Abstract
Understanding the biosynthetic pathway of protein glycosylation in various expression cell lines is important for controlling and modulating the glycosylation profiles of recombinant glycoproteins. We found that expression of erythropoietin (EPO) in a HEK293S N-acetylglucosaminyltransferase I (GnT I)−/− cell line resulted in production of the Man5GlcNAc2 glycoforms, in which more than 50% were core-fucosylated, implicating a clear GnT I-independent core fucosylation pathway. Expression of GM-CSF and the ectodomain of FcγIIIA receptor led to ∼30% and 3% core fucosylation, suggesting that the level of core fucosylation also depends on the nature of the recombinant proteins. To elucidate the GnT I-independent core fucosylation pathway, we generated a stable HEK293S GnT I−/− cell line with either knockdown or overexpression of FUT8 by a highly efficient lentivirus-mediated gene transfer approach. We found that the EPO produced from the FUT8 knockdown cell line was the pure Man5GlcNAc2 glycoform, whereas that produced from the FUT8-overexpressing cell line was found to be fully core-fucosylated oligomannose glycan (Man5GlcNAc2Fuc). These results provide direct evidence that FUT8, the mammalian α1,6-fucosyltransferase, is the sole enzyme responsible for the GnT I-independent core fucosylation pathway. The production of the homogeneous core-fucosylated Man5GlcNAc2 glycoform of EPO in the FUT8-overexpressed HEK293S GnT I−/− cell line represents the first example of production of fully core-fucosylated high-mannose glycoforms.
Keywords: erythropoietin, fucosyltransferase, glycoprotein, glycoprotein biosynthesis, glycosylation
Introduction
Glycosylation, the covalent attachment of glycans to proteins, can profoundly affect the intrinsic properties and biological functions of proteins (1, 2). For the recombinant production of therapeutic glycoproteins such as monoclonal antibodies, it is particularly important to control glycosylation to achieve the optimal therapeutic efficacy (3–5). The biosynthesis of asparagine-linked (i.e. N-linked) glycoproteins involves multiple steps at different sites, which includes the initial attachment of a large precursor N-glycan, Glc3Man9GlcNAc2,to the nascent protein by the oligosaccharyltransferase at the endoplasmic reticulum, followed by the trimming of the precursor to Man9GlcNAc2 and Man8GlcNAc2 at the ER and then further trimming and processing to complex type N-glycans in the Golgi apparatus. A crucial step in this process is the transfer of a GlcNAc, in a β1,2 linkage, to the three-arm mannose residue of the Man5GlcNAc2 intermediate by N-acetylglucosaminyltransferase I (GnT I).2 This step is considered a prerequisite for two key further steps of N-glycosylation: the core fucosylation involving the transfer of an α1,6-linked fucose to the innermost GlcNAc by an α1,6 fucosyltransferase (FUT8) as well as the further trimming of the Man5GlcNAc2 intermediate to form complex-type glycans (6–8) (Fig. 1). Nevertheless, trace amount of core-fucosylated high-mannose glycans were detected from lysosomal proteins, like human β-glucuronidase (9), porcine cathepsin D (10), rat liver alkaline phosphatase (11), and bovine α-mannosidase (12). It was once proposed that those minor core-fucosylated, high-mannose glycans found in lysosomal proteins were derived from hydrolysis of core-fucosylated, hybrid-type N-glycans by N-acetylglucosaminidase (Fig. 1). However, the discovery of core-fucosylated Man5GlcNAc2 glycan from natural proteins of a GnT I knockout CHO cell lines by Lin et al. (13) provided the first evidence suggesting the presence of a GnT I-independent pathway for core fucosylation of Man5GlcNAc2 and paucimannose glycans in mammalian systems. Later on, Crispin et al. (14) reported the detection of minor fractions (∼5%) of core-fucosylated, high-mannose glycoforms in recombinant glycoproteins produced in GnT I knockout CHO and human HEK293S cell lines, confirming the presence of a GnT I-independent fucosylation pathway. Along this line, what remains to be characterized is the enzymes involved in the core-fucosylation of the Man5GlcNAc2 and paucimannose glycans in the GnT I-independent fucosylation pathway. The failure of in vitro fucosylation of the Man5GlcNAc2 or Man3GlcNAc2 substrate, either crude extract (6, 15, 16) or purified mammalian fucosyltransferase (FUT8) (7, 17), implicated the presence of enzymes other than FUT8 for core fucosylation of high-mannose N-glycans.
FIGURE 1.
Schematic of possible pathways for the generation of fucosylated Man5GlcNAc2 glycoforms. α-Man II, α-mannosidase II.
In this study, we demonstrate that core fucosylation of high-mannose-type glycans is a common phenomenon when glycoproteins were expressed in the HEK293S GnT I−/− cell line, but the degree of core fucosylation could vary significantly depending on the context of glycoproteins. We further show that expression of erythropoietin (EPO) from the FUT8-overexpressed and FUT8 knockdown cell lines yield fully core-fucosylated (Man5GlcNAc2Fuc) and completely non-fucosylated (Man5GlcNAc2) glycoforms, respectively. Our data suggest that FUT8, the mammalian α1,6-fucosyltransferase, is the sole enzyme responsible for the GnT I-independent core fucosylation of high-mannose N-glycans in N-glycoprotein biosynthesis.
