Abstract
The FXYD proteins are a family of small membrane proteins that share an invariant four amino acid signature motif F-X-Y-D and act as tissue-specific regulatory subunits of the Na,K-ATPase. FXYD5 (also termed dysadherin or RIC) is a structurally and functionally unique member of the FXYD family. As other FXYD proteins, FXYD5 specifically interacts with the Na,K-ATPase and alters its kinetics by increasing Vmax. However, unlike other family members FXYD5 appears to have additional functions, which cannot be readily explained by modulation of transport kinetics. Knockdown of FXYD5 in MDA-MB-231 breast cancer cells largely decreases expression and secretion of the chemokine CCL2 (MCP-1). A related effect has also been observed in renal cell carcinoma cells. The current study aims to further characterize the relationship between the expression of FXYD5 and CCL2 secretion. We demonstrate that transfection of M1 epithelial cell line with FXYD5 largely increases lipopolysaccharide (LPS) stimulated CCL2 mRNA and secretion of the translated protein. We have completed a detailed analysis of the molecular events leading to the above response. Our key findings indicate that FXYD5 generates a late response by increasing the surface expression of the TNFα receptor, without affecting its total protein level, or mRNA transcription. LPS administration to mice demonstrates induced secretion of CCL2 and TNFα in FXYD5-expressing lung peripheral tissue, which suggests a possible role for FXYD5 in normal epithelia during inflammation.
Keywords: cytokine induction, inflammation, lipopolysaccharide (LPS), plasma membrane, translocation
Introduction
FXYD is a family of type I plasma membrane proteins characterized by the extracellular motif Phe-Xxx-Tyr-Asp. All seven mammalian members of this family specifically interact with the Na,K-ATPase and they have been described to act as tissue specific regulators or auxiliary subunits of the Na,K-ATPase whose role is to adjust the pump's activity to the cell environment (1). FXYD5 (also termed dysadherin or RIC) is a structurally and functionally unique member of the FXYD family. As other FXYD proteins, FXYD5 specifically interacts with the Na,K-ATPase and alters its kinetics by increasing Vmax (2, 3). However, in addition to regulating Na,K-ATPase kinetics, FXYD5 appears to have other functions. FXYD5 has been identified as a cancer-associated protein whose expression inhibits E-cadherin and promotes metastasis (4). Clinical studies have demonstrated correlations between the abundance of FXYD5 and the progression of malignancies and survival chances of patients with various human cancers (for review see Ref. 5). Silencing FXYD5 decreases individual and collective cell motility, while its overexpression has the opposite effect (6–8). In addition, the expression of FXYD5 has been associated with changes in cytoskeletal organization, altered cell shape and impairment of tight and adherence junctions (6–10). The above observations are consistent with the fact that the extracellular domain of FXYD5 is much longer than that of other FXYD proteins and presumably undergoes excessive O-glycosylation (4, 11).
Previously, it was reported that knockdown of FXYD5 in MDA-MB-231 breast cancer cells largely decreases expression and secretion of the chemokine CCL2 (12). A related effect has also been observed in renal cell carcinoma cells (7). CCL2 (also termed MCP-12 for monocyte chemotactic protein-1) is a small cytokine belonging to the CC chemokine family. It participates in monocyte recruitment during infection or under other inflammatory conditions (13). Since it is known that many tumors secrete CCL2, which attracts tumor-associated macrophages that promote tumor growth, angiogenesis, and metastasis (14–16), this finding has been connected to the effect of FXYD5 on tumorgenicity.
FXYD5 is up-regulated in cystic fibrosis, which is characterized by an excessive airway inflammatory response (10). It has been suggested that the increased levels of FXYD5 contributes to a MCP-1 autoregulatory loop downstream of NF-κB (12). Moreover, FXYD5 is one of the genes up-regulated in chronic inflammation and immune system overactivity described in the aging process of the human brain (17).
In the current study, we show that the presence of FXYD5 in normal epithelial cells predisposes to a more inflammatory phenotype. In M1 cells, FXYD5 largely increases the LPS- induced expression of CCL2 mRNA and secretion of the translated protein. This effect however requires extracellular stimuli such as LPS (lipopolysaccharide), and it is mediated by TNFα.
Experimental Procedures
Expression and Silencing of FXYD5 in Cultured Cells
The mouse kidney collecting duct cell line M1 (18), was purchased from ATCC. Cells were cultured in a 1:1 mixture of DMEM and F12 medium (Biological Industries, Beit Haemek, Israel) supplemented with 5% fetal calf serum, 5 μm dexamethasone, and antibiotics (penicillin, streptomycin). Transfection of FXYD5 and silencing of FXYD4 were previously described (9). H1299 cells (human non small cell lung carcinoma) were cultured in RPMI 1640 (Biological Industries, Beit Haemek, Israel) supplemented with 10% FCS, 1 mm sodium pyruvate, and antibiotics (penicillin, streptomycin). Silencing of endogenous FXYD5 in H1299 cells was previously described (19).
Detection of CCL2 Protein
Cells were grown until confluent in 12-well plates and allowed to continue proliferation for an additional 4 days to allow polarization of the epithelial layer. The medium was replaced by 0.5 ml of full medium ± LPS, TNFα, and other reagents. After an additional 24-h incubation, medium was removed, and aliquots were assayed for secreted CCL2 using either mouse or human MCP-1 ELISA Kit (Thermo Scientific), according to the manufacturer's instructions.
Real-time Relative Reverse Transcription PCR
RNA was isolated from cultured cells using an RNAeasy kit (Qiagen) and reverse transcribed from the poly(A)+ tail using a Super-Script II Reverse Transcriptase kit (Invitrogen). PCR reactions were set up using the Power SYBR Green PCR kit (Applied Biosystems) according to the manufacturer's instructions. Data were normalized to the abundance of GAPDH mRNA. The genes indicated below were then amplified using the forward and reverse primers: CCL2 mouse 5′-cctgtcatgcttctgggcctgc, CCL2 mouse 3′-ggggcgttaactgcatctggctg, CCL2 human 5′-cgctcagccagatgcaatcaatgc, CCL2 human 3′-ctcgcgagcctctgcactgaga, TNFR1 5′-ccgggagaagagggatagctt, TNFR1 3′-tcggacagtcactcaccaagt, TNFα mouse 5′-gcctcttctcattcctgcttg, TNFα mouse 3′-ctgatgagagggaggccatt, TLR4 mouse 5′-atggcatggcttacaccacc, TLR4 mouse 3′- gaggccaattttgtctccaca.
Reagents and Antibodies
Lipopolysaccharides (LPS) from Escherichia coli 055:B5 was from Sigma-Aldrich (cat. no. L6529), FITC-LPS was from Sigma-Aldrich (cat. no. L8666). Recombinant Mouse TNFα was from PROSPEC (cat. no. cyt-252). The NF-κB activation inhibitor 6-amino-4-(4-phenoxyphenylethylamino) quinazoline (QNZ) was from Calbiochem (cat. no. 481407). Wortmannin was from Sigma-Aldrich (cat. no. W1628). Antibodies against FXYD5 and TNFR-1 were purchased from Santa Cruz Biotechnology (cat. no. sc-166782 and sc-1070), respectively. Anti-TNFα was purchased from Cell Signaling (cat. no. 3707).
