Abstract
The circadian system in Neurospora is based on the transcriptional/translational feedback loops and rhythmic frequency (frq) transcription requires the WHITE COLLAR (WC) complex. Our previous paper has shown that frq could be transcribed in a WC-independent pathway in a strain lacking the histone H3K36 methyltransferase, SET-2 (su(var)3–9-enhancer-of-zeste-trithorax-2) (1), but the mechanism was unclear. Here we disclose that loss of histone H3K36 methylation, due to either deletion of SET-2 or H3K36R mutation, results in arrhythmic frq transcription and loss of overt rhythmicity. Histone acetylation at frq locus increases in set-2KO mutant. Consistent with these results, loss of H3K36 methylation readers, histone deacetylase RPD-3 (reduced potassium dependence 3) or EAF-3 (essential SAS-related acetyltransferase-associated factor 3), also leads to hyperacetylation of histone at frq locus and WC-independent frq expression, suggesting that proper chromatin modification at frq locus is required for circadian clock operation. Furthermore, a mutant strain with three amino acid substitutions (histone H3 lysine 9, 14, and 18 to glutamine) was generated to mimic the strain with hyperacetylation state of histone H3. H3K9QK14QK18Q mutant exhibits the same defective clock phenotype as rpd-3KO mutant. Our results support a scenario in which H3K36 methylation is required to establish a permissive chromatin state for circadian frq transcription by maintaining proper acetylation status at frq locus.
Keywords: circadian rhythm, clock gene, gene transcription, histone modification, Neurospora, SET-2 pathway, WC-independent frq
Introduction
Despite evolutionary distance, circadian clock oscillators are conserved among the organisms to perform their cellular and behavioral activities. The eukaryotic circadian clock oscillator contains positive and negative elements that form auto-regulatory negative feedback loops (2–8).
In the Neurospora circadian negative feedback loop, the GATA-family transcription factors WHITE COLLAR-1 (WC-1)3 and WHITE COLLAR-2 (WC-2) serve as positive elements. Typically, WC-1 and WC-2 form a heterodimer through their Per-Arnt-Sim (PAS) domain and rhythmically bind the promoter of the clock gene frequency (frq) to activate its transcription. FREQUENCY (FRQ) and its partner FRQ-interacting RNA helicase (FRH) act as negative elements. FRQ is the core factor in Neurospora oscillator, which determines the normal clock rhythm (9–16). The translated FRQ interacts with FRH and forms the FFC (FRQ-FRH complex) to inhibit its own transcription by suppressing the activity of the WC complex. FRQ is progressively phosphorylated and degraded through the ubiquitin-proteasome pathway. Degradation of FRQ derepresses the WC complex, initiating a new circle of frq transcription (14, 17–19). The circadian oscillator creates a robust rhythmic system with a period of ∼22 h in Neurospora crassa (17, 20). Rhythmic activation and repression of frq transcription result in cyclic changes of frq mRNA abundance. In Neurospora, histone modifiers and chromatin remodelers are required for rhythmic transcription of frq gene and clock-controlled genes (1, 9, 22–24). However, the mechanism of transcriptional regulation of frq by different histone modifications is not fully understood.
Although frq transcription is WC-dependent, our previous studies have shown that frq can be transcribed in a WC-independent manner in the absence of transcriptional corepressors, RCO-1 or RCM-1 (1, 25). Unlike the WC-dependent frq transcription, WC-independent frq transcription is constitutive, which disrupts the rhythmical WC-dependent frq transcription through interference the negative feedback loop. Moreover, our results indicate that histone modifications are required to suppress the WC-independent frq transcription. In rco-1KO mutant, the histone modifications at frq locus are changed, including increased H3K4me3 and H3K9ac, and decreased H3K36me3.
SET-2 is the histone H3K36 methyltransferase in Neurospora, which is required for growth and development (26). The homolog of SET-2 in yeast Set2, it directly regulates gene transcription through interacting with phosphorylated RNA polymerase II CTD. During transcription elongation, Set2-mediated H3K36me3 is recognized by the histone deacetylase complex Rpd3S. Rpd3S complex removes the acetyl modification from the histones and maintains the gene body in a hypoacetylation status (27–30). Meanwhile, Set2-mediated H3K36me3 affects the interaction between histone H3 and its chaperones and suppresses histone exchange in nucleosomes of transcribed genes. Histone modifications directly affect the chromatin structures, which play important roles in regulating gene transcription. Soluble histones are predominantly acetylated and increased histone exchange lead to the accumulation of histone acetylation on chromatin. Thus, loss of H3K36me3 results in increased histone acetylation at the open reading frame (ORF) of genes and cryptic transcription from promoters within ORFs (31, 32). Our previous study indicates that RCO-1 functions to regulate H3K36 methylation levels at the frq locus to suppress WC-independent frq transcription. Consistent with the decreased H3K36me3 and increased H3K9ac at frq locus in rco-1KO mutant, deletion of set-2 gene results in WC-independent frq transcription and impaired circadian rhythm (1), suggesting that crosstalk of different histone modifications correlates with the rhythmic transcription of frq gene. However, the mechanism that SET-2 represses the WC-independent frq transcription remains unknown.
Here we show that SET-2 is rhythmically recruited to the frq gene body and leads to rhythmic H3K36 methylation at frq locus. Loss of SET-2 results in elevated histone acetylation at frq ORF, which makes the chromatin more accessible for WC-independent frq transcription and disrupts the circadian clock rhythm. Thus, SET-2 is implicated in establishing a permissive chromatin state for circadian transcription of frq gene.
Experimental Procedures
Strains and Culture Conditions
The 87-3 (bd, a) was used as the wild-type strain in this study. The bd ku70RIP strain got previously (14) was used as the host strain for generating the rpd-3 or eaf-3 knock-out mutants and H3K36R or H3K9QK14QK18Q knock-in mutants. The wc-1RIP, wc-2RIP, and set-2KO strains made before (1, 11, 33, 34) were also included in this study. The newly created strains were rpd-3KO wc-1RIP, eaf-3KO wc-2RIP, H3K9QK14QK18Q wc-1RIP double mutants. Liquid culture conditions were the same as recommended (35).
Race Tube Assay
Race tube medium contained 1×Vogel's salts, 0.1% glucose, 0.17% arginine, 50 ng/ml biotin, and 1.5% agar. Conidia of different strains were inoculated at one end of each race tube and were grown under constant light (LL) for 1 day to synchronize the clock. Race tubes were then transferred to constant darkness (DD) and the position of the advancing mycelia front was marked at 24-h intervals on the tube. When growth was completed, tubes were scanned and the period of each strain was calculated (2).
Generation of H3K36R and H3K9QK14QK18Q Mutant Strains
H3K36R and H3K9QK14QK18Q mutant strains were generated as described previously (36). A knock-in cassette containing the mutated histone H3 gene and a hygromycin resistance gene (hph) inserted downstream of the 3′-untranslated region (UTR) of the H3 gene was transformed into a ku70RIP genetic background strain and hygromycin-resistant transformants were selected by hygromycin B. The homokaryotic strains were obtained by microconidia purification and confirmed by DNA sequencing. The H3K9QK14QK18Q wc-1RIP double mutants were obtained by crossing.
