Abstract
Liver X receptors (LXR) are oxysterol-activated nuclear receptors that play a central role in reverse cholesterol transport through up-regulation of ATP-binding cassette transporters (ABCA1 and ABCG1) that mediate cellular cholesterol efflux. Mouse models of atherosclerosis exhibit reduced atherosclerosis and enhanced regression of established plaques upon LXR activation. However, the coregulatory factors that affect LXR-dependent gene activation in macrophages remain to be elucidated. To identify novel regulators of LXR that modulate its activity, we used affinity purification and mass spectrometry to analyze nuclear LXRα complexes and identified poly(ADP-ribose) polymerase-1 (PARP-1) as an LXR-associated factor. In fact, PARP-1 interacted with both LXRα and LXRβ. Both depletion of PARP-1 and inhibition of PARP-1 activity augmented LXR ligand-induced ABCA1 expression in the RAW 264.7 macrophage line and primary bone marrow-derived macrophages but did not affect LXR-dependent expression of other target genes, ABCG1 and SREBP-1c. Chromatin immunoprecipitation experiments confirmed PARP-1 recruitment at the LXR response element in the promoter of the ABCA1 gene. Further, we demonstrated that LXR is poly(ADP-ribosyl)ated by PARP-1, a potential mechanism by which PARP-1 influences LXR function. Importantly, the PARP inhibitor 3-aminobenzamide enhanced macrophage ABCA1-mediated cholesterol efflux to the lipid-poor apolipoprotein AI. These findings shed light on the important role of PARP-1 on LXR-regulated lipid homeostasis. Understanding the interplay between PARP-1 and LXR may provide insights into developing novel therapeutics for treating atherosclerosis.
Keywords: ABC transporter, atherosclerosis, cholesterol regulation, gene expression, nuclear receptor, transcription corepressor, ABCA1, ADP-ribosylation, LXR, PARP-1
Introduction
Atherosclerosis, characterized by the buildup of cholesterol-laden macrophages in the arterial wall, is the most common cause of cardiovascular disease (1). Normally, arterial macrophages ingest lipoprotein-derived cholesterol and transfer it to HDL for delivery to the liver and excretion from the body through bile. This process is known as reverse cholesterol transport and has an atheroprotective function because it promotes clearance of lipid particles from the body (2, 3). However, persistent hyperlipidemia promotes atherogenesis by overwhelming the efflux process and leading to the formation of lipid-laden macrophage foam cells that are retained within the arterial wall (4, 5).
LXRs2 belong to the nuclear receptor family of transcription factors and are activated physiologically by oxysterol cholesterol metabolites and cholesterol precursors (6–8). The LXRs, LXRα and LXRβ, act as key regulators of lipid homeostasis by controlling the expression of a number of genes involved in cholesterol absorption, transport, and elimination (9). Although the expression of LXRα is restricted to macrophages and tissues involved in lipid metabolism, LXRβ is ubiquitously expressed (10). The transcriptional activity of LXR is dependent on formation of a heterodimer, with retinoid X receptors, that binds to a DNA motif termed LXR response element (LXRE) present in the promoter of LXR target genes (6). The heterodimer in its unliganded state inhibits gene expression by forming a complex with corepressors such as silencing mediator of retinoic acid and thyroid hormone receptor and nuclear receptor corepressor (NCoR) (11). Ligand binding causes a conformational change in the receptor that releases the corepressors and recruits coactivators such as E1A-associated protein p300 (Ep300) and activating signal cointegrator 2 (ASC2) to induce target gene expression (12, 13).
LXR signaling in macrophages imparts antiatherogenic effects through its prominent role in reverse cholesterol transport (14, 15). Increases in cellular concentrations of cholesterol and oxysterol activate LXRs to up-regulate membrane ATP-binding cassette transporters (ABCA1 and ABCG1) and extracellular cholesterol acceptor, apolipoprotein E (apoE) (16–19). LDL receptor−/− and ApoE−/− mice that are predisposed to hypercholesterolemia and atherosclerosis exhibit regression of plaques and reduced atherosclerosis upon systemic administration of an LXR agonist (20–22). Moreover, ApoE−/− mice deficient in both LXRα and LXRβ die within 10 weeks of age because of extensive accumulation of cholesterol in peripheral tissue macrophages (9). Recently, ABCA1 was shown to be a critical mediator of the anti-inflammatory effects of LXR (23). Given the beneficial roles of LXRs in modulating ABCA1 gene expression, there remains a lack of understanding of the cellular factors controlling LXR activity in a gene-specific manner.
Nuclear receptors serve as the best examples of transcriptional regulation through the targeted recruitment of protein complexes that reversibly alter chromatin to activate or repress gene expression (24). The nature of nuclear receptor interactions with transcriptional cofactors can be influenced by conformational changes in the receptor induced by ligands, post-translation modifications, and DNA elements to selectively affect target gene expression. We hypothesized that LXR activity could be modulated in a gene-specific manner by altering the function of receptor-associated transcriptional regulators that selectively act at certain target genes but not others. Here we have performed a mass spectrometry-based proteomics study and identified poly(ADP-ribose) polymerase-1 (PARP-1) as a novel LXR interacting protein that selectively regulates LXR-dependent ABCA1 expression in macrophages. PARP-1, the founding member of the PARP superfamily, is a highly expressed and ubiquitous nuclear protein that regulates many nuclear processes (25). It is an enzyme responsible for the majority of cellular ADP-ribosylation (26), which is a reversible post-translational modification that occurs via covalent transfer of poly-ADP-ribose units from NAD+ to glutamine, asparagine, lysine, and/or arginine amino acids of target proteins (27). PARP-1 catalyzes the polymerization of ADP-ribose units to form linear or branched ADP-ribose chains on target proteins, thus modifying them to various masses. Although PARP-1 was originally known for its roles in DNA repair pathways, it is now well established that PARP-1 also regulates gene transcription both under basal and signal-activated conditions (25). In this study, we demonstrate that in macrophages LXR transcriptional activity at the ABCA1 gene can be increased by reducing PARP-1 levels or by inhibiting the catalytic activity of PARP-1, thereby facilitating cholesterol efflux.
Experimental Procedures
Cell Culture
HEK293T cells obtained from the ATCC were infected with recombinant retroviruses that were produced by transfecting LZRSpBMN-GFP or LZRSpBMN-GFP/LXRα into 293GP cells to create stable lines expressing vector only (293T-Vo) or FLAG-tagged human LXRα (293T-LXRα), respectively. After infection, the cells positive for green fluorescence protein expression were sorted by fluorescence-activated cell sorting and cultured in DMEM (Corning) containing 10% FBS and 1 unit/ml penicillin and 1 μg/ml streptomycin. HEK293 and RAW 264.7 cells from ATCC and RAW 264.7 cells stably expressing FLAG-tagged human LXRα previously described (28) were also maintained under the same culture conditions. The cells were routinely tested for mycoplasma and were mycoplasma-free. Bone marrow-derived macrophages were prepared from monocytes harvested from the tibia and femur of 6–8-week-old C57BL/6 male mice. Bone marrow cells were incubated in red blood cell lysis buffer (Sigma) to remove red blood cells. The cells were then resuspended and maintained for a week in DMEM containing 20% FBS and 10 ng/ml macrophage colony-stimulating factor (PeproTech Inc., Rocky Hill, NJ) to differentiate them into unactivated (M0) macrophages.
Affinity Purification of FLAG-LXRα Protein Complexes
HEK293T-LXRα or HEK 293T-Vo cells were lysed by incubating cells in hypotonic buffer (10 mm HEPES, 1.5 mm MgCl2, 10 mm KCl, pH 7.9) and passing them 10 times through a 25-gauge syringe needle. The crude nuclear pellet obtained after centrifugation was resuspended in 10 mm HEPES, 25% glycerol, 420 mm NaCl, 1.5 mm MgCl2, 0.2 mm EDTA solution at pH 7.9. Supernatant containing the nuclear proteins was collected after centrifugation. We then brought the final concentration of the solution to 300 mm salt and 1% Triton X-100 by adding HEMG0 buffer containing 25 mm HEPES, 12.5 mm MgCl2, 10% glycerol, 1 mm EDTA, and Triton X-100. FLAG-LXRα was immunoprecipitated from the nuclear extracts using agarose beads conjugated to FLAG antibody (Sigma). The beads were incubated with the samples for 6 h and were washed three times in HEMG0 buffer with 300 mm KCl and then in HEMG0 buffer with 150 mm KCl and two times in TBS. The beads were incubated in 50 μl of TBS with 0.5 mg/ml FLAG peptide (Sigma F3165) for 1 h, with gentle mixing of the solution every 10 min (29, 30). The proteins in the supernatant were precipitated with TCA overnight. The TCA precipitate was processed and then subjected to analysis by Multidimensional Protein Identification Technology and LTQ and LTQ orbitrap mass spectrometry as described previously (31).
Mass Spectrometry
TCA precipitate was resuspended in 8 m urea, and the extracts were processed with ProteasMAX (Promega, Madison, WI) following the manufacturer's instructions. The samples were subsequently reduced by incubation with 5 mm tris-(2 carboxyethyl) phosphine at room temperature for 20 min and alkylated in the dark by treatment with 10 mm iodoacetamide for an additional 20 min. The proteins were digested overnight at 37 °C with sequencing grade modified trypsin (Promega). The reaction was stopped by acidification with formic acid.
Multidimensional Protein Identification Technology and LTQ Mass Spectrometry
The protein digest was pressure-loaded onto a 250-μm inner diameter capillary packed with 2.5 cm of 10-μm Jupiter C18 resin (Phenomenex, Torrance, CA) followed by an additional 2.5 cm of 5-μm Partisphere strong cation exchanger (Whatman, Clifton, NJ). The column was washed with buffer solution containing 5% acetonitrile, 0.1% formic acid, and 95% water. Next a 100-μm inner diameter capillary with a 5-μm pulled tip packed with 15 cm of 4-μm Jupiter C18 resin (Phenomenex) was attached to the filter union, and the entire split column (desalting column-filter union-analytical column) was placed in line with an Agilent 1100 quaternary HPLC (Palo Alto, CA) and analyzed using a modified five-step separation as described previously (63). The buffer solutions used were buffer A (5% acetonitrile, 0.1% formic acid), buffer B (80% acetonitrile, 0.1% formic acid), and buffer C (500 mm ammonium acetate, 5% acetonitrile, 0.1% formic acid). Step 1 consisted of a 75-min gradient from 0–100% buffer B, and steps 2–5 had a similar profile except 100% buffer A for 3 min, X% buffer C for 5 min, gradient from 0 to 15% buffer B for 10 min, and a 105-min gradient from 10 to 55% buffer B (except for step 5, which %B was increased from 10% to 100%). The 5-min buffer C percentages (X) were 10, 40, 60, and 100%, respectively, for the five-step analysis. As peptides eluted from the microcapillary column, they were electrosprayed directly into an LTQ mass spectrometer (ThermoFinnigan, Palo Alto, CA). For LTQ analysis, as peptides eluted from the microcapillary column, they were electrosprayed directly into an LTQ two-dimensional ion trap mass spectrometer (ThermoFinnigan) with the application of a distal 2.4-kV spray voltage. A cycle of one full scan mass spectrum (400–2000 m/z) followed by seven data-dependent MS/MS spectra at a 35% normalized collision energy was repeated continuously throughout each step of the multidimensional separation. Application of mass spectrometer scan functions and HPLC solvent gradients were controlled by the Xcalibur data system.