Surface Biotinylation and Western Blotting
Surface biotinylation procedure was done as described in Ref. 19. In brief: confluent monolayers were surface biotinylated by a 10-min incubation at 4 °C with 1.5 mg/ml EZ-Link sulfo-NHS-SS-biotin (Pierce cat. no. 21331) in PBS without calcium or magnesium. The unbound biotin was quenched with 100 mm glycine in PBS, and cells were lysed by rocking for 1 h at 4 °C in RIPA buffer supplemented with protease inhibitors (20 mm Tris·HCl, pH 7.4, 2 mm EDTA, 2 mm EGTA, 1% Triton X-100, 0.1% SDS, 1 mm PMSF, 20 mg/ml leupeptin, and 20 mg/ml pepstatin A), unless stated otherwise. Cell debris was removed by centrifugation at 5,000 × g for 5 min, 2–10% of the volume was taken as “total protein” sample, and the rest (∼700 μl) was incubated overnight at 4 °C with 100 μl streptavidin agarose resin slurry (Pierce cat. no. 20353). The agarose beads were then washed, and streptavidin-bound proteins were eluted by incubation with SDS sample buffer (cell surface fraction). Total and cell surface proteins were resolved electrophoretically on 7.5% acrylamide Tris-glycine gels (Bio Rad cat no. 161-0171) and blotted onto PVDF membranes (Bio Rad cat. no. 170-4157)
Animals
8–10-week-old male C57BL/6 mice were purchased from Charles River Laboratories. All experiments were approved by the Northwestern University Animal Care and Use Committee.
LPS Administration and Collection of Bronchoalveolar Lavage Fluid (BAL)
Mice were treated with a single intraperitoneal injection of PBS or LPS (6 mg/kg in 50 μl of PBS) as described (20). Lung proteins were obtained by homogenizing lung tissue collected from the peripheral 1–2 mm of each lobe as previously described (21, 22). BAL was performed through a 20-gauge angiocath ligated into the trachea via tracheostomy. 1 ml of PBS was slowly instilled into the lungs and then carefully aspirated three times (23). BAL was centrifuged for 5 min at 1500 rpm to remove cells and used to determine cytokines. Mouse CCL2 and TNFα were determined using ELISA kits from Thermo Scientific and eBiosciences, respectively.
Promoter-reporter Assay
The assay was done by the Dual-Luciferase Reporter Assay System (Promega). 12-well plates of parental and M1+FXYD5 cells were transfected with a total of 1.8 μg/well of nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB)-LUC promoter-reporter plasmid, together with 360 ng/well of pRL-TK (Renilla) plasmid, using JetPEI transfection reagent (Polyplus transfection) according to the manufacturer's instructions. The plasmids were obtained from the Forchheimer Plasmid Collection of the Weizmann Institute. After transfection, cells were incubated for 48 h and lysed according to the manufacturer's instructions. Luminescence was measured by a Modulus Microplate Luminometer (Promega).
The Kinetics of LPS-FITC Binding to M1 Cells
Confluent M1 WT and M1+FXYD5 cells were pre-incubated for 30 min at 37 °C with LPS-FITC (1 μg/ml) in growth medium. Pre-incubated cells were washed with PBS, and then 100 nm LPS was added for another 30 min. The cells were recorded by confocal microscopy (Olympus) with 5-min intervals. The binding of LPS-FITC to M1 cells was analyzed by ImageJ.
Statistics
Data are expressed as means ± S.E., and significance was calculated by one way ANOVA + Dunnetts's multiple comparisons test using Kaleidagraph version 4.1.
Results
FXYD5 Increases LPS Induced CCL2 Secretion in M1 Cells
Our laboratory has studied the effects of FXYD5 in the M1 model epithelium (9, 19). M1 cells originate from the FXYD5-expressing mouse kidney collecting duct, but the isolated cell line lacks FXYD5. Transfecting them with FXYD5 cDNA (M1+FXYD5) results in its association with the Na,K-ATPase and evokes effects of FXYD5 on cell-cell contact, cytoskeletal organization, and cell adhesion and polarization (9, 19).
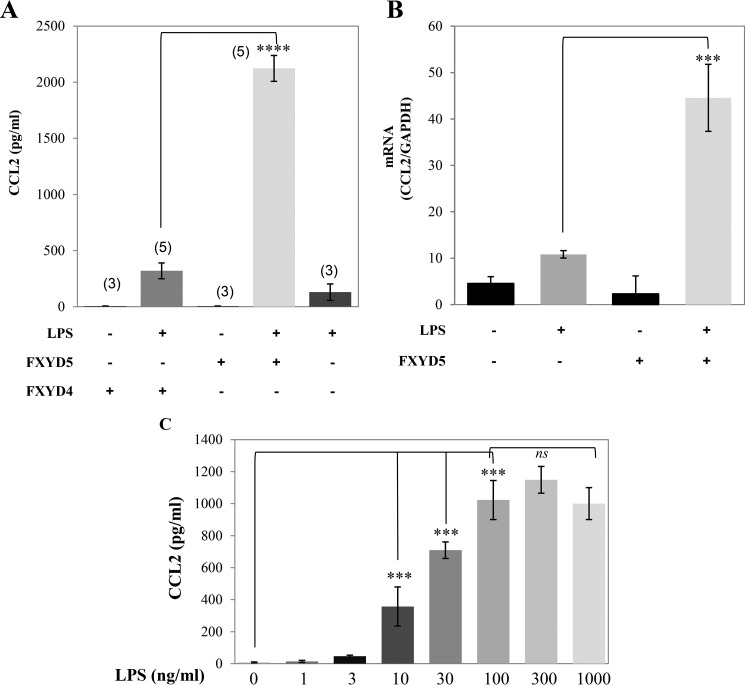
Untreated M1 cells do not secrete a significant amount of CCL2 irrespective of the expression of FXYD5. Such secretion could largely be induced by treating cells with 100 ng/ml LPS for 24 h (Fig. 1A). LPS-induced CCL2 secretion was FXYD5- dependent, as M1+FXYD5 cells secreted at least 5-fold more CCL2 than the non-transfected cells. This was also matched by a higher abundance of CCL2 mRNA (Fig. 1B). Previously we have demonstrated that M1 cells constitutively express FXYD4, which is suppressed by the expression of FXYD5 (19). To exclude the possibility that the above effect is due to FXYD4 down-regulation rather than FXYD5 expression, we have also measured LPS-induced CCL2 secretion in FXYD4 silenced cells. Such silencing does not cause up-regulation of any other FXYD protein (19). As can be seen in Fig. 1A, inhibiting the expression of FXYD4 in M1 cells does not elevate CCL2 secretion. Fig. 1C summarizes the LPS dose response in M1+FXYD5 cells. Maximal induction of CCL2 in M1+FXYD5 cells is achieved at 100–300 ng/ml, similar to that previously reported (24).
FIGURE 1.
FXYD5 increases LPS-induced CCL2 secretion in M1 cells. Wild type, M1+FXYD5, and FXYD4 silenced M1 cells were treated with either 100 ng/ml LPS or diluent. A, medium was removed 24 h later and assayed for CCL2 as described under “Experimental Procedures.” Means ± S.E. of at least three independent experiments are depicted. The asterisk indicates a significant difference between the two test groups, as analyzed by ANOVA; ****, p < 0.0001, ns, non-significant. B, cells were lysed, total RNA extracted, and assayed for the abundance of CCL2 and GAPDH mRNA by RT-PCR as described under “Experimental Procedures.” Data are represented by arbitrary units normalized to GAPDH. Means ± S.E. of at least three independent experiments are depicted. The asterisk indicates a significant difference between the two test groups, as analyzed by ANOVA; ***, p < 0.001. C, M1+FXYD5 cells were incubated with LPS with the indicated concentration for 24 h. CCL2 secretion was quantified as described above. Means ± S.E. of at least three independent experiments are depicted. The asterisk indicates a significant difference between the two test groups, as analyzed by ANOVA; ****, p < 0.0001.
