Abstract
Type 1 diabetes mellitus is caused by the killing of insulin-producing β cells by CD8+T cells. The disease progression, which is chronic, does not follow a course like responses to conventional antigens such as viruses, but accelerates as glucose tolerance deteriorates. To identify the unique features of the autoimmune effectors that may explain this behavior, we analyzed diabetogenic CD8+ T cells that recognize a peptide from the diabetes antigen IGRP (NRP-V7-reactive) in prediabetic NOD mice and compared them to others that shared their phenotype (CD44+CD62LloPD-1+CXCR3+) but negative for diabetes antigen tetramers and to LCMV (lymphocytic choriomeningitis)-reactive CD8+ T cells. There was an increase in the frequency of the NRP-V7-reactive cells coinciding with the time of glucose intolerance. The T cells persisted in hyperglycemic NOD mice maintained with an insulin pellet despite destruction of β cells. We compared gene expression in the three groups of cells compared with the other two subsets of cells, and the NRP-V7-reactive cells exhibited gene expression of memory precursor effector cells. They had reduced cellular proliferation and were less dependent on oxidative phosphorylation. When prediabetic NOD mice were treated with 2-deoxyglucose to block aerobic glycolysis, there was a reduction in the diabetes antigen versus other cells of similar phenotype and loss of lymphoid cells infiltrating the islets. In addition, treatment of NOD mice with 2-deoxyglucose resulted in improved β cell granularity. These findings identify a link between metabolic disturbances and autoreactive T cells that promotes development of autoimmune diabetes.
Keywords: autoimmunity, beta cell (B-cell), immunology, T-cell, type 1 diabetes
Introduction
Type 1 diabetes (T1D)2 in humans is an autoimmune disease involving the destruction of insulin-producing β cells in the islets of Langerhans by CD8+ T cells (1–6). In humans, the disease develops over a period of years after the first appearance of autoantibodies that identify individuals who are at risk. The kinetics of progression to T1D differs from those of other cell-mediated immune responses. In responding to foreign pathogens, there is rapid expansion of antigen-specific effector T cells followed by contraction and the appearance of memory T cells. Immune targets are destroyed within weeks. Primarily based on serologic data from studies of discordant triplets and relatives at risk for the disease, β cell destruction in T1D is thought to occur over a period of years, although we and others have postulated that there was waxing and waning of the process. More recent data has suggested that the progression is not uniform. The time required for metabolic changes in glucose tolerance was thought to reflect the need to destroy a large β cell mass before glucose tolerance deteriorates, but our studies of β cell killing in NOD mice and humans suggests instead that the progression of β cell killing may be rapid in the peridiagnosis period. In NOD mice, which first develop insulitis at 5–6 weeks of age, the rate of β cell killing does not show a significant increase until after11 weeks of age coincident with the appearance of glucose intolerance. In our studies of β cell killing in relatives of patients with T1D, only 25% of the 49 measurements that were obtained approximately every 6 months for up to 4.14 years before diagnosis showed increased levels, indicating that the destruction of β cells is an uncommon event. However, in at-risk subjects who were closer to diagnosis and manifest dysglycemia, 81% of the measurements in 27 subjects showed elevated levels of β cell death. These data showed that β cell killing is significantly greater when glucose intolerance is present.
These observations led us to consider whether there were interactions between the pathogenic immune cells and host factors that may affect the progression of disease. To understand these interactions, we focused our studies on CD8+ T cells that are reactive to an epitope of glucose-6-phosphatase catalytic subunit related protein (IGRP) and are known to cause β cell killing. These CD8+ T cells are the best characterized among pathogenic T cells (2–4, 7–15). In human autopsy studies, CD8+ T cells reactive to an epitope of IGRP have been found in pancreata from patients several years after the onset of disease (6). IGRP-reactive CD8+ T cells can be found in NOD mice by staining with MHC-I tetramer loaded with NRP-V7 peptide (5, 12, 16). They can cause diabetes when they receive help from CD4+ T cells (13). We analyzed gene expression among these cells to identify mechanisms that might account for the kinetics of disease and interactions between the host and the pathogenic immune response. We found that compared with CD8+ T cells that share the phenotype but not the antigen specificity of the IGRP-reactive T cells as well as viral antigen-reactive CD8+ T cells, the expansion and effector function of the NRP-V7-reactive T cells are dependent on glucose availability. We show that blocking glucose metabolism may selectively reduce the frequency of these cells and others that infiltrate the pancreatic islets. Our work suggests that elevated glucose may drive a feed-forward mechanism in which modest hyperglycemia provides fuel to effector T cells and leads to the maintenance of pathogenic T cells and accelerated β cell destruction.