Analysis of Tandem Mass Spectra
Protein identification and quantification analyses were done with Integrated Proteomics Pipeline (IP2, Integrated Proteomics Applications, Inc., San Diego, CA) using ProLuCID, DTASelect2, and Census. Tandem mass spectra were extracted into ms1 and ms2 files from raw files using Raw Extract 1.9.9 and were searched against IPI human protein database (version 3_57_01, released on January 1, 2009; plus sequences of known contaminants such as keratin and porcine trypsin concatenated to a decoy database in which the sequence for each entry in the original database was reversed using ProLuCID/Sequest. LTQ data were searched with 3000.0 milli-amu precursor tolerance, and the fragment ions were restricted to a 600.0-ppm tolerance. All searches were parallelized and performed on the Garibaldi 64-bit LINUX cluster with 2848 cores at the Scripps Research Institute. Search space included all fully and half-tryptic peptide candidates with no missed cleavage restrictions. Carbamidomethylation (+57.02146) of cysteine was considered a static modification, and we require two peptides per protein and at least one tryptic terminus for each peptide identification. The ProLuCID search results were assembled and filtered using the DTASelect program (version 2.0), with a false discovery rate of 0.05; under such filtering conditions, the estimated false discovery rate was less than 1% at the protein level in all analyses.
Preparation of Whole Cell Extracts and Immunoprecipitation
The cells were washed twice in PBS and lysed in Triton lysis buffer (50 mm HEPES, pH 7.6, 150 mm NaCl, 1 mm EDTA, pH 8.0, 1 mm EGTA, pH 8.0, 1 mm NaF, 1% Triton X-100, 10% glycerol) with protease inhibitor mixture (Cell Signaling). Protein concentrations were measured by using a Bradford assay (Bio-Rad). For immunoprecipitation, the lysates were incubated with FLAG antibody-conjugated agarose beads or protein G magnetic beads (Invitrogen) cross-linked to either 5 μg of LXRα antibody (Abcam ab41902) or mouse IgG (Sigma-Aldrich) overnight at 4 °C. The beads were washed once in the lysis buffer and three times in TBS. Proteins associated with the antibody were eluted in TBS by competition with FLAG peptides as described above for FLAG beads and by boiling at 95 °C in the presence of 5× Laemmli sample buffer and β-mercaptoethanol for 5 min.
Western Blotting
Protein samples were mixed with 5× Laemmli sample buffer containing β-mercaptoethanol and boiled at 95 °C for 5 min to denature the proteins. Proteins were separated on SDS-PAGE polyacrylamide gels and transferred to PVDF membranes (Millipore). The membranes were incubated with blocking solution (5% BSA in TBS, pH 7.4) for 1 h and incubated in primary antibody solubilized in blocking solution overnight at 4 °C. Antibodies used were anti-ABCA1 (Novus 400–105), anti-HSP90 (BD 610419), anti-LXRα (Abcam ab41902), anti-LXRβ (Santa Cruz Biotechnology sc-1001), anti-FLAG (Sigma F3165), anti-α-tubulin (Covance MMS-489P), anti-PARP-1 (Cell Signaling 9542), and anti-poly-ADP-ribose (anti-PAR) (Enzolifesciences ALX-804–220-R100). The membranes were washed three times in TBS with 0.1% Tween (TBS-T) for 10 min and incubated with horseradish peroxidase-conjugated secondary antibody at room temperature for 1 h. The membranes were washed in TBST. The blots were developed with ECL detection reagents (E2400; Denville Scientific, Holliston, MA). Quantification of Western blots was done by using ImageJ software.
Quantitative Real Time PCR
Total RNA from macrophages was extracted by using a Qiagen RNeasy mini kit as described in the manufacturer's protocol. 500 μg to 1 μg RNA was reverse transcribed to synthesize cDNA by using the random primer mix in the first strand cDNA synthesis kit for real time PCR following the manufacturer's instructions. cDNA amplification and quantification was done by using the SYBR Green Taq Ready Mix (USB-Affymetrix) and MyiQ single-color real time PCR detection system from Bio-Rad. The primers used for qPCR are listed below. mRNA expression was quantified relative to control genes, cyclophilin A, or RPL19. Primers used for mRNA analysis are mouse Abca1 forward, 5′-GGA CAT GCA CAA GGT CCT GA-3′; Abca1 reverse, 5′-CAG AAA ATC CTG GAG CTT CAA A-3′; mouse Abcg1 forward, 5′-CCC TCA AAG CCG TAT CTG AC-3′; mouse Abcg1 reverse, 5′-TTG ACA CCA TCC CAG CCT AC-3′; mouse SREBP-1c forward, 5′-GGA GCC ATG GAT TGC ACA TT-3′; mouse SREBP-1c reverse, 5′-GCT TCC AGA GAG GCC AG-3′; mouse cyclophilin A forward, 5′-GGC CGA TGA CGA GCC C-3′; mouse cyclophilin A reverse, 5′-TGT CTT TGG AAC TTT GTC TGC AA-3′; human RPL19 forward, 5′-CAC AAG CTG AAG GCA GAC AA-3′; human RPL19 reverse, 5′-GCG TGC TTC CTT GGT CTT AG-3′; human ABCA1 forward, 5′-AAC TCT ACA TCT CCC TTC CCG-3′; human ABCA1 reverse, 5′-CTC CTG TCG CAT GTC ACT CC-3′; human nascent ABCA1 forward, 5′-TTA ATG GGG CTG GAA AAT CA-3′; and human nascent ABCA1 reverse, 5′-GCA AAA TAC AAG CCA CTT-3′.
CRISPR-Cas9-mediated PARP-1 Deletion
A guide sequence 5′-CGA GTC GAG TAC GCC AAG AGC GG-3′ targeting exon 1 of human PARP-1 gene was designed by using CRISPR design tool. The sequence was cloned into the expression plasmid pSpCas9 (BB)-2A-GFP (32), which bears a guide RNA backbone, Cas9, and GFP. The resulting plasmid was transfected into HEK293 cells. After 24 h, single cells were sorted for the expression of GFP into 96-well plates by FACS. Clones deficient in PARP-1 were identified by Western blot using PARP-1 antibody.
In Vitro Poly(ADP-ribosyl)ation Assay
To examine poly(ADP-ribosyl)ation of LXRα, 100 ng of recombinant full-length human LXRα (Protein One, Rockville, MD) was incubated with 40 ng of recombinant human PARP-1 (Active Motif, Carlsbad, CA; 31238) in the reaction buffer containing 100 mm NaCl, 10 mm MgCl2, 10 μm ZnCl2, 10% glycerol, 300 μm β- NAD+, 1 mm dithiothreitol, 20 mm Tris (pH 7.9), and 100 μg/ml sonicated salmon sperm DNA for 30 min. The reaction was terminated by the addition of 5× SDS sample buffer containing β-mercaptoethanol, and the mixture was boiled at 95 °C for 5 min. The samples were then resolved by SDS-PAGE, and poly(ADP-ribosyl)ation was visualized by immunoblotting with anti-PAR antibody.
Chromatin Immunoprecipitation
DNA-protein complexes were cross-linked by incubating RAW-LXRα cells in 1% formaldehyde for 10 min at room temperature. The cross-linking reaction was stopped by adding glycine at the concentration of 0.125 m. The cells were washed twice with PBS and lysed with Farnham lysis buffer (5 mm PIPES, pH 8.0, 85 mm KCl, 0.5% Nonidet P-40). Crude nuclear pellet was collected and resuspended in radioimmune precipitation assay buffer containing 0.5% SDS and homogenized by applying 10 strokes with a 25-gauge syringe. Chromatin was sonicated using the Bioruptor (Diagenode; Twin UCD-400) to achieve 500-bp fragments. 5 μg of LXRα antibody (Abcam; ab41902), PARP-1 antibody (Cell Signaling; 9542), rabbit IgG, or mouse IgG (Sigma-Aldrich) was incubated with the sonicated lysates overnight for immunoprecipitation. The lysates were then incubated with protein G magnetic beads (Invitrogen) for 2 h at 4 °C. The beads were washed with LiCl wash buffer (100 mm Tris, pH 7.5, 500 mm LiCl, 1% Nonidet P-40, 1% sodium deoxycholate) and TE (10 mm Tris-HCl, pH 7.5, 0.1 mm Na2EDTA) at 4 °C. Cross-linking was reversed in elution buffer (1% SDS, 0.1 m NaHCO3) by incubating samples overnight in a 65 °C water bath. DNA was purified using PrepEase DNA clean-up kit (USB) and amplified by real time PCR using the Hot Start SYBR green (USB). The primers used for amplifications are mouse Abca1 LXRE (ChIP) forward, 5′-GGG GAA AGA GGG AGA GAA CAG-3′; Abca1 LXRE (ChIP) reverse, 5′-GAA TTA CTG GTT TTT GCC GC-3′; mouse Abca1 control non-LXR binding region forward, 5′-AGG GAA AGC TCT CTG GAG CAT-3′; mouse Abca1 control non-LXR binding region reverse, 5′-CAG GAA TTT CTC CAT CCT TTG AGT-3′; mouse Abcg1 promoter LXRE forward, 5′-CCA TTA GCT GAC TGT GAG CAT-3′; mouse Abcg1 promoter LXRE reverse, 5′-GGG CAG GCA AGT GGT TGT CAC AT-3′; mouse SREBP-1c LXRE forward, 5′-AGG CTC TTT TCG GGG ATG-3′; and mouse SREBP-1c LXRE reverse, 5′-TGG GGT TAC TGG CGG TCA-3′.