LPS-induced CCL2 Secretion Is Mediated by NF-κB
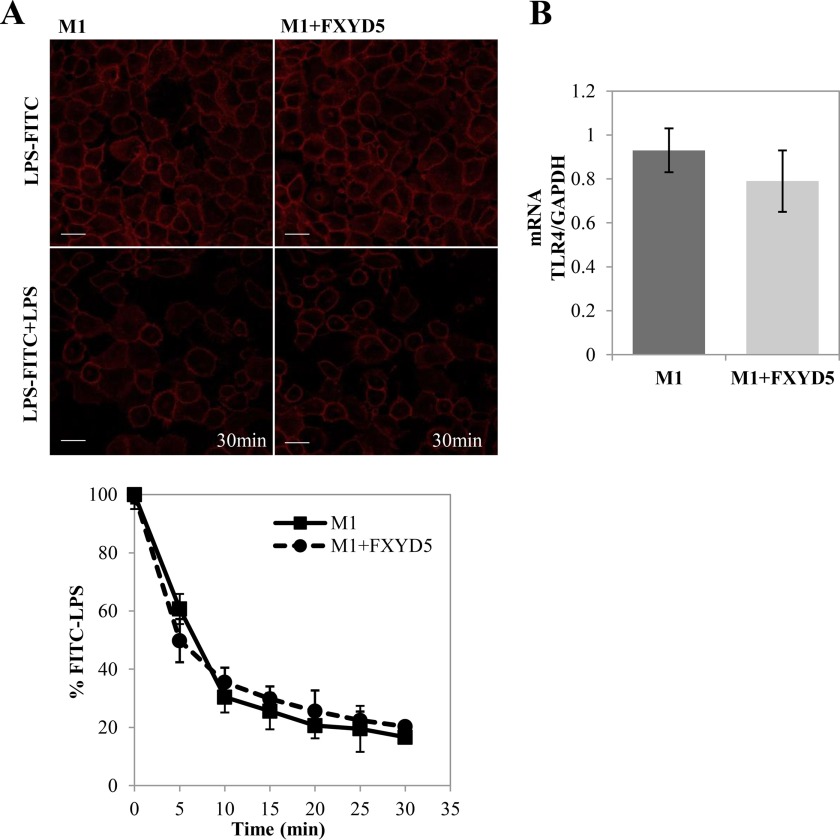
LPS activation and initiation of pro-inflammatory events occur rapidly after LPS interacts with the Toll-like receptor 4 (TLR4) (25). As a first step in understanding the differential CCL2 secretion, we analyzed the surface binding of LPS in M1 WT versus M1+FXYD5 cells by measuring the kinetics of competitive LPS binding with FITC-LPS conjugate. The curves observed in Fig. 2A show no variance in FITC-LPS release between both cells lines. Also, no significant changes were observed in TLR4 mRNA (Fig. 2B). The fact that FXYD5 has no effect on LPS-TLR4 interaction suggests that the step affected by this protein is postreceptor binding.
FIGURE 2.
LPS-FITC binding to M1 cells. A, M1 WT and M1+FXYD5 cells were incubated for 30 min with FITC-LPS. Non-fluorescent LPS was added and competitive kinetics were observed by fluorescent signal decay under confocal microscopy (×20 magnification). Representative images of 0 min and 30 min time points are depicted (left panel). Scale bar, 10 μm. Total fluorescence of each image was calculated and plotted (bottom). Data points are means ± S.E. of 4 fields for each cell type. B, M1 WT and M1+FXYD5 cells were lysed, total RNA extracted, and assayed for the abundance of TLR4 mRNA by RT-PCR as described under “Experimental Procedures.” Data represented by arbitrary units normalized to GAPDH. Means ± S.E. of three experiments are depicted.
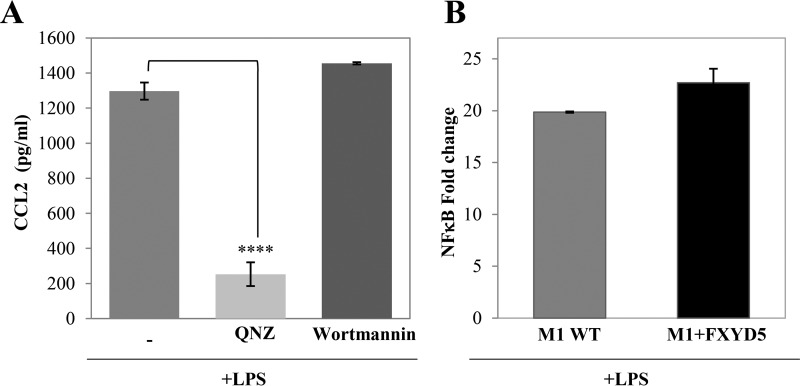
LPS activates a broad range of signaling pathways including the NF-κB and MAPK cascades (26), and also the PI3K pathway (27). To elucidate whether FXYD5 regulates the LPS-induced CCL2 secretion by modulating any of these pathways, we pre-incubated M1+FXYD5 cells with QNZ (NF-κB inhibitor), and with Wortmannin (PI3K inhibitor). The results in Fig. 3A demonstrate that while Wortmannin had no effect on CCL2 secretion, QNZ reduces it almost completely, suggesting that CCL2 secretion in M1+FXYD5 is mediated through NF-κB pathway. However, NF-κB was equally induced in WT M1 and M1+FXYD5 cells when assessed by an NF-κB dual luciferase reporter assay, shortly after LPS activation (Fig. 3B). These results suggest that while NF-κB is necessary for the LPS-induced CCL2 secretion, its initial activation is not dependent on the presence of FXYD5.
FIGURE 3.
LPS-induced CCL2 secretion is mediated by NF-κB. A, confluent monolayers of M1+FXYD5 cells received either 100 nm of the indicated inhibitor or diluent. 30-min later the cultures received 100 ng/ml LPS and were incubated for 24 h. CCL2 secretion was analyzed in the culture medium. The asterisk indicates a significant difference between the two test groups, as analyzed by ANOVA; ****, p < 0.0001. B, M1 and M1+FXYD5 cells were transfected with NF-κB-LUC and Renilla plasmids. After 24 h the cells were treated with 100 ng/ml LPS for 30 min at 37 °C. Luciferase activity was normalized to Renilla expression. Means ± S.E. of at least three independent experiments are depicted.
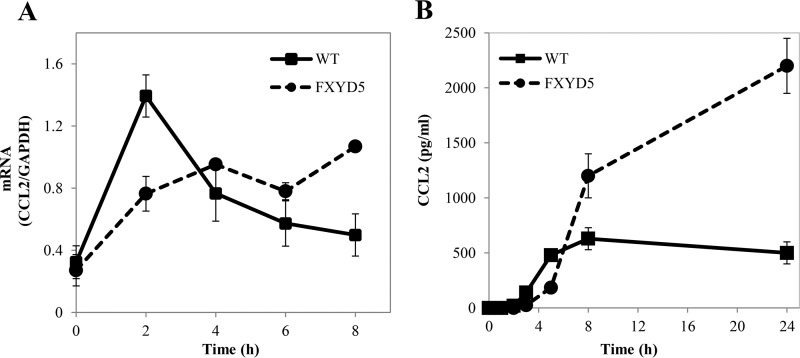
FXYD5-dependent CCL2 Secretion Is a Result of Late Gene Activation
The fact that FXYD5 has no influence on the main LPS stimulatory steps suggests that its differential regulation is on downstream mediators. Closer examination of the kinetics of the LPS-induced increase in CCL2 mRNA indicates that in non-transfected cells LPS evokes a relatively fast increase in mRNA, which peaks at 2 h and then decays back to background level. In M1+FXYD5 cells, on the other hand, the effect builds up slower, but is more sustained (Fig. 4A). The mRNA data are supported by CCL2 protein secretion, as shown in Fig. 4B. While in M1 WT cells, CCL2 accumulates earlier and remains constant, in M1+FXYD5 cells, the secretion occurs later, but remains continuous, as shown by protein accumulation up to 24 h. The above data suggest an additional, FXYD5-dependent activation phase, only in M1+FXYD5 cells.
FIGURE 4.
FXYD5-dependent CCL2 secretion is a result of late gene activation. WT M1 and M1+FXYD5 cells received 100 ng/ml LPS for the indicated periods of time. A, cells were lysed and total RNA was isolated and assayed for the abundance of CCL2 mRNA by RT-PCR. Data are represented by arbitrary units normalized to GAPDH. B, medium was collected and assayed for CCL2 as described under “Experimental Procedures.” Means ± S.E. of three experiments are depicted.
