Assays of IFT-B Subunits
We applied the VIP assay to examine binary interactions between the 14 known constituent proteins of the IFT-B complex and two proteins (TTC26 and Cluap1) that were candidate constituents at the beginning of our study (see Fig. 1A) (2, 4, 18). All but one of the subunits we examined were of human origin; in the experiments shown in Figs. 4–6 and supplemental Figs. S3 and S4, we used mouse Cluap1 (19) (see supplemental Tables S1 and S2). To examine protein-protein interactions by VIP assays, we constructed expression vectors for these IFT-B proteins fused to EGFP and either tRFP or mCherry and then co-expressed any of the 256 possible combinations of the EGFP- and tRFP/mCherry-fused proteins in HEK293T cells by co-transfection of the expression plasmids. As described previously (17), lysates prepared from the transfected cells were subjected to immunoprecipitation with GST-tagged anti-GFP nanobody (or, as noted, with GST-tagged anti-mCherry nanobody), prebound to glutathione-Sepharose beads, and the green and red fluorescent signals on beads bearing the immunoprecipitates were directly observed under a fluorescence microscope. Unless otherwise noted, the beads images were acquired under constant conditions in the same series of experiments. If a given protein (IFT-X) interacts with another protein (IFT-Y) in the transfected cells, both the green and red signals are detectable on the precipitated beads. By contrast, if IFT-X does not interact with IFT-Y, only the green signal is detected (supplemental Fig. S1; also see Ref. 17). The expression level and/or stability of certain fluorescent fusion proteins are often affected by co-expressed proteins; consequently, we routinely evaluate binary interactions as “positive” only when red signals are detected on the surface/perimeter of the precipitated beads in reciprocal combinations of EGFP- and tRFP/mCherry-fused proteins under identical conditions. Therefore, as in other protein-protein interaction assays, the absence of a positive VIP result does not necessarily imply that the two proteins in question cannot interact with each other. In addition, convenience is the most important aspect of the VIP assay. Therefore, we did not quantify the strength of the interactions by standardizing the assay according to the EGFP signals because the expression levels vary among individual fluorescent fusion proteins, and their stability is often affected by co-expressed proteins, as described above.
FIGURE 1.
All-by-all VIP assays of IFT-B proteins. A, schematic representation of structure and domain organization of IFT-B proteins. Coil, coiled-coil region; GTPase, GTPase domain; NN-CH, divergent calponin homology domain; GIFT, GldG/IFT domain; TPR, tetratricopeptide repeat domain; WD40, WD40 repeat domain. B and C, HEK293T cells cultured in 6-well plates were transfected with a combination of expression vectors for EGFP- and tRFP/mCherry-fused IFT-B proteins as indicated and incubated for 24 h. After expression of the green and red fluorescent fusion proteins in transfected cells was confirmed under a microscope, lysates were prepared from the transfected cells and precipitated with GST-tagged anti-GFP nanobody prebound to glutathione-Sepharose beads. The green (B) and red (C) fluorescence signals on the precipitated beads were observed, and the bead images were acquired using a BZ-8000 microscope. D, an incomplete map of interactions among IFT-B subunits, predicted from the data shown in C. In A and D, subunits of the core and peripheral subcomplexes are colored yellow and light blue, respectively.
FIGURE 4.
Domains responsible for interactions between IFT-B peripheral subunits. A, determination of Cluap1 domains responsible for its interactions with IFT20 and IFT80. Lysates prepared from HEK293T cells co-transfected with expression vectors for the indicated EGFP-fused Cluap1 construct and either IFT20-tRFP or IFT80-mCherry were processed for the VIP assay. B, determination of the IFT80 domain responsible for its interaction with Cluap1. Lysates prepared from HEK293T cells co-transfected with expression vectors for an EGFP-fused IFT80 construct indicated and either an FL or an NN-CH domain construct of tRFP-Cluap1 were processed for the VIP assay. C, determination of the IFT57 domains responsible for its interactions with IFT20 and IFT172. Lysates prepared from HEK293T cells co-transfected with expression vectors for the indicated EGFP-fused IFT57 construct and either IFT20-tRFP or mCherry-IFT172 were processed for the VIP assay. D, determination of the IFT172 domains responsible for its interaction with IFT57. Lysates prepared from HEK293T cells co-transfected with expression vectors for the indicated EGFP-fused IFT172 construct and either an FL or an NN-CH domain construct of tRFP-IFT57 were processed for the VIP assay. E, determination of the domains responsible for the IFT54 interactions with IFT20 and IFT74. Lysates prepared from HEK293T cells co-transfected with expression vectors for an EGFP-fused IFT54 construct indicated and either IFT20-tRFP or mCherry-IFT74 were processed for the VIP assay. F, determination of the IFT172 domains responsible for its interaction with IFT80. Lysates prepared from HEK293T cells co-transfected with expression vectors for the indicated EGFP-fused IFT80 and mCherry-fused IFT172 constructs were processed for the VIP assay. G, schematic representation of domain interactions among the IFT-B peripheral subunits.
FIGURE 5.
Detailed analyses of peripheral subunit interactions. A and B, formation of complex composed of four coiled-coil peripheral subunits. HEK293T cells cultured in 6-cm dishes were transfected with expression vectors for the indicated combinations of EGFP- and tRFP-fused peripheral subunit constructs. A, 24 h after transfection, lysates were prepared from cells, precipitated with GST-tagged anti-GFP nanobody (anti-GFP-NB) prebound to glutathione-Sepharose beads, and processed for the VIP assay. B, proteins bound to the precipitated beads (top and third panels) or input proteins (second and bottom panels) were subjected to immunoblotting with anti-tRFP antibody (top two panels) or anti-GFP antibody (bottom two panels). C and D, six peripheral subunits can form a complex en bloc. C, lysates prepared from HEK293T cells transfected with expression vectors for the indicated combinations of mCherry- and EGFP-fused peripheral subunit constructs were precipitated with GST-tagged anti-mCherry nanobody (anti-mCherry-NB) prebound to glutathione-Sepharose beads and processed for the VIP assay. D, proteins bound to the precipitated beads (top and third panels) or input proteins (second and bottom panels) were subjected to immunoblotting with anti-GFP antibody (top two panels) or anti-mRFP antibody, which can react with mCherry (bottom two panels). IP, immunoprecipitation; IB, immunoblotting.
FIGURE 6.