Transfection of Plasmids and siRNA and Pharmacological Inhibition of PARP-1
RAW-LXRα cells were transfected with constructs that express human PARP-1 (pCMV6-PARP-1; OriGene Technologies, Rockville, MD) or the vector construct (pCMV6-Entry) as a control using Lipofectamine LTX (Life Technologies) following the manufacturer's instructions. HEK293 cells were transfected with pCMV6-FLAG-mLXRα, pCMV6-FLAG-mLXRβ, or their respective vector control with Lipofectamine 2000. For knockdown experiments, RAW-LXRα cells were transfected with 50 nm On-Targetplus siControl nontargeting pool or the On-Targetplus Smartpool for mouse PARP-1 (GE Dharmacon, Lafayette, CO) with Hiperfect transfection reagent (Qiagen) for 72 h as per the manufacturer's instructions. The cells were cultured overnight in 1% FBS prior to treatment with vehicle or agonists. For PARP inhibition studies, the cells were pretreated with 3-aminobenzamide (3-AB) for 16 h in 1% FBS. The cells were then treated with ligands for 1, 4, and 8 h before harvesting them for ChIP assay, RNA, and protein analysis, respectively, unless otherwise noted.
Bodipy-Cholesterol Efflux Assay
RAW-LXRα cells (8 × 104/well) were seeded into 48-well plates and allowed to adhere overnight. The cells were incubated with a homogenized solution containing BODIPY cholesterol (810255P; Avanti Polar Lipids Inc., Alabaster, AL), methyl-β-cyclodextrin, and unlabeled cholesterol for 1 h. The cells were washed with Minimum Essential Medium Eagle's HEPES Modification (MEM) (Gibco) and equilibrated for 18 h with DMEM containing 0.2% BSA supplemented with vehicle or 5 mm 3-AB in the presence or absence of 5 μm T0901317. The cells were washed with MEM-HEPES medium and then incubated with efflux medium (MEM-HEPES with 0.2% BSA) containing the extracellular acceptor protein apoA-I (10 μg/ml) for 4 h. The fluorescence intensities were measured in the efflux medium, and the cell lysates were prepared by solubilizing cells in 1% cholic acid. The fluorescence intensities were recorded by using a Molecular Devices M2 plate reader (excitation, 482 nm; emission, 515 nm). Cholesterol efflux was calculated by dividing the fluorescence intensity of the media by the sum of fluorescence values of the media and the lysate. Background efflux in the absence of acceptors was subtracted from this value to measure efflux to apoA-I.
Radioactive Cholesterol Efflux Assay
Primary bone marrow-derived macrophages (BMDMs) were prepared from WT, LXRα−/−LXRβ−/− (33), or ABCA1−/− (34) mice and seeded into 48-well plates at 2.5 × 105 cells. The cells were loaded with [3H]cholesterol (0.5 μCi/ml [3H]cholesterol for 24 h and equilibrated in 2 mg/ml BSA medium overnight. Cholesterol efflux to apoA-I (50 μg/ml) or to the media (2 mg/ml BSA) in the absence of acceptors was carried out for 24 h. Equilibration and cholesterol efflux were performed in the presence or absence of 5 mm 3-AB. Efflux was measured by scintillation counting and is expressed as a percentage of [3H]cholesterol in medium/([3H]cholesterol in medium + [3H]cholesterol in cells) × 100%. Specific efflux to apoA-I was calculated by subtracting the effluxes of the wells without apoA-I from those containing apoA-I.
Statistical Analysis
The values are represented as means ± S.E. from three independent experiments unless otherwise noted. The significance of differences was estimated by using the two-tailed Student's t test (*, p < 0.05; **, p < 0.005; ***, p < 0.0005). All statistical analyses were performed with Prism software (GraphPad Software, Inc., La Jolla, CA). Western blots are representative of three independent experiments.
Results
PARP-1 Interacts with LXRs
We performed shotgun proteomic mass spectrometry (35) to identify LXRα interacting proteins from nuclear extracts of HEK293T cells expressing FLAG-tagged human LXRα (293T-LXRα). The number of peptides identified in the FLAG immunoprecipitate was compared between HEK293 control cells expressing the empty vector (293T-Vo) and 293T-hLXRα cells. A protein was considered a specific LXRα interactor if it was identified in the immunoprecipitated material from LXRα expressing cells but not control cells in replicate analyses.
We identified a number of established LXRα-associated proteins such as retinoid X receptor, the obligatory heterodimeric partner of LXR, and NCoR, thus validating the approach (Table 1). Importantly, a number of proteins that have roles as transcriptional regulators were also revealed as potential LXRα interactors. These included TAF15, a TATA-binding protein-associated factor (36); NAT10, an acetyltransferase that is known to acetylate histones (37, 38); SMARCB1 (39); SMARCE1 (40); components of chromatin remodeling SWI/SNF complex; and PARP-1. Given that PARP-1 impacts the transcriptional activity of several nuclear receptors including estrogen receptor α (41), retinoic acid receptor (42), and farnesoid X receptor (43), we focused on the relationship between PARP-1 and LXRα in controlling transcription of target genes important for cholesterol homeostasis.
TABLE 1.
Identity of LXRα-associated proteins by mass spectrometry
Listed are nuclear LXRα-associated proteins recovered by FLAG immunoprecipitations from LXRα-expressing (LXRα) and control cells not expressing LXRα (Control) identified by mass spectroscopy. Proteins are listed with their international protein index (Accession); spectral counts, which are a reflection of protein abundance; and description.
| Accession | LXRα | Control | Description |
|---|---|---|---|
| IPI00301894 | 54 | 0 | NR1H3 cDNA FLJ56172, highly similar to LXRα |
| IPI00449049 | 6 | 0 | PARP1 Poly [ADP-ribose] polymerase 1 |
| IPI00549205 | 5 | 0 | RAD50 isoform 2 |
| IPI00029159 | 5 | 0 | MRE11A cDNA FLJ38069 fis, clone CTONG2015434 |
| IPI00607575 | 4 | 0 | RXRB retinoid X receptor, β |
| IPI00219897 | 4 | 0 | ACSL4 long chain fatty acid-CoA ligase 4 |
| IPI00293946 | 4 | 0 | UBXN4 UBX domain-containing protein 4 |
| IPI00300127 | 4 | 0 | NAT10 N-acetyltransferase 10 |
| IPI00172656 | 3 | 0 | FAF2 FAS-associated factor 2 |
| IPI00009747 | 3 | 0 | LSS lanosterol synthase |
| IPI00017669 | 3 | 0 | SMARCE1 isoform 1 |
| IPI00020194 | 2 | 0 | TAF15 TATA-binding protein-associated factor 2N |
| IPI00297241 | 2 | 0 | URB1 Nucleolar preribosomal-associated protein 1 |
| IPI00923606 | 2 | 0 | EPRS glutamyl-prolyl tRNA synthetase |
| IPI00026824 | 2 | 0 | HMOX2 heme oxygenase 2 |
| IPI00745019 | 2 | 0 | SMARCB1 isoform B |
| IPI00289344 | 2 | 0 | NCOR1 isoform 1 of nuclear receptor corepressor 1 |
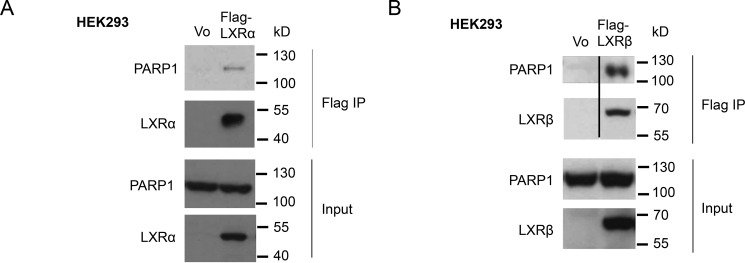
To validate the interaction of LXRα with PARP-1, we performed coimmunoprecipitation experiments. Indeed, endogenous PARP-1 coimmunoprecipitated with ectopically expressed LXRα in HEK293 nuclear extracts (Fig. 1A). Unsurprisingly, because LXRβ is similar to LXRα, displaying 78% amino acid sequence identity in the DNA and ligand-binding domains (44), we found that PARP-1 also interacted with LXRβ (Fig. 1B). Thus, both LXRs bind to PARP-1.
FIGURE 1.
Interaction of PARP-1 with LXRs. A and B, vector alone, FLAG-tagged LXRα (A), or LXRβ (B) was transiently expressed in HEK293 cells. Whole cell extracts were prepared, and FLAG-tagged protein complexes were immunoprecipitated with FLAG antibody and eluted by FLAG peptides. The eluted proteins were analyzed by Western blotting for LXRα (A) or LXRβ (B) and PARP-1. The lysates used for immunoprecipitation were Western blotted with PARP-1 antibody, as well as LXRα or LXRβ antibody. The blots in the top two panels in B were spliced as marked by the black line to show only the lanes that are relevant.