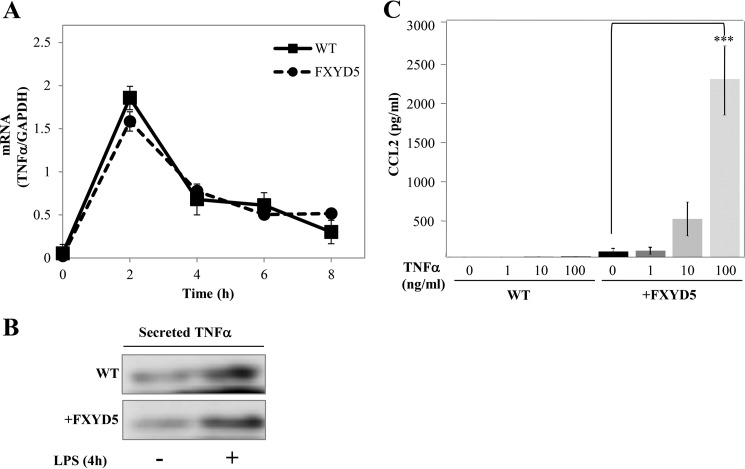
LPS induces a wide range of parallel signaling pathways that can trigger a potential autocrine stimulatory loop or act as feedback inhibitors to control late LPS-responsive genes, and our data suggest that one of those pathways may be regulated by the presence of FXYD5. Tumor necrosis factor α (TNFα) is a potent pro-inflammatory cytokine with important roles in control of immune and inflammatory responses as well as cell cycle proliferation and apoptosis (28) whose expression is also increased by LPS (29). Fig. 5A shows that the levels of TNFα mRNA are elicited equally by LPS treatment in WT M1 and M1+FXYD5 cells. The above data are matched by secreted protein, detected in growth medium (Fig. 5B). M1 WT and M1+FXYD5 cells were incubated with increasing concentrations of TNFα and analyzed for secreted CCL2. The effect of TNFα on CCL2 secretion was only observed in M1+FXYD5, and not in WT M1 cells (Fig. 5C).
FIGURE 5.
LPS-induced TNFα stimulates CCL2 only in M1+FXYD5 cells. A, WT M1 and M1+FXYD5 cells received 100 ng/ml LPS for the indicated periods of time. Cells were lysed, and total RNA was isolated and assayed for the abundance of TNFα mRNA by RT-PCR. Data are represented by arbitrary units normalized to GAPDH. B, following 4 h of LPS stimulation, medium was collected and concentrated. Medium samples from LPS stimulated and non-stimulated cells were electrophoretically resolved, and TNFα secretion was analyzed with anti TNFα (1:1000) by Western blotting. C, wild type (WT) M1 and M1+FXYD5 cells were incubated for 24 h with the indicated concentrations of TNFα, and the CCL2 concentration in the medium was determined. Values are means ± S.E. of three independent experiments. The asterisk indicates a significant difference between the two test groups, as analyzed by ANOVA; ***, p < 0.001.
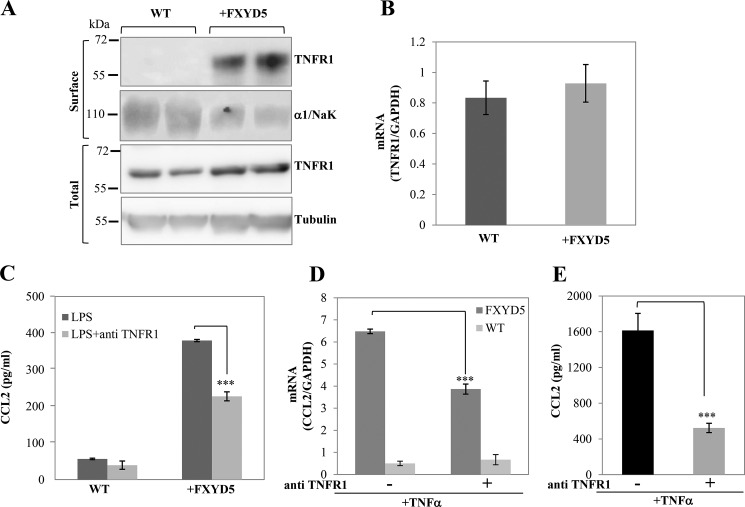
We then aimed to identify the FXYD5 differentiated step in TNFα mediated CCL2 secretion. TNFα exerts its biological functions via interactions with two distinct receptors, designated TNFR1 and TNFR2 (30). The TNFα effects on NF-κB activation occurs predominantly through TNFR1 (31). We therefore analyzed the expression pattern of TNFR1 in WT M1 versus M1+FXYD5 cells. Fig. 6A shows that TNFR1 is expressed approximately at the same level in total cell lysates (lower panel); however it is translocated to the plasma membrane only in the presence of FXYD5 (upper panel). In agreement with the results, the levels of TNFR1 mRNA are comparable in WT M1 and M1+FXYD5 (Fig. 6B).
FIGURE 6.
FXYD5 promotes plasma membrane expression of TNFR1. A, M1 and M1+FXYD5 were surface labeled with biotin, and total and surface-biotinylated proteins were extracted as described under “Experimental Procedures.” Western blots were analyzed with TNFR1-specific antibody. Tubulin and α1 subunit of Na,K-ATPase (α1/NaK) are shown as the loading controls for the total and surface expressed proteins, respectively. Two independent protein preparations from WT and M1+FXYD5 cells are depicted. B, RT-PCR was performed to measure TNFR1 mRNA abundance. Data are represented by arbitrary units normalized to GAPDH. C, CCL2 secretion following treatment with TNFR1 blocking antibody. Prior to LPS treatment, WT M1 and M1+FXYD5 cells were incubated for 1 h with 10 μg/ml TNFR1 blocking antibody or diluent. Medium was removed, and 0.5 ml of fresh medium was added ± 100 ng/ml LPS and incubated for 8 h. Medium was removed, and aliquots were analyzed for secreted CCL2. Means ± S.E. of three different experiments are depicted. The asterisk indicates a significant difference between the two test groups, as analyzed by ANOVA, ***, p < 0.001. D, following TNFα stimulation, anti-TNFR1 treated (TNFα+ab) and non-treated (TNFα) cells were lysed, total RNA extracted and assayed for the abundance of CCL2 mRNA by RT-PCR as described under “Experimental Procedures.” Data represented by arbitrary units normalized to GAPDH. Means ± S.E. of three experiments are depicted. The asterisk indicates a significant difference between the two test groups, as analyzed by ANOVA; ***, p < 0.001. E, CCL2 secretion following treatment with TNFR1-blocking antibody. Prior to TNFα treatment, M1+FXYD5 cells were incubated for 1 h with 10 μg/ml TNFR1 blocking antibody or diluent. Medium was removed, and 0.5 ml of fresh medium was added +100 ng/ml TNFα and incubated for 8 h. Medium was removed, and aliquots were analyzed for secreted CCL2. Means ± S.E. of three different experiments are depicted. The asterisk indicates a significant difference between the two test groups, as analyzed by ANOVA; ***, p < 0.001.
To further verify that the FXYD5-induced increase in CCL2 secretion is a result of the second phase activation mediated through TNFα receptor, we blocked the receptor with anti-TNFR1 antibody (E-20) (32) (Fig. 6C). WT M1 and M1+FXYD5 cells were pre-incubated either with anti-TNFR1 for 1 h or with diluent, followed by 8 h LPS treatment. In M1+FXYD5 cells, in the presence of the TNFR1 antibody the production of CCL2 was decreased by more than 40%, while in M1 WT cells, in agreement with the absence of the receptor, the addition of the antibody does not significantly modify the secretion of CCL2. The above data are matched by inhibition of CCL2 mRNA, observed 4 h following LPS stimulation (Fig. 6D). The partial reduction of CCL2 secretion could be explained by incomplete inhibition of TNFR1 receptor by the antibody, due to experimental limitation. This was supported by partial activation of the TNFR1 receptor by TNFα in the presence of the same concentration of anti-TNFR1 (Fig. 6E). Nevertheless, the existence of additional pathways that stimulate CCL2 secretion in M1+FXYD5 cannot be completely ruled out.