Experimental Procedures
Mice and Reagents
NOD and NOD/scid mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and maintained in our facility under specific pathogen free conditions. All animal use protocols were approved by the Yale University Institutional Animal Care and Use Committee.
Infection with LCMV
NOD mice 8 weeks of age were infected with 2–3 × 105 pfu of V-Armstrong (intraperitoneal), 2–3 × 106 pfu of LCMV clone 13 (intravenous), and 23,104 cfu of recombinant Listeria monocytogenes (intravenous) expressing the Gp33 and Gp276 epitopes (rLM33, a gift from Dr. H. Shen) (17).
Identification of Antigen-specific T Cells
H-2-Kd/peptide monomers were prepared by FPLC as previously described, and heavy and light chain proteins were refolded in a solution containing a high concentration of NRP-V7 (KYNKANVFL) mimotope, negative control peptide TUM (KYQAVTTTL), or in mice infected with LCMV, Gp33, and Gp276 peptides (Genscript, Piscataway, NJ) as described previously (3, 4, 18). Single cell suspensions were prepared from the pancreatic, mesenteric, axillary/brachial, and inguinal lymph nodes, spleen, liver, and pancreas. Islets of Langerhans were hand-picked from 12-week-old NOD mice, and single cell suspensions were analyzed after dissociation with EDTA.
Flow Analysis
Flow cytometry acquisition was performed on a FACS LSRII cytometer and analyzed with FlowJo v7 software (Asland OR); non-viable cells were excluded. The cells were stained with antibodies to CD8α, CD44, CD127, PD-1, CD62L, CXCR3 and after fixation and permeabilization with Cytofix/Cytoperm (BD Biosciences) antibodies to T-bet, Eomes, and KLRG1.
Gene Expression by Microarray
Single cell suspensions of splenocytes were prepared from pools of 4–6, 10–18-week-old NOD mice or NOD mice 9 days after infection with LCMV virus. The cells were labeled with live/dead fixable stain, tetramer, and antibodies to CD44, CD62L, PD-1, and CXCR3. These antibodies also used sort tetramer+ CD8+ T cells and tetramer− CD8+ T cells of the same phenotype (phenotype+). Cell pellets were prepared in buffer RLT (Qiagen, Valencia, CA) and stored at −80 °C. They were pooled, and RNA was isolated using RNeasy kit. cRNA was prepared and analyzed using Gene Chip Murine Genome U74Av2 Array (Affymetrix, Santa Clara, CA).
Gene Array Analysis
Raw microarray expression data were transformed (variance-stabilizing transformation) and normalized (quantile method) using the Lumi package from R and Bioconductor (19, 20) (The R Project for Statistical Computing). After quality control there were 11 samples including replicates; 5 for the NRP-V7-reactive cells, 3 for the phenotype+, and 3 for LCMV-reactive cells. The expression data were further filtered by removing transcripts with a detection p value cutoff >0.01. Genes with multiple probe sets were collapsed by choosing the probe set with the highest average expression across samples. Differentially expressed genes were identified by an absolute-fold change (>2) and by a statistically significant change in expression (<0.05), as determined by LIMMA using a Benjamini-Hochberg false discovery rate (FDR) (19). Biological and technical replicates were accounted for using the correlation parameter in LIMMA.
