Abstract
Thrombin is produced from the C-terminal half of prothrombin following its proteolytic activation. The N-terminal half, released as the propiece Fragment 12 (F12), is composed of an N-terminal γ-carboxyglutamate domain (Gla) followed by two kringles (K1 and K2). The propiece plays essential roles in regulating prothrombin activation and proteinase function. The latter results from the ability of F12 to reversibly bind to the (pro)catalytic domain through K2 with high affinity and highly favorable thermodynamic constants when it is a zymogen in comparison to proteinase. Such discrimination is lost for K2 binding after proteolytic removal of the N-terminal Gla-K1 region of F12. The Ca2+-stabilized structure of the Gla domain is not required for F12 to bind the zymogen form more favorably. Enhanced binding to zymogen versus proteinase correlates with the ability of the propiece to enforce zymogen-like character in the proteinase. This is evident in variants of meizothrombin, an intermediate of prothrombin activation that contains the propiece covalently attached. This phenomenon is also independent of the Gla domain. Thus, the presence of K1 in covalent linkage with K2 in the propiece governs the ability of K2 to bind the (pro)catalytic domain in favor of zymogen, thereby enforcing zymogen-like character in the proteinase.
Keywords: allosteric regulation, prothrombin, serine protease, thermodynamics, thrombin
Introduction
Thrombin, a trypsin-like serine proteinase, plays a preeminent role in hemostasis and the prevention of blood loss (1, 2). It activates platelets and cleaves soluble fibrinogen to form insoluble fibrin, both essential components of the blood clot (3, 4). It acts as a feedback activator of steps in the coagulation cascade to enhance flux toward its own formation (3, 4). When bound to the cofactor, thrombomodulin on the endothelium, it catalyzes the activation of protein C and initiates the anticoagulant reactions responsible for decreasing flux through the coagulation cascade (5). Thus, thrombin functions as a central regulator of coagulation that derives from its ability to act on multiple biological substrates (6).
Two anion binding exosites (ABE12 and ABE2) play important roles in the binding of substrates and ligands to thrombin (7, 8). The role played by ABE1 in ligating a range of thrombin substrates and ligands has been established by numerous biochemical and structural studies (6, 7). In contrast, far fewer ligands have been identified for ABE2, found approximately on the opposite face of the proteinase domain from ABE1 (6). Although ABE2 was originally defined on the basis of heparin binding, the propiece of the zymogen precursor that is cleaved away when thrombin is produced represents an important protein ligand for this site (9, 10).
Prothrombin, the zymogen precursor, requires cleavage at two sites to be converted to thrombin (Scheme 1). Cleavage at Arg271 releases the N-terminal propiece (F12), whereas cleavage at Arg320 is analogous to the cleavage at Arg15 in chymotrypsinogen and yields the proteinase. Thrombin is composed of the catalytic domain in disulfide linkage with a short light chain (Scheme 1). When Arg320 is not cleaved, the C-terminal procatalytic domain corresponds to the zymogen, prethrombin 2 (P2). The F12 propiece is composed of an N-terminal domain (Gla), bearing γ-carboxyglutamic acid residues, which is stabilized by Ca2+ and allows for membrane binding. Two kringle domains (K1 and K2) follow separated by a linker (Scheme 1). The presence of a thrombin-sensitive cleavage site at position 155, within the linker, facilitates the separation of F12 into the N-terminal fragment 1 (F1, Gla-K1) and fragment 2 (F2) bearing K2. A series of x-ray structures establish that it is F2 that contacts the ABE2 site within the (pro)catalytic domain, and the F1 region extends away as schematically illustrated (Scheme 1)(10–12).
SCHEME 1.
Prothrombin activation and cleaved derivatives. Upper panel, cleavage of prothrombin at Arg320 and Arg271 yields F12 and thrombin (IIa). The propiece, F12, is composed of the N-terminal Gla domain followed by K1 and K2. Thrombin-mediated cleavage at Arg155 separates F12 into the F1 region (Gla-K1) and F2 (K2). The Gla domain can be released by chymotryptic cleavage at Tyr43. The folded structure of the Gla domain is destabilized by EDTA or by the lack of γ-carboxyglutamate modifications (dG). Lower panel, after single cleavage at Arg271, the procatalytic domain (P2) can bind reversibly to the indicated derivatives of the propiece through K2. Thrombin produced by cleavage at both Arg271 and Arg320 can also bind propiece derivatives through K2. The (pro)catalytic domain is shown in gray for the zymogen and in red for the proteinase. Cleavage at Arg320 but not Arg271 yields the proteinase mIIa with the indicated propiece derivatives covalently attached.
Thrombin and P2 can reversibly bind F12 despite prior cleavage at Arg271 (Scheme 1) (9, 13). F12 binds the zymogen P2 with ∼30-fold higher affinity than it does to thrombin (13). Binding of F12 imparts membrane binding function and accounts for the membrane-dependent cleavage of the F12-P2 complex to thrombin by prothrombinase, the membrane-bound enzyme complex responsible for prothrombin activation (13). The ∼30-fold weaker interaction of F12 with thrombin releases the bulk of the proteinase product from the membrane surface freeing it to act on its multiple substrates in a membrane-independent manner (13).
The differential affinity of F12 for zymogen and proteinase is dwarfed by profound and compensating differences in enthalpy and entropy for the binding of the propiece to P2 and thrombin (14). Thus, F12 binding to the zymogen is highly thermodynamically favored in comparison to the proteinase. This is evident from the finding that F12 binding to ABE2 enforces zymogen-like character in thrombin (14). Despite irreversible cleavage at Arg320, thrombin can reversibly interconvert between zymogen-like and proteinase-like states depending on the complement of ligands bound to ABE2, the active site, and the Na+ binding site in the proteinase domain (14).
Although these ideas provide a unifying framework for the consideration of thrombin allostery, their physiologic relevance is questionable because of the modest affinity of F12 for the proteinase (13, 14). Binding affinity is not at issue in the case of meizothrombin (mIIa) that is cleaved only at Arg320 and retains covalent linkage with F12 (Scheme 1). Accordingly, despite cleavage at the equivalent of Arg15 in chymotrypsinogen, mIIa displays prominent zymogen-like character (15). It exists in approximately equally populated zymogen-like and proteinase-like forms that interconvert slowly (15). This has major implications for the sequential cleavage of prothrombin activation by prothrombinase in which mIIa is produced as a major intermediate and for the understanding of the basis of its skewed spectrum of biological functions in comparison to thrombin (15, 16).
Surprisingly, despite the fact that it is K2 in the propiece that contacts ABE2, F2 binding to the zymogen and proteinase are energetically equivalent and weak (13). Thus, provided the thermodynamic arguments have merit, F2 will not be expected to elicit zymogen-like features in the proteinase. This discrepancy in the binding characteristics of F12 and F2 suggests that the N-terminal F1 domain somehow enforces the zymogen favoring ability of K2 and the following residues in their interaction with ABE2. The possibility that the F2 domain is fundamentally altered when F12 is cleaved at Arg155 is not obvious from available x-ray structures (10). It is also inconsistent with evidence suggesting that F1 and F2 are independent folding domains within F12 or prothrombin (17). On the other hand, several lines of evidence suggest that the Ca2+-dependent folding of the Gla domain has distant effects on the proteinase domain (18–20). It, therefore, seems plausible that the folded structure of the Gla domain regulates the binding of F12 through the F2 region to the proteinase domain. The thermodynamic and kinetic approaches presented here shed unexpected light on the function of the F1 domain as zymogenizer within the propiece.