FXYD5 Effect on CCL2 Secretion Is Not Exclusive to M1 Cells
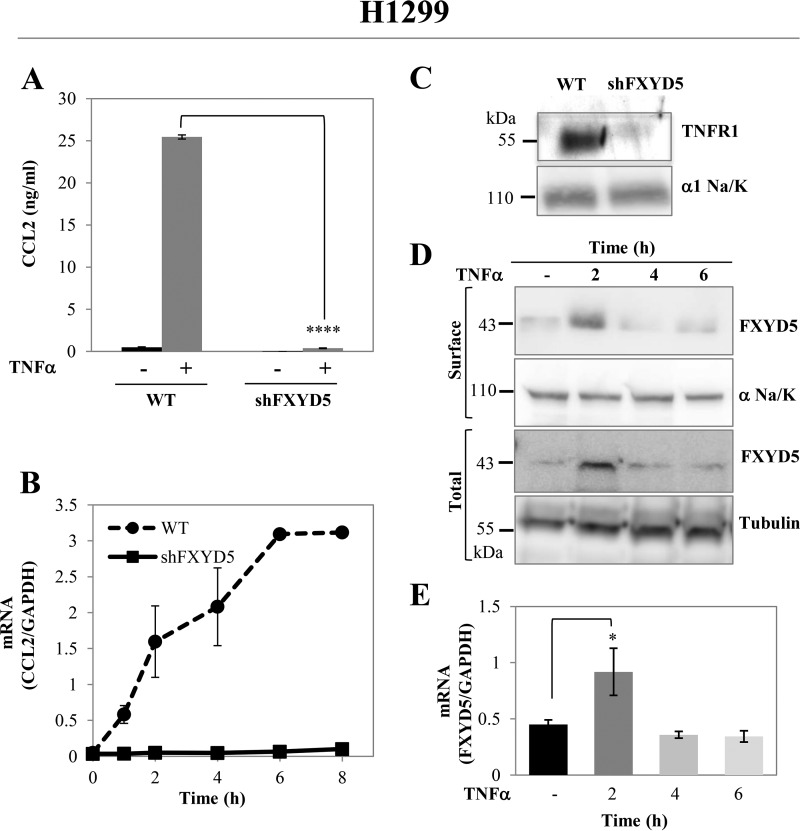
Next, we tested the generality of the above phenomenon by measuring CCL2 secretion in the non-small lung carcinoma H1299 cells, which natively express FXYD5, and in H1299 cells where FXYD5 was silenced by transfection with a specific shRNA. H1299 cells were not responsive to external LPS irrespective of the FXYD5 expression (data not shown). However CCL2 secretion was strongly induced by TNFα, and silencing of FXYD5 almost completely abolished it, as shown by both decrease in secreted protein and mRNA (Fig. 7, A and B). In agreement with the results in M1 cells, the absence of FXYD5 in H1299 significantly reduces TNFR1 expression at the plasma membrane (Fig. 7C). Furthermore, since H1299 cells natively express FXYD5, we assessed FXYD5 abundance following TNFα stimulation. Surface labeling of FXYD5 was a problem until now, since it could not be biotinylated or stained by antibodies, probably because of post-transcriptional modification of the extracellular domain, such as glycosylation. To overcome this difficulty, after biotinylation, we performed protein extraction, with nonionic detergent (C12E10), which was shown to preserve protein-protein interactions. The C12E10 buffer (1 mg/ml C12E10, 100 mm RbCl, 1 mm EDTA, 50 mm Tris, pH 7.5) was previously used by our laboratory for coimmunoprecipitation assays of Na,K-ATPase and FXYD proteins (33). This maneuver allowed us to isolate FXYD5 from cell surface as a complex with surface biotinylated pump.
FIGURE 7.
FXYD5 effect on TNFα receptor is not exclusive to M1 cells. A, TNFα induced CCL2 secretion in H1299. WT and FXYD5 silenced H1299 cells were incubated in the presence or absence of 100 ng/ml TNFα for 24 h. Medium was removed, and aliquots were analyzed for secreted CCL2. The asterisk indicates a significant difference between the two test groups, as analyzed by ANOVA; ****, p < 0.0001. B, time course of TNFα-dependent CCL2 mRNA transcription. 12-well plates of H1299 WT and shFXYD5 cells received 100 ng/ml TNFα for the indicated periods of time. Cells were then lysed, and total RNA was isolated and assayed for the abundance of CCL2 mRNA by RT-PCR. Data are represented by arbitrary units normalized to GAPDH. Means ± S.E. of three experiments are depicted. C, 10-cm plates of M1 and M1+FXYD5 cells were grown till confluence and surface biotinylated proteins were extracted as described under “Experimental Procedures.” Samples were resolved by electrophoresis and blotted with antibodies to TNFR1. The α1 subunit of Na,K-ATPase (α1/NaK) is shown as the loading controls for surface expressed proteins. The figure shows a representative Western blot. D, TNFα induced FXYD5 expression in H1299. 10-cm plates of H1299 WT and shFXYD5 cells received 100 ng/ml TNFα for the indicated periods of time. For total fraction: cells were lysed in RIPA buffer, for surface FXYD5: the cells were biotinylated and extracted in C12E10 buffer (1 mg/ml C12E10, 50 mm Tris, pH 7.5, 1 mm EDTA, 150 mm NaCl). The protein extracts were resolved by Western blotting and assayed for the abundance of FXYD5 with anti FXYD5 (1:100). Specific bands were quantified and normalized to tubulin or α1/NaK expression. A representative Western blot is shown. E, 12-well plates of H1299 WT and shFXYD5 cells received 100 ng/ml TNFα for the indicated periods of time. Cells were lysed and total RNA was isolated and assayed for the abundance of FXYD5 mRNA by RT-PCR. Data are represented by arbitrary units normalized to GAPDH. Data are expressed as means ± S.E. The asterisk indicates a significant difference between the two test groups, as analyzed by ANOVA; *, p ≤ 0.05.
As observed in Fig. 7D, FXYD5 total and surface protein is significantly up-regulated after 2 h of treatment with TNFα, and then its level returns back to baseline. FXYD5 mRNA up-regulation was also noticed Fig. 7E, suggesting alterations at transcriptional level.
FXYD5 Is Up-regulated in LPS-stimulated Mice Lung Epithelium
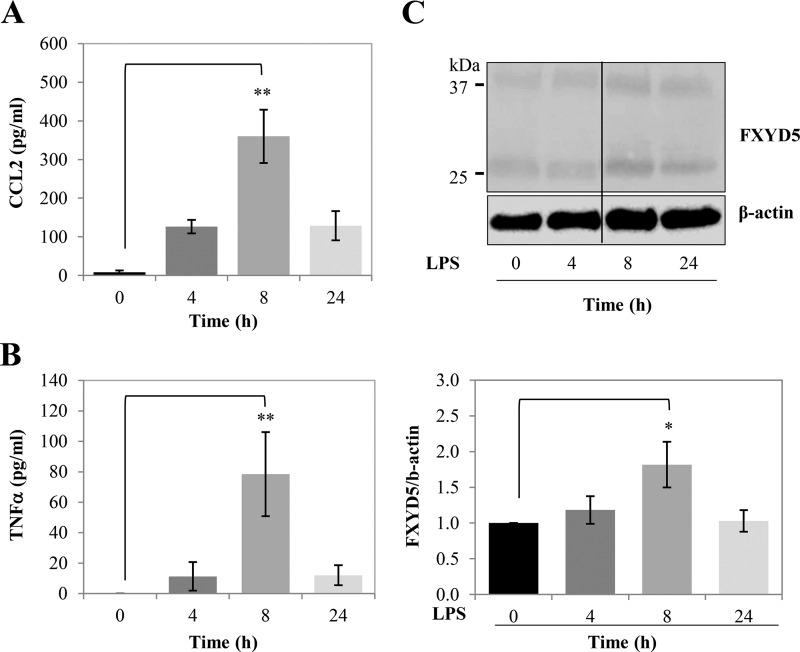
Finally we examined the effects of LPS on the secretion of CCL2 and TNFα in vivo. In our previous publication (2), we have shown that FXYD5 can be found in normal cells and that it is particularly abundant in epithelial cells in kidney, intestine, and lung. These epithelia are directly exposed to different levels of bacterial pathogens, present as contaminants in food or in airborne particles. As expected, LPS induced CCL2 and TNFα secretion in mice respiratory system. In Fig. 8, mice were treated with LPS according to the protocol described under “Experimental Procedures.” BAL fluid was collected at the indicated periods of time and tested for the presence of CCL2 (Fig. 8A) and TNFα (Fig. 8B). In parallel, membranes from lung tissue were analyzed for the presence of FXYD5 by Western blotting (Fig. 8C). The results demonstrate secretion of both CCL2 and TNFα following LPS stimulation, matched by an increase in FXYD5 protein in the plasma membrane.