Essential role of Cluap1 in ciliogenesis and in peripheral subcomplex construction. A–E, rescue of ciliogenesis defects in Cluap1−/− MEFs by exogenous Cluap1 expression. Cluap1−/− MEFs were transfected with an empty EGFP vector (A–A″) or an expression vector for EGFP-fused FL Cluap1 (B–B″), Claup1ΔC (C–C″), or Cluap1ΔN (D–D″) (see Fig. 4A) and then incubated for 24 h under starvation conditions. The cells were immunostained for acetylated α-tubulin (A′–D′). Regions indicated by arrows are enlarged in insets. E, cells exogenously expressing EGFP or an EGFP-Claup1 construct in the experiments shown in A–D were classified as ciliated or non-ciliated, and percentages of ciliated cells are represented as bar graphs. Values are means ± S.D. (error bars) of three independent experiments. In each experiment, 30–50 cells with EGFP signals were observed. The original data shown in supplemental Table S3 were analyzed using a two-tailed, unpaired Student's t test. *, p < 0.002; **, p < 0.001. F, comparison of levels of IFT-B proteins between WT and Cluap1−/− MEFs. Lysates from WT or Cluap1−/− MEFs were subjected to immunoblotting analysis for Cluap1 (top panel), IFT88 (second panel), IFT57 (third panel), or actin (bottom panel). G–R, localization of IFT-B proteins in WT and Cluap1−/− MEFs. WT MEFs (G–I and M–O) or Cluap1−/− MEFs (J–L and P–R) were cultured under standard conditions (G–L) or starved for 24 h (M–R) and subjected to triple immunostaining for any of Cluap1 (G, J, M, and P), IFT88 (H, K, N, and Q), and IFT57 (I, L, O, and R); γ-tubulin (G′–R′); acetylated α-tubulin (G″–R″); and merged images (G‴–R‴). Regions indicated by arrows are enlarged in insets.
Fig. 1, B and C, shows signals of EGFP and tRFP/mCherry, respectively, immunoprecipitated with GST-tagged anti-GFP-nanobody in all-by-all VIP assays of the IFT-B proteins, and Fig. 1D schematically shows the IFT-B subunit interaction map predicted from the data shown in Fig. 1C. Several of the detected binary interactions were suggested by previous biochemical studies of Chlamydomonas proteins, such as IFT25-IFT27 (9, 11), IFT46-IFT52 (7, 11), IFT52-IFT88 (7, 11), and IFT74-IFT81 (6, 10, 11), and some were later confirmed by x-ray crystallography (13–15). In addition, some of the other interactions (IFT20-IFT54 and IFT20-IFT57) were suggested by studies using the yeast two-hybrid system or biochemical assays (22–24).
Our predicted interaction map of the mammalian IFT-B complex is compatible with previous biochemical studies of the Chlamydomonas IFT-B complex: (i) High salt treatment of the purified IFT-B complex dissociates weakly associated subunits (IFT20, IFT57, IFT80, and IFT172) from a salt-resistant core subcomplex containing IFT27/46/52/74/81/88 (6); (ii) IFT22 and IFT25 are included in the core subcomplex (7); and (iii) the core subcomplex can be divided into two parts, IFT46/52/70/88 and IFT22/25/27/74/81 (11, 13).
The map predicted from the binary interaction data suggests several associations not reported previously. (i) TTC26 exhibited a robust interaction with a core subunit, IFT46. While this study was in progress, other groups showed that TTC26 is included in the IFT-B complex (25, 26). (ii) Cluap1 is an integral component of the predicted peripheral subcomplex and connects IFT20 to IFT80. (iii) IFT20/54/57/80/172 and Cluap1 constitute the peripheral subcomplex. (iv) The predicted peripheral subcomplex is likely to be connected to the core subcomplex via an interaction between IFT54 and IFT74 (also see Fig. 3F).
FIGURE 3.
Visible three-hybrid assays of IFT-B peripheral subunits. HEK293T cells grown on 6-cm dishes were transfected with sets of expression vectors as follows. A, IFT80-EGFP, IFT20-tRFP, and either tBFP-Cluap1 (top) or tBFP-IFT22 (bottom); B, EGFP-Cluap1, tRFP-IFT57, and either IFT20-tBFP (top) or tBFP-IFT22 (bottom); C, EGFP-IFT172, IFT20-tRFP, and either tBFP-IFT57 (top) or tBFP-IFT22 (bottom); D, EGFP-IFT54, tRFP-Cluap1, and either IFT20-tBFP (top) or tBFP-IFT22 (bottom); E, EGFP-IFT54, tRFP-IFT57, and either IFT20-tBFP (top) or tBFP-IFT22 (bottom); F, IFT20-EGFP, tRFP-IFT74, and either tBFP-IFT54 (top) or tBFP-IFT22 (bottom). After expression of the green and red fluorescent fusion proteins in transfected cells was confirmed, lysates were prepared from the transfected cells and precipitated with GST-anti-GFP nanobody prebound to glutathione-Sepharose beads. The green (left), blue (middle), and red (right) fluorescence signals on the precipitated beads were observed, and bead images were acquired using an A1R-MP confocal laser-scanning microscope.
One-to-many and Many-to-many Subunit Interactions in the Core Subcomplex
Important issues to be addressed in the predicted interaction map (Fig. 1D) based on the binary interaction data (Fig. 1C) included the following: (i) IFT22 or IFT70 did not exhibit any obvious interaction with other IFT-B subunits; (ii) the IFT25-IFT27 dimer was not connected to any component of the core subcomplex; and (iii) a part of the core subcomplex containing IFT46/52/88/TTC26 was not connected to any other core component. To address these issues, we exploited one of the advantages of the VIP assay over other qualitative protein-protein interaction assays; namely the VIP assay can easily determine one-to-many protein interactions (17) and, in principle, many-to-many protein interactions, whereas such interactions can be detected by conventional co-immunoprecipitation and immunoblotting analysis of epitope-tagged proteins only with tremendous effort.
A recent study by Lorentzen and colleagues (13) showed that Chlamydomonas IFT22 can be pulled down using a combination of the central linker regions of IFT74 and IFT81. Therefore, we first confirmed this one-to-two subunit interaction using the VIP assay. Lysates prepared from cells co-expressing tRFP-IFT22 and either EGFP-IFT74 or -IFT81 or a combination of EGFP-IFT74 and -IFT81 were processed for immunoprecipitation with GST-anti-GFP nanobody prebound to glutathione-Sepharose beads. As shown in Fig. 2A, the red signal was detected only when both EGFP-IFT74 and -IFT81 were co-expressed with tRFP-IFT22. Note that the green signal of EGFP-IFT81 in the immunoprecipitate was weaker when the protein was expressed alone than when it was co-expressed with EGFP-IFT74, suggesting that IFT81 is stabilized by formation of a heterodimer with IFT74. Co-immunoprecipitation of tRFP-IFT22 with EGFP-IFT74 and -IFT81 was confirmed by processing the immunoprecipitates for conventional immunoblotting (Fig. 2B). Thus, IFT22 can interact with the IFT74-IFT81 heterodimer but not with IFT74 or IFT81 alone.
FIGURE 2.