Experimental Procedures
Reagents
Human plasma used for protein isolation was a gift of the Plasmapheresis Unit of the Hospital of the University of Pennsylvania. d-Phenylalanyl-l-proline-l-arginine chloromethyl ketone (FPRck, Calbiochem), dansylarginine-N-(3-ethyl-1,5-pentanediyl)-amide (DAPA, Hematologic Technologies), and Nα-dansyl-(p-guanidino)-phenylalanine-piperidide (I-2581,DiaPharma), tosyl-l-lysine chloromethyl ketone, and tosyl-l-phenylalanine chloromethyl ketone (Sigma) were from the indicated suppliers. Concentrations of stock solutions prepared in water were determined using E330m = 4010 m−1·cm−1 (DAPA) and E342m = 4105 m−1·cm−1 (I-2581).
Proteins
Prothrombin was isolated from human plasma by established procedures (21, 22). Bovine chymotrypsin (Worthington) was treated with tosyl-lysine chloromethyl ketone dialyzed into 20 mm Hepes, 0.15 m NaCl, pH 7.4, and stored frozen in aliquots. Recombinant ecarin was expressed and purified as described (23). Recombinant prothrombin with alanine substituting the catalytic serine (IIA195, chymotrypsin numbering) or bearing glutamine substitutions for arginine as positions 155, 271, and 284 (IIQQQ, sequential numbering of the mature protein) were expressed as either fully carboxylated or uncarboxylated (dG) forms and purified as before (13, 23). The stated degree of carboxylation was confirmed by amino acid analysis of γ-carboxyglutamate content after base hydrolysis and ion exchange HPLC with post-column derivatization (24).
Preparative proteolysis of plasma prothrombin was used to generate F12, F2, and prethrombin 1 followed by their purification (25). Uncarboxylated F12 (dG-F12), P2A195, and IIaA195 were generated from dG-IIA195 and purified as described (14, 23). IIQQQ lacking the first 43 residues (IIQQQ-Δ43) was generated by preparative cleavage of fully carboxylated IIQQQ with chymotrypsin (26). The cleavage mixture (11 ml) contained 13.9 μm IIQQQ and 140 nm chymotrypsin in 20 mm Tris, 0.15 m NaCl, pH 7.4. After digestion for 1 h at 25 °C, chymotrypsin was inactivated by the addition of 20 μm tosyl-l-phenylalanine chloromethyl ketone, diluted with 50 ml of 20 mm Tris, pH 7.4, and applied at 5 ml/min to a 10 × 100-mm column of Poros HQM (Thermo Fisher). After washing with the same buffer, bound protein was eluted with a gradient of increasing NaCl (0–600 mm NaCl in 20 mm Tris, pH 7.4, 5 ml/min, 12 min). Fractions containing IIQQQ-Δ43 free of precursor and the cleaved peptide were pooled and precipitated with solid (NH4)2SO4 to 80% saturation. Precipitated protein was collected by centrifugation, dissolved in 50% (v/v) glycerol, and stored at −20 °C. The chemical identity of IIQQQ-Δ43 was established by N-terminal sequence analysis performed by Dr. Jan Pohl (Centers for Disease Control). While the bulk of the product was cleaved at Tyr43, a minor second sequence (∼10% yield) was observed consistent with some cleavage at Trp41.
Protein concentrations were determined using the following molecular weights and extinction coefficients (E2800.1%): all full-length prothrombin variants, 72,000, 1.47 (25); IIaA195 or P2A195, 37,500, 1.89 (27); F12 or dG-F12, 34,800, 1.2 (25); F2, 1.28, 12,800 (25); prethrombin 1, 1.78, 50,000 (25); IIQQQ-Δ43, 1.69, 66,600; ecarin, 88,000, 1.0.
Calorimetry
Isothermal titration calorimetry (ITC) was performed using an iTC200 (Microcal) at 25 °C. All protein species were treated with 10 μm p-amidinophenylmethylsulfonyl fluoride (Sigma) before dialysis for 18 h versus 2 changes of 4 liters of buffer composed of 20 mm Hepes, 0.15 m NaCl containing either 5 mm Ca2+ or 5 mm EDTA, pH 7.4, at 4 °C. For two experiments, buffering in the second dialysis step was done with 20 mm Bistris propane instead of Hepes. After dialysis the samples were concentrated by centrifugal ultrafiltration if needed (Ultracel-10K, Amicon) and centrifuged to remove particulates before use. The cell (204 μl) contained either P2A195 or IIaA195 (14.6–30 μm), and the injection syringe (45 μl) contained F12 or F2 (224–431 μm). Heat flow was measured with continuous stirring, with injections of titrant at 180-s intervals. Typically, the first injection was 0.5 μl followed by 20 injections of 2 μl each. Heat flow due to ligand dilution and buffer mismatch was determined from an identical series of injections of titrant into dialysate. Samples were taken both before and after the experiment for analysis by SDS-PAGE to confirm that the heat flow measurements were not compromised by proteolytic degradation of the proteins.
Rapid Kinetic Studies
Stopped flow fluorescence measurements were performed at 25 °C using a SX20 stopped flow instrument with a 20-μl cell (Applied Photophysics). The binding of DAPA or I-2581 to mIIa variants was measured after rapid mixing of probe with proteinase using λEX = 280 nm and monitoring broadband fluorescence (λEX > 500 nm) using a long pass filter (LWP-500, Miles Girot) in the emission beam.
As previously described, mIIa variants were prepared by cleaving the appropriate prothrombin variants with recombinant ecarin in situ and maintaining the cleaved product on ice for the ∼4-h experiment duration (15). This approach minimizes possible perturbations in the zymogen/proteinase equilibrium that could result from the use of ion exchange to repurify the cleaved product (15). IIA195 or IIQQQ were used to generate mIIaA195 or mIIaQQQ and dG-IIA195 or dG-IIQQQ to yield the corresponding uncarboxylated mIIa forms. Prethrombin 1 was cleaved by ecarin to yield mIIa-ΔF1, and IIQQQ-Δ43 was used to produce mIIaQQQ-Δ43. For cleavage, 5 μm concentrations of the zymogen variant in 20 mm Hepes, 0.15 m NaCl, 5 mm Ca2+, 0.1% (w/v) PEG-8000, 5 μm CoCl2, pH 7.4, was treated with 100 nm ecarin for 10 min at 25 °C and transferred to ice. For experiments done in EDTA, the cleavage mixture lacked Ca2+, and the reaction mixture was supplemented with 5 mm EDTA after cleavage was complete. SDS-PAGE analysis confirmed quantitative conversion to the appropriate mIIa species, and stability over the experimental period was established by gel analysis after the ∼4-h experiment duration.