Materials and Methods
Cell Culture
Human HEK293S GnT I−/− (18) (a gift from Dr. Kelly Moremum, University of Georgia) and its derivative cell lines were used for the expression of EPO and other glycoproteins. They were cultured in suspension in serum-free medium of a 50:50 mixture of EX-Cell (Sigma-Aldrich) and FreeStyleTM 293 medium (Life Technologies), incubated at 37 °C and 8% CO2, and shaken at 140 rpm/min. The cells were passaged every 3–4 days with an initial seeding density of 5 × 105 cells/ml.
Creation of EPO-S126V- and GM-CSF-expressing Constructs
The EPO cDNA sequence with a His6 tag was synthesized and then cloned into the pcDNA3 mammalian expressing vector (Life Technologies), resulting in the pcDNA-EPO plasmid. To eliminate the O-glycosylation site, serine 126 was mutated to valine (S126V) by site-directed mutagenesis with a QuikChange II mutagenesis kit (Agilent Technologies) according to the instructions of the manufacturer. The GM-CSF-expressing construct was created using similar procedures but without mutagenesis. The expressing construct for the ectodomain of FcγIIIa receptor was a gift from Dr. Eric Sundberg (University of Maryland School of Medicine). All glycoproteins contained a His tag to facilitate purification.
Transfection and Expression of EPO-S126V and Other Expressing Constructs in HEK293S GnT I−/− Cells
The endotoxin-free expressing plasmids for transfection were prepared with the NucleoBond® Xtra Maxi EF kit (Clontech). Transient transfection was performed following a protocol modified from Hacker et al. (19) using PEI (1 mg/ml, 25-kDa linear PEI, Polysciences). One day before the transfection, the cells were seeded at a density of 5 × 105/ml. The next day, the cells were spun down and resuspended in FreeStyleTM 293 medium at a density of 2 × 107/ml. The expressing plasmid was added into the cell suspension to a final concentration of 25 μg/ml and gently swirled, followed by the addition of PEI solution (62.5 μg/ml final concentration). After gentle swirling to mix, the transfection was kept at 37 °C for 3 h. Finally, the transfected cells were diluted with EX-Cell/FreeStyleTM 293 medium to 1 × 106 cells/ml, and the histone deacetylase inhibitor valproic acid was added to a final concentration of 2.2 mm. The transfected cells were kept in culture for another 3 days until harvest.
Purification of High-mannose-type EPO and Other Proteins
The His-tagged EPO and other proteins were purified by nickel-affinity chromatography. The supernatant of the cell culture (50–150 ml) was centrifuged at low speed (4000 rpm for 15 min) and then filtered through a 0.22 μm membrane to remove cell debris. The clarified supernatant was loaded on a HisTrap HP 1-ml column (GE Healthcare) that was equilibrated with PBS (pH 7.4) with 20 mm imidazole. The column was washed with 10 ml of PBS (pH 7.4) containing 50 mm imidazole and then eluted with 5 ml of PBS (pH 7.4) containing 200 mm imidazole.
Enzymatic Transformation
Purified high-mannose-type EPO was treated with several specific enzymes for further characterization. PNGase F and Endo H (New England Biolabs) treatments were performed under native conditions according to the instructions of the manufacturer. α1,6-Fucosidase from Lactobacillus casei was expressed and purified following the published methods (20). Treatment with Endo H and α1,6-fucosidase was carried out by incubation of 10 μg of recombinant glycoproteins with the respective enzyme (enzyme/substrate, 1/100, w/w) in 20 μl of PBS buffer (pH 7.4). The mixture was incubated at 37 °C overnight.
Release of total N-Glycans
The total N-glycans of EPO and other glycoproteins were released from 10 μg of glycoprotein by 100 units of PNGase F (New England Biolabs) in 20 μl of 50 mm phosphate buffer (pH 7.5) with incubation at 37 °C for 2 h. The released N-glycan was isolated with porous graphite carbon solid phase extraction as reported by Packer et al. (21).
Mass Spectrometry Analysis
LC electron spray ionization MS was used to analyze intact glycoproteins. The LC electron spray ionization MS was performed on an LXQ system (Thermo Scientific) with a C8 column (Poroshell 300SB-C8, 1.0 × 75 mm, 5 μm, Agilent). The released N-glycans were analyzed using a Bruker Autoflex III MALDI-TOF mass spectrometer (Bruker Daltonics) with 2,5-dihydroxybenzoic acid (10 mg in 10% ethanol, 10 mm NaCl) as the matrix in reflector-positive mode.
HPLC Analysis of Fmoc-labeled N-Glycan
N-(9-Fluorenyl)methoxycarbonyl (Fmoc) labeling of the N-glcyans released from EPO by PNGase F treatment was performed as described by Kamoda et al. (22). The labeled glycan was analyzed with reverse-phase HPLC on a C8 column (Poroshell 300SB-C8, 1.0 × 75 mm, 5 μm, Agilent) eluted with 5–90% acetonitrile in 30 min. The ratio between the fucosylated and non-fucosylated glycans was estimated by their peak areas of absorbance at 266 nm.