PARP-1 Represses LXR-mediated ABCA1 Expression
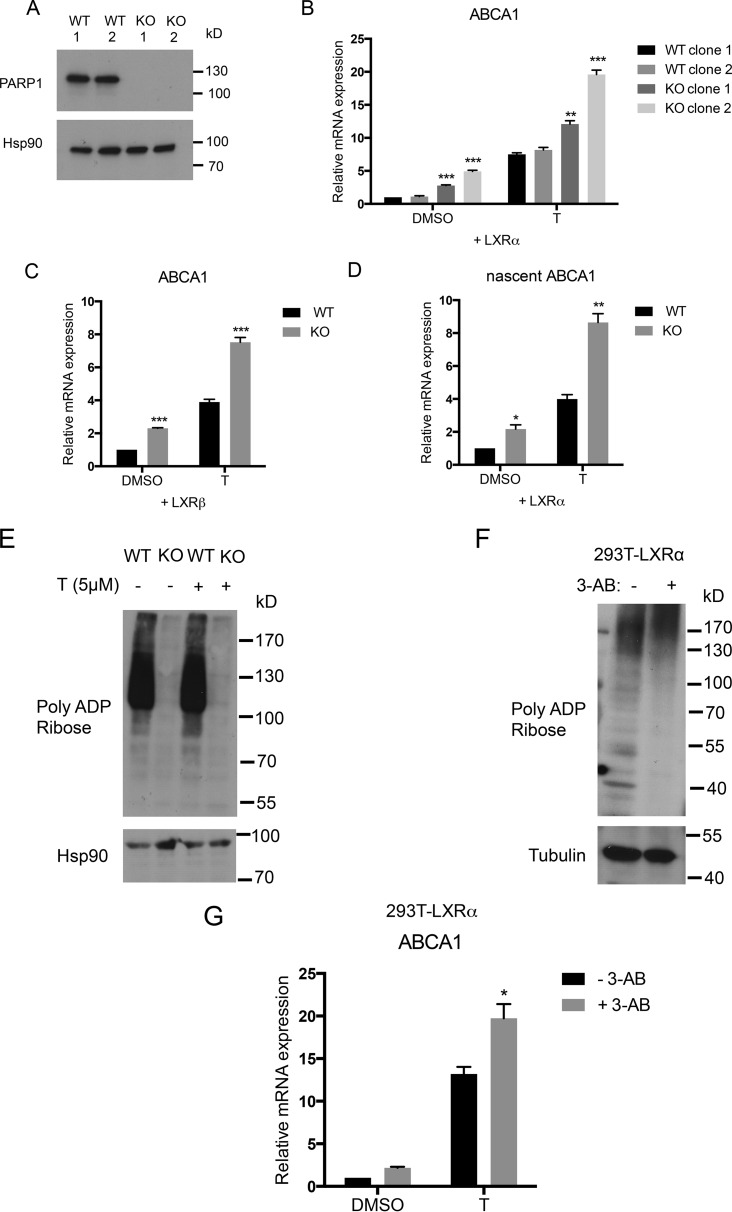
We next investigated the function of PARP-1 in LXR-mediated transcriptional regulation by using HEK293 cells deficient in PARP-1 (293-PARP-1-KO) generated by the CRISPR/Cas9 system (Fig. 2A). LXRα or LXRβ was ectopically expressed in cell lines generated from two independent clones each of wild-type HEK293 cells (293-WT) and 293-PARP-1-KO cells, and expression levels of the LXR target gene ABCA1 were examined. Interestingly, PARP-1-deficient cells expressing LXRα showed significantly higher ABCA1 than WT cells (Fig. 2B). Similarly, we observed increased ABCA1 expression in LXRβ expressing 293-PARP-1-KO cells compared with 293-WT cells (Fig. 2C; data not shown for the second clone). The effect of PARP-1 deletion was also reflected in the newly formed nascent mRNA levels, thereby confirming that the change in ABCA1 expression is a result of a transcriptional event (Fig. 2D).
FIGURE 2.
Effects of modulating PARP-1 level and activity on LXR-mediated ABCA1 expression in HEK293 cells. A, PARP-1 protein levels were measured by Western blot in the whole cell lysates of 293-WT and 293-PARP-1-KO cells generated by using CRISPR/Cas9. Clones 1 and 2 represent cell lines generated from two independent CRISPR clones. B and C, 293-WT and 293-PARP-1-KO cells were transiently transfected with LXRα (B) or LXRβ (C) and treated with DMSO or 5 μm T0901317 (T) for 4 h. ABCA1 steady state mRNA levels were measured by qPCR. The expression levels of ABCA1 were normalized to RPL19 levels. D, ABCA1 nascent mRNA levels were measured by qPCR in WT and KO cells that were transiently transfected with LXRα. E, protein extracts prepared from 293-WT and 293-PARP-1-KO cells in parallel with RNA samples in D were analyzed for global PARylation by immunoblotting with anti-PAR antibody. F, global PARylation levels were measured in whole cell extracts from 293T-LXRα cells pretreated with 0 mm or 5 mm of pan-PARP inhibitor, 3-AB for 16 h. G, 293T-LXRα cells were pretreated with 5 mm 3-AB and were stimulated with vehicle (DMSO) or 5 μm T for 4 h. ABCA1 mRNA expression levels were measured by qPCR similarly as above. Experiments were performed three times, and the values were averaged. The error bars represent S.E. Significance was determined using the two-tailed Student's t test. *, p < 0.05; **, p < 0.005; ***, p < 0.0005. For B, significance was determined by comparing values with WT clone 1.
Using an antibody that recognizes the poly(ADP-ribose) modification, we found that in the absence of PARP-1, global poly(ADP-ribosyl)ation (PARylation) was markedly reduced in whole cell extracts (Fig. 2E). PARP activity can be inhibited by the NAD+ analog 3-AB that competes with NAD+ for the catalytic site of PARP (45). To examine whether PARP-1 catalytic activity was involved in modulating LXRα transcriptional activity at the ABCA1 gene, we incubated 293T-LXRα cells with 3-AB. Treatment with 3-AB led to a significant reduction in the global PARylation levels (Fig. 2F). Interestingly, inhibition of PARP also resulted in increased ABCA1 mRNA expression, suggesting a role for PARP-1 catalytic activity in LXR-dependent ABCA1 expression (Fig. 2G).
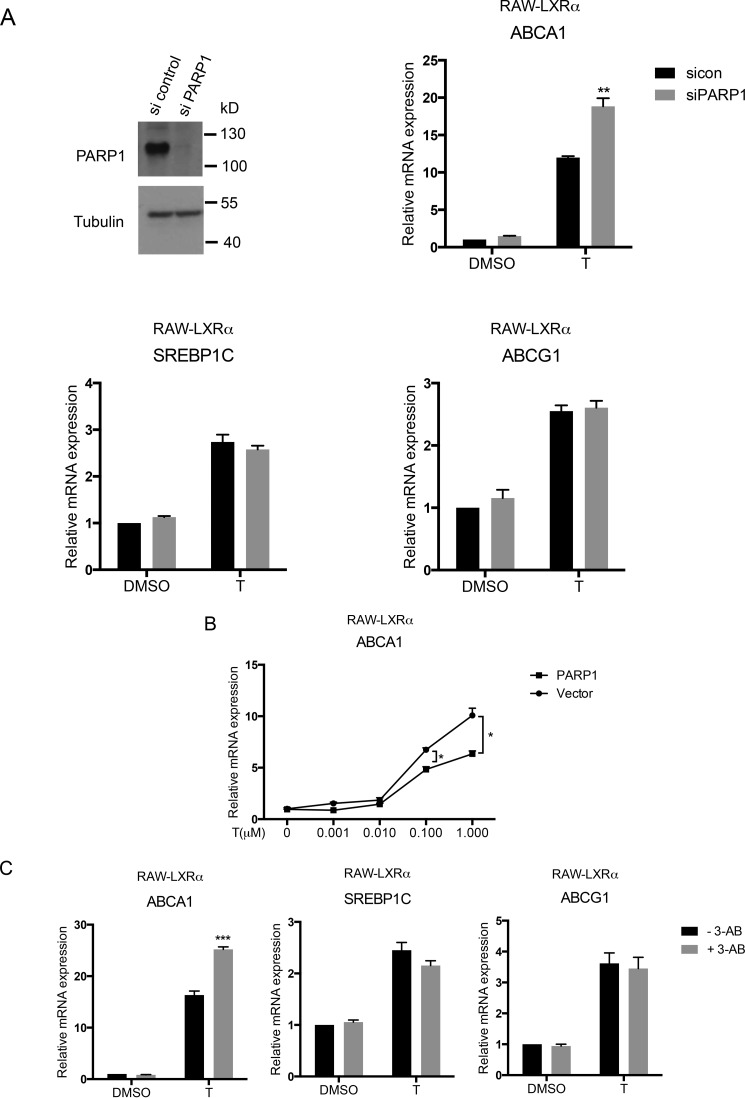
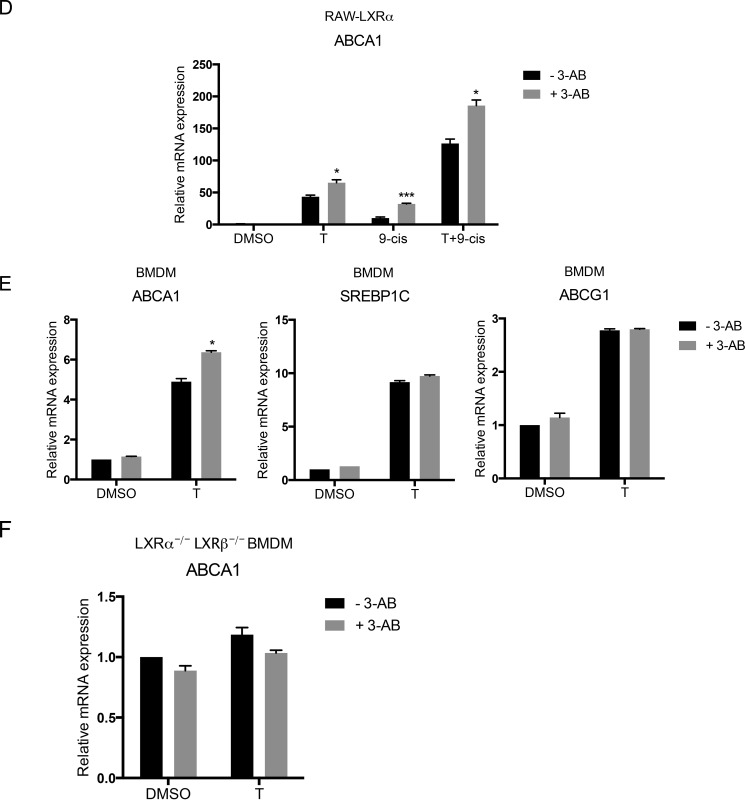
We next examined whether the regulation of ABCA1 gene expression by PARP-1 was also found in a functionally relevant macrophage cell line. We utilized RAW 264.7 macrophages stably expressing LXRα (RAW-LXRα) and modulated PARP-1 levels in these cells. PARP-1 was depleted using siRNA in RAW-LXRα cells, and the expression of LXR target genes including ABCA1, ABCG1, and SREBP-1c was examined. As in the HEK293 cell model, depletion of PARP-1 increased ABCA1 expression upon treatment with the synthetic LXR ligand T0901317 (Fig. 3A). In contrast, ABCG1 and SREBP-1c mRNA levels remained unchanged upon PARP-1 depletion (Fig. 3A). Consistent with this observation, PARP-1 overexpression in RAW-LXRα significantly reduced ABCA1 mRNA expression induced by T0901317 (Fig. 3B). Moreover, inhibition of PARP by 3-AB in the RAW-LXRα also showed increased LXR-dependent ABCA1 mRNA expression (Fig. 3C). However, the inhibitor treatment did not affect ABCG1 and SREBP-1c mRNA expression (Fig. 3C). In addition to LXR ligands, we treated cells with 9-cis-retinoic acid, which transactivates LXR via its heterodimeric partner retinoid X receptor or with 9-cis in combination with T0901317. 3-AB diminished expression of ABCA1 under all of these ligand conditions (Fig. 3D). Importantly, primary BMDMs treated with 3-AB also showed similar effects on the expression of LXR target genes. Although ABCA1 mRNA levels were significantly enhanced with 3-AB, SREBP1c and ABCG1 levels did not change (Fig. 3E). Moreover, BMDMs cultured from mice deficient in LXRα and LXRβ (LXRα−/− LXRβ−/−) were insensitive to the inhibitor treatment with regards to ABCA1 expression (Fig. 3F), suggesting that 3-AB-induced increase in ABCA1 expression is dependent on LXRs. Thus, our results indicate that PARP-1, via its catalytic activity, negatively regulates LXR-dependent transcription in a gene-specific manner.