Stopped flow measurements were conducted by rapid mixing of equal volumes of enzyme solution (0.6 μm mIIa variant) with 10 different concentrations of either DAPA or I-2581 (1–16 μm) in the same buffer. Traces were acquired over 2 time scales, typically 2000 points in 0.2 s followed by 1000 points in 6.0 s, to permit description of the rapid and slow phases of the transient. Probe dissociation was measured by trapping free proteinase using FPRck (15). For these studies, 0.6 μm mIIa variant was premixed with 4 μm probe and reacted with equal volumes of 400 or 800 μm FPRck in the same buffer. Traces describing the decrease in fluorescence associated with probe dissociation were collected using 10,000 data points over ∼10 t½ of the transient. Despite the fact that mIIaA195 cannot covalently react with FPRck, the inhibitor evidently binds with sufficient affinity to trap free enzyme. Successful isolation of the intrinsic dissociation rate constant for the probe was inferred from the independence of the time constant for fluorescence decay on the concentration of FPRck.
Data Analysis
Heat traces from ITC measurements were initially analyzed by singular value decomposition using the program NITPICK to normalize for baseline variation and noise and yield integrated areas for each injection (28). After subtraction of the small integrated heats obtained from the control experiment, the isotherms were analyzed using SEDPHAT (29). Fitted values of Kd, ΔH, and baseline offset as well as calculated values of ΔG and ΔS were obtained using the A + B hetero-association model assuming a stoichiometry of 1. Uncertainty in this assumed value, probably arising from systematic errors in assigning protein concentrations, was accommodated by fitting a term for the non-binding fraction of incompetent protein in the cell or in the syringe. This fraction was typically <10%. Confidence limits (67%) of the fitted terms were determined by Monte Carlo iterations, and these errors were propagated to the calculated values (30).
Between 10 and 20 replicate stopped flow traces collected for each experimental condition were overlaid to identify and eliminate the rare and obvious outliers. The remaining traces were averaged, and the first 2 ms of data were eliminated on the basis of a conservative estimate of the instrument dead time. The data were globally analyzed using KinTek Explorer 5.2 (KinTek Corp.) according to Scheme 2 (mIIa-ΔF1) or Scheme 3 (all other mIIa variants) with locally fitted values for baseline offset and amplitude. Analysis according to Scheme 2 yielded rate constants for the two equilibria and calculated values for fe, fE, and KE,P. Analysis according to Scheme 3 yielded fitted values for three sets of rate constants assuming identical fluorescence yields for e.P and E.P. This assumption principally impacts the rate constants for the equilibrium between e.P and E.P. Consequently, we only report the equilibrium constant for this interconversion step (KConf) regardless of the fitted rate constants. This version of KinTek automatically enforces the relationship Kconf × Ke,P = ([e]/[E]) × KE,P calculated from the fitted rate constants. Confidence limits from the fitted rate constants were propagated to the calculated values.
SCHEME 2.
Reaction of mIIa-ΔF1 with active site probe (P). The proteinase is proposed to exist in a pre-equilibrium between e and E, of which E binds P with high affinity to form E.P resulting in an increase in fluorescence intensity. Probe binding affinity is given by KE,P = k−2/k+2.
SCHEME 3.
Reaction of other mIIa forms with active site probe (P). The proteinase is proposed to exist in pre-equilibrium between e and E. P binds E with high affinity to form E.P, whereas binding of P to e is of weaker affinity. The fluorescence yields of e.P and E.P are assumed to be identical. Interconversion between e.P and E.P is described by KConf = e.P/E.P. Probe binding affinities are given by KE,P = k−2/k+2 and Ke,P = k−3/k+3.
For structural superimpositions, the regions corresponding to residues 156–271, or as much of it was available, were extracted from the PDB files for 3NXP (31), 5EDM, 5EDK (12), and 4NZQ (11). Pairwise alignment and superimposition of these fragments to obtain RMSD was performed using PyMol (Schrodinger, LLC).
Results
Propiece Binding
Previous studies have established the ability of F12 but not F2 to thermodynamically distinguish between zymogen and proteinase forms (13). Given the literature in the field, we were near-certain that this arose from long range effects of the folded Gla domain within F12. This assumption was tested by ITC measurements of the binding of F12 to P2 and thrombin in the presence of EDTA (Fig. 1). Despite the established ability of EDTA to destabilize the Ca2+-dependent folded structure of the Gla domain within F12, we were surprised to find that the large differences in affinity and in the thermodynamic terms describing the binding of F12 to P2 versus thrombin were preserved (Fig. 1).
FIGURE 1.
Binding of F12 to zymogen or to proteinase in the presence of EDTA. Integrated heats from heat flow traces were obtained by ITC after sequential injections of 288 μm F12 into 19.5 μm P2A195 (●) or 431 μm F12 into 20.2 μm IIaA195 (○). Measurements were performed in 20 mm Hepes, 0.15 m NaCl, 5 mm EDTA, pH 7.4. The lines are drawn according to the fitted constants listed in Table 1.
More comprehensive ITC measurements further revealed inconsistency between the initial assumption and the findings (Table 1). Within experimental error, F12 binding to P2 was consistently of ∼40-fold higher affinity than its binding to thrombin. This difference was evident when measured with fully carboxylated propiece in the presence of Ca2+ or EDTA or with dG-F12 in the presence of Ca2+ (Table 1). Higher affinity binding was accompanied by differences of large magnitude in ΔH (∼12 kcal/mol) and TΔS (∼10 kcal/mol) in the interaction of F12 to P2 versus thrombin (Table 1). In contrast, F2 bound to P2 and thrombin with equivalent thermodynamic parameters (Table 1). These findings rule out a significant contribution from the Ca2+-stabilized folded structure of the Gla domain in regulating the ability of F12 to preferentially bind ABE2 in the zymogen through K2. The resulting function of the propiece in enforcing zymogen-like character in the proteinase might also parallel these structural requirements.
TABLE 1.
Thermodynamic constants for propiece binding to zymogen or proteinasea
a Constants obtained by isothermal titration calorimetry for the indicated interaction are listed ±67% confidence limits. In two instances, results of duplicate experiments with different protein preparations are listed to provide a measure of interexperiment variability in the fitted constants.
b ΔS at the measurement temperature of 298 K.
c Heat silent and -incompetent fraction of protein either in the cell or syringe.
d Difference in ΔG, ΔH and ΔS for binding to thrombin and to P2.
Zymogen-like Character in Proteinase
Meizothrombin variants provide a facile approach to investigate the ability of the covalently attached propiece to instill zymogen-like features in the proteinase. This is evident in the complex binding kinetics of active site probes to mIIa (15). Meizothrombin is highly susceptible to autolysis and usually requires inhibitors for stabilization (32). Because its zymogen-like character could be perturbed by further purification or the use of inhibitors after cleavage at Arg320, we have employed ecarin and the appropriate precursors that either yield a catalytically inactive product or bear arginine to glutamine substitutions to make the product cleavage-resistant.