Generation of Antiserum against SET-2
GST-SET-2 (containing SET-2 amino acids 308–568) fusion protein was expressed in BL21 cells and the recombinant protein was purified and used as the antigen to generate rabbit polyclonal antiserum as described previously (25, 36, 37).
Chromatin Immunoprecipitation
ChIP assay was performed as described previously (1, 25, 36, 38). Briefly, Neurospora tissues under the experimental conditions were fixed with 1% formaldehyde for 15 min at 25 °C with shaking, the fixation reaction were stopped by adding 125 mm glycine with shaking for another 5 min. The cross-linked tissues are grinded and resuspended at 1 g/8 ml in lysis buffer containing protease inhibitors (1 mm PMSF, 1 μg/ml leupeptin, and 1 μg/ml pepstatin A). Chromatin was sheared by sonication to ∼200-500-bp fragments. 1 ml of protein (2 mg/ml) was used for each immunoprecipitation reaction, and 10 μl was kept as the input DNA. ChIP assay was carried out with 10 μl of SET-2 antibody, 5 μl of H3 antibody (CST 2650), 3 μl of H3K36me3 antibody (abcam ab9050), 4 μl of H3ac antibody (Millipore 06-599) or 10 μl of H4ac antibody (Millipore 06-598). Immunoprecipitated DNA was quantified using real-time PCR. The primer sequences used are C-Box (5′-GTCAAGCTCGTACCCACATC-3′ and 5′-CCGAAAGTATCTTGAGCCTCC-3′), PLRE (5′-CGGACGACGGCTGGCCAATTAG-3′ and 5′-TCGTGCTCTCTTGCTCACTTTCC-3′), TSS (5′- GAGGAACCAGAACGTAGCAG-3′ and 5′-GCAGGATAAACGGAGAAATGAC-3′), ORF 5′ (5′-TTACTTCATCTTCCGCACTGG-3′ and 5′-GGCAGGGTTACGATTGGATT-3′), ORF m (5′-GGACACCTTTCATTACAAACCG-3′ and 5′-TCCGCTAAAATCCCACTTCG-3′), ORF 3′ (5′-GATACCGAGACTGATGTGCG-3′ and 5′-AGCATGTCCACCTCTTTTCC-3′), 3′ UTR (5′-GAGAGCAAAAGGAACGCATTG-3′ and 5′-CTCCCCTGAAAATGGCAAAAG-3′), ncu05093 (5′-CAAACACAGCAACACTCCAG-3′ and 5′-CAGCCAATACCTCTATCCCAG-3′). ChIP-qPCR (quantitative PCR) data were normalized by the input and presented as percentage of input DNA. Each experiment was independently performed at least three times.
Protein and RNA Analyses
Protein extraction, quantification and Western blot analysis were performed as described previously (39, 40). Western blot analyses were performed by using antibodies against the proteins of interest. Equal amounts of total protein (40 μg) were loaded in each protein lane. After electrophoresis, proteins were transferred onto PVDF membrane, and Western blot analysis was performed. RNA was extracted as described previously and then analyzed by Northern blotting (26). For Northern blot analysis, equal amounts of total RNA (20 μg) were loaded onto 1.3% agarose gels for electrophoresis, the RNAs were transferred to nylon membrane. The membranes were probed with RNA probes specific for frq or ccg-1 gene.
Luciferase Reporter Assay
The luciferase reporter assay was performed as reported previously (41). The 87-3 (bd, a), frq-luc strain was used as control strain in this study. The rpd-3KO or H3K9QK14QK18Q strains were crossed with the 87-3 (bd, a), frq-luc strain to obtain the rpd-3KO, frq-luc strain and H3K9QK14QK18Q, frq-luc strain. The luciferase reporter construct was transformed into H3K36R (bd, a) strain to obtain H3K36R, frq-luc strain. LumiCycle (ACTIMETRICS) and the AFV (autoclaved FGS-Vogel's) medium (1×FGS, 1×Vogel's medium, 50 μg/liter biotin, and 1.8% agar) containing 50 μm firefly luciferin (BioSynt L-8200 d-luciferin firefly [synthetic] potassium salt) were used for the luciferase assay. Conidia suspensions were placed on AFV medium and grown in constant light (LL) overnight to synchronize the clock. The cultures were then transferred to constant darkness, and luminescence was recorded in real time using a LumiCycle after 1 day in DD (under our experimental condition, luciferase signals are highly variable during the first day in the LumiCycle and become stabilized afterward, which is likely due to an artifact caused by the light-dark transfer of the cultures). The data were then normalized with LumiCycle Analysis software by subtracting the baseline luciferase signal, which increases as cell grows.
Results
SET-2 Is Rhythmically Recruited to frq Gene and Results in Rhythmic H3K36me3 at frq Locus
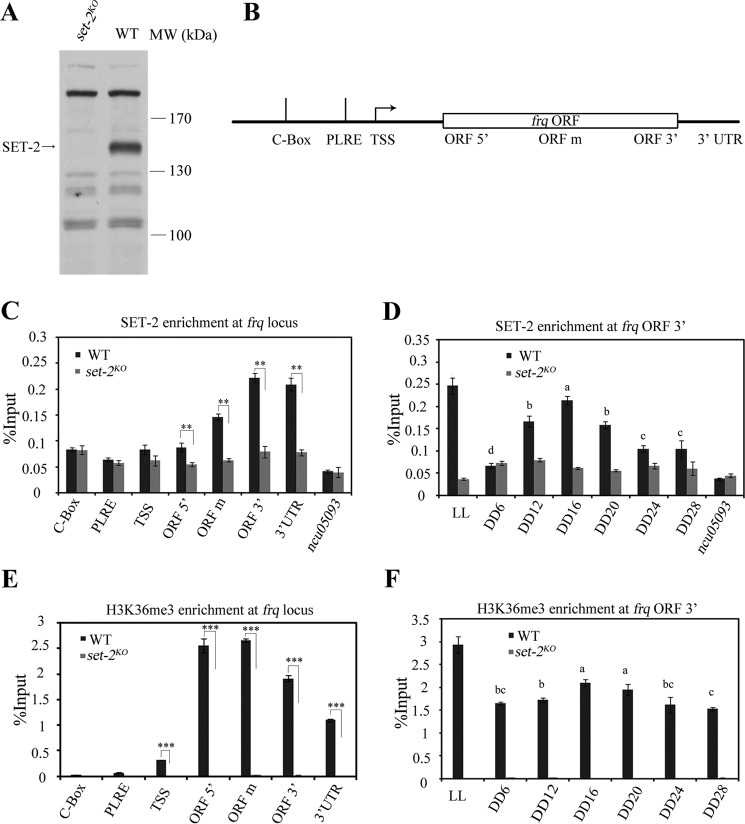
Our previous results showed that SET-2 plays an important role in Neurospora circadian system (1). However, the underlying mechanism is not clear. Several studies in yeast exhibited that Set2 is recruited to the open reading frame (ORF) of actively transcribed genes by interacting with phosphorylated CTD of RNA polymerase II (27, 42). To test whether SET-2 directly regulates frq transcription, chromatin immunoprecipitation (ChIP) assay was performed by using the SET-2-specific polyclonal antibody (Fig. 1A). As there are nonspecific bands recognized by SET-2 antibody, set-2KO strain was used as the negative control in the ChIP assay. At DD16 (constant darkness), a time point when frq transcription peaks, the signal of SET-2 toward the 3′-end of frq gene in the wild-type strain was significantly higher than that in the set-2KO strain, while no SET-2 enrichment was detected at the promoter regions of frq and ncu05093 genes (used as a negative control here) in both strains. These results indicate that SET-2 is recruited to frq locus during transcription (Fig. 1, B and C). Moreover, the enrichment of SET-2 at the 3′ region of frq ORF was rhythmic in different circadian time points (Fig. 1D). In agreement with these results, H3K36 trimethylation (H3K36me3) was rhythmic at increased levels toward the 3′ end of frq in the wild-type strain in DD (Fig. 1, E and F). These results suggest that SET-2 is enriched at frq locus and directly regulates frq transcription.