Gene Set Enrichment Analysis
Gene set enrichment analysis (GSEA) (21) obtained from the Broad Institute website was used to identify active pathways. Gene sets were first filtered based on size (min = 15, max = 500), leaving 781 pathway gene sets used in the analysis. Probes were collapsed to genes based on the gene symbol, choosing the probe with the maximum average value if multiple probes existed, resulting in 13,340 total genes. Gene set enrichment analysis was run on the gene list ranked by the signal-to-noise metric comparing NRP-V7-reactive and phenotype+ cells. The normalized enrichment score was calculated for each gene set, and statistical significance was determined using 5000 permutations of the gene set phenotype labels. Both the p value and FDR were calculated for each set. Gene sets with FDR < 0.05 were considered differentially expressed.
Cell Culture Studies
Splenocytes harvested from 11 weeks or older NOD mice were labeled with cell tracer violet then stimulated with 0.01 μg/ml anti-CD3 in culture in media supplemented with glucose and 50 units/ml IL-2 but in the presence of 4.1 nm, 1.37 nm, or 0.15 nm oligomycin. After 24 h, cells were washed to remove anti-CD3 then re-cultured overnight in glucose and IL-2 supplemented culture medium at the concentrations discussed above then harvested and analyzed for cellular proliferation by flow cytometry. Uptake of the glucose analog, 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG) (Sigma) was measured by flow cytometry in splenocytes from NOD mice that were cultured in 20 mm glucose for 48 h.
Treatment with 2-Deoxyglucose (2DG)
To determine the role of metabolic pathways, 11–13-week-old NOD mice were treated with 2DG (500 mg/kg, intraperitoneally) for 5 days, and the frequency of tetramer+ and phenotype+ T cells in the spleen and lymph nodes was analyzed by flow cytometry. The relative proportion of tetramer+ versus phenotype+ CD8+ T cells was compared in the treated and untreated mice.
Analysis of Islet Cells
The granularity of β cells and islet-infiltrating T cells was analyzed by flow cytometry. Islets from prediabetic NOD mice were hand-picked after collagenase digestion of the pancreas and dispersed into single cells using described methods (22). Before flow analysis the cells were stained with Fluoro-Zn and TMRE. Electronic gates were first placed around endocrine cells based on forward and side scatter Zn+TMRE+ cells. The granularity of the β cells were distinguished using side scatter (23) Infiltrating lymphocytes could be identified by forward and side scatter characteristics and comparison to islet cells from NOD/scid mice.
Statistical Analysis
Unless indicated data are presented as the mean ± S.E. For comparisons of two groups, Student's t tests or Mann Whitney tests were performed as indicated using GraphPad Prism (version 6.03). We analyzed changes over time with a two-way ANOVA (GraphPad, v6). Comparisons of secondary lymphoid structures and changes over time were done with a mixed linear model using SAS (v9.3, Cary, NC). p values < 0.05 were considered statistically significant. Designations throughout the manuscript are: *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Results
Appearance of Diabetogenic CD8+ T Cells during Progression of Diabetes
We measured the frequency of the IGRP-reactive, NRP-V7-reactive T cells in the secondary lymphoid structures (spleen, pancreatic, mesenteric, axillary, and brachial lymph nodes) in NOD mice at different ages by tetramer staining. The cells were first detected in some mice in the pancreatic lymph nodes at 6 weeks of age but consistently in the pancreatic lymph nodes at 10 weeks of age. The frequency of the cells increased with time (Fig. 1, p = 0.0017). They were most abundant in the pancreatic LN (0.063 ± 0.09%) > mesenteric LN (0.06 ± 0.09%) > spleen (0.06 ± 0.09) than peripheral lymph nodes (axillary and inguinal: 0.03 ± 0.06%). At 14 weeks of age, there was a significantly greater proportion of NRP-V7-reactive cells in the pancreatic lymph nodes compared with other secondary lymphoid structures (mesenteric (p = 0.0008), axillary and inguinal (p < 0.0001)), but not compared with the spleen (p = 0.45). These findings suggest that diabetogenic CD8 T cells are present before 10 weeks of age, but there is a dramatic increase in the frequency of the cells that closely aligns with the beginning of impaired glucose tolerance (24).
FIGURE 1.