One-to-many and many-to-many subunit interactions in the core subcomplex. HEK293T cells cultured in 6-well plates were transfected with expression vectors for the indicated combinations of EGFP- and tRFP/mCherry-fused IFT-B core subunits. Twenty-four hours after transfection, lysates were prepared from the cells and precipitated with GST-tagged anti-GFP nanobody (anti-GFP-NB) prebound to glutathione-Sepharose beads and then processed for the VIP assay (A, C, E, and G) or immunoblotting analysis using antibodies against tRFP/mRFP (top two panels) or GFP (bottom two panels) (B, D, F, and H). A and B, IFT74-IFT81 interaction with IFT22; C and D, IFT52-IFT88 interaction with IFT70; E and F, IFT74-IFT81 interaction with IFT25-IFT27; G and H, IFT81 interaction with IFT46-IFT52. IP, immunoprecipitation; IB, immunoblotting.
Unexpectedly, IFT70 did not exhibit an interaction with any of the IFT-B subunits. This was surprising because when co-expressed in E. coli, Chlamydomonas IFT70 was co-purified with an IFT52 fragment and as an IFT46/52/70/88 tetramer (11), although a more recent study suggested that IFT52 forms a trimeric complex with IFT70 and IFT88 (13). Therefore, we considered the possibility of a one-to-many interaction. As shown in Fig. 2, C and D, our VIP assay and subsequent immunoblotting analysis unequivocally showed that human IFT70 interacted strongly with the IFT52-IFT88 dimer. A faint band corresponding to mCherry-IFT70 was detected when this protein was co-expressed with EGFP-IFT52 (Fig. 2D, top), suggesting a weak interaction between IFT52 and IFT70.
Taking into account the biochemical studies of Cole and colleagues (6, 7), Lorentzen and colleagues (13) proposed that the C-terminal coiled-coil region of the IFT74-IFT81 heterodimer serves as an attachment site for IFT25-IFT27 and IFT46-IFT52, although at that time, there was no direct evidence for these two-to-two subunit interactions. To test this idea, we used the VIP assay to investigate these potential two-to-two subunit interactions. Regarding the proposed interaction between IFT25-IFT27 and IFT74-IFT81, the red signal was detected when EGFP-IFT74 and -IFT81 and tRFP-IFT25 and -IFT27 were co-expressed but not in the absence of any one of the four components (Fig. 2E). The results of the VIP assay were confirmed by conventional immunoblotting (Fig. 2F). Together, the VIP and immunoblotting data verified the proposed two-to-two subunit interaction between IFT25-IFT27 and IFT74-IFT81.
Regarding the proposed interaction between IFT46-IFT52 and IFT74-IFT81, the red signals were detected in the presence of mCherry-IFT46 and -IFT52 and either EGFP-IFT81 or a combination of EGFP-IFT74 and -IFT81, but not in the presence of mCherry-IFT46 and -IFT52 and EGFP-IFT74 alone (Fig. 2G). The VIP data were confirmed by immunoblotting (Fig. 2H). These results unequivocally demonstrate the one-to-two subunit interaction between IFT81 and the IFT46-IFT52 dimer. All of the data presented in Fig. 2 are largely compatible with previous pioneering models of IFT-B core assembly proposed by Cole and colleagues (7) and Richey and Qin (27).
Detailed Analyses of Peripheral Subcomplex Architecture
A novel point suggested by our predicted IFT-B subunit interaction map based on the binary interaction data is that IFT20/54/57/80/172 and Cluap1 constitute the peripheral subcomplex; the former five proteins were reported to dissociate from the Chlamydomonas IFT-B complex under high salt conditions (6), and Cluap1 was suggested to be an IFT-B component, although it was unknown how Cluap1 was incorporated into the IFT-B complex (23, 28–30). To confirm that Cluap1 is indeed included in the predicted peripheral subcomplex and validate the hierarchy of the subunit interactions in the predicted map of the peripheral subcomplex, we exploited another advantage of the VIP assay; using EGFP-, tRFP/mCherry-, and tBFP-fused proteins, the visible three-hybrid assay can determine not only interactions themselves but also the hierarchy of the interactions (17).
As shown in Fig. 3A, IFT20-tRFP was co-immunoprecipitated with IFT80-EGFP in the presence of co-expressed tBFP-Cluap1 but not when tBFP-Cluap1 was replaced by tBFP-IFT22, demonstrating that Cluap1 is an integral component of the peripheral subcomplex and serves to connect IFT20 and IFT80. In a similar manner, tRFP-IFT57 was co-immunoprecipitated with EGFP-Cluap1 in the presence of co-expressed IFT20-tBFP (Fig. 3B), and IFT20-tRFP was co-immunoprecipitated with EGFP-IFT172 in the presence of tBFP-IFT57 (Fig. 3C). The ternary interaction data shown in Fig. 3, A–C, together demonstrate the linear interactions between the five peripheral subunits, IFT172-IFT57-IFT20-Cluap1-IFT80.
We next examined the predicted branch of IFT54 in the peripheral subcomplex. tRFP-Cluap1 (Fig. 3D) or tRFP-IFT57 (Fig. 3E) was co-immunoprecipitated with EGFP-IFT54 only in the presence of co-expressed IFT20-tBFP, demonstrating the predicted three-way interactions among the four subunits ITF54, IFT57, IFT20, and Cluap1, with IFT20 at the center.
We next confirmed the predicted connection between the core and peripheral subcomplexes by visible three-hybrid assay. As shown in Fig. 3F, tRFP-IFT74 was efficiently co-immunoprecipitated with IFT20-EGFP in the presence of tBFP-IFT54 but not in the presence of tBFP-IFT22, suggesting that the linear IFT20-IFT54-IFT74 interactions connect the core and peripheral subcomplexes. However, as described below, the IFT54-IFT74 interaction appears not to make a major contribution to the core-peripheral connection.
Domains Involved in Subunit Interactions in the Peripheral Subcomplex
Among the six subunits in the predicted peripheral subcomplex, some share similar domain organizations (see Fig. 1A). For example, IFT54, IFT57, and Cluap1 have divergent NDC80-NUF2 calponin homology (NN-CH) domains in their N-terminal regions and coiled-coil regions in their C-terminal regions (4, 31); IFT80 and IFT172 have WD40 repeats in their N-terminal regions (4). The conservation of domain organizations suggests that common domains participate in interactions among these proteins.
We applied the VIP assay to determine the domains of Cluap1 involved in its interactions with IFT20 and IFT80 (Fig. 4A). Truncation of the C-terminal region, which follows the coiled-coil region, did not affect the interaction of Cluap1 with IFT20 or IFT80 (Fig. 4A, ΔC, third row). Removal of the N-terminal NN-CH domain abolished the Cluap1 interaction with IFT80 (ΔN, fourth row), and results obtained with an additional C-terminal truncation indicated that the coiled-coil region of Cluap1 is responsible for its interaction with IFT20 (CC, sixth row). The N-terminal deletion appeared to strengthen the interaction of the remaining Cluap1 region with IFT20 (compare the second and fourth rows), although we did not further investigate this phenomenon in this study. The N-terminal NN-CH domain was involved in the Cluap1 interaction with IFT80 (NN-CH, fifth row). We next attempted to determine which region of IFT80 participates in its interaction with the Cluap1 NN-CH domain. As shown in Fig. 4B, the WD40 domain of IFT80 is responsible for its interaction with the NN-CH domain of Cluap1.