FIGURE 1.
SET-2 is recruited to frq gene and regulates its transcription by catalyzing H3K36me3. A, Western blot analysis showing a specific band present in the wild-type strain but not the set-2KO strain. The arrow points out the SET-2 protein band detected by our SET-2 antibody. WT, wild-type; MW, molecular weight. B, schematic depiction of the frq locus. C, ChIP analysis showing the enrichment of SET-2 at the frq locus in DD16. ChIP experiment performed on chromatin isolated at DD16 when frq expression is maximal by using the SET-2 antibody. The amount of DNA associated with SET-2 was determined by qPCR. D, rhythmic association of SET-2 at the 3′-end of frq gene in different time points. Samples were grown for the indicated hours in constant darkness (DD) prior to harvesting and processing for ChIP. LL, constant light. E, ChIP analysis shows that the H3K36me3 levels are increased at the 3′-end of frq in the wild-type strain. F, rhythmic changes of H3K36 trimethylation at the 3′-end of frq gene in different time points. The set-2KO strain was used as a negative control in the ChIP assay, no SET-2 binding at ncu05093 promoter in C and D also acts as a negative control. Error bars show the mean ± S.D. (n = 3). Significance in C and E was assessed by using a two-tailed t test. *, p < 0.05, **, p < 0.01; ***, p < 0.001. The statistical analysis was also performed on wild-type from DD6 to DD24 in D and F, bars with different letters in D and F are statistically significant, p < 0.05, one-way Anova and Tukey's HSD test.
SET-2-mediated H3K36me Is Required for Neurospora Circadian Rhythm
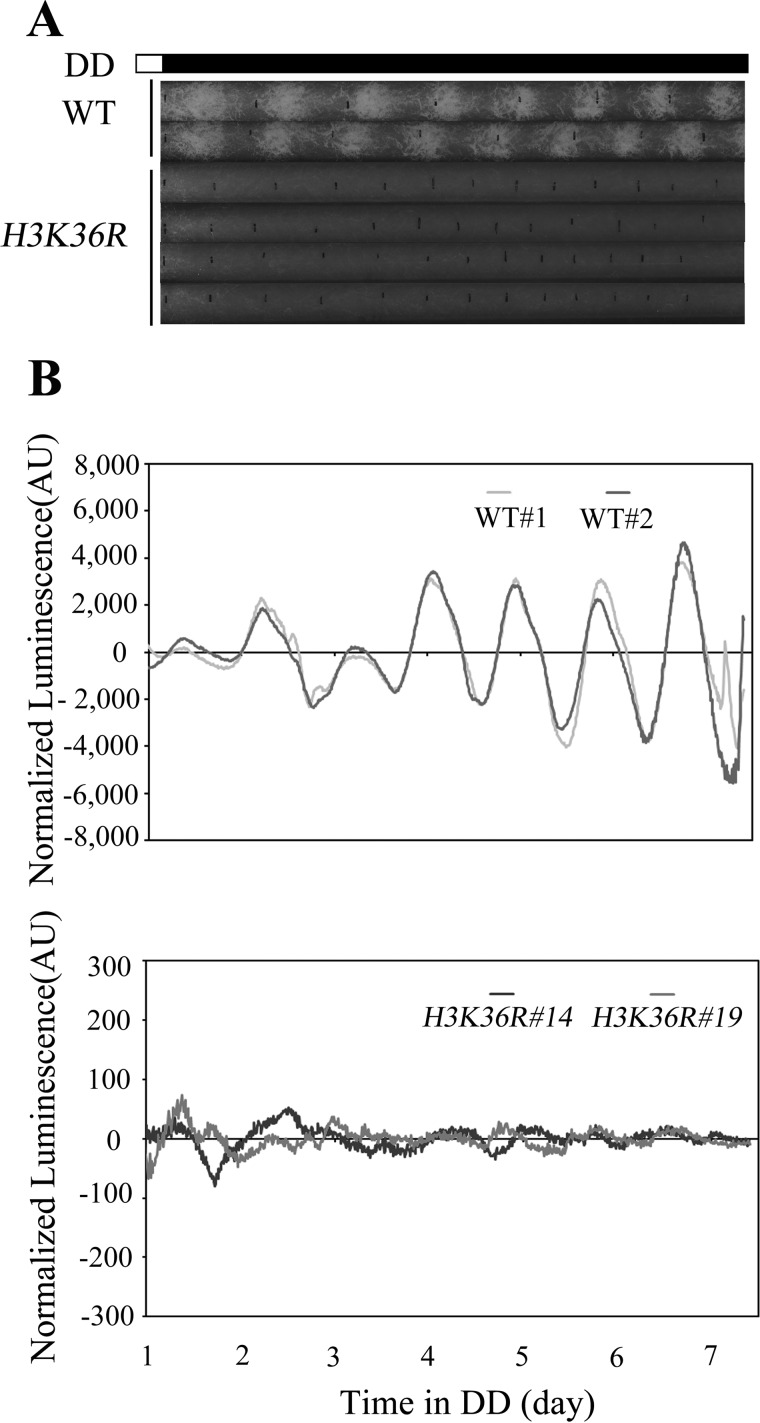
To explore whether the effect of SET-2 on circadian clock is mediated by histone methylation, histone H3K36R mutant strains were generated by substituting H3 lysine 36 with arginine at its endogenous locus (14, 36). Comparison of the H3K36R strain to wild-type on race tubes indicated an apparent defect of conidiation formation and a reduced hyphae growth rate in H3K36R mutants (Fig. 2A). To further define the function of H3K36 methylation, we introduced a frq promoter-driven luciferase reporter into the H3K36R strain to examine the frq promoter activity (43). As in the set-2KO mutant (1), the robust bioluminescence rhythm of the wild-type was abolished in H3K36R strains (Fig. 2B). These results suggest that H3 lysine 36 is the crucial physiological substrate of SET-2, and the methylation on this residue is required for the Neurospora circadian rhythm.
FIGURE 2.