FIGURE 8.
FXYD5 is up-regulated in LPS-stimulated mice. A and B, LPS-stimulated secretion of CCL2 and TNFα in lung BALF. Mice were treated with a single intraperitoneal injection of 50 μl of PBS or LPS (6 mg/kg) in 50 μl of PBS and BAL was collected at the indicated times as described under “Experimental Procedures.” The asterisk indicates a significant difference between the two test groups, as analyzed by ANOVA; **, p ≤ 0.01. C, lungs were lysed, and the presence of FXYD5 was analyzed by Western blotting (upper panel). Densitometry analysis of the bands was performed, and the FXYD5 signal was normalized to β-actin (lower panel). Data are expressed as means ± S.E. n = 3. The asterisk indicates a significant difference between the two test groups, as analyzed by ANOVA; *, p ≤ 0.05.
Discussion
Systemic inflammation constitutes a major cause of morbidity and mortality as it is involved in a host of different infectious and noninfectious diseases. In this study, using LPS as stimulator, we described that the presence of FXYD5 in normal epithelial cells increases the production of CCL2, suggesting a more inflammatory phenotype. Our key finding showed differential localization of the TNFR1 receptor in FXYD5-expressing cells, suggesting that FXYD5 can contribute to the inflammatory state through TNFα signaling in normal cells. We have also characterized the above effect in two different cell lines and in vivo.
The primary model system used in this study was the kidney collecting duct cell line M1, as FXYD5 is expressed in these ducts under normal conditions (2). In non-stimulated cells, CCL2 expression and secretion cannot be detected, either in cells containing or lacking FXYD5. However, LPS treatment leads to the production of the cytokine CCL2, which is much greater in cells expressing FXYD5. The renal epithelial cells are directly exposed to bacteria and viruses known to induce CCL2, so the secretion of CCL2 in M1 was expected. However, the strong dependence of this effect on the expression of FXYD5 may suggest that up-regulating CCL2 secretion in response to bacterial or viral infection is one of the functions of FXYD5 in normal tissue. Previously, we have shown that the expression of FXYD5 in M1 cells impairs tight junctions, as measured by an increase of the paracellular permeability and redistribution of tight junction proteins (19). Thus, a simplified explanation to the LPS-induced FXYD5-dependent CCL2 secretion in M1 cells is that the expression of this protein makes LPS more accessible to their receptors. However, this is unlikely to be the case, since the LPS receptor-TLR4 is apical. Furthermore, we showed a similar labeling of FITC-LPS on both parental and M1+FXYD5 cells, providing direct evidence that both cell lines possess LPS binding capability.
Several experiments have been carried out in an attempt to elucidate the molecular events mediating the above response. Preliminary experiments using QNZ demonstrated that LPS induced CCL2 secretion in M1 cells is mediated by NF-κB pathway. However, promoter-reporter studies showed that despite the significant difference in amount of secreted CCL2 between M1 WT and M1+FXYD5 cells, LPS-mediated NF-κB activation in both is similar.
A detailed kinetic analysis of CCL2 mRNA transcription showed that CCL2 accumulation in M1+FXYD5 cells is due to a second phase of gene expression. This result suggests involvement of a secondary regulator that generates additional, more prolonged, CCL2 mRNA transcription and protein secretion in FXYD5-expressing cells. In both, M1 WT and M1+FXYD5 cells, LPS exposure increased the expression of TNFα. However, we found a striking difference in the cellular response to TNFα between M1 WT and M1+FXYD5 cells. We showed that TNFα is an early response cytokine induced by LPS in both parental and M1+FXYD5 cells. Our results establish an autocrine loop, in which TNFα promotes the production of CCL2, only in M1+FXYD5 cells. Our data demonstrate that FXYD5 increases the production of CCL2 by increasing the abundance of the TNFα receptor in the plasma membrane. This key finding was confirmed first by our results showing that M1 WT cells are much less responsive to TNFα stimuli than M1+FXYD5 cells in terms of CCL2 secretion. Second, more than 40% of LPS-induced CCL2 secretion in M1+FXYD5 cells was neutralized by TNFR1 blocking antibody. The partial inhibition of CCL2 secretion by anti-TNFR1 could indicate that additional factors may be involved in this process. It also may point to the early translated CCL2, which is mediated exclusively by LPS. In addition, incomplete abolishment may be explained by experimental limitations, such as incomplete inhibition of the receptor by the antibody.
Finally, the results obtained in M1 cells were validated in another cellular system. We found that the secretion of CCL2 stimulated by TNFα was completely abolished by FXYD5 silencing, along with down-regulation of surface TNFR1 in H1299 cells that natively express FXYD5. Furthermore, we showed up-regulation in FXYD5 total and surface expression immediately upon TNFα stimulation, which highlights FXYD5 role in TNFα-mediated signaling not only during inflammation, but under any homeostatic and pathophysiological conditions.
The presence of FXYD5 did not modify the total protein levels of TNFR1 or interfere with its transcription suggesting that FXYD5 may regulate targeting of the TNFR1 to the plasma membrane. TNFR1, in the absence of stimuli, is predominantly localized at the Golgi apparatus (34). The FXYD5-dependent increase in surface receptor, without external stimuli, could occur due to an interference with the TNFR1 cytoplasmic domain, which is responsible for its intracellular retention (35). Alteration of this process by FXYD5 or in any other step of TNFR1 membrane traffic pathway may have an important effect on the ability of a cell to respond to TNFα.
The underlying mechanisms mediated by FXYD5, that control TNFR1 localization and hence, severity of inflammation, are not defined yet. FXYD5 lacks intrinsic enzymatic activity; therefore its downstream signaling depends upon sequential recruitment of cytosolic adaptor proteins or by co-interaction with additional membrane proteins, as Na,K-ATPase. The interaction of FXYD5 with Na,K-ATPase has long been established (2, 3). It was previously reported that in addition to maintaining the electrochemical gradient, Na,K-ATPase may promote intracellular signaling processes that effect cell proliferation and apoptosis (36, 37). It can effect cell-cell contact and motility (38, 39) and also form a hormone-receptor complex capable of inducing phosphorylation (40). Thus, FXYD5 may regulate TNFR1 expression at the plasma membrane via interaction with the pump.
To further establish the significance of the FXYD5 role during inflammation, we induced infection mimicking conditions in mice. Intraperitoneal injection of LPS resulted in the appearance of both CCL2 and TNFα in mice BAL fluid. Secretion of CCL2 and TNFα during infection has long been established both in vivo and in vitro (41). We now provide the first indication of a correlation between LSP-mediated CCL2 secretion and elevation in FXYD5 protein abundance in vivo. The in vivo kinetics of TNFα and CCL2 secretion correlates with FXYD5 up-regulation in normal lung tissue, which by itself might suggest involvement of FXYD5 in the infection process. The clearance of TNFα and CCL2 from mice lung after 24 h is not in agreement with the in vitro studies in M1 cells, which display CCL2 accumulation. This inconsistency might have a simple explanation. The experimental setting in vivo has a much more complicated structure and tighter regulation then that observed in culture cells. In addition, FXYD5 high expression in M1 cells is constitutively induced by stable transfection, as opposed to native FXYD5, which resides under strict cellular regulation.