Stopped flow studies examined the binding of the active site probes, I-2581 and DAPA, by measuring the increase in energy transfer between aromatic side chains and the dansyl moiety in the inhibitor (15, 33). The rise in fluorescence after mixing of I-2581 and dG-mIIaQQQ was multiphasic and could be empirically described by a 3-exponential rise (Fig. 2). Approximately 50% of the change was rapid and occurred with a t½ of ∼15 ms. The remaining change was much slower (t½ ∼ 400 ms). These findings parallel those previously made with fully carboxylated variants of mIIa (15). Thus, the compromised structural integrity of the Gla domain in dG-mIIaQQQ does not affect the complex kinetics of active site probe binding previously attributed to its zymogen-like character (15). In contrast, binding of I-2581 to mIIa-ΔF1 was rapid and could be adequately described by a single exponential (t½ ∼ 8 ms) in a manner analogous to that previously observed for thrombin (15). Thus proteolytic removal of the F1 region has a major impact on the kinetics of active site probe binding. Parallel results were obtained with dG-mIIaA195 and mIIaA195-ΔF1 with active site binding monitored using either I-2581 or DAPA (below). Therefore, the discrepancies between full-length mIIa and mIIa-ΔF1 are neither mutation- nor probe-specific.
FIGURE 2.
Stopped-flow traces of the binding of I-2581 to dG-mIIaQQQ and mIIa-ΔF1. Fluorescence was measured using λEX = 280 nm and λEM >500 nm after rapid mixing of dG-mIIaQQQ (upper trace) or mIIa-ΔF1 (lower trace) with I-2581 in 20 mm Hepes, 0.15 m NaCl, 5 mm CaCl2, 5 μm CoCl2, 0.1% (w/v) PEG8000, pH 7.4, at 25 °C. The final concentrations of proteinase and I-2581 were 0.3 μm and 2 μm. The lower trace is fit to a single exponential rise with Offset = 1.87, Amp = 1.9 ± 0.01, and kobs = 86.5 ± 0.35 s−1. The upper trace is fitted according to a 3 exponential rise with Offset = 1.74, Amp1 = 1.43 ± 0.02, kobs,1 = 96.8 ± 1.8 s−1, Amp2 = 1.11 ± 0.02, kobs,2 = 25.6 ± 0.5 s−1, Amp3 =1.29 ± 0.01, kobs,3 = 1.7 ± 0.01 s−1.
Probe Binding to mIIa-ΔF1
Mechanistic insights into probe binding to mIIa-ΔF1 were sought from more comprehensive kinetic studies. In previous studies with thrombin, despite the appearance of simple binding kinetics, fitting was improved by including an initial pre-equilibrium between two forms of proteinase (e and E), wherein the predominant E species binds probe with high affinity (Scheme 2) (15). For consistency, we have retained this model to analyze the behavior of mIIa-ΔF1 variants despite the fact that fitting statistics were changed but not improved in a compelling way from the simpler model describing the bimolecular interaction of probe with a homogenous population of enzyme.
Traces obtained at increasing concentrations of I-2581 were analyzed in combination with measurements of probe dissociation by trapping free proteinase with FPRck (Fig. 3). The data could be adequately described by Scheme 2, illustrated using data and fitted curves for a selected number of probe concentrations for clarity (Fig. 3). Provided Scheme 2 applies here, the fitted constants (Table 2) indicate that ∼90% of added mIIa-ΔF1 is found as E in the initial pre-equilibrium. The small fraction present as e, relatively rapidly equilibrates with E and is forced to EP at probe concentrations well above KE,P. This is in reasonable agreement with the proposal that ∼15% of thrombin is zymogen-like and can be stabilized in the proteinase state by active site ligation (14). The rate constants for the second step yield the equilibrium dissociation constant for probe binding to E that is in line with the directly measured value for the binding of I-2581 to thrombin (34).
FIGURE 3.
Kinetics of I-2581 binding to mIIa-ΔF1. Main panel, fluorescence traces were obtained after rapid mixing of mIIa-ΔF1 with increasing concentrations of I-2581, and averages of 10–20 replicates are illustrated. Final concentrations were 0.3 μm proteinase and 1, 2, 4, 6, and 8 μm I-2581 (bottom to top). Inset, probe dissociation kinetics was measured by mixing equal volumes of a mixture containing 0.6 μm mIIa-ΔF1 and 4 μm I-2581 with 800 μm FPRck. The black lines in both panels are drawn according to a global fit using Scheme 2 and the fitted constants listed in Table 2. A subset of traces used for global analysis is shown for clarity.
TABLE 2.
Constants for the binding of active site-directed fluorescent probes to meizothrombin variants
Constants ± 95% confidence limits were determined from global fitting according to Scheme 2 (mIIa-ΔF1) or Scheme 3 (all other mIIa variants). Symbolic constants correspond to those listed in the two schemes.
| Species | Probe | k+1 | k−1 | fea | fE | k+2 | k−2 | KE,Pb | k+3 | k−3 | Ke,P | KConf |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| s−1 | s−1 | μm−1.s−1 | s−1 | nm | μm−1.s−1 | s−1 | nm | |||||
| mIIa-ΔF1 | I-2581 | 43.3 ± 0.9 | 3.10 ± 0.3 | 0.07 ± 0.01 | 0.93 ± 0.09 | 49.7 ± 1.1 | 1.2 ± 0.01 | 24.1 ± 0.2 | NAc | NA | NA | NA |
| mIIa-ΔF1 | DAPA | 26.7 ± 0.3 | 5.25 ± 0.4 | 0.16 ± 0.01 | 0.84 ± 0.06 | 90.3 ± 1.1 | 0.44 ± 0.01 | 4.9 ± 0.1 | NA | NA | NA | NA |
| mIIaQQQd | I-2581 | 1.67 ± 0.01 | 1.93 ± 0.02 | 0.54 | 0.46 | 57.3 ± 0.2 | 0.54 ± 0.01 | 9.3 ± 0.01 | 1.76 ± 0.01 | 2.30 ± 0.23 | 1310 ± 110 | 0.008 ± 0.001 |
| mIIaQQQd | DAPA | 1.70 ± 0.17 | 1.89 ± 0.08 | 0.53 | 0.47 | 92.5 ± 0.6 | 0.26 ± 0.01 | 2.84 ± 0.05 | 2.33 ± 0.07 | 0.58 ± 0.06 | 250 ± 20 | 0.013 ± 0.003 |
| dG-mIIaQQQ | I-2581 | 2.76 ± 0.03 | 1.87 ± 0.02 | 0.40 ± 0.01 | 0.60 ± 0.01 | 24.6 ± 0.2 | 0.33 ± 0.01 | 13.4 ± 0.4 | 1.59 ± 0.27 | 3.94 ± 0.44 | 2480 ± 350 | 0.004 ± 0.001 |
| dG-mIIaQQQ | DAPA | 1.95 ± 0.02 | 1.70 ± 0.02 | 0.47 ± 0.01 | 0.53 ± 0.01 | 41.5 ± 0.2 | 0.20 ± 0.01 | 4.8 ± 0.24 | 1.56 ± 0.02 | 1.07 ± 0.17 | 686 ± 109 | 0.006 ± 0.001 |
| dG-mIIaA195 | I-2581 | 1.59 ± 0.10 | 1.71 ± 0.06 | 0.52 ± 0.02 | 0.48 ± 0.02 | 30.0 ± 0.2 | 0.24 ± 0.03 | 8.0 ± 1.0 | 1.76 ± 0.03 | 1.49 ± 0.19 | 847 ± 108 | 0.010 ± 0.001 |
| dG-mIIaA195 | DAPA | 1.42 ± 0.10 | 1.95 ± 0.05 | 0.58 ± 0.02 | 0.42 ± 0.01 | 33.8 ± 0.4 | 0.26 ± 0.02 | 7.7 ± 0.6 | 3.22 ± 0.05 | 3.78 ± 0.23 | 1174 ± 72 | 0.009 ± 0.001 |
| mIIaQQQ+ EDTA | I-2581 | 1.54 ± 0.03 | 1.64 ± 0.03 | 0.52 ± 0.01 | 0.48 ± 0.01 | 18.8 ± 0.1 | 0.24 ± 0.03 | 12.8 ± 1.6 | 1.68 ± 0.13 | 1.64 ± 0.11 | 976 ± 71 | 0.014 ± 0.001 |
| mIIaQQQ-Δ43 | I-2581 | 2.35 ± 0.07 | 2.15 ± 0.06 | 0.48 ± 0.01 | 0.52 ± 0.02 | 25.8 ± 0.4 | 0.31 ± 0.01 | 12.0 ± 0.3 | 1.67 ± 0.08 | 1.19 ± 0.3 | 713 ± 181 | 0.015 ± 0.004 |
a Fraction of enzyme in the e and E forms in the initial equilibrium are denoted by fe and fE.