H3K36 methylation is required for circadian rhythm. Race tube assay of the wild-type and H3K36R strains is shown in A, vertical black lines on race tubes mark daily growth fronts of the strains. The conidiation rhythm in H3K36R strains is lost compared with wild-type strains. Four independent H3K36R strains were shown in the figure. B, analyses of luciferase activity in wild-type, frq-luc, and H3K36R, frq-luc strains show that the luciferase activity rhythm is impaired in the H3K36R, frq-luc strains. Three independent H3K36R, frq-luc strains were used to test luciferase activity, and the result of two strains was shown in the figure. The low normalized luciferase signal levels in the H3K36R, frq-luc strain reflect the low-amplitude fluctuation of luciferase activity. Different strains' conidia suspension was placed on AFV medium and luminescence was recorded in real time using a LumiCycle. Raw data were normalized to subtract the baseline calculated by LumiCycle analysis software.
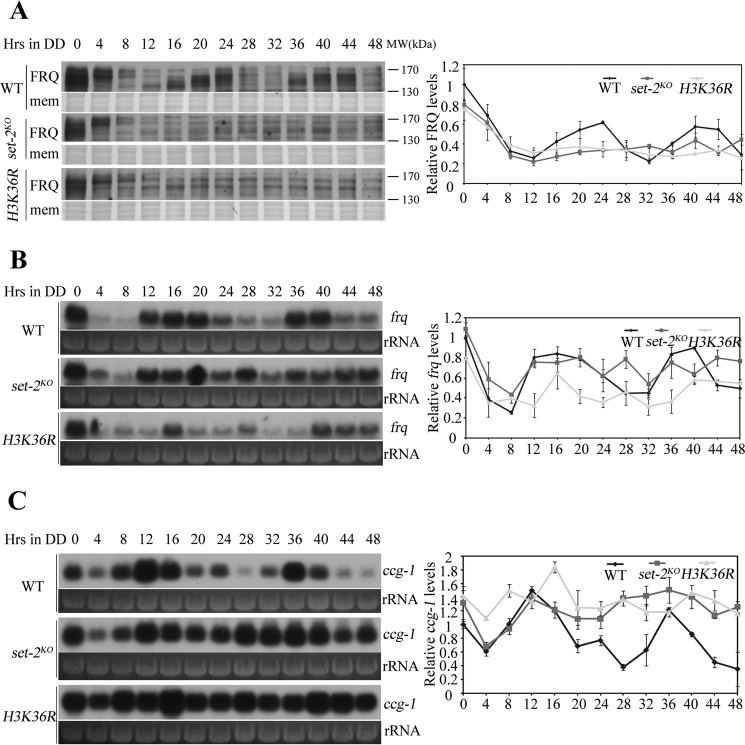
To determine the role of H3K36 methylation in circadian clock, the circadian rhythmicity in set-2KO and H3K36R strains were molecularly analyzed by examining the expression of FRQ protein and frq mRNA in constant darkness (DD). As shown in Fig. 3A, the wild-type strain exhibited robust rhythms at FRQ protein level and FRQ phosphorylation profile, but both rhythms were severely disrupted either in set-2KO or in H3K36R strains. Same as FRQ protein results, the circadian rhythm of frq mRNA abundance was abolished in the set-2KO and H3K36R mutants (Fig. 3B). Defects of the core circadian oscillator would affect the rhythmic expression of genes in the output pathway. Indeed, the rhythm of clock-controlled gene ccg-1 was eliminated in set-2KO and H3K36R strains (Fig. 3C). Taken together, these data demonstrate that H3K36 methylation is required for sustained clock rhythmicity in Neurospora.
FIGURE 3.
H3K36 methylation is essential for the Neurospora circadian clock. A, Western blot analysis shows that the circadian oscillation of FRQ in set-2KO and H3K36R is impaired when compared with wild-type strain. Samples were grown in constant darkness (DD) for indicated hours before harvest. The Coomassie Blue-stained membranes (mem) represent total protein in each sample and act as a loading control. Quantification of the FRQ protein is shown on the right side of Western blot. Northern blot analysis shows that frq (B) and ccg-1 (C) transcription are arrhythmic in set-2KO and H3K36R strains. Ribosome RNA (rRNA) bands stained by ethidium bromide shown below the Northern blot act as a loading control for each sample. Quantification of frq and ccg1 are shown on the right side of Northern blot. These experiments were performed at least three times, and one representative result is shown here.
Deletion of SET-2 Results in Hyperacetylation of frq Open Reading Frame (ORF)
Why H3K36me3 is required for proper frq transcription? In Saccharomyces cerevisiae, Set2-mediated H3K36me3 maintains a hypoacetylation state of transcribed genes (28, 29, 31, 32), especially for genes with long sequence and transcribed less often (44). Based on these studies, we wondered whether SET-2 affects the histone acetylation level at frq locus. As shown in Fig. 4, A and B, deletion of set-2 gene resulted in increased H3 and H4 acetylation levels over frq ORF, especially atits 3′ end. These results suggest that SET-2-mediated H3K36 methylation suppresses histone acetylation at frq ORF region.
FIGURE 4.
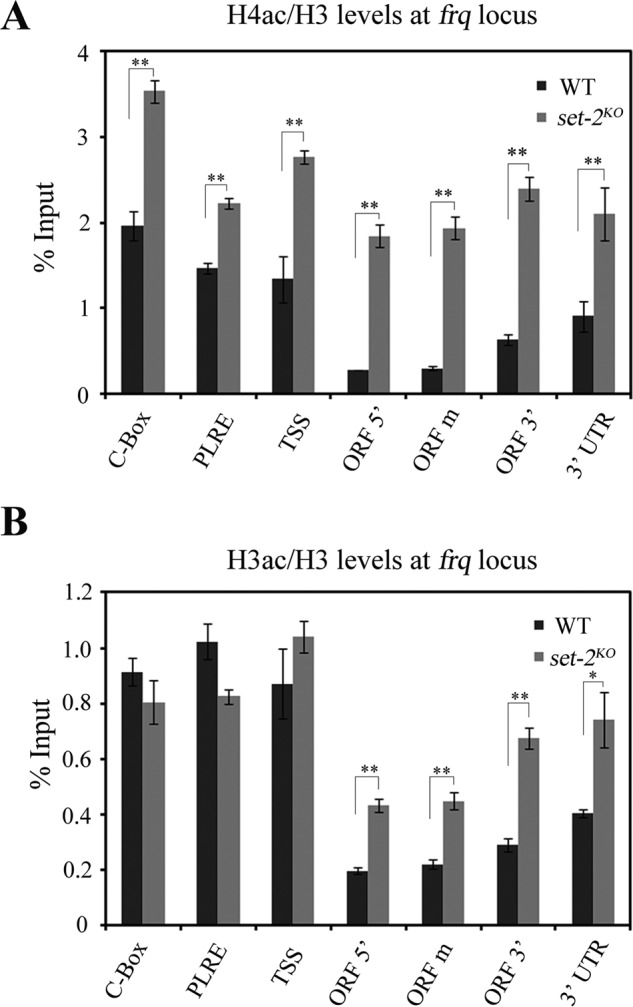
SET-2 directs the histone acetylation state of frq ORF. ChIP analyses of H4ac (A) and H3ac (B) occurring at the frq locus. Both H4ac and H3ac levels at frq ORF region are increased in set-2KO strains compared with wild-type. ChIP experiment was performed on chromatin isolated at DD16.The amount of DNA associated with H4ac or H3ac was determined by qPCR. Significance was assessed by using a two-tailed t test. *, p < 0.05; **, p < 0.01. Error bars show the mean ± S.D. (n = 3).