Similar interaction modes were observed for IFT57, IFT20, and IFT172. The NN-CH domain and coiled-coil region of IFT57 are involved in its interaction with IFT172 and IFT20, respectively (Fig. 4C), and IFT172 interacts with the IFT57 NN-CH domain through its WD40 domain (Fig. 4D). Thus, common NN-CH-WD40 and coiled-coil-coiled-coil interactions are conserved in the linear interactions of IFT172-IFT57-IFT20-Cluap1-IFT80.
We then sought to determine which domains of IFT54 are involved in its interactions with IFT20 and IFT74. As shown in Fig. 4E, the coiled-coil region of IFT54 is responsible for its interactions with both IFT20 and IFT74.
In the binary interaction assays shown in Fig. 1, we repeatedly detected an interaction between IFT80-EGFP and mCherry-IFT172, although we could not detect this interaction with a reciprocal combination of green and red fluorescent protein fusions (i.e. between EGFP-IFT172 and IFT80-mCherry). We used the deletion constructs of IFT80 and IFT172 described above (Fig. 4, B and D, respectively) to investigate the potential IFT80-IFT172 interaction. As shown in Fig. 4F, we detected an interaction between a full-length (FL) construct of IFT80 fused to EGFP with a FL or tetratricopeptide repeat construct of IFT172 fused to mCherry. However, we could not detect an interaction between a WD40 repeat construct of IFT80 and IFT172. The VIP assay data were confirmed by immunoblotting (supplemental Fig. S2). Thus, a region encompassing the WD40 domain and the C-terminal region appears to be involved in the interaction of IFT80 with the tetratricopeptide repeat domain of IFT172.
Fig. 4G shows a schematic representation of the domain interactions among the subunits of the peripheral subcomplexes predicted from the data shown in Fig. 4, A–F. The domain interaction map, in conjunction with the visible three-hybrid assay data shown in Fig. 3, predicts that IFT20, the smallest IFT-B protein containing a coiled-coil region, serves as a hub in the predicted peripheral subcomplex. Because coiled coils exhibit remarkable diversity with regard to the number and arrangement of associated helices and can consist of 2–6 strands adopting a parallel or antiparallel helix orientation (32), we next examined whether coiled coils of the four peripheral proteins (IFT57/54/20/Cluap1) could simultaneously interact with one another. To this end, we simultaneously transfected HEK293T cells with expression vectors for the four coiled-coil constructs (IFT54CC fused to EGFP and Cluap1ΔN, IFT57CC, and IFT20 fused to tRFP) and subjected cell lysates to immunoprecipitation with GST-anti-GFP nanobody prebound to glutathione-Sepharose beads (Fig. 5A), followed by immunoblotting with anti-tRFP antibody (Fig. 5B, top). As shown in Fig. 5B, EGFP-IFT54CC co-immunoprecipitated tRFP-Cluap1ΔN and tRFP-IFT57CC in the presence (lane 2) but not in the absence (lane 3) of IFT20-tRFP. Based on these results, it is likely that these coiled-coil proteins can simultaneously associate to form a four-stranded structure.
Together, the data shown in Fig. 5, A and B, as well as those in Fig. 3, A–E, indicate that the six proteins (IFT172/80/57/54/20/Cluap1) can form a peripheral subcomplex en bloc. To confirm this, we adopted the following strategy. HEK293T cells were simultaneously transfected with expression vectors for the six proteins, IFT54 fused to mCherry and the other five fused to EGFP, and processed for immunoprecipitation using GST-tagged anti-mCherry nanobody prebound to glutathione-Sepharose beads (supplemental Fig. S3A), followed by immunoblotting with anti-GFP antibody or with anti-mRFP antibody, which can recognize mCherry (supplemental Fig. S3B). If these proteins form a hexapartite complex, we would expect to detect five protein bands in GST-anti-mCherry nanobody immunoprecipitates by immunoblotting with anti-GFP antibody. As shown in supplemental Fig. S3B, lane 2, however, we detected only four bands. Among them, bands corresponding to EGFP-IFT172, IFT80-EGFP, and IFT20-EGFP could be clearly assigned, whereas the other band appeared to represent an overlap of the EGFP-IFT57 and EGFP-Cluap1 bands.
To discriminate the bands corresponding to EGFP-IFT57 and EGFP-Cluap1, we exploited an EGFP-Cluap1ΔC construct, which lacks the C-terminal region of Cluap1 but retains its ability to interact with other peripheral subunits (Fig. 4A). As shown in Fig. 5D, mCherry-IFT54 co-immunoprecipitated EGFP-tagged IFT172, IFT80, IFT57, and Cluap1ΔC in the presence (lane 2) but not in the absence (lane 3) of IFT20-EGFP. These results make it likely that these peripheral subunits form a hexapartite complex.
Homodimerization of IFT20, IFT57, and Cluap1 via Their Coiled-coil Regions
The all-by-all VIP assay data shown in Fig. 1 indicated that IFT20, IFT57, and Cluap1 exhibited homophilic interactions, in addition to heteromeric interactions with other peripheral subunits. Because the domain interaction experiments shown in Fig. 4 indicated that the three peripheral subunits exhibit heteromeric interactions with one another via their coiled-coil regions, we next investigated the possibility that these regions also engage in homophilic interactions. As shown in supplemental Fig. S4, A and B, Cluap1 and IFT57 did indeed show homophilic interactions through their coiled-coil regions. IFT20 also exhibited a homophilic interaction (supplemental Fig. S4C), but we did not attempt to determine the responsible region(s) of the protein. Thus, IFT20, IFT57, and Cluap1 can form homodimers, although we did not further pursue the physiological relevance of these homophilic interactions in this study.
Cluap1 Is Essential for Ciliogenesis and Peripheral Subcomplex Formation
We previously reported that although Cluap1-knock-out mice were embryonic lethal, it was possible to establish MEFs from knock-out embryos (19). Because Cluap1−/− MEFs exhibited ciliogenesis defects (19) (also see Fig. 6, A–A″), we investigated whether exogenous expression of Cluap1 constructs could rescue the defects. When a FL construct of Cluap1 fused to EGFP was transfected into Cluap1−/− MEFs, ciliogenesis was significantly recovered (Fig. 6, B–B″ and E); the Cluap1 construct itself was localized at the basal body and in the cilium. Exogenous expression of a Cluap1ΔC construct, which lacks a C-terminal region but retains the abilities to bind to both IFT20 and IFT80 (see Fig. 4A), restored ciliogenesis (Fig. 6, C–C″ and E). However, expression of a construct lacking the ability to bind IFT80 (Cluap1ΔN; see Fig. 4A) failed to induce formation of cilia in Cluap1−/− MEFs (Fig. 6, D–D″ and E); we also attempted to examine whether expression of a Cluap1NN-CH construct, which lacks the ability to bind IFT20 (see Fig. 4A), could restore ciliogenesis, but the attempts were unsuccessful due to serious cytotoxicity of its expression in MEFs. Together, these observations indicate that incorporation of Cluap1 into the IFT-B peripheral subcomplex is essential for IFT-B functions and is therefore required for normal ciliogenesis.