b KE,P = k-2/k+2 is the equilibrium dissociation constant for P binding to E. Ke,P = k-3/k+3 is the equilibrium dissociation constant for P binding to e and KConf = [eP]/[EP]. Propagation of errors in the rate constants was used to estimate confidence limits in the calculated equilibrium constants (30).
c NA, not applicable.
Comparable findings were made in kinetic studies of DAPA binding to mIIa-ΔF1 (Table 2). The rate constants for the pre-equilibrium and distributions between e and E forms were in tolerable agreement with those obtained with I-2581. The calculated value for KE,P was also in line with the measured equilibrium dissociation constant for the binding of DAPA to thrombin (35). The results with both active site probes consistently suggest that mIIa-ΔF1 behaves much like thrombin (15). Both forms are mostly stabilized in a proteinase-like form that can bind active site ligands with high affinity.
Full-length mIIa Variants
The enhanced information content of multiphasic traces obtained for active site probe binding to full-length mIIa variants allows expansion of the Scheme 2 to the more general case with a complete thermodynamic cycle (Scheme 3). This scheme illustrates the differential binding of probe to the e and E forms in the initial pre-equilibrium as well as reversible interconversion between probe-bound enzyme forms (Scheme 3).
Analysis was performed as before using traces obtained with dG-mIIaQQQ and increasing concentrations of I-2581 combined with measurements of probe dissociation (Fig. 4). The data could be adequately described by Scheme 3 with the fitted constants listed in Table 2. The fitted terms indicate that e and E are approximately equally populated and interconvert slowly (t½ ∼ 0.6 s). The rate constants for probe binding to the two forms yield values for KE,P and Ke,P indicating that P binds E with >150-fold higher affinity than it does to e (Table 2). This large difference in affinity for the active site probe forms the basis for the suggestion that e is zymogen-like, whereas the E form is proteinase-like. The present finding closely parallels those reported for fully carboxylated mIIa (15). Thus, although mIIa-ΔF1 behaves similarly to thrombin, the full-length form of mIIa, regardless of the carboxylation status of its N-terminal domain, has a large fraction that is zymogen-like which equilibrates slowly with the proteinase-like form.
FIGURE 4.
Kinetics of I-2581 binding to dG-mIIaQQQ. Fluorescence traces are illustrated for the same experimental conditions listed in Fig. 3, except the proteinase was dG-mIIaQQQ. The black lines in both panels are drawn according to a global fit using Scheme 3 and the fitted constants listed in Table 2. A subset of traces used for global analysis is shown for clarity.
Similar and consistent conclusions can be drawn from the analysis of comparable data sets measuring the binding kinetics of either DAPA or I-2581 to dG-mIIaQQQ or dG-mIIaA195 (Table 2). Comparable results were also obtained with fully carboxylated mIIaQQQ in the presence of EDTA (Table 2). Thus, equivalent conclusions result from different approaches to destabilize the Gla domain combined with the use of catalytically inactive and catalytically competent mIIa variants and two chemically dissimilar active site probes. Congruence between these multiple approaches rules out an important role for the stabilized Gla domain in mediating the zymogen-like character of mIIa that arises from covalent linkage between the catalytic domain and the propiece.
Kinetic Behavior Correlates with Thermodynamics of Binding
The idea that the covalently linked propiece in mIIa might enforce zymogen-like behavior in the proteinase extends from the finding that F12 binding to the zymogen, P2, is thermodynamically more favorable than its binding to thrombin. However, there are no structures available for P2/F12, IIa/F12, or for full-length mIIa containing the F1 domain. Thus, there is no absolute logical requirement for the covalently linked propiece in mIIa to elicit comparable effects as might be predicted from binding studies performed with free F12.
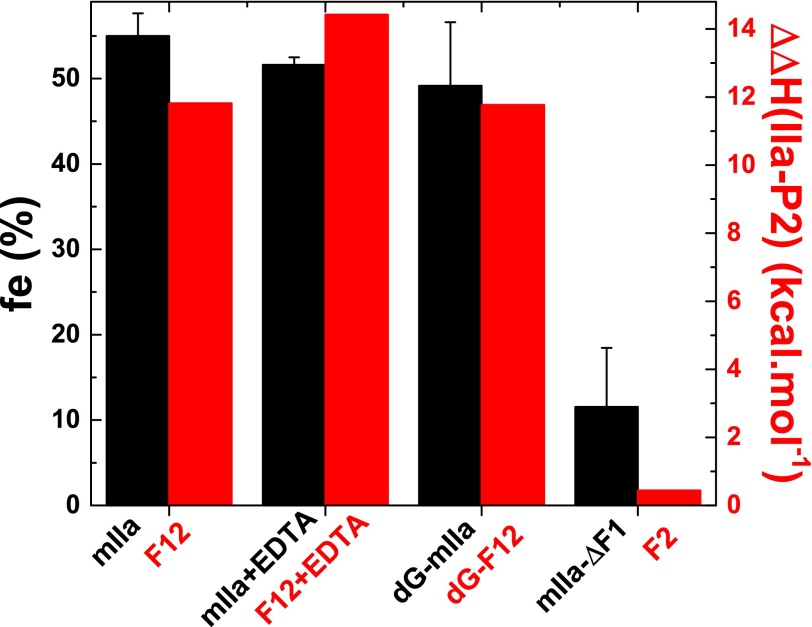
The interrelationship of the two ideas was sought from a comparison of the differential energetics of propiece variants binding zymogen and proteinase with the fraction of zymogen-like species evident in the appropriate mIIa variants (Fig. 5). There was excellent correlation between ΔΔH favoring P2 over thrombin and the fraction of appropriate mIIa variant in the zymogen-like state (Fig. 5). This comparison graphically illustrates the zymogen favoring role of the N-terminal F1 region within F12 and the fact that this role is independent of the Ca2+-stabilized structure of the Gla domain.