Rpd3S-mediated Deacetylation of frq Coding Region Suppresses WC-independent frq Expression
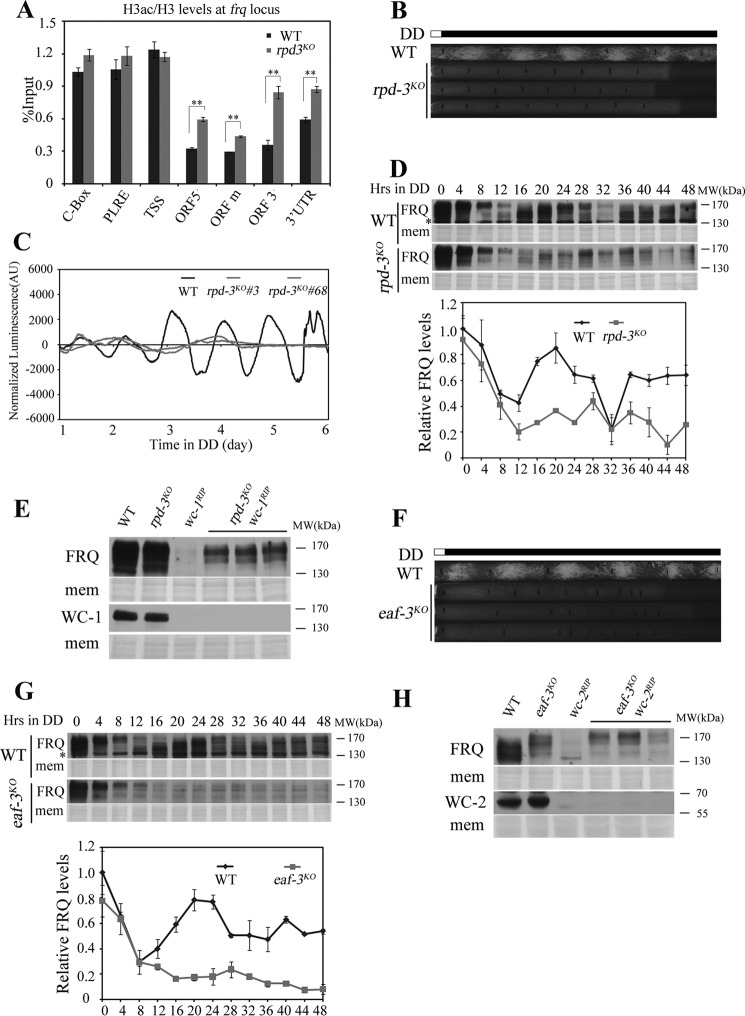
The deacetylase complex Rpd3S can remove histone acetylation at the coding region of transcribed genes through recognizing histone H3K36me (28, 29). To explore whether H3K36me-dependent deacetylation of frq coding region is required for proper frq transcription, we deleted the histone deacetylase gene rpd-3 (ncu00824) from the Neurospora genome. As illustrated in the set-2KO strain, the histone H3 acetylation levels were elevated after deletion of rpd-3 (Fig. 5A). The race tube assay showed that disruption of rpd-3 gene resulted in loss of circadian conidiation rhythm (Fig. 5B). We also noticed the slow growth rate of rpd-3KO mutants with sparse conidia. So the luciferase reporter driven by frq promoter was introduced into the rpd-3KO strain to confirm its phenotype at the molecular level. Fig. 5C showed that the robust circadian rhythm of luciferase activity in the wild-type was abolished in rpd-3KO strains. Western blot analysis confirmed the disrupted clock function of the rpd-3KO mutant as both the FRQ protein level and FRQ phosphorylation profile rhythms were lost (Fig. 5D). The impaired circadian phenotype prompts us to detect WC-independent frq expression in the rpd-3KO strain. The rpd-3KO wc-1RIP double mutant strains were generated, and FRQ expression was observed in these double mutants but not in wc-1RIP single mutant (Fig. 5E). These results indicate that histone deacetylase RPD-3 is required for suppressing WC-independent frq expression.
FIGURE 5.
Rpd3S-mediated deacetylation of frq coding region suppresses WC-independent frq expression. A, H3ac at the frq locus was examined by ChIP analysis in wild-type and rpd-3KO strains at DD16. The H3 acetylation levels at frq ORF region are higher in rpd-3KO strain than wild-type. Significance was assessed by using a two-tailed t test. *, p < 0.05; **, p < 0.01. Error bars show the mean ± S.D. (n = 3). B, conidiation rhythmicity is lost in rpd-3KO strains in the race tube assay. Vertical black lines on race tubes mark daily growth fronts of the strains. Three independent rpd-3KO strains run on race tube were shown in the figure. C, luciferase reporter assay showing the normalized frq promoter activity of wild-type, frq-luc and rpd-3KO, frq-luc strains. Three independent rpd-3KO, frq-luc strains were used to test luciferase activity and the result of two strains was shown in the figure. D, Western blot analysis shows the circadian oscillation of FRQ in rpd-3KO is impaired when compared with wild-type strain. Samples were grown in darkness (DD) for indicated hours before harvest. The Coomassie Blue-stained membranes (mem) represent total protein in each sample. Asterisks indicate nonspecific bands. The experiment was performed independently three times and one representative result was shown in the figure. E, Western blot analyses were performed using antibodies against FRQ or WC-1 in the wild-type, rpd-3KO, wc-1RIP, and rpd-3KO wc-1RIP strains. Samples were grown under constant light before harvest and three different rpd-3KO wc-1RIP double mutants were used. The Coomassie Blue-stained membranes (mem) represent total protein in each sample. F, race tube assay of wild-type and eaf-3KO strains. Three independent eaf-3KO strains were shown in the figure. G, Western blot analysis showing the circadian oscillation of FRQ in the wild-type and eaf-3KO strains. Both FRQ protein and phosphorylation rhythm in wild-type are abolished in the eaf-3KO strain. Samples were grown in constant darkness (DD) for indicated hours before harvest. The Coomassie Blue-stained membranes (mem) represent total protein in each sample. Asterisks indicate nonspecific bands. The experiment was performed independently three times and one representative result was shown in the figure. H, Western blot analyses were performed using antibodies against FRQ or WC-2 in the wild-type, eaf-3KO, wc-2RIP, and eaf-3KO wc-2RIP strains. Samples were grown under constant light before harvest and three different eaf-3KO wc-2RIPdouble mutants were used. The Coomassie Blue-stained membranes (mem) represent total protein in each sample.