Generation of Stable Cell Lines
The two stable cell lines with either overexpression or knockdown of FUT8 were created with high-efficiency lentiviral transduction. For the overexpression cell line, FUT8 gene was cloned into a lentiviral vector, pLV-CT (a gift from Cellomics Technology, Halethorpe, MD), under the control of the CMV immediate early promoter. The vector contains a puromycin resistance gene for the screening of positive cell clones. For the knockdown cell lines, three validated shRNA lentiviral vectors that highly efficiently knock down the FUT8 gene and target different region of its mRNA were purchased from Sigma-Aldrich (TRCN0000229959, 97%; TRCN0000229960, 88%; and TRCN0000229961, 94% knockdown). Both overexpressing and shRNA lentiviral vectors were packaged with the pMD2.G envelope and psPAX2 helper plasmids (Addgene, Cambridge, MA). The lentiviruses were packaged and titrated according to Oberbek et al. (23). To generate the pooled stable cell lines, HEK293S GnT I−/− cells were transduced with either the overexpressing or shRNA viruses at a multiplicity of infection of 2 in the presence of 8 μg/ml Polybrene (Clontech). Eight hours after transduction, the fresh medium with 1 μg/ml puromycin (Clontech) was added to screen the puromycin-resistant clones. The pooled stable cells were expanded with 2 weeks of continuous culture.
Results
Characterization of EPO Produced from the HEK293S GnT I−/− Cell Line
As part of our project for producing homogeneous glycoforms of therapeutic glycoproteins via a chemoenzymatic approach (24–26), we attempted to express EPO using a human GnT I knockout cell suspension culture system in the hope of producing the homogenous EPO-Man5GlcNAc2 glycoform as the precursor. The natural human EPO has three conserved N-glycosylation sites and carries one O-glycan at the Ser-126 site. To focus on N-glycosylation in the production of well defined glycoforms of EPO, we eliminated the natural O-glycan by mutating Ser-126 to valine (S126V mutation). An early study also indicated that O-glycosylation was not essential for either the stability or bioactivity of EPO (27). As a result, all EPO glycoforms in this study are expressed as the S126V mutant. The expressed EPO was purified to homogeneity with nickel-nitrilotriacetic acid affinity chromatography. SDS-PAGE analysis showed that the purified protein migrated as a band around 27 kD (Fig. 2A), which is a little bit larger than its expected molecular weight of 24 kDa, probably caused by the hydration of glycans. After treatment with PNGase F, the deglycosylated protein shifted to a lower position of around 19 kDa (Fig. 2A), as expected for the removal of all the three high-mannose N-glycans. Unexpectedly, MALDI-TOF MS analysis of the N-glycans released by PNGase F showed two major molecular ions: one with an m/z of 1257.3 that matches N-glycan Man5GlcNAc2 (calculated, molecular weight = 1257.4 Da) and the other with an m/z of 1403.5 that is in agreement with a Man5GlcNAc2 glycan plus a fucose moiety (Man5GlcNAc2Fuc; calculated, molecular weight = 1403.4 Da) (Fig. 2B). The relative intensity indicates that the putative fucosylated Man5GlcNAc2 species accounts for more than 50% of total N-glycans. For further quantification of the two N-glycans, the glycans released by PNGase F treatment were tagged in situ with an Fmoc group (by reaction with Fmoc chloride).The Fmoc glycans were isolated and quantified by HPLC analysis following a procedure reported previously (22). Based on the UV absorbance, the ratio between the fucosylated and non-fucosylated Man5GlcNAc2 was found to be 58:42, which was similar to the relative intensity estimated from the MALDI-TOF MS analysis (Fig. 2B, inset). LC-MS analysis of the PNGase F-treated EPO gave a species of 19,366 Da (deconvolution data), which is consistent with the polypeptide backbone of EPO (calculated, 19,363 Da) (Fig. 2C). LC-MS analysis of the intact recombinant EPO revealed a mixture of EPO glycoforms carrying three Man5GlcNAc2 glycans with varied (no to three) additions of a species of 146 Da (matching the molecular mass of fucose) (Fig. 2D).
FIGURE 2.
Enzymatic and mass analysis of EPO glycoforms produced from the HEK293S GnT I−/− cell line. A, SDS-PAGE analysis of the recombinant EPO before and after enzymatic treatment. Lane 1, recombinant EPO (untreated); lane 2, recombinant EPO treated with PNGase F; lane 3, recombinant EPO treated with Endo H. MW, molecular weight. B, MALDI-TOF MS analysis of the N-glycans released from the recombinant EPO by PNGase F treatment. Inset, reverse phase-HPLC analysis of Fmoc-labeled EPO N-glycan. C, LC-MS analysis of the recombinant EPO after deglycosylation with PNGase F. D, LC-MS analysis of the intact recombinant EPO. E, LC-MS analysis of the recombinant EPO after deglycosylation with Endo H. F, LC-MS analysis of the recombinant EPO after treatment with Endo H and the α1,6-fucosidase from L. casei. The datasets are representative of two independent experiments.