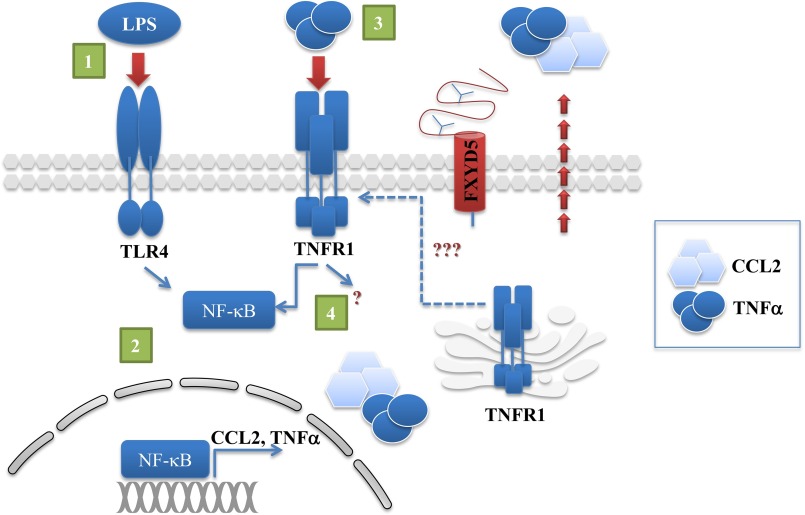
The finding that there is a relation between the expression of FXYD5 and CCL2 secretion after LPS treatment indicates that the presence of FXYD5 generates a more pro-inflammatory phenotype and might play a role in immune processes, which is a novel concept. Finally, we offer a mechanism that indicates involvement of the TNFα receptor as a key element mediating FXYD5-dependent LPS-induced CCL2 secretion (Illustrated in Fig. 9). LPS binding to TLR4 receptor in M1 WT and M1+FXYD5 cells immediately activates NF-κB, which generates gene transcription of CCL2 and TNFα. In turn, secreted TNFα activates FXYD5 facilitated plasma membrane TNFR1, and generates additional, more prolonged CCL2 secretion in M1+FXYD5 cells, possibly through more than one signaling pathway.
FIGURE 9.
Schematic representation of signaling events leading to CCL2 accumulation in FXYD5-expressing cells. 1, LPS binding to TLR4 receptor triggers NF-κB activation. 2, NF-κB nuclear translocation induces transcription and secretion of CCL2 and TNFα. 3, FXYD5 promotes the expression of the TNFR1 at the plasma membrane. 4, TNFα binds to surface TNFR1 generating an autocrine loop leading to prolonged CCL2 secretion.
In the current study (Fig. 9) we characterized FXYD5-dependent CCL2 secretion following LPS stimulation; however, the importance of our results is that any condition, which involves TNFα secretion, will be affected by FXYD5 expression. Moreover, epithelial tissues with a greater expression of FXYD5 may be prone to larger inflammatory responses. Further investigation will undoubtedly reveal insights into the FXYD5 role in inflammation and may provide additional targets for therapy.
Author Contributions
I. L.-G. designed and performed mRNA experiments, analyzed data, and wrote the manuscript. C. A. designed and performed protein secretion experiments, analyzed data, and revised the manuscript. L. A. D. designed and performed in vivo experiments, analyzed data, and contributed to the manuscript. H. G. conceived and coordinated the project.
This work was supported by a grant from the U.S.-Israel Binational Science Foundation (to L. A. D. and H. G.) and National Institutes of Health Grant HL113350. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- MCP
- monocyte chemotactic protein
- LPS
- lipopolysaccharide
- BAL
- bronchoalveolar lavage fluid.
References
- 1. Garty H., and Karlish S. J. D. (2006) Role of FXYD proteins in ion transport. Annu. Rev. Physiol. 68, 431–459 [DOI] [PubMed] [Google Scholar]
- 2. Lubarski I., Pihakaski-Maunsbach K., Karlish S. J. D., Maunsbach A. B., and Garty H. (2005) Interaction with the Na,K-ATPase and tissue distribution of FXYD5 (related to ion channel). J. Biol. Chem. 280, 37717–37724 [DOI] [PubMed] [Google Scholar]
- 3. Lubarski I., Karlish S. J. D., and Garty H. (2007) Structural and functional interactions between FXYD5 and the Na+-K+-ATPase. Am. J. Physiol. Renal Physiol. 293, F1818–F26 [DOI] [PubMed] [Google Scholar]
- 4. Ino Y., Gotoh M., Sakamoto M., Tsukagoshi K., and Hirohashi S. (2002) Dysadherin, a cancer-associated cell membrane glycoprotein, down-regulates E-cadherin and promotes metastasis. Proc. Natl. Acad. Sci. U.S.A. 99, 365–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nam J.-S., Hirohashi S., and Wakefield L. M. (2007) Dysadherin: a new player in cancer progression. Cancer Lett. 255, 161–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shimamura T., Yasuda J., Ino Y., Gotoh M., Tsuchiya A., Nakajima A., Sakamoto M., Kanai Y., and Hirohashi S. (2004) Dysadherin expression facilitates cell motility and metastatic potential of human pancreatic cancer cells. Cancer Res. 64, 6989–6995 [DOI] [PubMed] [Google Scholar]
- 7. Schüler Y., Lee-Thedieck C., Geiger K., Kaiser T., Ino Y., Aicher W. K., and Klein G. (2012) Osteoblast-secreted factors enhance the expression of dysadherin and CCL2-dependent migration of renal carcinoma cells. Int. J. Cancer 130, 288–299 [DOI] [PubMed] [Google Scholar]
- 8. Lee Y.-K., Lee S.-Y., Park J.-R., Kim R.-J., Kim S.-R., Roh K.-J., and Nam J.-S. (2012) Dysadherin expression promotes the motility and survival of human breast cancer cells by AKT activation. Cancer Sci. 103, 1280–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lubarski I., Asher C., and Garty H. (2011) FXYD5 (dysadherin) regulates the paracellular permeability in cultured kidney collecting duct cells. Am. J. Physiol. Renal Physiol. 301, F1270–F80 [DOI] [PubMed] [Google Scholar]
- 10. Miller T. J., and Davis P. B. (2008) FXYD5 modulates Na+ absorption and is increased in cystic fibrosis airway epithelia. Am. J. Physiol. Lung Cell Mol. Physiol. 294, L654–L64 [DOI] [PubMed] [Google Scholar]
- 11. Tsuiji H., Takasaki S., Sakamoto M., Irimura T., and Hirohashi S. (2003) Aberrant O-glycosylation inhibits stable expression of dysadherin, a carcinoma-associated antigen, and facilitates cell-cell adhesion. Glycobiology 13, 521–527 [DOI] [PubMed] [Google Scholar]
- 12. Nam J.-S., Kang M.-J., Suchar A. M., Shimamura T., Kohn E. A., Michalowska A. M., Jordan V. C., Hirohashi S., and Wakefield L. M. (2006) Chemokine (C-C motif) ligand 2 mediates the prometastatic effect of dysadherin in human breast cancer cells. Cancer Res. 66, 7176–7184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yadav A., Saini V., and Arora S. (2010) MCP-1: chemoattractant with a role beyond immunity: a review. Clin. Chim. Acta 411, 1570–1579 [DOI] [PubMed] [Google Scholar]
- 14. Schaer D. A., Lesokhin A. M., and Wolchok J. D. (2011) Hiding the road signs that lead to tumor immunity. J. Exp. Med. 208, 1937–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baay M., Brouwer A., Pauwels P., Peeters M., and Lardon F. (2011) Tumor cells and tumor-associated macrophages: secreted proteins as potential targets for therapy. Clin. Dev. Immunol. 2011, 565187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee H.-W., Choi H.-J., Ha S.-J., Lee K.-T., and Kwon Y.-G. (2013) Recruitment of monocytes/macrophages in different tumor microenvironments. Biochim. Biophys. Acta. 1835, 170–179 [DOI] [PubMed] [Google Scholar]