We next addressed whether lack of Cluap1 affects the stability of other IFT-B components. When lysates from wild-type (WT) and Cluap1−/− MEFs were subjected to immunoblotting analysis to examine levels of IFT-B proteins, the IFT88 level was not changed in Cluap1−/− MEFs compared with WT MEFs (Fig. 6F). On the other hand, the IFT57 level was substantially decreased in Cluap1−/− MEFs compared with WT MEFs. Although we could not examine other IFT-B components due to limitation of availability of antibodies with applicable quality, these results suggest that lack of Cluap1 could affect the stability of other peripheral subunits.
We then compared localization of IFT-B subunits in WT and Cluap1−/− MEFs. In WT MEFs cultured under standard conditions, Cluap1 (Fig. 6, G–G‴), IFT88 (Fig. 6, H–H‴), and IFT57 (Fig. 6, I–I‴) were localized around the centrosome labeled with anti-γ-tubulin. In striking contrast, in Cluap1−/− MEFs, pericentrosomal staining for IFT57 was not detectable (Fig. 6, L–L‴), whereas IFT88 was retained around the centrosome (Fig. 6, K–K‴). When cultured under starvation conditions, Cluap1, IFT88, and IFT57 (Fig. 6, M–O) were found within cilia in WT MEFs, whereas in Cluap1−/− MEFs, IFT88 (Q), but not IFT57 (R), was found around the basal body/centrosome. These observations make it likely that formation of an intact peripheral subcomplex is required for proper localization around the centrosome and subsequent entry into cilia of its subunits, although it is unlikely that peripheral subunits are essential for core subcomplex formation.
Composite Interactions Participate in the Connection between the Core and Peripheral Subcomplexes
The data shown in Figs. 1C, 3F, and 4E suggest that the IFT54-IFT74 interaction participates in the connection between the core and peripheral subcomplexes. However, we further examined the core-peripheral interface because interactions among coiled-coil proteins could lead to false positive results.
As shown in supplemental Fig. S5, A and B, the interaction between IFT54 and IFT74 was not affected by the presence of IFT20, in line with the visible three-hybrid data shown in Fig. 3F. However, unexpectedly, the IFT54-IFT74 interaction was abolished in the presence of co-expressed IFT81 (supplemental Fig. S5, C and D). Because IFT74 forms a robust heterodimer with IFT81 through their coiled-coil regions (14), the data suggest that another interaction(s) mediates the connection between the core and peripheral subcomplexes.
To address this issue, we exploited another advantage of our flexible VIP assay system, the subtraction VIP assay, by which we previously revealed one-subunit versus multisubunit interactions in the exocyst complex (17). When all of the core subunits fused to EGFP were co-expressed with all of the peripheral subunits fused to tRFP/mCherry in HEK293T cells, immunoprecipitation of the cell lysates with GST-anti-GFP nanobody yielded red fluorescent signals (Fig. 7A, second row). It is expected that omitting one or more of the peripheral subunits abolishes the red signal if the peripheral subunit(s) makes a crucial contribution to the core-peripheral connection. As shown in Fig. 7A, the red fluorescent signal was extremely diminished when IFT57 or Cluap1 was omitted in a set of subtraction VIP assays, suggesting that these two subunits meditate the interaction of the peripheral subcomplex with the core subcomplex. Similarly, a set of subtraction VIP assays for the core subunits suggested that IFT52 and IFT88 mediate the interaction of the core subcomplex with the peripheral subcomplex (Fig. 7B). It is also noteworthy that omitting IFT54 (Fig. 7A) or IFT74 (Fig. 7B) did not apparently diminish the red signal, suggesting that neither of them mainly contributes to the core-peripheral connection.
FIGURE 7.
Determination of interface between the core and peripheral subcomplexes. A and B, subtraction VIP assays to determine IFT-B subunits involved in the core-peripheral connection. HEK293T cells were transfected with expression vectors for all of the core subunits fused to EGFP (IFT22/25/27/46/52/70/74/81/88/TTC26) and all (IFT20/54/57/80/172/Cluap1) but one (as indicated) of the peripheral subunits fused to tRFP/mCherry (A) or those for all of the peripheral subunits (IFT20/54/57/80/172/Cluap1) fused to EGFP and all (IFT22/25/27/46/52/70/74/81/88/TTC26) but one (as indicated) of the core subunits fused to tRFP/mCherry (B) and processed for the VIP assay as described in the legend to Fig. 2. C–F, HEK293T cells were transfected with expression vectors for the indicated combinations of EGFP-fused IFT52/IFT88 and tRFP-fused IFT57/Cluap1 and then processed for the VIP assay (C and D) or immunoblotting analysis using antibodies against tRFP (top two panels) or GFP (bottom two panels) as described in the legend to Fig. 2. IP, immunoprecipitation; IB, immunoblotting.
To examine whether these four subunits indeed participate in the core-peripheral connection, we then performed VIP assays for one-to-two and two-to-two subunit interactions. As shown in Fig. 7, C and D, the red fluorescent signal was observed when all of IFT52, IFT88, IFT57, and Cluap1 were expressed simultaneously (third row) but not when at least one of the four subunits was omitted.
We then attempted to confirm the VIP data by conventional immunoblotting. As shown in Fig. 7E, a doublet band for tRFP-IFT57 and -Cluap1 was detected when EGFP-IFT52 and -IFT88 were co-expressed (top), although a faint band (probably for tRFP-IFT57) was also observed when either EGFP-IFT52 or -IFT88 was co-expressed. To discriminate between the bands for tRFP-IFT57 and -Cluap1, we then used tRFP-Cluap1ΔC (see Fig. 4A). As shown in Fig. 7F, strong bands for tRFP-IFT57 and -Cluap1ΔC were detected when EGFP-IFT52 and -IFT88 were coexpressed (top); again, a faint band for tRFP-IFT57 was detected when either EGFP-IFT52 or -IFT88 was co-expressed. These results together indicate that the core and peripheral subcomplexes are connected by composite interactions involving IFT52, IFT88, IFT57, and Cluap1. During the revision process of this paper, Lorentzen and colleagues (33) reported that Chlamydomonas IFT57-Cluap1 was pulled down with GST-IFT52-IFT88. Thus, we and Lorentzen and colleagues independently reached essentially the same conclusion about the interface between the core and peripheral subcomplexes (see Fig. 8).