To determine where the putative fucose moiety is attached, we performed a comparative LC electron spray ionization MS analysis of the intact recombinant EPO before and after treatment with Endo H as well as with mixtures of Endo H and an α-1,6-fucosidase from L. casei (20). Treatment of EPO with Endo H, which hydrolyzes high-mannose-type N-glycan at the chitobiose core to leave the innermost GlcNAc still attached to the protein, gave four ion species (m/z): 19,970, 20,117, 20,263, and 20,407 (Fig. 2E). The m/z 19,970 species is the molecular ion of EPO carrying three GlcNAc moieties (each at a respective N-glycosylation site). The next three ion species each had an extra 146 Da added, suggesting that the fucose moiety is attached at the innermost GlcNAc moiety of the N-glycans in the recombinant EPO. Finally, treatment of the Endo H-deglycosylated EPO with a recombinant α1,6-fucosidase from L. casei that is specific for hydrolyzing α1,6-fucosidic linkage to GlcNAc resulted in the removal of all fucose moieties in the Endo H-treated EPO, as reflected by the generation of a single molecular ion at m/z 19,969 that is corresponded to the EPO carrying three GlcNAc moieties (calculated, molecular weight = 19,972 Da) (Fig. 2F). These results confirm that the new N-glycan is the Man5GlcNAc2 carrying an α1,6-fucose moiety at the innermost Asn-linked GlcNAc residue.
Expression, Purification, and Glycan Analysis of Other Glycoproteins in the HEK293S GnT I−/− Cell Line
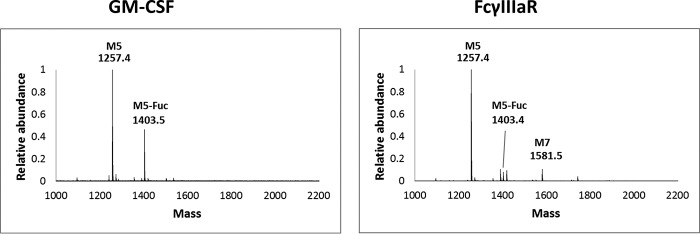
After the observation of more than 50% fucosylation of the high-mannose glycan of EPO in HEK293S GnT I−/− cells in serum-free suspension culture, we were wondering whether this was an observation unique for EPO or whether it would be a general phenomenon applicable to most glycoproteins expressed in this cell line and/or such a culture condition. To answer this question, we expressed two other glycoproteins, GM-CSF and the ectodomain of FcγIIIa receptor. GM-CSF contains two conserved N-glycosylation sites, whereas FcγIIIa potentially has five. As shown in the N-glycan analysis of the two recombinant glycoproteins (Fig. 3), GM-CSF also had a significant amount of Man5GlcNAc2Fuc species (estimated as 30% of total glycan), whereas FcγIIIa contained a relatively low level of fucosylated oligomannose glycan (∼3% total glycan). Taken together, these results suggested that core fucosylation of high-mannose N-glycans in the recombinant glycoproteins expressed from the HEK293S GnT I−/− cell line appeared as a common phenomenon, but the extent of core fucosylation also varied depending on the nature of the proteins.
FIGURE 3.
MALDI-TOF analysis of the N-glycans released by PNGase F treatment of the recombinant glycoproteins produced in the HEK293S GnT I−/− cell line. A, N-glycans released from the recombinant GM-CSF. B, N-glycans released from the recombinant FcγIIIa receptor. The datasets are representative of two independent experiments.
Generation of FUT8-overexpressing and Knockdown Cell Lines and Production/Characterization of EPO from These Cell Lines
After confirming the core fucosylation of high-mannose glycan in the GnT I knockout cell line, we sought to identify the enzyme catalyzing such an unusually high transfer of fucose to high-mannose-type glycan. In previous publications, FUT8 was speculated for such a GnT I independent reaction, but no direct experimental verification was carried out. In our study, a significant amount of Man5GlcNAc2Fuc was produced, particularly in the case of recombinant EPO, which may suggest the involvement of other unknown, more active fucosyltransferases or cofactors. To investigate this possibility, we first tried to knock down the FUT8 gene in HEK293S GnT I−/− cells and then analyze the glycoforms of EPO produced from such a cell line. If a glycotransferase other than FUT8 was involved, then knockdown of FUT8 should not have a significant impact on the glycoforms of EPO. We first did a quick test by co-transfecting the EPO-expressing plasmid with three shRNA plasmids that highly knock down FUT8 expression. After glycan analysis of purified EPO, the Man5GlcNAc2Fuc glycoform decreased from 58% to ∼20%, as quantified by HPLC analysis of the PNGase F-released N-glycans with in situ Fmoc tagging (data not shown). Considering just 30% transfection efficacy (30% of cells were delivered in transfected plasmids, as determined by parallel transfection with the EGFP reporter plasmid), this result strongly suggests that FUT8 is at least the major transferase for the fucose transfer. To acquire conclusive evidence, we generated stable cell lines that knocked down its endogenous expression (HEK293S GnT I−/− FUT8↓). For the creation of stable cell lines, we first tried to use a conventional transfection method. However, the low efficiency of transfection could not generate enough seed cells (>1 × 104/ml) to effectively expand the cell population. Alternatively, we chose lentiviral transduction with a transfer efficiency of more than 90% to generate the stable cell line. For the knockout cell line, we used shRNA lentiviruses that would be highly efficient in knocking down FUT8 expression.