FIGURE 3.
Effects of PARP-1 depletion, overexpression, and inhibition on expression of LXR target genes in macrophages. A, whole cell lysates from siRNA nontargeting control and siRNA PARP-1 transfected RAW-LXRα cells were analyzed by immunoblotting for the PARP-1 protein levels (top left). RAW-LXRα macrophages transfected with si control or si PARP-1 were treated with DMSO (D) or 5 μm T for 4 h, and ABCA1 (top right), SREBP1c (bottom left), and ABCG1 (bottom right) mRNA levels were measured by qPCR. The expression levels were normalized to cyclophilin A levels. B, RAW-LXRα cells were overexpressed with PARP-1 or vector control and treated with 0, 0.001, 0.01, 0.1, and 1 μm T0901317 (T), and ABCA1 mRNA levels were measured by qPCR. C–F, RAW-LXRα cells (C and D), WT primary BMDMs (E), and LXRα−/− LXRβ−/− BMDMs (F) were pretreated with 5 mm 3-AB and were stimulated with vehicle (DMSO), 5 μm T0901317 (T), 1 μm 9-cis-RA (9-cis), or a combination of T0901317 and 9-cis-retinoic acid (T+9-cis) as indicated for 4 h (C, E, and F) or 24 h (D). ABCA1, SREBP1c, and/or ABCG1 mRNA expression levels were measured by qPCR as indicated similarly as above. Experiments were performed three times, and the values were averaged. The error bars represent S.E. Significance was determined using the two-tailed Student's t test. *, p < 0.05; **, p < 0.005; ***, p < 0.0005.
PARP-1 Occupies the ABCA1 Promoter
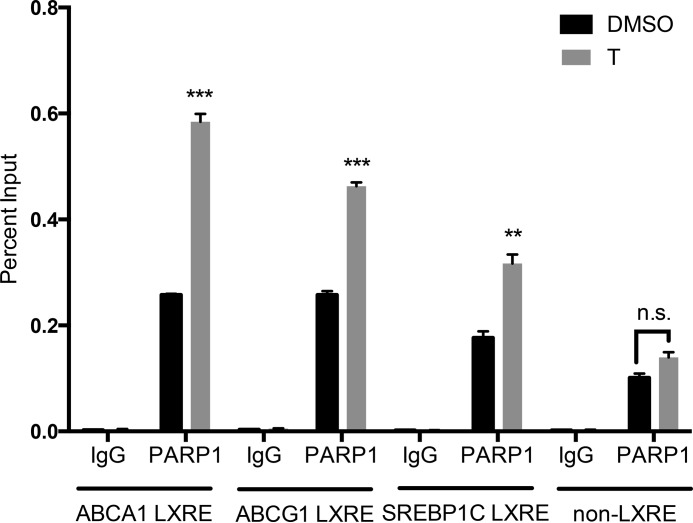
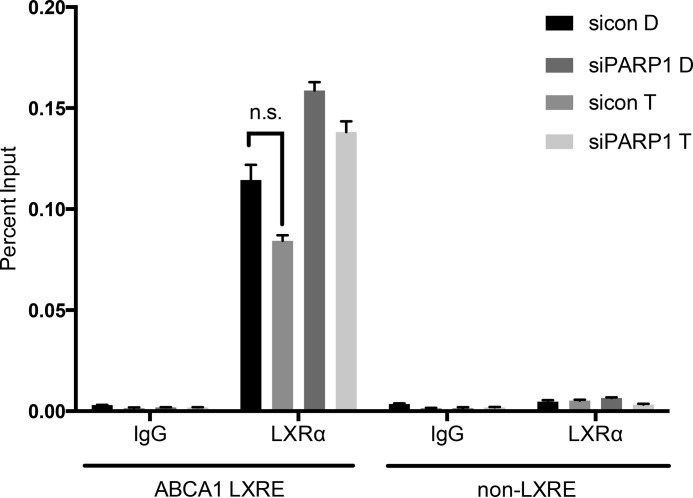
Because PARP-1 interacts with LXRs and impacts LXR-dependent gene expression, we investigated whether PARP-1 occupies the ABCA1 gene regulatory region containing the LXR binding site. We used primers spanning the LXRE on the ABCA1 gene 85 bp upstream of the transcription start site and a control non-LXR binding site 4 kb upstream of the transcription start site. We found that PARP-1 occupancy was significantly greater at the ABCA1 LXRE compared with that at the far upstream site (Fig. 4). Interestingly, PARP-1 occupancy at the region spanning the LXRE site in the ABCA1 regulatory region was significantly greater upon T0901317 stimulation than that under the basal condition, suggesting ligand-mediated recruitment of PARP-1. PARP-1 occupancy was also observed at the LXRE sites in the promoters of ABCG1 and SREBP-1c, genes that were insensitive to changes in PARP-1 expression. However, the ligand-mediated recruitment of PARP-1 was greatest at the ABCA1 locus. Thus, our results demonstrate occupancy of PARP-1 at the promoter proximal ABCA1 LXRE, which is further enhanced upon LXR ligand treatment. We also investigated whether PARP-1 depletion altered LXRα binding to the ABCA1 promoter through ChIP assay using an LXRα antibody. Depletion of PARP-1 did not change LXRα occupancy at the ABCA1 promoter LXRE, suggesting that the mechanism by which PARP-1 affects LXR-dependent gene regulation does not involve changes in LXRα binding to the ABCA1 promoter (Fig. 5).
FIGURE 4.
PARP-1 occupancy at the ABCA1 promoter. RAW-LXRα cells were incubated with DMSO or 5 μm T0901317 (T) for 1 h. Chromatin immunoprecipitation was performed using PARP-1 antibody or rabbit IgG. Precipitated DNA was quantified by qPCR using the primers spanning a non-LXRE site 4 kb upstream of the transcription start site of the ABCA1 gene, LXRE site at the ABCA1 promoter 85 bp upstream of the transcription start site, and ABCG1 promoter LXRE and SREBP-1c LXRE sites; normalized to total input chromatin levels; and measured as a percentage of input. The experiment was performed three times, and the values were averaged. The error bars represent S.E. Significance was determined using the two-tailed Student's t test. *, p < 0.05; **, p < 0.005; ***, p < 0.0005.
FIGURE 5.
Effects of depletion of PARP-1 on LXRα occupancy at ABCA1 promoter LXRE. RAW LXRα macrophages transfected with siRNA control (sicon) or siRNA PARP-1 (siPARP1) were treated with DMSO (D) or 5 μm T0901317 (T) for 1 h. Chromatin immunoprecipitation was performed using LXRα antibody or mouse IgG. Precipitated DNA was quantified by qPCR using primers spanning ABCA1 LXRE or a control non-LXRE site, normalized to total input chromatin levels, and measured as a percentage of input. The experiment was performed three times, and the values were averaged. The error bars represent S.E. The two-tailed Student's t test showed no significant (ns) differences in LXRα occupancy between si control and si PARP1 groups.
PARP-1 Poly(ADP-ribosyl)ates LXRα
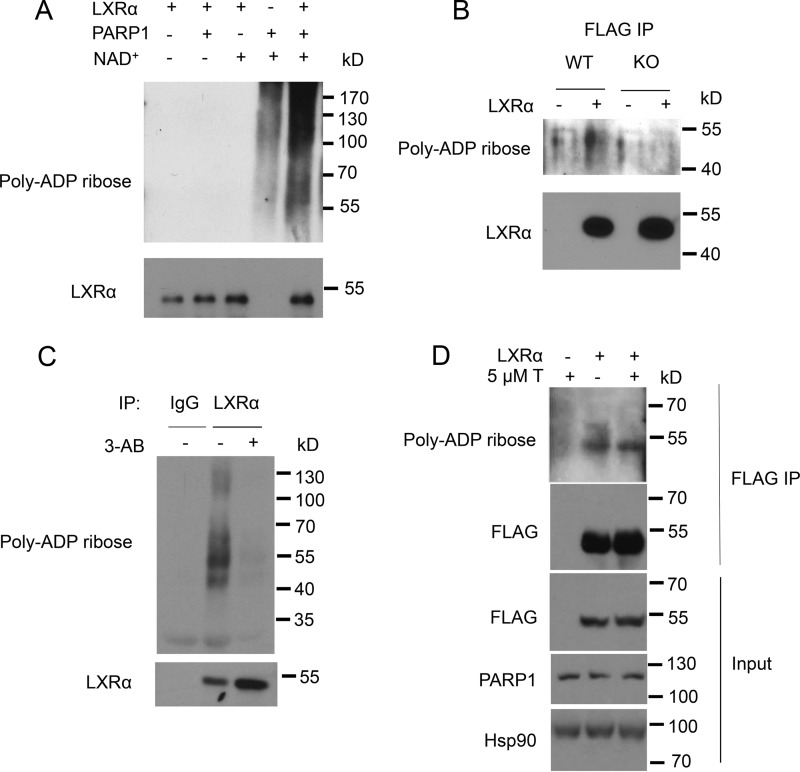
Our results strongly indicate that the catalytic activity of PARP-1 controls LXR-dependent ABCA1 expression. Given that PARP-1 modifies its target proteins through PARylation, we assessed whether the enzymatic activity of PARP-1 mediated ADP-ribosylation of LXRα. Recombinant human LXRα was incubated with recombinant human PARP-1 in the presence or absence of the donor molecule NAD+ in a cell-free system. In the presence of PARP-1 and NAD+, poly(ADP-ribosyl)ated LXRα was detected as a higher molecular mass smear that originated at 50 kDa, the molecular mass of LXRα, indicative of the addition of the poly (ADP-ribose) polymers onto LXRα (Fig. 6A). Next, using immunoprecipitation we examined whether LXRα is a target for ADP-ribosylation by PARP-1 in mammalian 293-WT and 293-PARP-1-KO cells expressing FLAG-LXRα. LXRα ADP-ribosylation was evident in 293-WT cells expressing PARP-1, whereas it was not detected in PARP-1-deficient 293-PARP-1-KO cells (Fig. 6B). LXRα poly(ADP-ribosyl)ation was also detected in primary bone marrow-derived macrophages, which was reduced upon inhibition of PARP-1 by 3-AB (Fig. 6C). LXRα activation via the T0901317 ligand did not change the ADP-ribosylation status of the receptor (Fig. 6D). Thus, our results strongly suggest that LXRα is poly(ADP-ribosyl)ated by PARP-1.