Appearance of NRP-V7-reactive T cells in prediabetic NOD mice. NRP-V7-reactive T cells were identified with Class I MHC tetramer and measured by flow cytometry. There was an increase in the frequency of the cells with age (p < 0.0001), which varied by site (p = 0.009) (ANOVA, n = 3–5/group at each time point). Compared with the first measurement at 6 weeks, there was an increase in the frequency of tetramer+ cells in the mesenteric (*, p = 0.03), pancreatic (***, p < 0.001) lymph nodes, and spleen (***, p < 0.001) over 14 weeks.
Phenotype of NRP-V7-reactive CD8 T Cells
We determined the phenotype of the NRP-V7-reactive cells from NOD mice at the different anatomic sites over time (Fig. 2). The T cells isolated from the pancreatic and mesenteric lymph nodes and spleens at 10 and 12 weeks of age had similar phenotypes and were CD44+, CXCR3+, and PD-1+ CD62lo (Fig. 2a). Approximately half of the cells were KLRG1+. Similar to the findings of Trudeau et al. (25), the Ins1 reactive CD8+ T cells were present at a considerably lower frequency than the NRP-V7-reactive cells at all locations and time points. We were able to detect them in the spleen of NOD/scid mice that received splenocytes from diabetic NOD mice and the InsL9-reactive cells had a similar phenotype to the NPR-V7 reactive cells (Fig. 2b). Compared with the tetramer− CD8+ T cells in the spleen, pancreatic lymph nodes, and in the islets, the NRP-V7-reactive cells showed higher levels of expression of CD44, PD-1, and CXCR3 (all p < 0.0001) (Fig. 2c) (it was not possible to identify tetramer+ cells within the islets most likely because of modulation of the T cell receptor (TCR)). The phenotype of the tetramer+ cells was similar in the spleen, pancreatic, and mesenteric lymph nodes. The tetramer+ cells also expressed T-bet and Eomes (Fig. 2d) and produced IFNγ, CD107a, and TNF when activated with PMA and calcium ionophores (not shown). There were significant changes in cell surface markers on the NRP-V7-reactive T cells from the mesenteric and pancreatic lymph nodes over time (Fig. 2e). CD44 increased from 10 weeks until the onset of diabetes (p < 0.01), and CD69 also increased after diabetes onset (p < 0.01), suggesting cell activation. CXCR3 was at the highest levels just before diabetes onset but declined afterward. Other markers such as CD27, KLRG1, CD62L, PD-1, and CD127 did not show significant changes.
FIGURE 2.
Phenotype of tetramer+ T cells. Lymphocytes from spleen, mesenteric, and pancreatic lymph nodes from 10-week old NOD mice were stained with tetramer and antibodies to CD44, CD27, CD69, KLRG1, CXCR3, CD62L, PD-1, and CD127. a, the expression of these markers on tetramer+ (NRP-V7-reactive) and tetramer− CD8+ T cells are shown. b, the phenotype of InsL9-reactive and NRP-V7-reactive cells in the spleen after transfer of splenocytes into NOD/scid mice are compared. Data in a and b are with splenocytes from a single mouse representative of 6 mice. c, the phenotype of tetramer+ (Tet+) and tetramer− (Tet−) splenocytes and pancreatic LN (Panc LN) CD8+ T cells and intraislet CD8+ T cells from the same mice are shown. Because the TCR and CD8 were modulated on the intraislet lymphocytes, intracellular staining was done with the intraislet lymphocytes. The frequency of the NRP-V7-tetramer+ cells in the spleen and pancreatic LN was 0.19 ± 0.04% and 0.122 ± 0.04%. respectively. The relative expression of CXCR3, PD-1, and CD44 was highest on the tetramer+ cells in the spleen and pancreatic lymph nodes (****, p < 0.0001, mean ± S.E. from 3 mice are shown). d, intracellular staining of T-bet and EOMES of tetramer+ and tetramer− cells are shown. e, the phenotype of mesenteric lymph node CD8+ T cells was compared in prediabetic NOD mice of 10 and 12 weeks of age and diabetic NOD mice. The data are the mean ± S.E. from three mice (comparison by two-way ANOVA. *, p < 0.05; **, p < 0.01). f, comparison of the phenotype of NRP-V7 reactive and gp33 reactive CD8+ T cells from 10-week-old NOD mice and NOD mice 10 days after infection with LCMV. (**, p < 0.01; ****, p < 0.0001 by two way ANOVA).