FIGURE 8.
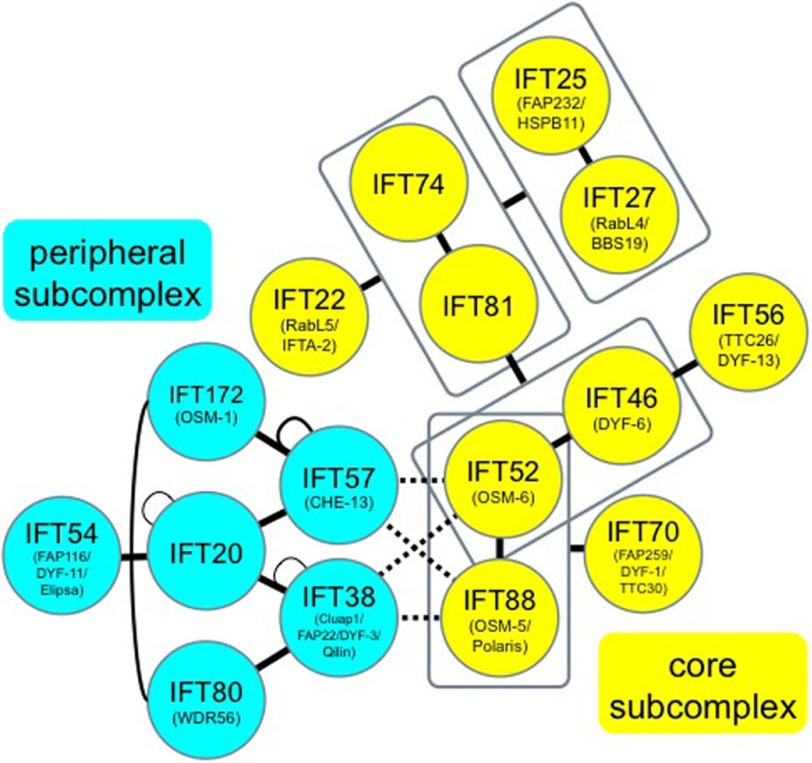
Architecture of the IFT-B complex predicted from the data presented in this study. Subunits of the core and peripheral subcomplexes are colored yellow and light blue, respectively. Broken lines indicate composite interactions involving IFT38, IFT52, IFT57, and IFT88. For other lines, see the key in Fig. 1D.
Discussion
Using the VIP assay, we revealed the overall molecular architecture of the IFT-B complex (Fig. 8). The complex can be divided into core and peripheral subcomplexes composed of 10 and 6 subunits, respectively, including proteins previously described as candidate IFT-B components. The detailed map predicted from our data revealed the following. (i) TTC26 is included in the core subcomplex via a robust interaction with at least one core subunit, IFT46 (26); hereafter, we refer to TTC26 as IFT56, as recently proposed by Marshall and colleagues (25). (ii) Cluap1 is an integral component of the peripheral subcomplex and serves to connect IFT20 and IFT80; hereafter, we refer to Cluap1 as IFT38, as proposed by Nachury and colleagues (16, 18). (iii) IFT38, with five other proteins (IFT20/54/57/80/172), constitutes a peripheral subcomplex. (iv) The core and peripheral subcomplexes are likely to be connected by composite interactions involving IFT38, IFT52, IFT57, and IFT88.
Although IFT20, IFT54, IFT57, IFT80, and IFT172 dissociate from the purified Chlamydomonas IFT-B complex under high salt conditions (6), it was not previously known whether these proteins constitute a subcomplex. Our analyses unequivocally show that the five IFT proteins constitute a peripheral subcomplex. Moreover, our data demonstrate that IFT38, previously referred to as Cluap1, qilin, DYF-3, or FAP22, depending on the species, is an indispensable component of the peripheral subcomplex, although it was suggested to be an IFT-B component (23, 28–30). The ciliogenesis defect of MEFs derived from an IFT38/Cluap1−/− mouse, which is embryonic lethal (19, 29), can be rescued by exogenous expression of full-length IFT38 but not a deletion mutant lacking IFT80-binding ability (Fig. 6), indicating that IFT38 is essential for proper IFT-B functions.
The architecture of the IFT-B peripheral subcomplex appears to be established on the basis of common domain-domain interactions (Fig. 4). For example, the NN-CH domains of IFT38 and IFT57 interact with the WD40 repeat domains of IFT80 and IFT172, respectively. On the other hand, the IFT20 coiled-coil region can bind simultaneously to the coiled-coil regions of IFT38, IFT54, and IFT57, because our visible three-hybrid assay unequivocally showed that IFT20 can form a complex by interacting simultaneously with at least two of IFT38, IFT54, and IFT57 (Fig. 3), thus serving as a hub of the peripheral subcomplex. Future studies should investigate how these coiled-coil regions interact simultaneously with one another to form a multipartite coiled-coil structure.
The interaction map of the mammalian IFT-B core subcomplex revealed by our VIP assay is largely consistent with the model of the Chlamydomonas core subcomplex proposed by Lorentzen and colleagues (4, 13) on the basis of their in vitro reconstitution, mapping of protein-protein interactions, and crystal structures, although their model lacked IFT56. However, some details differ between our interaction map of the core subcomplex and the one proposed by Lorentzen and colleagues (4, 13). They detected a direct interaction between IFT52 and IFT70, whereas we could detect an interaction between IFT70 and IFT52 only when the latter was co-expressed with IFT88. Another difference was that we detected an interaction of the IFT46-IFT52 dimer with IFT81 alone, whereas Lorentzen and colleagues (4, 13) proposed that the IFT46-IFT52 dimer interacts with the IFT74-IFT81 dimer. Although we do not currently know the exact reasons for these apparent discrepancies, they might result from the differences in the origins of the proteins (human in our study and Chlamydomonas in theirs).
The detailed interaction map provides some insight into the functional relevance of the IFT-B architecture. For example, Chlamydomonas ift46, ift52, and ift88 mutant strains have very short or no flagella (34–36), and the lack of IFT46 or IFT52 severely affects assembly of the IFT-B complex (27, 36). By contrast, in an ift56 mutant strain, although flagella are slightly shorter than those of the wild type and motility of flagella is mildly impaired, IFT-B assembly is not affected (25). In our IFT-B interaction map (Fig. 8), IFT46, IFT52, and IFT88 participate in interactions with more than one other core subunit, whereas IFT56 interacts only with IFT46. It is therefore likely that at least IFT46 and IFT52 are important for the assembly of overall core subcomplex, whereas IFT56 is dispensable for core assembly. This is consistent with the work of Lorentzen and colleagues (13), who showed that when co-expressed in insect cells, IFT22/25/27/46/52/70/74/81/88 were co-purified without additional expression of IFT56.