FIGURE 6.
Poly(ADP-ribosyl)ation of LXRα by PARP-1. A, recombinant LXRα (100 ng) and PARP-1 (40 ng) together or alone were incubated in PARylation buffer in the presence or absence of 300 μm NAD+ for 30 min. PARylation was stopped by the addition of SDS sample buffer. The samples were run on a 10% SDS-polyacrylamide gel. PARylation was detected by Western blot with anti-poly-ADP-ribose antibody. B, 293-WT and 293-PARP-1-KO cells were transiently transfected with FLAG-tagged LXRα or vector alone. LXRα was immunoprecipitated by FLAG antibody and immunoblotted with anti-PAR antibody. C, whole cell extracts from BMDMs treated with vehicle or 5 mm 3-AB were subjected to immunoprecipitation using control IgG or LXRα antibody. The samples were analyzed for total LXRα and PARylation by Western blot. D, LXRα was ectopically expressed in HEK293 cells, which were then treated with vehicle (DMSO) or 5 μm T0901317 (T) for 4 h. LXRα was immunoprecipitated and analyzed by Western blot for PARylation. Whole cell lysates were also analyzed for FLAG-tagged LXRα and PARP-1 protein levels by Western blot. Hsp90 was used as a loading control. The experiments were performed at least three times with similar results, and a representative experiment is shown.
Inhibition of PARP-1 Activity Increases Macrophage Cholesterol Efflux
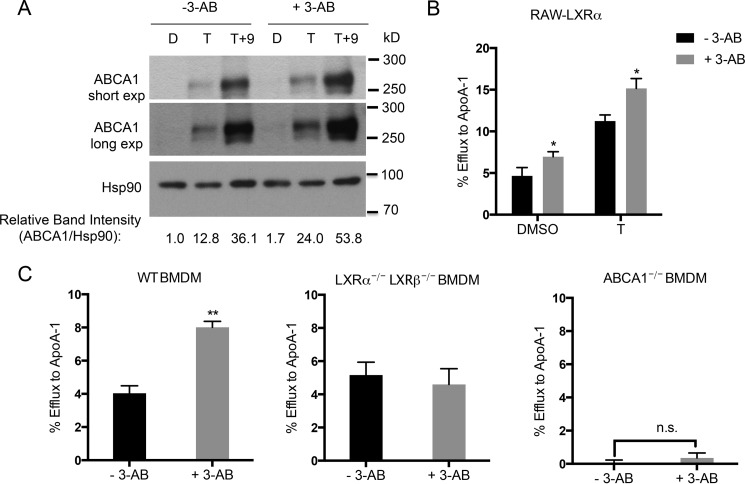
Having established that PARP-1 represses LXR-dependent expression of ABCA1 mRNA, we examined whether the changes in the mRNA translated into changes in ABCA1 protein expression. We incubated RAW-LXRα cells with either vehicle or PARP inhibitor in the absence or presence of ligands T0901317 or T0901317 together with 9-cis-retinoic acid and measured ABCA1 protein levels. We found that macrophages treated with the PARP inhibitor, 3-AB, showed markedly enhanced ABCA1 protein expression upon ligand treatment (Fig. 7A).
FIGURE 7.
Inhibition of PARP-1 increases cholesterol efflux in macrophages. A, ABCA1 protein levels were measured in the whole cell lysates from RAW-LXRα cells pretreated with vehicle or 5 mm 3-AB and stimulated with vehicle (D), 5 μm T0901317 (T), or a combination of 5 μm T0901317 and 1 μm 9-cis-retinoic acid (T+9). Two exposures (short and long) are shown. Hsp90 levels were measured as a loading control. The Western blot was quantitated using ImageJ software, and the ratio of ABCA1 to HSP90 is shown with DMSO and non-3-AB-treated sample set to 1. B, RAW-LXRα cells were labeled with medium containing BODIPY cholesterol, methyl-β-cyclodextrin, and unlabeled cholesterol for 1 h. Then the cells were equilibrated with vehicle or 3-AB in combination with DMSO or 5 μm T0901317 for 18 h before incubating for 4 h with the efflux medium containing extracellular acceptor apoA-I (10 μg/ml). apoA-I-mediated efflux was calculated as a percentage of total cholesterol cleared from the cells after accounting for the cholesterol taken out from the cells in the absence of acceptors. C, WT, Lxrα−/− Lxrβ−/−, and Abca1−/− primary BMDMs were loaded with [3H]cholesterol, and specific efflux to apoA-I was measured in the presence or absence of 5 mm 3-AB. The experiments in B and C were performed three times with similar results. Each efflux experiment was performed in triplicate, and a representative experiment is shown. The error bars represent S.D. Significance was determined using the two-tailed Student's t test. *, p < 0.05; **, p < 0.005.
In macrophages, ABCA1 facilitates the clearance of excess cellular free cholesterol by pumping out the cholesterol to acceptor proteins, primarily apoA-I. Given the increase in ABCA1 levels by PARP inhibition, we tested whether PARP-1 inhibition would enhance the cholesterol efflux capacity of macrophages. To test this, we used a BODIPY-cholesterol efflux assay, which utilizes fluorescently labeled cholesterol to measure efflux to extracellular acceptors. We found that ABCA1-mediated cholesterol efflux to apoA-I was significantly greater in RAW cells treated with the PARP inhibitor compared with the efflux in vehicle-treated cells (Fig. 7B). We also measured the ability of primary BMDMs treated with vehicle or PARP inhibitor to efflux cholesterol to apoA-I by cholesterol efflux assay using [3H]cholesterol. In the presence of the inhibitor, apoAI-directed cholesterol efflux was greatly enhanced (Fig. 7C). We found that both ABCA1 and LXRs were indispensable for the inhibitor-mediated increase in cholesterol efflux because macrophages from mice lacking LXRα and LXRβ (Lxrα−/− Lxrβ−/−) and ABCA1 (Abca1−/−) did not exhibit changes in cholesterol efflux with the addition of the inhibitor. Our data suggest that in macrophages, PARP-1 is an LXRα regulator that selectively represses ABCA1 expression, such that when PARP activity is reduced, cholesterol efflux is enhanced.
Discussion
PARP-1 has been reported to have roles as a promoter-specific coactivator or corepressor for several DNA binding transcriptional regulators independent of its role in the DNA damage response (25). In fact, PARP-1 has been shown to be a transcriptional coregulator for a number of nuclear receptors including thyroid hormone receptor, estrogen receptor α, retinoic acid receptor, and farnesoid X receptor (42, 43, 46). Here we report that PARP-1 interacts with both LXRα and LXRβ, and functions as a gene-specific corepressor of LXR-mediated gene expression. Whereas ABCG1 and SREBP-1C were unaffected by changes in PARP-1 expression or activity, the cholesterol transporter ABCA1 was up-regulated by either PARP-1 depletion or inhibition of its catalytic activity. Consistent with this finding, inhibition of PARP activity significantly increased ABCA1 protein levels and cholesterol efflux to apoA-I, an ABCA1-specific cholesterol acceptor in an ABCA1- and LXR-dependent manner. Thus, our study suggests that PARP inhibition in vivo would be anti-atherogenic by virtue of enhanced cholesterol efflux from macrophages.
Indeed, previous reports indicated that PARP-1 activation was pro-atherogenic, whereas PARP inhibition was anti-atherogenic. These conclusions were drawn from studies where factors that promoted atherosclerosis, such as oxidized LDL, hyperglycemia, and H2O2-stimulated PARP activity (47–50). PARP-1 was activated within atherosclerotic plaques of ApoE−/− mice fed a high fat diet (51). PARP-1 deletion, on the other hand, reduced atherosclerotic lesion formation in high fat diet-fed ApoE−/− mice (52), and PARP catalytic inhibitors reduced atherosclerotic plaque burden in mouse models of atherosclerosis (53). Thus, PARP inhibition in vivo is atheroprotective and is consistent with our data that PARP-1 inhibition, through increasing lipid efflux, impeded the accumulation of cholesterol in macrophages, a process that is central to the pathogenesis of atherosclerosis.
Although the mechanisms by which PARP-1 imparts its gene-specific effects on LXR-dependent gene expression are not understood, it was clear that the enzymatic activity of PARP-1 was required. This is distinct from PARP-1 coactivator function for NFκB, which requires PARP-1 cleavage and is independent of its catalytic activity (54). PARP-1 has been shown to modify proteins by transferring an ADP-ribose moiety to target substrates. Here, we demonstrated that LXRα was PARylated by PARP-1. Several post-translational modifications on LXRα have been previously described including phosphorylation (28), O-linked β-N-acetylglucosamine (O-GlcNAcylation) (55), acetylation (37), and sumoylation (56). These modifications on LXRα were shown to impact target gene expression through changes in LXRα stability, transactivation, and/or recruitment of transcriptional regulatory factors at specific target genes. By analogy, we suggest that PARylation of LXR by PARP-1 could affect coactivator-corepressor interactions at genes such as ABCA1. For example, whereas LXR occupancy was observed at the ABCA1 promoter in the absence and presence of ligand, the coregulator G-protein pathway suppressor 2 (GPS2), a component of the NCoR repression complex, was shown to occupy the ABCA1 promoter in the absence of ligand and was released upon ligand binding. At ABCG1, the opposite was true because both LXR and GPS2 were recruited by ligand (57). Thus, GPS2 in the context of ABCG1 was associated with transcriptional activation, whereas at ABCA1 GPS2 was correlated with transcriptional repression. It is conceivable that PARP-1 maintains the GPS2 containing NCoR repression complex at ABCA1 to modulate expression. Additional experiments will be required to identify the residue(s) of LXR that are PARylated and to interrogate cofactor occupancy at ABCA1 versus ABCG1 to elucidate the functional consequence of PARP-1 in LXR-dependent transcriptional regulation.
Elegant work from the Luger lab indicated that PARP-1 had histone chaperone function (58). This action of PARP-1, in principle, could modulate ABCA1 expression by facilitating a less open and more repressive chromatin state that precluded LXR binding. However, we did not observe any change in LXR occupancy at the ABCA1 LXRE under basal and ligand-stimulated conditions upon PARP inhibition (Fig. 4). Thus, PARP-1-dependent reduction of ABCA1 expression was not a result of altered LXR occupancy at the ABCA1 promoter.