- 17. Nikas J. B. (2013) Inflammation and immune system activation in aging: a mathematical approach. Sci. Rep. 3, 3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stoos B. A., Náray-Fejes-Tóth A., Carretero O. A., Ito S., and Fejes-Tóth G. (1991) Characterization of a mouse cortical collecting duct cell line. Kidney Int. 39, 1168–1175 [DOI] [PubMed] [Google Scholar]
- 19. Lubarski I., Asher C., and Garty H. (2014) Modulation of cell polarization by the Na+-K+-ATPase-associated protein FXYD5 (dysadherin). Am. J. Physiol. Cell Physiol. 306, C1080–8 [DOI] [PubMed] [Google Scholar]
- 20. Wang H. L., Akinci I. O., Baker C. M., Urich D., Bellmeyer A., Jain M., Chandel N. S., Mutlu G. M., and Budinger G. R. S. (2007) The intrinsic apoptotic pathway is required for lipopolysaccharide-induced lung endothelial cell death. J. Immunol. 179, 1834–1841 [DOI] [PubMed] [Google Scholar]
- 21. Dumasius V., Sznajder J. I., Azzam Z. S., Boja J., Mutlu G. M., Maron M. B., and Factor P. (2001) β(2)-adrenergic receptor overexpression increases alveolar fluid clearance and responsiveness to endogenous catecholamines in rats. Circ. Res. 89, 907–914 [DOI] [PubMed] [Google Scholar]
- 22. Azzam Z. S., Dumasius V., Saldias F. J., Adir Y., Sznajder J. I., and Factor P. (2002) Na,K-ATPase overexpression improves alveolar fluid clearance in a rat model of elevated left atrial pressure. Circulation 105, 497–501 [DOI] [PubMed] [Google Scholar]
- 23. Mutlu G. M., Green D., Bellmeyer A., Baker C. M., Burgess Z., Rajamannan N., Christman J. W., Foiles N., Kamp D. W., Ghio A. J., Chandel N. S., Dean D. A., Sznajder J. I., and Budinger G. R. S. (2007) Ambient particulate matter accelerates coagulation via an IL-6-dependent pathway. J. Clin. Invest. 117, 2952–2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anand A. R., Bradley R., and Ganju R. K. (2009) LPS-induced MCP-1 expression in human microvascular endothelial cells is mediated by the tyrosine kinase, Pyk2 via the p38 MAPK/NF-κB-dependent pathway. Mol. Immunol. 46, 962–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chow J. C., Young D. W., Golenbock D. T., Christ W. J., and Gusovsky F. (1999) Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274, 10689–10692 [DOI] [PubMed] [Google Scholar]
- 26. Oeckinghaus A., and Ghosh S. (2009) The NF-κB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 1, a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mendes Sdos S., Candi A., Vansteenbrugge M., Pignon M.-R., Bult H., Boudjeltia K. Z., Munaut C., and Raes M. (2009) Microarray analyses of the effects of NF-κB or PI3K pathway inhibitors on the LPS-induced gene expression profile in RAW264.7 cells: synergistic effects of rapamycin on LPS-induced MMP9-overexpression. Cell Signal. 21, 1109–1122 [DOI] [PubMed] [Google Scholar]
- 28. Tracey K. J., and Cerami A. (1993) Tumor necrosis factor, other cytokines and disease. Annu. Rev. Cell Biol. 9, 317–343 [DOI] [PubMed] [Google Scholar]
- 29. Amiot F., Fitting C., Tracey K. J., Cavaillon J. M., and Dautry F. (1997) Lipopolysaccharide-induced cytokine cascade and lethality in LT α/TNF α-deficient mice. Mol. Med. 3, 864–875 [PMC free article] [PubMed] [Google Scholar]
- 30. Locksley R. M., Killeen N., and Lenardo M. J. (2001) The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104, 487–501 [DOI] [PubMed] [Google Scholar]
- 31. McFarlane S. M., Pashmi G., Connell M. C., Littlejohn A. F., Tucker S. J., Vandenabeele P., and MacEwan D. J. (2002) Differential activation of nuclear factor-κB by tumour necrosis factor receptor subtypes. TNFR1 predominates whereas TNFR2 activates transcription poorly. FEBS Lett. 515, 119–126 [DOI] [PubMed] [Google Scholar]
- 32. Rowlands D. J., Islam M. N., Das S. R., Huertas A., Quadri S. K., Horiuchi K., Inamdar N., Emin M. T., Lindert J., Ten V. S., Bhattacharya S., and Bhattacharya J. (2011) Activation of TNFR1 ectodomain shedding by mitochondrial Ca2+ determines the severity of inflammation in mouse lung microvessels. J. Clin. Invest. 121, 1986–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Garty H., Lindzen M., Scanzano R., Aizman R., Füzesi M., Goldshleger R., Farman N., Blostein R., and Karlish S. J. D. (2002) A functional interaction between CHIF and Na-K-ATPase: implication for regulation by FXYD proteins. Am. J. Physiol. Renal Physiol. 283, F607–F15 [DOI] [PubMed] [Google Scholar]
- 34. Jones S. J., Ledgerwood E. C., Prins J. B., Galbraith J., Johnson D. R., Pober J. S., and Bradley J. R. (1999) TNF recruits TRADD to the plasma membrane but not the trans-Golgi network, the principal subcellular location of TNF-R1. J. Immunol. 162, 1042–1048 [PubMed] [Google Scholar]
- 35. Gaeta M. L., Johnson D. R., Kluger M. S., and Pober J. S. (2000) The death domain of tumor necrosis factor receptor 1 is necessary but not sufficient for Golgi retention of the receptor and mediates receptor desensitization. Lab. Invest. 80, 1185–1194 [DOI] [PubMed] [Google Scholar]
- 36. Abramowitz J., Dai C., Hirschi K. K., Dmitrieva R. I., Doris P. A., Liu L., and Allen J. C. (2003) Ouabain- and marinobufagenin-induced proliferation of human umbilical vein smooth muscle cells and a rat vascular smooth muscle cell line, A7r5. Circulation 108, 3048–3053 [DOI] [PubMed] [Google Scholar]
- 37. McConkey D. J., Lin Y., Nutt L. K., Ozel H. Z., and Newman R. A. (2000) Cardiac glycosides stimulate Ca2+ increases and apoptosis in androgen-independent, metastatic human prostate adenocarcinoma cells. Cancer Res. 60, 3807–3812 [PubMed] [Google Scholar]
- 38. Vagin O., Tokhtaeva E., and Sachs G. (2006) The role of the β1 subunit of the Na,K-ATPase and its glycosylation in cell-cell adhesion. J. Biol. Chem. 281, 39573–39587 [DOI] [PubMed] [Google Scholar]
- 39. Barwe S. P. (2005) Novel role for Na,K-ATPase in phosphatidylinositol 3-kinase signaling and suppression of cell motility. Mol. Biol. Cell 16, 1082–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dmitrieva R. I., and Doris P. A. (2003) Ouabain is a potent promoter of growth and activator of ERK1/2 in ouabain-resistant rat renal epithelial cells. J. Biol. Chem. 278, 28160–28166 [DOI] [PubMed] [Google Scholar]
- 41. Xaus J., Comalada M., Valledor A. F., Lloberas J., López-Soriano F., Argilés J. M., Bogdan C., and Celada A. (2000) LPS induces apoptosis in macrophages mostly through the autocrine production of TNF-α. Blood 95, 3823–3831 [PubMed] [Google Scholar]