The interaction map of the core subcomplex is also compatible with an auxiliary role of the IFT25-IFT27 pair, which is in contact with the IFT74-IFT81 pair but not with any other IFT-B subunits (Fig. 8); neither the IFT25 nor IFT27 gene is found in some ciliated organisms, including Caenorhabditis elegans and Drosophila melanogaster (16), and knock-out of IFT27 does not affect ciliogenesis or entry of other core subunits, except for IFT25, into cilia (37–39).
The interface between the core and peripheral subcomplexes appears to be intricate. We first showed a direct interaction between IFT74 from the core subcomplex and IFT54 form the peripheral subcomplex (Figs. 1C, 3F, and 4E). However, the IFT54-IFT74 interaction was abolished in the presence of IFT81 (supplemental Fig. S5, C and D), which can form a robust heterodimer with IFT74 (14). By exploiting a subtraction VIP assay, we then demonstrated that IFT52 and IFT88 from the core subcomplex and IFT38 and IFT57 from the peripheral subcomplex mediate the contacts between the two subcomplexes (Fig. 7). During the revision process of this paper, Lorentzen and colleagues (33) reached essentially the same conclusion about the core-peripheral interface of the Chlamydomonas IFT-B complex. Thus, the overall architecture, including the interface between the core and peripheral subcomplexes, of the IFT-B complex is largely conserved between human and Chlamydomonas, although there was a small difference; Chlamydomonas IFT38 and IFT57 can form a heterodimer (33), whereas the human peripheral subunits cannot directly interact with each other but are connected by IFT20 (Fig. 3B).
The results of this study provide an overview of the architecture of the IFT-B complex. To understand the mechanism of ciliary protein trafficking, it will also be necessary to determine how the IFT-B complex cooperates with the IFT-A complex, and possibly with the BBSome, to construct the IFT machinery, the so-called “IFT train.” In addition, the mode of interaction of the IFT-B complex with kinesin motors is another issue to be addressed in future studies. The VIP assay will provide a powerful tool for addressing these issues.
Author Contributions
Y. K. and M. T. designed and performed experiments and prepared the manuscript; Y. N., R. T., and S. N. performed experiments; H. H. designed experiments; and K. N. designed experiments and prepared the manuscript.
Acknowledgments
We thank Takashi Matsumoto for technical assistance. The cDNA clones of IFT54 and IFT70 were provided by RIKEN BRC, which is participating in the National Bio-Resources Project of MEXT, Japan, and the clone of IFT172 was provided by the Kazusa DNA Research Institute.
Note Added in Proof
In Fig. 7A in the original version of this article, published as a Paper in Press on March 15, 2016, the images of the IFT172 beads represented in the green and red channels did not correspond. This error has been corrected, and this correction does not affect the interpretation of the results or the conclusions of the paper.
This work was supported in part by Grants-in-Aid for Scientific Research on Innovative Areas “Cilia and Centrosome” from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (Grants 25113514 and 15H01211 to K. N.); Japan Society for Promotion of Science Grants 22390013 and 15H04370 (to K. N.) and 25860044 and 15K07929 (to Y. K.); and grants from the Uehara Memorial Foundation (to K. N.) and from the Takeda Science Foundation (to Y. K.). The authors declare that they have no conflicts of interest with the contents of this article.

This article contains supplemental Tables S1–S3 and Figs. S1–S5.
- IFT
- intraflagellar transport
- NN-CH
- NDC80-NUF2 calponin homology
- VIP
- visible immunoprecipitation
- mRFP
- monomer RFP
- EGFP
- enhanced GFP
- MEF
- mouse embryo fibroblast
- FL
- full-length
- tRFP
- TagRFP
- tBFP
- TagBFP.
References
- 1. Rosenbaum J. L., and Witman G. B. (2002) Intraflagellar transport. Nat. Rev. Mol. Cell Biol. 3, 813–825 [DOI] [PubMed] [Google Scholar]
- 2. Ishikawa H., and Marshall W. F. (2011) Ciliogenesis: building the cell's antenna. Nat. Rev. Mol. Cell Biol. 12, 222–234 [DOI] [PubMed] [Google Scholar]
- 3. Sung C.-H., and Leroux M. R. (2013) The roles of evolutionarily conserved functional modules in cilia-related trafficking. Nat. Cell Biol. 15, 1387–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taschner M., Bhogaraju S., and Lorentzen E. (2012) Architecture and function of IFT complex proteins in ciliogenesis. Differentiation 83, S12–S22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhogaraju S., Engel B. D., and Lorentzen E. (2013) Intraflagellar transport complex structure and cargo interactions. Cilia 2, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lucker B. F., Behal R. H., Qin H., Siron L. C., Taggart W. D., Rosenbaum J. L., and Cole D. G. (2005) Characterization of the intraflagellar transport complex B core: direct interaction of the IFT81 and IFT74/72 subunits. J. Biol. Chem. 280, 27688–27696 [DOI] [PubMed] [Google Scholar]
- 7. Lucker B. F., Miller M. S., Dziedzic S. A., Blackmarr P. T., and Cole D. G. (2010) Direct interactions of intraflagellar transport complex B proteins IFT88, IFT52, and IFT46. J. Biol. Chem. 285, 21508–21518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cole D. G., Diener D. R., Himelblau A. L., Beech P. L., Fuster J. C., and Rosenbaum J. L. (1998) Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J. Cell Biol. 141, 993–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang Z., Fan Z.-C., Williamson S. M., and Qin H. (2009) Intraflagellar transport (IFT) protein IFT25 is a phosphoprotein component of IFT complex B and physically interacts with IFT27 in Chlamydomonas. PLoS One 4, e5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fan Z.-C., Behal R. H., Geimer S., Wang Z., Williamson S. M., Zhang H., Cole D. G., and Qin H. (2010) Chlamydomonas IFT70/CrDYF-1 is a core component of IFT particle complex B and is required for flagellar assembly. Mol. Biol. Cell 21, 2696–2706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taschner M., Bhogaraju S., Vetter M., Morawetz M., and Lorentzen E. (2011) Biochemical mapping of interactions with the intraflagellar transport (IFT) B core complex: IFT52 binds directly to four other IFT-B subunits. J. Biol. Chem. 286, 26344–26352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Behal R. H., Miller M. S., Qin H., Lucker B. F., Jones A., and Cole D. G. (2012) Subunit interactions and organization of the Chlamydomonas reinhardtii intraflagellar transport complex A proteins. J. Biol. Chem. 287, 11689–11703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taschner M., Kotsis F., Braeuer P., Kuehn E. W., and Lorentzen E. (2014) Crystal structures of IFT70/52 and IFT52/46 provide insight into intraflagellar transport