Although PARP-1 binding was observed at LXR target genes that were sensitive and insensitive to PARP-1 activity, there was significantly greater PARP-1 recruitment upon ligand stimulation at the ABCA1 compared with the ABCG1 or SREBP-1c promoters. We suggest that LXR is being modified in an allosteric manner by DNA binding at the ABCA1 LXRE that resulted in a conformation favorable for PARP-1-LXR interaction to affect the regulation for this gene (59, 60). Because PARP-1 has a repressive effect on LXR-dependent gene expression, we speculate that enhanced PARP-1 recruitment acts as a negative feedback mechanism to control ABCA1 gene expression in response to ligand. Higher protein abundance of PARP-1 could potentially override such a feedback mechanism and reduce LXR occupancy at ABCA1.
In summary, our data revealed a novel interaction between LXR and PARP-1 and a role for PARP-1 in LXR-dependent expression of ABCA1 in cholesterol efflux. PARP inhibitors have garnered much attention as therapeutic agents in cancer (61). Several reports of PARP activation being critical in atherosclerotic plaque formation and destabilization support the notion that PARP inhibitors may exhibit therapeutic utility in the clinical management of atherosclerosis (62). In fact, the use of PARP inhibitors in mouse models of atherosclerosis has proven beneficial not only in preventing atherogenesis but also in promoting regression of preexisting plaques. It will be crucial to address what cell types and protein targets are affected by PARP-1 in atherosclerosis. In the context of this study, macrophage-specific PARP-1 knock-out mice will be essential in elucidating the contribution of PARP-1 in atherogenesis by modulating macrophage LXR signaling.
Author Contributions
E. S., E. A. F., and M. J. G. designed the study. E. S. and M. J. G. wrote the paper. E. S. performed the IP experiments and LXR in vitro PARylation studies and with M. A. H. performed the qPCR measurements for the LXR target genes. J. N. S. and J. R. Y. performed the mass spectrometry analysis. M. O. and E. S. performed the radioactive cholesterol efflux assay. E. S. and T. B. performed the bodipy cholesterol efflux assays. E. S. and S. L. created the PARP-1 KO cell line using CRISPR/Cas9 system. K. J. M. provided Abca1−/− mice and reagents for cholesterol efflux assays. All authors analyzed the results and approved the final version of the manuscript.
Acknowledgments
We thank members of the Garabedian lab for critically reading the manuscript. We thank Ines Pineda-Torra (University College London) for the HEK293T-LXRα and HEK 293T-Vo cells, and Jan-Åke Gustafsson (University of Houston) for the Lxrα−/−/Lxrβ −/− mice.
The work was supported in part by National Institutes of Health Grants T32GM007238 and UL1TR000038 from the National Center for the Advancement of Translational Science (to E. S.) and Grants P41GM103533 and R01MH067880 (to J. R. Y.), F32AG039127 and R00DC013805 (to J. N. S.), T32AI07180 and T32GM007308 (to M. A. H.), R01HL117226 (to M. J. G. and E. A. F.), and R01HL117334 (to K. J. M.); by a fellowship from the Vilcek Foundation (to E. S.); and by American Heart Association Fellowship 15POST25090199 (to M. O.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- LXR
- liver X receptor
- PARP
- poly(ADP-ribose) polymerase
- LXRE
- LXR response element
- NCoR
- nuclear receptor corepressor
- apo
- apolipoprotein
- qPCR
- quantitative PCR
- 3-AB
- 3-aminobenzamide
- BMDM
- bone marrow-derived macrophage.
References
- 1. Libby P., Ridker P. M., and Hansson G. K. (2011) Progress and challenges in translating the biology of atherosclerosis. Nature 473, 317–325 [DOI] [PubMed] [Google Scholar]
- 2. Duffy D., and Rader D. J. (2009) Update on strategies to increase HDL quantity and function. Nat. Rev. Cardiol. 6, 455–463 [DOI] [PubMed] [Google Scholar]
- 3. Gordon D. J., Probstfield J. L., Garrison R. J., Neaton J. D., Castelli W. P., Knoke J. D., Jacobs D. R. Jr., Bangdiwala S., and Tyroler H. A. (1989) High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 79, 8–15 [DOI] [PubMed] [Google Scholar]
- 4. Glass C. K., and Witztum J. L. (2001) Atherosclerosis. the road ahead. Cell 104, 503–516 [DOI] [PubMed] [Google Scholar]
- 5. Vainio S., and Ikonen E. (2003) Macrophage cholesterol transport: a critical player in foam cell formation. Ann. Med. 35, 146–155 [DOI] [PubMed] [Google Scholar]
- 6. Peet D. J., Turley S. D., Ma W., Janowski B. A., Lobaccaro J. M., Hammer R. E., and Mangelsdorf D. J. (1998) Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXRα. Cell 93, 693–704 [DOI] [PubMed] [Google Scholar]
- 7. Janowski B. A., Willy P. J., Devi T. R., Falck J. R., and Mangelsdorf D. J. (1996) An oxysterol signalling pathway mediated by the nuclear receptor LXRα. Nature 383, 728–731 [DOI] [PubMed] [Google Scholar]
- 8. Yang C., McDonald J. G., Patel A., Zhang Y., Umetani M., Xu F., Westover E. J., Covey D. F., Mangelsdorf D. J., Cohen J. C., and Hobbs H. H. (2006) Sterol intermediates from cholesterol biosynthetic pathway as liver X receptor ligands. J. Biol. Chem. 281, 27816–27826 [DOI] [PubMed] [Google Scholar]
- 9. Hong C., and Tontonoz P. (2014) Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat. Rev. Drug Discov. 13, 433–444 [DOI] [PubMed] [Google Scholar]
- 10. Zelcer N., and Tontonoz P. (2006) Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Invest. 116, 607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wagner B. L., Valledor A. F., Shao G., Daige C. L., Bischoff E. D., Petrowski M., Jepsen K., Baek S. H., Heyman R. A., Rosenfeld M. G., Schulman I. G., and Glass C. K. (2003) Promoter-specific roles for liver X receptor/corepressor complexes in the regulation of ABCA1 and SREBP1 gene expression. Mol. Cell. Biol. 23, 5780–5789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huuskonen J., Fielding P. E., and Fielding C. J. (2004) Role of p160 coactivator complex in the activation of liver X receptor. Arterioscl. Thromb. Vasc. Biol. 24, 703–708 [DOI] [PubMed] [Google Scholar]
- 13. Lee S., Lee J., Lee S. K., and Lee J. W. (2008) Activating signal cointegrator-2 is an essential adaptor to recruit histone H3 lysine 4 methyltransferases MLL3 and MLL4 to the liver X receptors. Mol. Endocrinol. 22, 1312–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Calkin A. C., and Tontonoz P. (2010) Liver X receptor signaling pathways and atherosclerosis. Arterioscl. Thromb. Vasc. Biol. 30, 1513–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Venkateswaran A., Laffitte B. A., Joseph S. B., Mak P. A., Wilpitz D. C., Edwards P. A., and Tontonoz P. (2000) Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXRα. Proc. Natl. Acad. Sci. U.S.A. 97, 12097–12102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chawla A., Boisvert W. A., Lee C. H., Laffitte B. A., Barak Y., Joseph S. B., Liao D., Nagy L., Edwards P. A., Curtiss L. K., Evans R. M., and Tontonoz P. (2001) A PPARγ-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol. Cell 7, 161–171 [DOI] [PubMed] [Google Scholar]
- 17. Costet P., Luo Y., Wang N., and Tall A. R. (2000) Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J. Biol. Chem. 275, 28240–28245 [DOI] [PubMed] [Google Scholar]
- 18. Schultz J. R., Tu H., Luk A., Repa J. J., Medina J. C., Li L., Schwendner S., Wang S., Thoolen M., Mangelsdorf D. J., Lustig K. D., and Shan B. (2000) Role of LXRs in control of lipogenesis. Genes Dev. 14, 2831–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schwartz K., Lawn R. M., and Wade D. P. (2000) ABC1 gene expression and ApoA-I-mediated cholesterol efflux are regulated by LXR. Biochem. Biophys. Res. Commun. 274, 794–802 [DOI] [PubMed] [Google Scholar]
- 20. Levin N., Bischoff E. D., Daige C. L., Thomas D., Vu C. T., Heyman R. A., Tangirala R. K., and Schulman I. G. (2005) Macrophage liver x receptor is required for antiatherogenic activity of LXR agonists. Arterioscl. Thromb. Vasc. Biol. 25, 135–142 [DOI] [PubMed] [Google Scholar]
- 21. Joseph S. B., McKilligin E., Pei L., Watson M. A., Collins A. R., Laffitte B. A., Chen M., Noh G., Goodman J., Hagger G. N., Tran J., Tippin T. K., Wang X., Lusis A. J., Hsueh W. A., Law R. E., Collins J. L., Willson T. M., and Tontonoz P. (2002) Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc. Natl. Acad. Sci. U.S.A. 99, 7604–7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tangirala R. K., Bischoff E. D., Joseph S. B., Wagner B. L., Walczak R., Laffitte B. A., Daige C. L., Thomas D., Heyman R. A., Mangelsdorf D. J., Wang X., Lusis A. J., Tontonoz P., and Schulman I. G. (2002) Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc. Natl. Acad. Sci. U.S.A. 99, 11896–11901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ito A., Hong C., Rong X., Zhu X., Tarling E. J., Hedde P. N., Gratton E., Parks J., and Tontonoz P. (2015) LXRs link metabolism to inflammation through Abca1-dependent regulation of membrane composition and TLR signaling. eLife 4, e08009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Collingwood T. N., Urnov F. D., and Wolffe A. P. (1999) Nuclear receptors: coactivators, corepressors and chromatin remodeling in the control of transcription. J. Mol. Endocrinol. 23, 255–275 [DOI] [PubMed] [Google Scholar]
- 25. Kraus W. L. (2008) Transcriptional control by PARP-1: chromatin modulation, enhancer-binding, coregulation, and insulation. Curr. Opin. Cell. Biol. 20, 294–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim M. Y., Zhang T., and Kraus W. L. (2005) Poly(ADP-ribosyl)ation by PARP-1: “PAR-laying” NAD+ into a nuclear signal. Genes Dev. 19, 1951–1967 [DOI] [PubMed] [Google Scholar]