Compared with immune responses to conventional (e.g. viral) antigens, the immune response leading to T1D is more prolonged and less robust. To understand the differences in the viral antigen and diabetes (auto)antigen-specific responses we also compared the NRP-V7-reactive cells to LCMV-reactive cells 9 days after infection of NOD mice with LCMV. We identified viral antigen-reactive CD8+ T cells with Class I MHC tetramers with the gp33 peptide 9 days after infection with the virus. The LCMV-reactive cells showed similar expression of CD44, CD27, CD62L, PD-1, and CXCR3 compared with the NRP-V7 reactive cells but expressed higher levels of CD25, CD69, and KLRG1 (p < 0.01, p < 0.0001, and p < 0.0001, respectively), consistent with the acute changes in effector T cells to viral infection (Fig. 2f).
Gene Expression in NRP-V7-reactive T Cells
To distinguish the features of the autoreactive, viral antigen-reactive CD8+ T cells from other cells in NOD mice that share the phenotype of these cells, we sorted CD8+ T cells that showed high expression of CD44, PD-1, and CXCR3 and low expression of CD62L and compared gene expression by microarray to the gp33 and NRP-V7 tetramer+ CD8+ T cells, sorted from the spleens mesenteric and pancreatic lymph nodes (data may be accessed at www.ncbi.nlm.nih.gov).
Fig. 3a displays the number of genes with significant differences between the NRP-V7-reactive cells and the polyclonal phenotype+ or LCMV-reactive cells (FDR < 0.05 and -fold change >1.8), and genes that show the same directional changes in comparison to both LCMV and phenotype+ cells. As expected from our phenotype analysis, gene expression in NRP-V7-reactive cells clustered more closely with phenotype+ than gp-33 reactive cells (Fig. 3b). We focused on expression of the 54 common genes that showed significant differences between the NRP-V7-reactive and phenotype+ cells to identify features associated with the pathogenicity of the NRP-V7-reactive cells and differentiated them from these other populations. There were several genes associated with differentiation pathways that differed between the NRP-V7-reactive and phenotype+ population. Among these, neuropilin 1 (NRP1), a marker expressed on tolerant CD8+ T cells and Tregs, was reduced as well as ICOS, which is associated with resolution of CD8-mediated lung injury in a transplant model (26–28). Consistent with our findings by flow cytometry (the mRNA expression levels of other transcription factors), Tbet was similar between NRP-V7-reactive, LCMV-reactive, and phenotype+ T cells, but Eomes was lower on the LCMV-reactive cells (not shown).
FIGURE 3.
Differential gene expression in sorted NRP-V7-reactive, phenotype+, and LCMV-reactive CD8+ T cells from NOD mice. a, the Venn diagram shows genes with significant differences between NRP-V7-reactive versus Phenotype+, and NRP-V7-reactive versus LCMV-reactive T cells (-fold change >1.8 and FDR < 0.05). There were 49 genes that showed common changes (i.e. both increase or both decrease) in the two comparisons. b, heat map of genes that show significant differences between NRP-V7-reactive and LCMV-reactive and phenotype+ cells. The NRP-V7-reactive CD8+ T cells cluster more closely with the phenotype+ than LCMV-reactive CD8+ T cells. c, 1 and 2, gene expression in five pools of NRP-V7+ T cells and three pools of phenotype+, or LCMV-reactive T cells were compared with gene signatures from memory precursor effector and short-lived effector CD8+ T cells (FDR < 0.05, -fold change >1.5). The ranked gene lists were compared with the ranked gene list of memory cells (MPECs) or SLECs. The NRP-V7 tetramer+ cells showed a significant enrichment of MPEC signature (p = 2.9 × 10−10 and p = 2.4 × 10–7, respectively), but the phenotype+ and LCMV-reactive T cells showed enrichment for SLEC (p = 2.9 × 10−10 and p = 1 × 10−14, respectively). The top five genes from both cell types are listed in red and green.