B core complex assembly. J. Cell Biol. 207, 269–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bhogaraju S., Cajanek L., Fort C., Blisnick T., Weber K., Taschner M., Mizuno N., Lamla S., Bastin P., Nigg E. A., and Lorentzen E. (2013) Molecular basis of tubulin transport within the cilium by IFT74 and IFT81. Science 341, 1009–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bhogaraju S., Taschner M., Morawetz M., Basquin C., and Lorentzen E. (2011) Crystal structure of the intraflagellar transport complex 25/27. EMBO J. 30, 1907–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shida T., Cueva J. G., Xu Z., Goodman M. B., and Nachury M. V. (2010) The major α-tubulin K40 acetyltransferase αTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc. Natl. Acad. Sci. U.S.A. 107, 21517–21522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Katoh Y., Nozaki S., Hartanto D., Miyano R., and Nakayama K. (2015) Architectures of multisubunit complex revealed by a visible immunoprecipitation assay using fluorescent fusion proteins. J. Cell Sci. 128, 2351–2362 [DOI] [PubMed] [Google Scholar]
- 18. Nachury M. V. (2014) How do cilia organize signalling cascades? Phil. Trans. R. Soc. B 10.1098/rstb.2013.0465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Botilde Y., Yoshiba S., Shinohara K., Hasegawa T., Nishimura H., Shiratori H., and Hamada H. (2013) Cluap1 localizes preferentially to the base and tip of cilia and is required for ciliogenesis in the mouse embryo. Dev. Biol. 381, 203–212 [DOI] [PubMed] [Google Scholar]
- 20. Fridy P. C., Li Y., Keegan S., Thompson M. K., Nudelman I., Scheid J. F., Oeffinger M., Nussenzweig M. C., Fenyö D., Chait B. T., and Rout M. P. (2014) A robust pipline for rapid production of versatile nanobody repertoires. Nat. Methods 11, 1253–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takahashi S., Kubo K., Waguri S., Yabashi A., Shin H.-W., Katoh Y., and Nakayama K. (2012) Rab11 regulates exocytosis of recycling vesicles at the plasma membrane. J. Cell Sci. 125, 4049–4057 [DOI] [PubMed] [Google Scholar]
- 22. Baker S. A., Freeman K., Luby-Phelps K., Pazour G. J., and Besharse J. C. (2003) IFT20 links kinesin II with a mammalian intraflagellar transport complex that is conserved in motile flagella and sensory cilia. J. Biol. Chem. 278, 34211–34218 [DOI] [PubMed] [Google Scholar]
- 23. Omori Y., Zhao C., Saras A., Mukhopadhyay S., Kim W., Furukawa T., Sengupta P., Veraksa A., and Malicki J. (2008) elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat. Cell Biol. 10, 437–444 [DOI] [PubMed] [Google Scholar]
- 24. Follit J. A., Xu F., Keady B. T., and Pazour G. J. (2009) Characterization of mouse IFT complex B. Cell Motil. Cytoskeleton 66, 457–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ishikawa H., Ide T., Yagi T., Jiang X., Hirono M., Sasaki H., Yanagisawa H., Wemmer K. A., Stainier D. Y. R., Qin H., Kamiya R., and Marshall W. F. (2014) TTC26/DYF13 is an intraflagellar transport protein required for transport of motility-related proteins into flagella. eLife 3, e01566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Swiderski R. E., Nakano Y., Mullins R. F., Seo S., and Bánfi B. (2014) A mutation in the mouse Ttc26 gene leads to impaired hedgehog signaling. PLoS Genet. 10, e1004689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Richey E. A., and Qin H. (2012) Dissecting the sequential assembly and localization of intraflagellar transport particle complex B in Chlamydomonas. PLoS One 7, e43118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee C., Wallingford J. B., and Gross J. M. (2014) Cluap1 is essential for ciliogenesis and photoreceptor maintenance in the vertebrate eye. Invest. Ophthalmol. Vis. Sci. 55, 4585–4592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pasek R. C., Berbari N. F., Lewis W. R., Kesterson R. A., and Yoder B. K. (2012) Mammalian clusterin associated protein 1 is an evolutionally conserved protein required for ciliogenesis. Cilia 1, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boldt K., Mans D. A., Won J., van Reeuwijk J., Vogt A., Kinkl N., Letteboer S. J. F., Hicks W. L., Hurd R. E., Naggert J., Texier Y., den Hollander A. I., Koenekoop R. K., Bennett J., Cremers F. P. M., et al. (2011) Disruption of intraflagellar protein transport in photoreceptor cilia causes Leber congenital amaurosis in humans and mice. J. Clin. Invest. 121, 2169–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schou K. B., Andersen J. S., and Pedersen L. B. (2014) A divergent calponin homology (NN-CH) domain defines a novel family: implications for evolution of ciliary IFT complex B proteins. Bioinformatics 30, 899–902 [DOI] [PubMed] [Google Scholar]
- 32. Lupas A. N., and Gruber M. (2005) The structure of α-helical coiled coils. Adv. Protein Chem. 70, 37–78 [DOI] [PubMed] [Google Scholar]
- 33. Taschner M., Weber K., Mourão A., Vetter M., Awasthi M., Stiegler M., Bhogaraju S., and Lorentzen E. (2016) Intraflagellar transport proteins 172, 80, 57, 54, 38, and 20 form a stable tubulin-binding IFT-B2 complex. EMBO J. 35, 773–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brazelton W. J., Amundsen C. D., Silflow C. D., and Lefebvre P. A. (2001) The bld1 mutation indentifies the Chlamydomonas osm-6 homolog as a gene required for flagellar assembly. Curr. Biol. 11, 1591–1594 [DOI] [PubMed] [Google Scholar]
- 35. Pazour G. J., Dickert B. L., Vucica Y., Seeley E. S., Rosenbaum J. L., Witman G. B., and Cole D. G. (2000) Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene Tg737, are required for assembly of cilia and flagella. J. Cell Biol. 151, 709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hou Y., Qin H., Follit J. A., Pazour G. J., Rosenbaum J. L., and Witman G. B. (2007) Functional analysis of an indivisual IFT protein: IFT46 is required for transport of outer dynein arms into flagella. J. Cell Biol. 176, 653–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang N., Li L., Eguether T., Sundberg J. P., Pazour G. J., and Chen J. (2015) Intraflagellar transport 27 is essential for hedgehog signaling but dispensable for ciliogenesis during hair follicle morphogenesis. Development 142, 2194–2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liew G. M., Ye F., Nager A. R., Murphy J. P., Lee J. S., Aguiar M., Breslow D. K., Gygi S. P., and Nachury M. V. (2014) The intraflagellar transport protein IFT27 promotes BBSome exit from cilia through the GTPase ARL6/BBS3. Dev. Cell 31, 265–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eguether T., San Agustin J. T., Keady B. T., Jonassen J. A., Liang Y., Francis R., Tobita K., Johnson C. A., Abdelhamed Z. A., Lo C. W., and Pazour G. J. (2014) IFT27 links the BBSome to IFT for maintenance of the ciliary signaling compartment. Dev. Cell 31, 279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]