- 27. Kraus W. L., and Hottiger M. O. (2013) PARP-1 and gene regulation: progress and puzzles. Mol. Aspects Med. 34, 1109–1123 [DOI] [PubMed] [Google Scholar]
- 28. Torra I. P., Ismaili N., Feig J. E., Xu C. F., Cavasotto C., Pancratov R., Rogatsky I., Neubert T. A., Fisher E. A., and Garabedian M. J. (2008) Phosphorylation of liver X receptor α selectively regulates target gene expression in macrophages. Mol. Cell. Biol. 28, 2626–2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nakatani Y., and Ogryzko V. (2003) Immunoaffinity purification of mammalian protein complexes. Methods Enzymol. 370, 430–444 [DOI] [PubMed] [Google Scholar]
- 30. Savas J. N., Makusky A., Ottosen S., Baillat D., Then F., Krainc D., Shiekhattar R., Markey S. P., and Tanese N. (2008) Huntington's disease protein contributes to RNA-mediated gene silencing through association with Argonaute and P bodies. Proc. Natl. Acad. Sci. U.S.A. 105, 10820–10825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mita P., Savas J. N., Ha S., Djouder N., Yates J. R. 3rd, and Logan S. K. (2013) Analysis of URI nuclear interaction with RPB5 and components of the R2TP/prefoldin-like complex. PLoS One 8, e63879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ran F. A., Hsu P. D., Wright J., Agarwala V., Scott D. A., and Zhang F. (2013) Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alberti S., Schuster G., Parini P., Feltkamp D., Diczfalusy U., Rudling M., Angelin B., Björkhem I., Pettersson S., and Gustafsson J. A. (2001) Hepatic cholesterol metabolism and resistance to dietary cholesterol in LXRβ-deficient mice. J. Clin. Invest. 107, 565–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aiello R. J., Brees D., and Francone O. L. (2003) ABCA1-deficient mice: insights into the role of monocyte lipid efflux in HDL formation and inflammation. Arterioscl. Thromb. Vasc. Biol. 23, 972–980 [DOI] [PubMed] [Google Scholar]
- 35. Domon B., and Aebersold R. (2010) Options and considerations when selecting a quantitative proteomics strategy. Nat. Biotechnol. 28, 710–721 [DOI] [PubMed] [Google Scholar]
- 36. Law W. J., Cann K. L., and Hicks G. G. (2006) TLS, EWS and TAF15: a model for transcriptional integration of gene expression. Brief. Funct. Genomic Proteomic 5, 8–14 [DOI] [PubMed] [Google Scholar]
- 37. Chi Y. H., Haller K., Peloponese J. M. Jr., and Jeang K. T. (2007) Histone acetyltransferase hALP and nuclear membrane protein hsSUN1 function in de-condensation of mitotic chromosomes. J. Biol. Chem. 282, 27447–27458 [DOI] [PubMed] [Google Scholar]
- 38. Kong R., Zhang L., Hu L., Peng Q., Han W., Du X., and Ke Y. (2011) hALP, A Novel Transcriptional U Three Protein (t-UTP), Activates RNA polymerase I transcription by binding and acetylating the upstream binding factor (UBF). J. Biol. Chem. 286, 7139–7148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Modena P., Lualdi E., Facchinetti F., Galli L., Teixeira M. R., Pilotti S., and Sozzi G. (2005) SMARCB1/INI1 tumor suppressor gene is frequently inactivated in epithelioid sarcomas. Cancer Res. 65, 4012–4019 [DOI] [PubMed] [Google Scholar]
- 40. Belandia B., Orford R. L., Hurst H. C., and Parker M. G. (2002) Targeting of SWI/SNF chromatin remodelling complexes to estrogen-responsive genes. EMBO J. 21, 4094–4103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang F., Wang Y., Wang L., Luo X., Huang K., Wang C., Du M., Liu F., Luo T., Huang D., and Huang K. (2013) Poly(ADP-ribose) polymerase 1 is a key regulator of estrogen receptor α-dependent gene transcription. J. Biol. Chem. 288, 11348–11357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pavri R., Lewis B., Kim T. K., Dilworth F. J., Erdjument-Bromage H., Tempst P., de Murcia G., Evans R., Chambon P., and Reinberg D. (2005) PARP-1 determines specificity in a retinoid signaling pathway via direct modulation of mediator. Mol. Cell 18, 83–96 [DOI] [PubMed] [Google Scholar]
- 43. Wang C., Zhang F., Wang L., Zhang Y., Li X., Huang K., Du M., Liu F., Huang S., Guan Y., Huang D., and Huang K. (2013) Poly(ADP-ribose) polymerase 1 promotes oxidative-stress-induced liver cell death via suppressing farnesoid X receptor α. Mol. Cell. Biol. 33, 4492–4503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ulven S. M., Dalen K. T., Gustafsson J. A., and Nebb H. I. (2005) LXR is crucial in lipid metabolism. Prostaglandins Leukot. Essent. Fatty Acids 73, 59–63 [DOI] [PubMed] [Google Scholar]
- 45. Virág L., and Szabó C. (2002) The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol. Rev. 54, 375–429 [DOI] [PubMed] [Google Scholar]
- 46. Miyamoto T., Kakizawa T., and Hashizume K. (1999) Inhibition of nuclear receptor signalling by poly(ADP-ribose) polymerase. Mol. Cell. Biol. 19, 2644–2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wei S. J., Xing J. H., Wang B. L., Xue L., Wang J. L., Li R., Qin W. D., Wang J., Wang X. P., Zhang M. X., and Chen Y. G. (2013) Poly(ADP-ribose) polymerase inhibition prevents reactive oxygen species induced inhibition of aldehyde dehydrogenase2 activity. Biochim. Biophys. Acta 1833, 479–486 [DOI] [PubMed] [Google Scholar]
- 48. Garcia Soriano F., Virág L., Jagtap P., Szabó E., Mabley J. G., Liaudet L., Marton A., Hoyt D. G., Murthy K. G., Salzman A. L., Southan G. J., and Szabó C. (2001) Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation. Nat. Med. 7, 108–113 [DOI] [PubMed] [Google Scholar]
- 49. Peng X., Li W., and Zhang W. (2012) Poly(ADP-ribose) polymerase 1 inhibition protects human aortic endothelial cells against LPS-induced inflammation response. Acta Biochem. Biophys. Sin. (Shanghai) 44, 911–917 [DOI] [PubMed] [Google Scholar]
- 50. Radovits T., Lin L. N., Zotkina J., Gero D., Szabó C., Karck M., and Szabó G. (2007) Poly(ADP-ribose) polymerase inhibition improves endothelial dysfunction induced by reactive oxidant hydrogen peroxide in vitro. Eur. J. Pharmacol. 564, 158–166 [DOI] [PubMed] [Google Scholar]
- 51. Oumouna-Benachour K., Hans C. P., Suzuki Y., Naura A., Datta R., Belmadani S., Fallon K., Woods C., and Boulares A. H. (2007) Poly(ADP-ribose) polymerase inhibition reduces atherosclerotic plaque size and promotes factors of plaque stability in apolipoprotein E-deficient mice: Effects on macrophage recruitment, nuclear factor-κB nuclear translocation, and foam cell death. Circulation 115, 2442–2450 [DOI] [PubMed] [Google Scholar]
- 52. Hans C. P., Feng Y., Naura A. S., Zerfaoui M., Rezk B. M., Xia H., Kaye A. D., Matrougui K., Lazartigues E., and Boulares A. H. (2009) Protective effects of PARP-1 knockout on dyslipidemia-induced autonomic and vascular dysfunction in ApoE−/− mice: effects on eNOS and oxidative stress. PLoS One 4, e7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xu S., Bai P., Little P. J., and Liu P. (2014) Poly(ADP-ribose) Polymerase 1 (PARP1) in atherosclerosis: from molecular mechanisms to therapeutic implications. Med. Res. Rev. 34, 644–675 [DOI] [PubMed] [Google Scholar]
- 54. Pétrilli V., Herceg Z., Hassa P. O., Patel N. S., Di Paola R., Cortes U., Dugo L., Filipe H. M., Thiemermann C., Hottiger M. O., Cuzzocrea S., and Wang Z. Q. (2004) Noncleavable poly(ADP-ribose) polymerase-1 regulates the inflammation response in mice. J. Clin. Invest. 114, 1072–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Anthonisen E. H., Berven L., Holm S., Nygård M., Nebb H. I., and Grønning-Wang L. M. (2010) Nuclear receptor liver X receptor is O-GlcNAc-modified in response to glucose. J. Biol. Chem. 285, 1607–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee J. H., Park S. M., Kim O. S., Lee C. S., Woo J. H., Park S. J., Joe E. H., and Jou I. (2009) Differential SUMOylation of LXRα and LXRβ mediates transrepression of STAT1 inflammatory signaling in IFN-γ-stimulated brain astrocytes. Mol. Cell 35, 806–817 [DOI] [PubMed] [Google Scholar]
- 57. Jakobsson T., Venteclef N., Toresson G., Damdimopoulos A. E., Ehrlund A., Lou X., Sanyal S., Steffensen K. R., Gustafsson J. A., and Treuter E. (2009) GPS2 is required for cholesterol efflux by triggering histone demethylation, LXR recruitment, and coregulator assembly at the ABCG1 locus. Mol. Cell 34, 510–518 [DOI] [PubMed] [Google Scholar]
- 58. Muthurajan U. M., Hepler M. R., Hieb A. R., Clark N. J., Kramer M., Yao T., and Luger K. (2014) Automodification switches PARP-1 function from chromatin architectural protein to histone chaperone. Proc. Natl. Acad. Sci. U.S.A. 111, 12752–12757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lefstin J. A., and Yamamoto K. R. (1998) Allosteric effects of DNA on transcriptional regulators. Nature 392, 885–888 [DOI] [PubMed] [Google Scholar]
- 60. van Tilborg M. A., Lefstin J. A., Kruiskamp M., Teuben J., Boelens R., Yamamoto K. R., and Kaptein R. (2000) Mutations in the glucocorticoid receptor DNA-binding domain mimic an allosteric effect of DNA. J. Mol. Biol. 301, 947–958 [DOI] [PubMed] [Google Scholar]
- 61. Lord C. J., and Ashworth A. (2008) Targeted therapy for cancer using PARP inhibitors. Curr. Opin. Pharmacol. 8, 363–369 [DOI] [PubMed] [Google Scholar]
- 62. Pacher P., and Szabó C. (2007) Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors. Cardiovasc. Drug Rev. 25, 235–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Washburn M. P., Wolters D., and Yates J. R. 3rd (2001) Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat. Biotechnol. 19, 242–247 [DOI] [PubMed] [Google Scholar]