In budding yeast, the recognition of methylated H3K36 by Rpd3S complex requires the Eaf3 subunit (28, 30). To examine whether the suppression of WC-independent frq expression by RPD-3 is dependent on Rpd3S complex, the eaf-3KO (ncu06787) strain was created. Like rpd-3KO strains, the conidiation rhythm was abolished in the eaf-3KOmutants (Fig. 5F), along with the loss of rhythmic FRQ expression (Fig. 5G). In addition, FRQ protein was detectable in eaf-3KO wc-2RIP double mutant strains (Fig. 5H), suggesting that the Rpd3S complex is involved in suppressing WC-independent frq expression.
Sustained Hyperacetylation of Histone H3 Results in Arrhythmic Circadian Clock
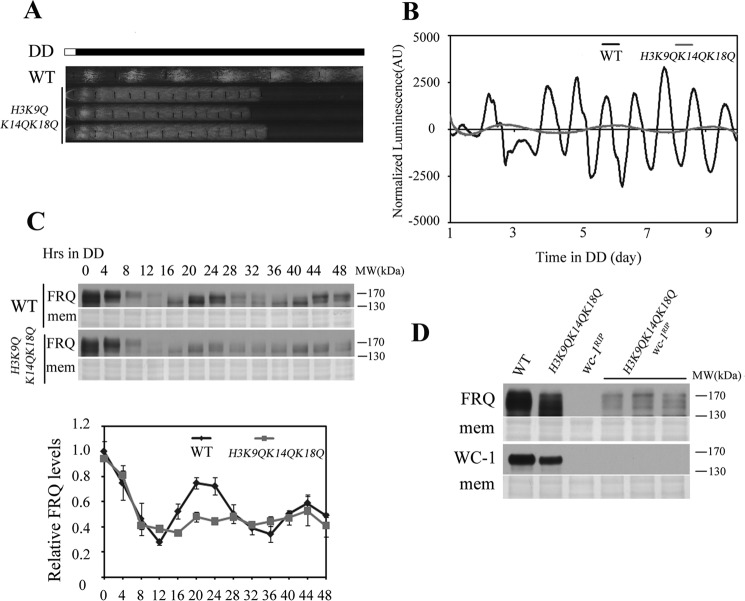
Previous study has shown that Neurospora RPD-3 affects the acetylation levels of histone H3 on Lys-9, Lys-14, and Lys-18 (45). To test whether histone acetylation at these sites is essential for circadian clock, the histone H3 lysine 9, 14, and 18 were substituted with glutamine to mimic hyperacetylated histone H3. In the race tube assay, H3K9QK14QK18Q mutant showed arrhythmic conidiation formation compared with wild-type strain (Fig. 6A). The luciferase assay showed that the rhythmic luciferase transcription driven by frq promoter in H3K9QK14QK18Q strains were dramatically impaired (Fig. 6B). Consistent with the luciferase data, rhythmic FRQ protein expression profile was also dampened (Fig. 6C), indicating that H3K9QK14QK18Q mutation affects Neurospora circadian clock. Similar to that in rpd-3KO wc-1RIP double mutants (Fig. 5E), WC-independent frq expression was detected in H3K9QK14QK18Q wc-1RIP strains (Fig. 6D). These results suggest that histone acetylation is important in maintaining Neurospora circadian clock by regulating WC-independent frq expression.
FIGURE 6.
The mimic acetylation states of histone H3 regulate frq transcription. A, H3K9QK14QK18Q strain lost the robust conidiation rhythm compared with WT in the race tube assay. Three independent H3K9QK14QK18Q strains were shown in the figure. B, analyses of luciferase activity in wild-type, frq-luc and H3K9QK14QK18Q, frq-luc strains. Three independent H3K9QK14QK18Q, frq-luc strains were used to test the luciferase activity and all strains lost the rhythmic luciferase activity compared with wild-type. One wild-type, frq-luc and one H3K9QK14QK18Q, frq-luc strain result was shown in the figure. C, Western blot analysis showing the circadian oscillation of FRQ in the wild-type and H3K9QK14QK18Q mutant strains. Samples were grown in constant darkness (DD) for indicated hours before harvest. The Coomassie Blue-stained membranes (mem) represent total protein in each sample. The experiment was performed independently at least three times, and one representative result was shown in the figure. D, Western blot analyses were performed using antibodies against FRQ or WC-1 in the wild-type, H3K9QK14QK18Q, wc-1RIP, and H3K9QK14QK18Q wc-1RIPstrains. Samples were grown on constant light before harvest and three different H3K9QK14QK18Q wc-1RIP strains were used. The Coomassie Blue-stained membranes (mem) represent total protein in each sample.
Discussion
Rhythmic frq transcription is not only a consequence but also a major basis for the circadian negative feedback loop in Neurospora. Our previous work has shown that there is WC-independent frq transcription in rco-1KO mutants (1). Moreover, the constitutive transcription of WC-independent frq interferes with the negative feedback loop and disrupts the circadian oscillator in rco-1KO mutants. Therefore, it is necessary to repress WC-independent frq transcription for normal clock function. Obviously, it is of great importance to find out how this is achieved in the wild-type strain.
In this study, we found that maintaining a proper histone acetylation status at frq locus is essential for the repression of the WC-independent frq transcription. First, loss of SET-2 led to increased histone acetylation levels at the frq locus and WC-independent frq transcription (1). Second, deletion of rpd-3, subunit of the Rpd3S complex, also resulted in hyperacetylation at frq locus. Last, frq could be expressed independent of WC complex in the H3K9QK14QK18Q mutant, which mimics hyperacetylation of histone H3. In mammals, histone acetylation was found to be important in regulating circadian gene transcription (46–49). However, what we found here is different from those studies. Previous studies mainly focus on the rhythmic changes of histone acetylation at the promoter region of clock genes (46–51), but in our system the histone acetylation at ORF of the clock gene is important for its transcription.
In yeast, Set-2 regulates the histone acetylation level of transcribed gene through different pathways. One is by recruiting the Rpd3S complex, and another one is by suppressing histone exchange through chromatin remodelers Isw1 (imitation switch protein 1) and Chd1 (chromo-domain helicase DNA-binding protein 1) (28, 29, 31, 32). In Neurospora, SET-2 functions through similar pathways to maintain a proper histone acetylation status at frq locus during transcription. As shown in this work, deletion of rpd-3 led to increased histone acetylation levels and WC-independent frq expression. Our previous study showed that chromo-domain helicase DNA-binding protein 1 (CHD-1) was also required for the repression of the WC-independent frq transcription in Neurospora (1), suggesting that histone exchange is also important in maintaining hypoacetylated histone pattern at frq locus. These results suggest that the Rpd3S complex and CHD-1 may work together to maintain a proper acetylation status across frq ORF.