FIGURE 5.
Thermodynamics of propiece binding and the zymogen-like character of mIIa. The fractional distribution of mIIa variants in the zymogen-like state inferred from rapid kinetic measurements is illustrated (left axis, black bars). Mean values ± S.E. for fe were taken from Table 2. The values for carboxylated mIIa were taken from Bradford and Krishnaswamy (15). The red bars scaled to the right axis illustrate the difference in enthalpy (ΔΔH) for the interaction of the relevant propiece under appropriate conditions to P2 and to thrombin, taken from Table 1.
Covalent Linkage with the Gla Domain
To address the possibility that covalent linkage with the Gla domain within F12 is sufficient to explain the findings, mIIaQQQ-Δ43 was prepared from purified IIQQQ-Δ43 generated by chymotryptic cleavage of prothrombin (see Scheme 1). Stopped flow studies indicated that I-2581 binding kinetics remained multiphasic and equivalent to mIIaQQQ retaining the Gla domain (Fig. 6). Global analysis according to Scheme 3 yielded constants that were in agreement with those observed for the full-length mIIa variants (Table 2). Thus, neither the Ca2+-stabilized structure of the Gla domain nor its presence is required to account for the zymogen-like character of mIIa. Sufficient material to perform ITC studies examining the binding of F12-Δ43 to P2, and thrombin could not be obtained. However, the agreement between the rapid kinetic measurements and the binding studies (Fig. 5) predicts that F12-Δ43 and F12 would behave equivalently. It logically follows that the presence of the K1 domain in covalent linkage with K2 allows the second to interact with ABE2 in such a way so as to zymogenize the catalytic domain.
FIGURE 6.
Kinetics of I-2581 binding to mIIaQQQ-Δ43. Fluorescence traces are illustrated for the same experimental conditions listed in Fig. 3, except the proteinase was dG-mIIaQQQ-Δ43. The black lines in both panels are drawn according to a global fit using Scheme 3 and the fitted constants listed in Table 2. A subset of traces used for the global analysis is shown for clarity.
Discussion
Our findings are consistent with an essential role for K1 but not the Gla domain within the F1 region in allowing F12 to bind in a much more favorable fashion to the zymogen than to the proteinase. As a result, such selectivity is unchanged by strategies to destabilize the folded structure of the Gla domain within F12 but not observed in F2 binding. These observations run counter to our initial expectation that thermodynamically favorable binding to the zymogen likely arises from long range effects of Gla domain folding on the interactions of the propiece with the catalytic domain. Thermodynamic preferences for binding, established using P2 and thrombin, translate into the ability of the covalently linked propiece to enforce prominent zymogen-like character in mIIa with the same structural requirements.
The details of the interaction between the F2 region and ABE2 in thrombin, mIIa-ΔF1, P2, prethrombin 1, and two versions of prothrombin are established in a series of x-ray structures (11, 12). In the three structures for the two prothrombin variants bearing deletions in the linker connecting K1 with K2, K1, and the Gla domain extend away from K2 and its interface with ABE2 without making additional contact with the precursor of the catalytic domain (11, 12). Within this context, our findings suggest that the zymogenizing role enforced by the F1 region of F12 arises from the ability of K1 to modulate K2 so as to significantly alter its binding to ABE2. Because the F1 region does not bind with detectable affinity to F2 or prethrombin 1, it follows that the proposed modulation requires K1 and K2 to be in a continuous polypeptide chain. Perhaps this requirement reflects weak interactions between K1 and K2 that are only meaningful when the two domains are connected but become experimentally irrelevant after cleavage at Arg155.
Although the foregoing provides a reasonable explanation for our findings, it cannot be readily reconciled with all structural information. The F2 domain in the prethrombin 1 structure (lacking the F1 domain) readily superimposes on the F2 domain in the three other structures of prothrombin retaining covalent linkage with K1 and the Gla domain (11, 12, 31). The pairwise root mean square deviations for these superimpositions ranged from 0.29–0.78 Å (mean 0.53 Å), suggesting the lack of a major effect of K1 or cleavage at Arg155 on the folding of K2. Indeed, the small differences in the Cα backbone are in regions of K2 that do not contact the (pro)catalytic domain. Perhaps this is a result of the fact that the prothrombin structures are of variants bearing large deletions in the linker between K1 and K2 (11, 12). This is also likely a contributor to interdomain flexing yielding different orientations of K1 in two different structures of the same prothrombin construct (11, 12). Although some of these reorientation effects have been attributed to structures obtained in the presence and absence of Ca2+, our findings indicate that the folded structure of the Gla domain has no bearing on ABE2 engagement by K2. Finally, a model of prothrombin has been proposed wherein K1 and K2 are widely separated in space by the flexible but unstructured linker region (36). These facets of the available structural information are also difficult to reconcile with the surmised effect of K1 in altering the ability of K2 to engage ABE2.
A recent analysis of available structures indicates that K2 engages the (pro)catalytic domain surface in two modes with overlapping but shifted contacts dependent on whether it is proteinase or zymogen (10). The residues in the catalytic domain, engaged by the propiece, are obviously a second and integral aspect of how thermodynamic selectivity for zymogen over proteinase is achieved. We were heartened to note that the K2 contacting surface in the catalytic domain is in the equivalent mode in structures of the complex of F2 with thrombin or P2 and in mIIa-ΔF1 (10). This is consistent with the finding that removal of the F1 region eliminates the ability of the propiece to selectively bind zymogen over proteinase or to impart a pronounced zymogen-like character in mIIa. The second and shifted mode is evident in the prothrombin variants containing both K1 and K2 with an intermediate mode evident for prethrombin 1 lacking Gla-K1 (10). Although the main interpretations of the binding surface analysis have focused on zymogen- or proteinase-like properties of the derivatives (10), it would appear that the findings are in line with some of the expectations of the present work. Several more structures of derivatives containing K1 or Gla-K1 are required to provide comprehensive comparisons. However, it seems reasonable to suggest that the zymogenizing role of K1 within the F1 domain arises from its ability to reposition and/or reconfigure K2, thereby altering its contacts with the (pro)catalytic domain in the zymogen relative to the proteinase. Precisely how this occurs without obvious changes in the conformation of K2 or without intimate interactions between K1 and K2 remains to be established.