Expression of EPO in the 293S GnT I−/− FUT8↓ cell line gave a homogeneous glycoform of EPO. LC-MS analysis of the intact recombinant EPO showed a single molecular ion of 23,009 (deconvolution data), which matches well with the glycoform of EPO carrying three Man5GlcNAc2 glycans (calculated, molecular weight = 23,011 Da) (Fig. 4A). MALDI-TOF MS analysis of the PNGase F-released N-glycan gave a single species at m/z 1257.2, which corresponds to Man5GlcNAc2 (Fig. 4B). There was no fucosylated glycan detected. The complete exclusion of fucosylated Man5GlcNAc2 in the FUT8 knockdown cell line suggests that FUT8 is the sole enzyme responsible for the attachment of fucose to the high-mannose glycans observed in the recombinant glycoproteins produced in the parent HEK293S GnT I−/− cell lines. Parallel to the creation of the knockdown cell line, we also created a FUT8-overexpressing GnT I knockout cell line (HEK293S GnT I−/− FUT8↑). The EPO expressed in this overexpressing cell line gave another homogeneous glycoform. LC-MS analysis showed a single molecular ion at m/z 23,448 after deconvolution, which matches an EPO carrying three homogeneous Man5GlcNAc2Fuc glycans (calculated, molecular weight = 23,449 Da) (Fig. 5C). MALDI-TOF MS analysis of the N-glycan released by PNGase F confirmed that all N-glycans attached were the fucosylated Man5GlcNAc2 (found, m/z 1403.8; calculated, molecular weight = 1403.4 Da) (Fig. 5D). The complete fucosylation of the N-glycans in the recombinant EPO produced in the 293S GnT I−/− FUT8↑ cell line suggests that Man5GlcNAc2-EPO would be an efficient substrate for FUT8 in vivo. Taken together, our experimental data provide clear evidence that the FUT8 is the sole enzyme for such a GnT I-independent fucosylation pathway. Overexpression of the glycosyltransferase led to complete fucosylation of the EPO Man5GlcNAc2 glycoform, whereas knockdown of this enzyme in the stable cell line totally eliminated the Man5GlcNAc2Fuc glycoform observed in the parent cell line. In addition, through this experiment, we successfully produced EPO with homogeneous core-fucosylated Man5GlcNAc2. To our knowledge, this is the first report of the production of recombinant glycoproteins with the pure Man5GlcNAc2Fuc glycoform.
FIGURE 4.
MS analysis of EPO glycoforms produced in the HEK293S GnT I−/− FUT8↓ and 293S GnT I−/− FUT8↑ cell lines. A, LC-MS analysis of the intact EPO glycoform produced in the HEK293S GnT I−/− FUT8↓ cell line. B, MALDI-TOF MS analysis of the N-glycans released by PNGase F treatment of the above EPO glycoform. C, LC-MS of the intact EPO glycoform produced in the HEK293S GnT I−/− FUT8↑ cell line. D, MALDI-TOF MS analysis of the N-glycans released by PNGase F treatment of the above EPO glycoform. The datasets are representative of two independent experiments.
FIGURE 5.
Microscopic pictures of HEK293S GnT I−/− FUT8↓ and HEK293S GnT I−/−FUT8↑ cells and their parent cell line HEK293S GnT I−/− in bright field light. The FUT8-overexpressing cell line showed enhanced cell-cell adhesion.
In the creation of two stable cell lines, we also observed an interesting morphology change in the HEK293S GnT I−/− FUT8↑ cell line. As shown in Fig. 5, the knockdown cell line demonstrated a similar phenotype as the parent HEK293S GnT I−/− cell line. In contrast, the overexpressing cell line created with a FUT8-overexpressing lentivirus showed significantly enhanced cell-cell adhesion. It has been reported that overexpression of FUT8 in human colon cancer cells will enhance the core fucosylation of E-Cadherin, promoting cell-cell adhesion (28). The promotion of cell-cell adhesion in hepatoma and other types of cells by core fucosylation of adhesion molecules was reported previously as well (29).
Discussion
Previously, small amounts of Man5GlcNAc2Fuc were detected from some natural and recombinant proteins. Early reports indicated that core-fucosylated Man4–5GlcNAc2 could be detected in recombinant glycoproteins such as tyrosine phosphatase μ and ligand of inducible co-stimulator produced in GnT1 knockout CHO and HEK293 cell lines (13, 14), but it appears as a minor component (<5%). Addition of the α-glucosidase I/II inhibitor N-butyldeoxynojirimycin hydrochloride (NB-DNJ) could increase the percentage of the Man5GlcNAc2Fuc glycoform in recombinant proteins produced from GnT I knockout CHO cell lines to 28% (14).