The SET-2 protein and its activity are conserved across species. Obviously, frq is not the only gene that SET-2 directly regulated. In 2005, Adhvaryu et al. have found that SET-2 is required for Neurospora growth and development (26), indicating that SET-2 regulate genes in these processes. Furthermore, previous study in Saccharomyces cerevisiae has shown that ∼25% of the total genome displayed a significant increase of H4ac following deletion of Set-2. The genes with longer sequence and less often transcribed display a stronger dependence on the Set2-Rpd3S pathway (44). These studies indicate that SET-2 may have a global effect on gene regulation. In this study, our results showed that similar yeast SET-2 pathway exists in Neuropora, but the function of this pathway on frq transcription is quite different from those described in yeast. One major function of the Set-2 pathway in yeast is to suppress cryptic transcription originated from transcribed ORF. However, cryptic transcripts from frq locus were not found in the set-2KO mutants (21). Instead, SET-2 is required to regulate the transcription of the full-length frq gene, which is supposed to be transcribed from its normal promoter (based on the length of frq mRNA in wild-type and set-2KO mutant strains).
Author Contributions
G. S., Z. Z., X. L., and Q. H. designed research; G. S., Z. Z., X. L., K. G., Q. L., and J. C. performed research; G. S., Z. Z., X. L., F. N. K., Y. W., and Q. H. analyzed data; Z. Z., G. S., and Q. H. wrote the paper.
Acknowledgment
We thank Yajun Wang for critical reading of the manuscript.
This project was supported by grants from a project supported by the State Key Program of National Natural Science of China (31330004) and National Basic Research Program of China (973 Program) Grant (2012CB947600) (to Q. H.). The authors declare that they have no conflicts of interest with the contents of this article.
- WC
- WHITE COLLAR
- frq
- frequency
- ORF
- open reading frame
- UTR
- untranslated region
- C-Box
- clock box
- PLRE
- proximal light-regulated element
- TSS
- transcription start site
- qPCR
- quantitative PCR.
References
- 1. Zhou Z., Liu X., Hu Q., Zhang N., Sun G., Cha J., Wang Y., Liu Y., and He Q. (2013) Suppression of WC-independent frequency transcription by RCO-1 is essential for Neurospora circadian clock. Proc. Natl. Acad. Sci. U.S.A. 110, E4867–E4874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baker C. L., Loros J. J., and Dunlap J. C. (2012) The circadian clock of Neurospora crassa. FEMS Microbiol. Rev. 36, 95–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bell-Pedersen D., Cassone V. M., Earnest D. J., Golden S. S., Hardin P. E., Thomas T. L., and Zoran M. J. (2005) Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat. Rev. Genet. 6, 544–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dunlap J. C. (1999) Molecular bases for circadian clocks. Cell 96, 271–290 [DOI] [PubMed] [Google Scholar]
- 5. Dunlap J. C. (2006) Proteins in the Neurospora circadian clockworks. J. Biol. Chem. 281, 28489–28493 [DOI] [PubMed] [Google Scholar]
- 6. Heintzen C., and Liu Y. (2007) The Neurospora crassa circadian clock. Adv. Genet. 58, 25–66 [DOI] [PubMed] [Google Scholar]
- 7. Liu Y., and Bell-Pedersen D. (2006) Circadian rhythms in Neurospora crassa and other filamentous fungi. Eukaryot. Cell 5, 1184–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Young M. W., and Kay S. A. (2001) Time zones: a comparative genetics of circadian clocks. Nat. Rev. Genet. 2, 702–715 [DOI] [PubMed] [Google Scholar]
- 9. Belden W. J., Loros J. J., and Dunlap J. C. (2007) Execution of the circadian negative feedback loop in Neurospora requires the ATP-dependent chromatin-remodeling enzyme CLOCKSWITCH. Mol. Cell 25, 587–600 [DOI] [PubMed] [Google Scholar]
- 10. Cheng P., Yang Y., Gardner K. H., and Liu Y. (2002) PAS domain-mediated WC-1/WC-2 interaction is essential for maintaining the steady-state level of WC-1 and the function of both proteins in circadian clock and light responses of Neurospora. Mol. Cell. Biol. 22, 517–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng P., Yang Y., and Liu Y. (2001) Interlocked feedback loops contribute to the robustness of the Neurospora circadian clock. Proc. Natl. Acad. Sci. U.S.A. 98, 7408–7413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crosthwaite S. K., Dunlap J. C., and Loros J. J. (1997) Neurospora wc-1 and wc-2: transcription, photoresponses, and the origins of circadian rhythmicity. Science 276, 763–769 [DOI] [PubMed] [Google Scholar]
- 13. Froehlich A. C., Loros J. J., and Dunlap J. C. (2003) Rhythmic binding of a WHITE COLLAR-containing complex to the frequency promoter is inhibited by FREQUENCY. Proc. Natl. Acad. Sci. U.S.A. 100, 5914–5919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. He Q., Cha J., He Q., Lee H. C., Yang Y., and Liu Y. (2006) CKI and CKII mediate the FREQUENCY-dependent phosphorylation of the WHITE COLLAR complex to close the Neurospora circadian negative feedback loop. Genes Dev. 20, 2552–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He Q., and Liu Y. (2005) Molecular mechanism of light responses in Neurospora: from light-induced transcription to photoadaptation. Genes Dev. 19, 2888–2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Loros J. J., and Dunlap J. C. (2001) Genetic and molecular analysis of circadian rhythms in Neurospora. Annu. Rev. Physiol. 63, 757–794 [DOI] [PubMed] [Google Scholar]
- 17. Garceau N. Y., Liu Y., Loros J. J., and Dunlap J. C. (1997) Alternative initiation of translation and time specific phosphorylation yield multiple forms of the essential circadian clock protein FREQUENCY. Cell 89, 469–476 [DOI] [PubMed] [Google Scholar]
- 18. Yang Y., Cheng P., and Liu Y. (2002) Regulation of the Neurospora circadian clock by casein kinase II. Genes Dev. 16, 994–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He Q., Cheng P., Yang Y., He Q., Yu H., and Liu Y. (2003) FWD1-mediated degradation of FREQUENCY in Neurospora establishes a conserved mechanism for circadian clock regulation. EMBO J. 22, 4421–4430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aronson B. D., Johnson K. A., Loros J. J., and Dunlap J. C. (1994) Negative feedback defining a circadian clock: autoregulation of the clock gene frequency. Science 263, 1578–1584 [DOI] [PubMed] [Google Scholar]