The zymogen to proteinase transition contributes to the presentation of the individual bonds within prothrombin and its singly cleaved activation intermediates for cleavage by prothrombinase (23). Consequently, it has a major effect on the sequential cleavages that convert prothrombin to thrombin, the intermediate produced, and the kinetic contribution of the two possible pathways for thrombin formation (37). Studies using dG variants of prothrombin and the possible intermediates (dG-mIIa and dG-F12/P2) have also established an essential role for membrane binding by the substrate for its oriented presentation to prothrombinase for cleavage (23). Although observed changes in the pathway for cleavage and the kinetics for the individual cleavage reactions were attributed to the role played by membrane binding by the substrate forms, effects arising from changes in the zymogen or proteinase-like character in the substrate forms lacking γ-carboxyglutamate residues could not definitively be ruled out (23). Our present studies negating a role for the Gla domain in regulating the zymogen-like or proteinase-like character of the intermediates now allow clearer attribution of the kinetic findings with the dG substrate variants to the role played by the substrate-membrane interaction in regulating substrate presentation to prothrombinase.
The enhanced preference of mIIa toward the anticoagulant pathway, resulting from its decreased catalytic efficiency for some procoagulant substrates in comparison to thrombin, has been the subject of numerous studies (19, 20, 38–40). The finding that mIIa shows pronounced zymogen-like character has led to the proposal that this partly results from the high affinity with which mIIa binds thrombomodulin through ABE1, which favors the proteinase-like state (15). Other substrates, which bind with weaker affinity, are not expected to rescue mIIa to the same extent (15). Although this provides an explanation for one contributing feature to the peculiarities of mIIa function, covalent occlusion of ABE2 by the propiece and the ability of mIIa to bind membranes could also explain its altered specificity. Indeed, membrane binding through the covalently linked Gla domain has been considered an important contributor to the specificity of mIIa because mIIa-ΔF1 lacking membrane binding function is more thrombin-like (19, 20). The present observations show that zymogen-like character is lost in mIIa-ΔF1 but preserved in mIIa-Δ43. Because membrane binding is eliminated in both cleaved derivatives, our work reveals new experimental strategies to distinguish the contribution of membrane binding versus zymogen-like character in explaining the skewed specificity profile of mIIa.
In summary, our findings shed new and unexpected light on how the N-terminal region of the F12 propiece plays a role as zymogenizer to modulate the interactions of F2 with ABE2 in the (pro)catalytic domain and favor the zymogen. This is revealed by parallels in the reversible binding of the propiece to zymogen and proteinase as well as by the zymogen-like character of mIIa in which it remains covalently attached. Contrary to our expectations, this zymogenizing role results from covalent linkage between K2 and K1 while the Gla domain is dispensable. These findings reveal new insights into the structural correlates of propiece function and how K1 and K2 cooperate to favor a zymogen-like form and regulate prothrombin cleavage as well as the enzymatic properties of the proteinase products.
Author Contributions
H. N. B. and S. K. performed the work.
Acknowledgments
We are grateful to Rodney Camire and William Church for critical evaluation of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants HL-074124 and HL-108933 (to S. K.) and by Project 1 of HL-12722. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- ABE1
- anion binding exosite 1
- ABE2
- anion binding exosite 2
- DAPA
- dansylarginine-N-(3-ethyl-1,5-pentanediyl)-amide
- dG-F12
- F12 lacking γ-carboxyglutamate modifications
- dG-II
- prothrombin lacking γ-carboxyglutamate modifications
- mIIa
- meizothrombin
- IIQQQ
- prothrombin variant containing Gln in place of Arg155, Arg271, and Arg284
- mIIaQQQ
- meizothrombin prepared from IIQQQ
- dG-mIIaQQQ
- meizothrombin lacking γ-carboxyglutamate modifications prepared from IIQQQ
- dG-mIIaA195
- meizothrombin lacking γ-carboxyglutamate modifications prepared from IIA195
- FPRck
- d-phenylalanyl-l-proline-l-arginine chloromethyl ketone
- F12
- fragment 1.2
- IIA195
- prothrombin variant containing Ala in place of catalytic Ser195
- IIaA195
- thrombin prepared from IIA195
- I-2581
- Nα-dansyl-(p-guanidino)-phenylalanine-piperidide
- ITC
- isothermal titration calorimetry
- mIIa-ΔF1
- meizothrombin variant lacking the fragment 1 region
- mIIa-Δ43
- meizothrombin variant lacking residues 1–43
- P2
- prethrombin 2
- P2A195
- prethrombin 2 prepared from IIA195
- Gla
- N-terminal 4-carboxyglutamate domain
- Bistris propane
- 1,3-bis[tris(hydroxymethyl)methylamino]propane.
References
- 1. Mann K. G., Jenny R. J., and Krishnaswamy S. (1988) Cofactor proteins in the assembly and expression of blood clotting enzyme complexes. Annu. Rev. Biochem. 57, 915–956 [DOI] [PubMed] [Google Scholar]
- 2. Mann K. G., Nesheim M. E., Church W. R., Haley P., and Krishnaswamy S. (1990) Surface-dependent reactions of the vitamin K-dependent enzyme complexes. Blood 76, 1–16 [PubMed] [Google Scholar]
- 3. Mann K. G. (2003) Thrombin formation. Chest 124, 4S–10S [DOI] [PubMed] [Google Scholar]
- 4. Jenny N. S., Lundblad R. L., and Mann K. G. (2006) Thrombin. In Hemostasis and Thrombosis. Basic Principles and Clinical Practice (Colman R. W., Marder V. J., Clowes A. J., George J. N., and Goldhaber S. Z., eds.) pp 193–213, Lippincott Williams & Wilkins, Philadelphia [Google Scholar]
- 5. Esmon C. T. (2003) The protein C pathway. Chest 124, 26S–32S [DOI] [PubMed] [Google Scholar]
- 6. Lane D. A., Philippou H., and Huntington J. A. (2005) Directing thrombin. Blood 106, 2605–2612 [DOI] [PubMed] [Google Scholar]
- 7. Bock P. E., Panizzi P., and Verhamme I. M. (2007) Exosites in the substrate specificity of blood coagulation reactions. J. Thromb. Haemost. 5, 81–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huntington J. A. (2005) Molecular recognition mechanisms of thrombin. J. Thromb. Haemost. 3, 1861–1872 [DOI] [PubMed] [Google Scholar]
- 9. Arni R. K., Padmanabhan K., Padmanabhan K. P., Wu T. P., and Tulinsky A. (1993) Structures of the noncovalent complexes of human and bovine prothrombin fragment 2 with human PPACK-thrombin. Biochemistry 32, 4727–4737 [DOI] [PubMed] [Google Scholar]