In this study, we observed unusually high core fucosylation of Man5GlcNAc2 in recombinant EPO (more than 50%) and recombinant GM-CSF (roughly 30% core fucosylation). On the other hand, the core fucosylation in recombinant FcγIIIa receptor was detectable, but at a relatively low level (<3%). The detection of core-fucosylated Man5GlcNAc2 glycoforms in all three recombinant glycoproteins expressed in the HEK293S GnT I−/− cell line, together with the previously reported detection of core-fucosylated high-mannose glycoforms in GnT I−/− CHO and HEK293S cell lines (13, 14), strongly suggests that the GnT I-independent core fucosylation of high-mannose glycoforms produced in the GnT I knockout mammalian cell lines is a common phenomenon. However, the extent of core fucosylation varies and may depend on the microenvironment of the glycosylation sites, the nature of the expressed glycoproteins, and the cell culture conditions.
To examine whether FUT8 was the only fucosyltransferase responsible for the fucosylation of the Man5GlcNAc2 glycoform or whether there were other enzymes or factors involved, we established stable cell lines with FUT8 overexpression or knockdown. Our experimental data demonstrate that knockdown of FUT8 completely eliminated the core fucosylation of the Man5GlcNAc2 glycoform of EPO. On the other hand, overexpression of FUT8 gave full core fucosylation of the Man5GlcNAc2 glycoform of EPO. These results provide direct evidence indicating that FUT8 is the sole α1,6-fucosyltransferase responsible for the GnT I-independent core fucosylation of high-mannose N-glycans. This conclusion is also consistent with the fact that FUT8, the mammalian α1,6-fucosyltransferase, appears to be the single gene so far discovered for core fucosylation of glycoproteins in mammalian systems (30). Nevertheless, at least one question remains to be answered. So far, all in vitro attempts to fucosylate high-mannose-type N-glycans by FUT8 have failed because the attachment of a terminal GlcNAc β1,2 linked to the mannose residue on the α1,3 branch of the N-glycan core appears to be a prerequisite for both the core fucosylation of Man5GlcNAc2 and its further processing by α1,2-mannosidase (7, 13, 17).
The unsuccessful in vitro core fucosylation of high-mannose N-glycans by FUT8 seems contradictory to the suggestion that FUT8 was the sole enzyme to fucosylate high-mannose-type glycoforms in vivo. It is possible that other factors, such as molecular chaperones, might be involved in the in vivo systems. However, another possibility, that core fucosylation of Man5GlcNAc2 is heavily dependent on the context of the protein, cannot be excluded. In fact, in vivo core fucosylation of high-mannose glycan occurs in the context of proteins, whereas all in vitro FUT8 core fucosylations of Man5GlcNAc2 or Man3GlcNAc2 were examined either with free N-glycans or simple glycopeptides. The fact that significantly different levels of core fucosylation were observed among different glycoproteins when expressed in the GnT I−/− CHO cell line suggest the importance of protein contexts in core fucosylation. For example, the EPO Man5GlcNAc2 glycoform appears to be an excellent substrate for FUT8, at least in vivo, as indicated by the fact that over 50% of the Man5GlcNAc2 glycoform is core-fucosylated in the parent HEK293S GnT I−/− cell line, whereas expression of recombinant GM-CSF and FcγIIIa receptor under the same cell culture conditions gave 30% and 3% core-fucosylated glycoforms, respectively. One explanation is that the N-glycosylation sites in EPO are easily accessible by either glycosyltransferase or glycosidase. This is supported by the fact that most of the N-glycans in recombinant EPO (produced from HEK293 cells) are well processed tri- and tetra-antennary glycans on all three glycosylation sites (31).
In the normal cells, when the N-acetylglucosaminyltransferase I and α1,6 fucosyltransferase are both present, the transfer action of GlcNAc by GnT I could go much faster than the attachment of a core fucose by FUT8 because Man5GlcNAc2 is known to be an excellent substrate for GnT I. However, in GnT I knockout cells, the Man5GlcNAc2 could be accumulated for fucose transfer by FUT8. Moreover, the easy access of the glycosylation sites of EPO is likely to make this transfer much more feasible than that of other proteins with less accessible glycosylation sites. It would be worthwhile to revisit the in vitro substrate specificity and activity of FUT8 with different N-glycans in the context of different proteins. Understanding the details of the glycosylation pathways is essential for the precise engineering of recombinant glycoproteins to produce the desired homogeneous glycoforms for therapeutic applications without contaminations.
The core-fucosylated Man5GlcNAc2 glycoform of EPO produced from the FUT8-overexpressing cell line represents the first example of a successful production of fully fucosylated high-mannose-type recombinant glycoproteins. It has been reported that EPO glycoforms produced from tobacco showed a 2-fold increase in cytoprotective effect compared with that produced from CHO cells. Glycan analysis of the EPO expressed in tobacco cells revealed a significant amount of high-mannose EPO (32). In this perspective, the homogeneous Man5GlcNAc2 and Man5GlcNAc2Fuc glycoforms of EPO obtained here may have potential values in the study of the cytoprotective effect of EPO.