- 21. Xue Z., Ye Q., Anson S. R., Yang J., Xiao G., Kowbel D., Glass N. L., Crosthwaite S. K., and Liu Y. (2014) Transcriptional interference by antisense RNA is required for circadian clock function. Nature 514, 650–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Belden W. J., Lewis Z. A., Selker E. U., Loros J. J., and Dunlap J. C. (2011) CHD1 remodels chromatin and influences transient DNA methylation at the clock gene frequency. PLoS Genet. 7, e1002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raduwan H., Isola A. L., and Belden W. J. (2013) Methylation of histone H3 on lysine 4 by the lysine methyltransferase SET1 protein is needed for normal clock gene expression. J. Biol. Chem. 288, 8380–8390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cha J., Zhou M., and Liu Y. (2013) CATP is a critical component of the Neurospora circadian clock by regulating the nucleosome occupancy rhythm at the frequency locus. EMBO Rep. 14, 923–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu X., Li H., Liu Q., Niu Y., Hu Q., Deng H., Cha J., Wang Y., Liu Y., and He Q. (2015) Role for protein kinase A in the Neurospora circadian clock by regulating white collar-independent frequency transcription through phosphorylation of RCM-1. Mol. Cell. Biol. 35, 2088–2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Adhvaryu K. K., Morris S. A., Strahl B. D., and Selker E. U. (2005) Methylation of histone H3 lysine 36 is required for normal development in Neurospora crassa. Eukaryot. Cell 4, 1455–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li J., Moazed D., and Gygi S. P. (2002) Association of the histone methyltransferase Set2 with RNA polymerase II plays a role in transcription elongation. J. Biol. Chem. 277, 49383–49388 [DOI] [PubMed] [Google Scholar]
- 28. Carrozza M. J., Li B., Florens L., Suganuma T., Swanson S. K., Lee K. K., Shia W. J., Anderson S., Yates J., Washburn M. P., and Workman J. L. (2005) Histone H3 methylation by Set2 directs deacetylation of coding regions by Rpd3S to suppress spurious intragenic transcription. Cell 123, 581–592 [DOI] [PubMed] [Google Scholar]
- 29. Keogh M. C., Kurdistani S. K., Morris S. A., Ahn S. H., Podolny V., Collins S. R., Schuldiner M., Chin K., Punna T., Thompson N. J., Boone C., Emili A., Weissman J. S., Hughes T. R., Strahl B. D., Grunstein M., Greenblatt J. F., Buratowski S., and Krogan N. J. (2005) Cotranscriptional set2 methylation of histone H3 lysine 36 recruits a repressive Rpd3 complex. Cell 123, 593–605 [DOI] [PubMed] [Google Scholar]
- 30. Li B., Gogol M., Carey M., Lee D., Seidel C., and Workman J. L. (2007) Combined action of PHD and chromo domains directs the Rpd3S HDAC to transcribed chromatin. Science 316, 1050–1054 [DOI] [PubMed] [Google Scholar]
- 31. Smolle M., Venkatesh S., Gogol M. M., Li H., Zhang Y., Florens L., Washburn M. P., and Workman J. L. (2012) Chromatin remodelers Isw1 and Chd1 maintain chromatin structure during transcription by preventing histone exchange. Nat. Struct. Mol. Biol. 19, 884–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Venkatesh S., Smolle M., Li H., Gogol M. M., Saint M., Kumar S., Natarajan K., and Workman J. L. (2012) Set2 methylation of histone H3 lysine 36 suppresses histone exchange on transcribed genes. Nature 489, 452–455 [DOI] [PubMed] [Google Scholar]
- 33. Collett M. A., Dunlap J. C., and Loros J. J. (2001) Circadian clock-specific roles for the light response protein WHITE COLLAR-2. Mol. Cell. Biol. 21, 2619–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. He Q., Cheng P., Yang Y., Wang L., Gardner K. H., and Liu Y. (2002) White collar-1, a DNA binding transcription factor and a light sensor. Science 297, 840–843 [DOI] [PubMed] [Google Scholar]
- 35. Aronson B. D., Johnson K. A., and Dunlap J. C. (1994) Circadian clock locus frequency: protein encoded by a single open reading frame defines period length and temperature compensation. Proc. Natl. Acad. Sci. U.S.A. 91, 7683–7687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang S., Li W., Qi S., Gai K., Chen Y., Suo J., Cao Y., He Y., Wang Y., and He Q. (2014) The highly expressed methionine synthase gene of Neurospora crassa is positively regulated by its proximal heterochromatic region. Nucleic Acids Res. 42, 6183–6195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu H., Wang J., Hu Q., Quan Y., Chen H., Cao Y., Li C., Wang Y., and He Q. (2010) DCAF26, an adaptor protein of Cul4-based E3, is essential for DNA methylation in Neurospora crassa. PLoS Genet. 6, e1001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao Y., Shen Y., Yang S., Wang J., Hu Q., Wang Y., and He Q. (2010) Ubiquitin ligase components Cullin4 and DDB1 are essential for DNA methylation in Neurospora crassa. J. Biol. Chem. 285, 4355–4365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang J., Hu Q., Chen H., Zhou Z., Li W., Wang Y., Li S., and He Q. (2010) Role of individual subunits of the Neurospora crassa CSN complex in regulation of deneddylation and stability of cullin proteins. PLoS Genet. 6, e1001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou Z., Wang Y., Cai G., and He Q. (2012) Neurospora COP9 signalosome integrity plays major roles for hyphal growth, conidial development, and circadian function. PLoS Genet. 8, e1002712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou M., Guo J., Cha J., Chae M., Chen S., Barral J. M., Sachs M. S., and Liu Y. (2013) Non-optimal codon usage affects expression, structure and function of clock protein FRQ. Nature 495, 111–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kizer K. O., Phatnani H. P., Shibata Y., Hall H., Greenleaf A. L., and Strahl B. D. (2005) A novel domain in Set2 mediates RNA polymerase II interaction and couples histone H3 K36 methylation with transcript elongation. Mol. Cell. Biol. 25, 3305–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gooch V. D., Mehra A., Larrondo L. F., Fox J., Touroutoutoudis M., Loros J. J., and Dunlap J. C. (2008) Fully codon-optimized luciferase uncovers novel temperature characteristics of the Neurospora clock. Eukaryot. Cell. 7, 28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li B., Gogol M., Carey M., Pattenden S. G., Seidel C., and Workman J. L. (2007) Infrequently transcribed long genes depend on the Set2/Rpd3S pathway for accurate transcription. Genes Dev. 21, 1422–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Smith K. M., Dobosy J. R., Reifsnyder J. E., Rountree M. R., Anderson D. C., Green G. R., and Selker E. U. (2010) H2B- and H3-specific histone deacetylases are required for DNA methylation in Neurospora crassa. Genetics 186, 1207–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Asher G., Gatfield D., Stratmann M., Reinke H., Dibner C., Kreppel F., Mostoslavsky R., Alt F. W., and Schibler U. (2008) SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134, 317–328 [DOI] [PubMed] [Google Scholar]
- 47. Hirayama J., Sahar S., Grimaldi B., Tamaru T., Takamatsu K., Nakahata Y., and Sassone-Corsi P. (2007) CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature 450, 1086–1090 [DOI] [PubMed] [Google Scholar]
- 48. Katada S., and Sassone-Corsi P. (2010) The histone methyltransferase MLL1 permits the oscillation of circadian gene expression. Nat. Struct. Mol. Biol. 17, 1414–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nakahata Y., Kaluzova M., Grimaldi B., Sahar S., Hirayama J., Chen D., Guarente L. P., and Sassone-Corsi P. (2008) The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134, 329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Curtis A. M., Seo S. B., Westgate E. J., Rudic R. D., Smyth E. M., Chakravarti D., FitzGerald G. A., and McNamara P. (2004) Histone acetyltransferase-dependent chromatin remodeling and the vascular clock. J. Biol. Chem. 279, 7091–7097 [DOI] [PubMed] [Google Scholar]
- 51. Etchegaray J.-P., Lee C., Wade P. A., and Reppert S. M. (2003) Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature 421, 177–182 [DOI] [PubMed] [Google Scholar]