- 10. Adams T. E., and Huntington J. A. (2016) Structural transitions during prothrombin activation: on the importance of fragment 2. Biochimie 122, 235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pozzi N., Chen Z., Pelc L. A., Shropshire D. B., and Di Cera E. (2014) The linker connecting the two kringles plays a key role in prothrombin activation. Proc. Natl. Acad. Sci. U.S.A. 111, 7630–7635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pozzi N., Chen Z., and Di Cera E. (2016) How the Linker Connecting the two kringles influences activation and conformational plasticity of prothrombin. J. Biol. Chem. 291, 6071–6082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kamath P., and Krishnaswamy S. (2008) Fate of membrane-bound reactants and products during the activation of human prothrombin by prothrombinase. J. Biol. Chem. 283, 30164–30173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kamath P., Huntington J. A., and Krishnaswamy S. (2010) Ligand binding shuttles thrombin along a continuum of zymogen- and proteinase-like states. J. Biol. Chem. 285, 28651–28658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bradford H. N., and Krishnaswamy S. (2012) Meizothrombin is an unexpectedly zymogen-like variant of thrombin. J. Biol. Chem. 287, 30414–30425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krishnaswamy S. (2013) The transition of prothrombin to thrombin J. Thromb. Haemost. 11, 265–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bloom J. W., and Mann K. G. (1979) Prothrombin domains: circular dichroic evidence for a lack of cooperativity. Biochemistry 18, 1957–1961 [DOI] [PubMed] [Google Scholar]
- 18. Armstrong S. A., Husten E. J., Esmon C. T., and Johnson A. E. (1990) The active site of membrane-bound meizothrombin. A fluorescence determination of its distance from the phospholipid surface and its conformational sensitivity to calcium and factor Va. J. Biol. Chem. 265, 6210–6218 [PubMed] [Google Scholar]
- 19. Doyle M. F., and Mann K. G. (1990) Multiple active forms of thrombin. IV. Relative activities of meizothrombins. J. Biol. Chem. 265, 10693–10701 [PubMed] [Google Scholar]
- 20. Côté H. C., Stevens W. K., Bajzar L., Banfield D. K., Nesheim M. E., and MacGillivray R. T. (1994) Characterization of a stable form of human meizothrombin derived from recombinant prothrombin (R155A, R271A, and R284A). J. Biol. Chem. 269, 11374–11380 [PubMed] [Google Scholar]
- 21. Baugh R. J., and Krishnaswamy S. (1996) Role of the activation peptide domain in human factor X activation by the extrinsic Xase complex. J. Biol. Chem. 271, 16126–16134 [DOI] [PubMed] [Google Scholar]
- 22. Orcutt S. J., Pietropaolo C., and Krishnaswamy S. (2002) Extended interactions with prothrombinase enforce affinity and specificity for its macromolecular substrate. J. Biol. Chem. 277, 46191–46196 [DOI] [PubMed] [Google Scholar]
- 23. Bradford H. N., Orcutt S. J., and Krishnaswamy S. (2013) Membrane binding by prothrombin mediates its constrained presentation to prothrombinase for cleavage. J. Biol. Chem. 288, 27789–27800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Price P. A. (1983) Analysis for γ-carboxyglutamic acid. Methods Enzymol. 91, 13–17 [DOI] [PubMed] [Google Scholar]
- 25. Mann K. G., Elion J., Butkowski R. J., Downing M., and Nesheim M. E. (1981) Prothrombin. Methods Enzymol. 80, 286–302 [DOI] [PubMed] [Google Scholar]
- 26. Pollock J. S., Shepard A. J., Weber D. J., Olson D. L., Klapper D. G., Pedersen L. G., and Hiskey R. G. (1988) Phospholipid binding properties of bovine prothrombin peptide residues 1–45. J. Biol. Chem. 263, 14216–14223 [PubMed] [Google Scholar]
- 27. Lundblad R. L., Kingdon H. S., and Mann K. G. (1976) Thrombin. Methods Enzymol. 45, 156–176 [DOI] [PubMed] [Google Scholar]
- 28. Keller S., Vargas C., Zhao H., Piszczek G., Brautigam C. A., and Schuck P. (2012) High-precision isothermal titration calorimetry with automated peak shape analysis. Anal. Chem. 84, 5066–5073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Houtman J. C., Brown P. H., Bowden B., Yamaguchi H., Appella E., Samelson L. E., and Schuck P. (2007) Studying multisite binary and ternary protein interactions by global analysis of isothermal titration calorimetry data in SEDPHAT: application to adaptor protein complexes in cell signaling. Protein Sci. 16, 30–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bevington P. R., and Robinson K. D. (1992) Data Reduction and Error Analysis for the Physical Sciences, pp. 161–167, 2nd Ed., McGraw-Hill, New York [Google Scholar]
- 31. Chen Z., Pelc L. A., and Di Cera E. (2010) Crystal structure of prethrombin-1. Proc. Natl. Acad. Sci. U.S.A. 107, 19278–19283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Doyle M. F., and Haley P. E. (1993) Meizothrombin: active intermediate formed during prothrombinase-catalyzed activation of prothrombin. Methods Enzymol. 222, 299–312 [DOI] [PubMed] [Google Scholar]
- 33. Krishnaswamy S., Church W. R., Nesheim M. E., and Mann K. G. (1987) Activation of human prothrombin by human prothrombinase: influence of factor Va on the reaction mechanism. J. Biol. Chem. 262, 3291–3299 [PubMed] [Google Scholar]
- 34. Lu G., Chhum S., and Krishnaswamy S. (2005) The affinity of protein C for the thrombin·thrombomodulin complex is determined in a primary way by active site-dependent interactions. J. Biol. Chem. 280, 15471–15478 [DOI] [PubMed] [Google Scholar]
- 35. Nesheim M. E., Prendergast F. G., and Mann K. G. (1979) Interactions of a fluorescent active-site-directed inhibitor of thrombin: dansylarginine N-(3-ethyl-1,5-pentanediyl)amide. Biochemistry 18, 996–1003 [DOI] [PubMed] [Google Scholar]
- 36. Lechtenberg B. C., Murray-Rust T. A., Johnson D. J., Adams T. E., Krishnaswamy S., Camire R. M., and Huntington J. A. (2013) Crystal structure of the prothrombinase complex from the venom of Pseudonaja textilis. Blood 122, 2777–2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bianchini E. P., Orcutt S. J., Panizzi P., Bock P. E., and Krishnaswamy S. (2005) Ratcheting of the substrate from the zymogen to proteinase conformations directs the sequential cleavage of prothrombin by prothrombinase. Proc. Natl. Acad. Sci. U.S.A. 102, 10099–10104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tans G., Nicolaes G. A., Thomassen M. C., Hemker H. C., van Zonnenveld A.-J., Pannekoek H., and Rosing J. (1994) Activation of human factor V by meizothrombin. J. Biol. Chem. 269, 15969–15972 [PubMed] [Google Scholar]
- 39. Matafonov A., Sarilla S., Sun M. F., Sheehan J. P., Serebrov V., Verhamme I. M., and Gailani D. (2011) Activation of factor XI by products of prothrombin activation. Blood 118, 437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Orfeo T., Brufatto N., Nesheim M. E., Xu H., Butenas S., and Mann K. G. (2004) The factor V activation paradox. J. Biol. Chem. 279, 19580–19591 [DOI] [PubMed] [Google Scholar]