Author Contributions
Q. Y. and L. X. W. designed the experiments, analyzed the experimental data, and wrote and edited the manuscript. Q. Y. performed the experiments. Both authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We thank the members of the Wang laboratory for helpful discussions and technical assistance, Dr. Kelly Moremen (University of Georgia) for the human HEK293S GnT I−/− cell line, Dr. Eric Sundberg (University of Maryland School of Medicine) for the construct encoding the ectodomain of FcγIIIa receptor, and Dr. Rui Zhang (Cellomics Technologies) for the pLV-CT lentiviral vector.
This work was supported by National Institutes of Health Grant R01 GM080374. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- GnT I
- N-acetylglucosaminyltransferase I
- EPO
- erythropoietin
- Endo H
- endo-β-N-acetylglucosaminidase H
- Fmoc
- N-(9-fluorenyl)methoxycarbonyl.
References
- 1. Dwek R. A. (1996) Glycobiology: toward understanding the function of sugars. Chem. Rev. 96, 683–720 [DOI] [PubMed] [Google Scholar]
- 2. Helenius A., and Aebi M. (2001) Intracellular functions of N-linked glycans. Science 291, 2364–2369 [DOI] [PubMed] [Google Scholar]
- 3. Walsh G., and Jefferis R. (2006) Post-translational modifications in the context of therapeutic proteins. Nat. Biotechnol. 24, 1241–1252 [DOI] [PubMed] [Google Scholar]
- 4. Jefferis R. (2009) Glycosylation as a strategy to improve antibody-based therapeutics. Nat. Rev. Drug Discov. 8, 226–234 [DOI] [PubMed] [Google Scholar]
- 5. Dalziel M., Crispin M., Scanlan C. N., Zitzmann N., and Dwek R. A. (2014) Emerging principles for the therapeutic exploitation of glycosylation. Science 343, 1235681. [DOI] [PubMed] [Google Scholar]
- 6. Longmore G. D., and Schachter H. (1982) Product-identification and substrate-specificity studies of the GDP-l-fucose:2-acetamido-2-deoxy-β-d-glucoside (FUC goes to Asn-linked GlcNAc) 6-α-l-fucosyltransferase in a Golgi-rich fraction from porcine liver. Carbohydr. Res. 100, 365–392 [DOI] [PubMed] [Google Scholar]
- 7. Voynow J. A., Kaiser R. S., Scanlin T. F., and Glick M. C. (1991) Purification and characterization of GDP-L-fucose-N-acetyl β-d-glucosaminide α-1,6-fucosyltransferase from cultured human skin fibroblasts: requirement of a specific biantennary oligosaccharide as substrate. J. Biol. Chem. 266, 21572–21577 [PubMed] [Google Scholar]
- 8. Schachter H., Narasimhan S., Gleeson P., and Vella G. (1983) Control of branching during the biosynthesis of asparagine-linked oligosaccharides. Can. J. Biochem. Cell Biol. 61, 1049–1066 [DOI] [PubMed] [Google Scholar]
- 9. Howard D. R., Natowicz M., and Baenziger J. U. (1982) Structural studies of the endoglycosidase H-resistant oligosaccharides present on human β-glucuronidase. J. Biol. Chem. 257, 10861–10868 [PubMed] [Google Scholar]
- 10. Takahashi T., Schmidt P. G., and Tang J. (1983) Oligosaccharide units of lysosomal cathepsin D from porcine spleen: amino acid sequence and carbohydrate structure of the glycopeptides. J. Biol. Chem. 258, 2819–2830 [PubMed] [Google Scholar]
- 11. Endo T., Fujiwara T., Ikehara Y., and Kobata A. (1996) Comparative study of the sugar chains of alkaline phosphatases purified from rat liver and rat AH-130 hepatoma cells: occurrence of fucosylated high-mannose-type and hybrid-type sugar chains. Eur. J. Biochem. 236, 579–590 [DOI] [PubMed] [Google Scholar]
- 12. Faid V., Evjen G., Tollersrud O. K., Michalski J. C., and Morelle W. (2006) Site-specific glycosylation analysis of the bovine lysosomal α-mannosidase. Glycobiology 16, 440–461 [DOI] [PubMed] [Google Scholar]
- 13. Lin A. I., Philipsberg G. A., and Haltiwanger R. S. (1994) Core fucosylation of high-mannose-type oligosaccharides in GlcNAc transferase I-deficient (Lec1) CHO cells. Glycobiology 4, 895–901 [DOI] [PubMed] [Google Scholar]
- 14. Crispin M., Harvey D. J., Chang V. T., Yu C., Aricescu A. R., Jones E. Y., Davis S. J., Dwek R. A., and Rudd P. M. (2006) Inhibition of hybrid- and complex-type glycosylation reveals the presence of the GlcNAc transferase I-independent fucosylation pathway. Glycobiology 16, 748–756 [DOI] [PubMed] [Google Scholar]
- 15. Wilson J. R., Williams D., and Schachter H. (1976) The control of glycoprotein synthesis: N-acetylglucosamine linkage to a mannose residue as a signal for the attachment of L-fucose to the asparagine-linked N-acetylglucosamine residue of glycopeptide from α1-acid glycoprotein. Biochem. Biophys. Res. Commun. 72, 909–916 [DOI] [PubMed] [Google Scholar]
- 16. Shao M. C., Sokolik C. W., and Wold F. (1994) Specificity studies of the GDP-[L]-fucose: 2-acetamido-2-deoxy-β-[D]-glucoside (Fuc->Asn-linked GlcNAc) 6-α-[L]-fucosyltransferase from rat-liver Golgi membranes. Carbohydr. Res. 251, 163–173 [DOI] [PubMed] [Google Scholar]
- 17. Li L., Liu Y., Ma C., Qu J., Calderon A. D., Wu B., Wei N., Wang X., Guo Y., Xiao Z., Song J., Sugiarto G., Li Y., Yu H., Chen X., and Wang P. G. (2015) Efficient chemoenzymatic synthesis of an N-glycan isomer library. Chem. Sci. 6, 5652–5661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reeves P. J., Callewaert N., Contreras R., and Khorana H. G. (2002) Structure and function in rhodopsin: high-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc. Natl. Acad. Sci. U.S.A. 99, 13419–13424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hacker D. L., Kiseljak D., Rajendra Y., Thurnheer S., Baldi L., and Wurm F. M. (2013) Polyethyleneimine-based transient gene expression processes for suspension-adapted HEK-293E and CHO-DG44 cells. Protein Expr. Purif. 92, 67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rodríguez-Díaz J., Monedero V., and Yebra M. J. (2011) Utilization of natural fucosylated oligosaccharides by three novel α-L-fucosidases from a probiotic Lactobacillus casei strain. Appl. Environ. Microbiol. 77, 703–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Packer N. H., Lawson M. A., Jardine D. R., and Redmond J. W. (1998) A general approach to desalting oligosaccharides released from glycoproteins. Glycoconj. J. 15, 737–747 [DOI] [PubMed] [Google Scholar]
- 22. Kamoda S., Nakano M., Ishikawa R., Suzuki S., and Kakehi K. (2005) Rapid and sensitive screening of N-glycans as 9-fluorenylmethyl derivatives by high-performance liquid chromatography: a method which can recover free oligosaccharides after analysis. J. Proteome Res. 4, 146–152 [DOI] [PubMed] [Google Scholar]
- 23. Oberbek A., Matasci M., Hacker D. L., and Wurm F. M. (2011) Generation of stable, high-producing CHO cell lines by lentiviral vector-mediated gene transfer in serum-free suspension culture. Biotechnol. Bioeng. 108, 600–610 [DOI] [PubMed] [Google Scholar]
- 24. Huang W., Giddens J., Fan S. Q., Toonstra C., and Wang L. X. (2012) Chemoenzymatic glycoengineering of intact IgG antibodies for gain of functions. J. Am. Chem. Soc. 134, 12308–12318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang L. X., and Lomino J. V. (2012) Emerging technologies for making glycan-defined glycoproteins. ACS Chem. Biol. 7, 110–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang L. X., and Amin M. N. (2014) Chemical and chemoenzymatic synthesis of glycoproteins for deciphering functions. Chem. Biol. 21, 51–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Delorme E., Lorenzini T., Giffin J., Martin F., Jacobsen F., Boone T., and Elliott S. (1992) Role of glycosylation on the secretion and biological activity of erythropoietin. Biochemistry 31, 9871–9876 [DOI] [PubMed] [Google Scholar]
- 28. Osumi D., Takahashi M., Miyoshi E., Yokoe S., Lee S. H., Noda K., Nakamori S., Gu J., Ikeda Y., Kuroki Y., Sengoku K., Ishikawa M., and Taniguchi N. (2009) Core fucosylation of E-cadherin enhances cell-cell adhesion in human colon carcinoma WiDr cells. Cancer Sci. 100, 888–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miyoshi E., Noda K., Ko J. H., Ekuni A., Kitada T., Uozumi N., Ikeda Y., Matsuura N., Sasaki Y., Hayashi N., Hori M., and Taniguchi N. (1999) Overexpression of α1–6 fucosyltransferase in hepatoma cells suppresses intrahepatic metastasis after splenic injection in athymic mice. Cancer Res. 59, 2237–2243 [PubMed] [Google Scholar]
- 30. Juliant S., Harduin-Lepers A., Monjaret F., Catieau B., Violet M. L., Cérutti P., Ozil A., and Duonor-Cérutti M. (2014) The α1,6-fucosyltransferase gene (fut8) from the Sf9 lepidopteran insect cell line: insights into fut8 evolution. PLoS ONE 9, e110422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ohta M., Kawasaki N., Hyuga S., Hyuga M., and Hayakawa T. (2001) Selective glycopeptide mapping of erythropoietin by on-line high-performance liquid chromatography-electrospray ionization mass spectrometry. J. Chromatogr. A 910, 1–11 [DOI] [PubMed] [Google Scholar]
- 32. Kittur F. S., Bah M., Archer-Hartmann S., Hung C. Y., Azadi P., Ishihara M., Sane D. C., and Xie J. (2013) Cytoprotective effect of recombinant human erythropoietin produced in transgenic tobacco plants. PLoS ONE 8, e76468. [DOI] [PMC free article] [PubMed] [Google Scholar]