To identify the functional phenotype of these cells, we compared the enrichment of the most differentially expressed (FDR < 0.05) NRP-V7 and phenotype+ signature genes to the ranked gene list of memory cells (MPECs) or short-lived effector cells (SLECs) (29). The NRP-V7 tetramer+ cells showed a significant enrichment of MPEC signature (p = 1.6 × 10−7) when compared with phenotype+ cells, whereas the phenotype+ cells showed a significant enrichment of SLEC signature (p = 2.9 × 10−10) (Fig. 3c). Similarly, when we compared the enrichment of the most differentially expressed NRP-V7 and LCMV signature genes, NRP-V7- and LCMV-reactive cells showed significant enrichment of MPEC (p = 2.4 × 10−7) and SLEC (p = 1 × 10-14) signatures, respectively (Fig. 3c).
Differences in the Expression of Genes from Proliferative and Metabolic Pathways in NRP-V7-reactive Cells and Other CD8+ T Cells That Share Their Phenotype
Using Gene set enrichment analysis, we identified 106 pathways that were significantly altered in the NRP-V7-reactive versus phenotype+ CD8 T cells (FDR < 0.05) (Fig. 4) and then examined these pathways in the LCMV-reactive cells. There were three pathways enhanced in the NRP-V7 versus phenotype+ cells. Cellular proliferation and DNA replication pathways were increased in the phenotype+ cells and gp-33 reactive cells versus NRP-V7-reactive cells (Table 1). Ex vivo, we confirmed these findings and found greater proliferation of the phenotype+ versus NRP-V7-reactive T cells from mice at 10 weeks of age as identified by a lower percentage of Ki67+ tetramer+ versus phenotype+ T cells from the same mice (Fig. 5) (p < 0.01).
FIGURE 4.
Differentially expressed pathways in NRP-V7 versus phenotype+ T cells. All pathways show a significant difference (FDR < 0.05). The relative pathway activity is shown.
TABLE 1.
Pathway analysis of NRP-V7-reactive CD8+ T cells
The enrichment and p value for the indicated pathways are shown.
| Pathway | NRP-V7 vs. phenotype positive |
NRP-V7 vs. LCMV |
||
|---|---|---|---|---|
| Enrichment value | p value | Enrichment value | p value | |
| Cell proliferation pathways | ||||
| REACTOME_DNA_REPLICATION | −5.11 | <0.0001 | −5.89 | <0.0001 |
| REACTOME_MITOTIC_M_M_G1_PHASES | −4.84 | <0.0001 | −5.40 | <0.0001 |
| REACTOME_SYNTHESIS_OF_DNA | −3.56 | <0.0001 | −4.92 | <0.0001 |
| REACTOME_CELL_CYCLE_CHECKPOINTS | −3.41 | <0.0001 | −4.40 | <0.0001 |
| REACTOME_CELL_CYCLE_MITOTIC | −4.57 | <0.0001 | −5.17 | <0.0001 |
| REACTOME_G1_S_TRANSITION | −2.96 | <0.0001 | −3.34 | <0.0001 |
| KEGG_CELL_CYCLE | −2.61 | <0.0001 | −3.19 | <0.0001 |
| Metabolic pathways | ||||
| KEGG_OXIDATIVE_PHOSPHORYLATION | −2.89 | =0.003 | −3.29 | <0.0001 |
| REACTOME_TCA_CYCLE_AND_RESPIRATION | −3.31 | <0.0001 | −3.87 | <0.0001 |
| KEGG_GLYCOLYSIS_GLUCONEOGENESIS | −2.06 | =0.022 | −2.36 | =0.048 |
| KEGG_AMINO_SUGAR_AND_NUCLEOTIDE_ | −2.44 | =0.003 | −2.96 | =0.005 |
| REACTOME_METABOLISM_OF_AMINO_ACID | −2.48 | =0.003 | −2.97 | <0.0001 |
| REACTOME_METABOLISM_OF_CARBOHYDRATE | −1.91 | =0.042 | −2.11 | <0.0001 |
| REACTOME_GLUCOSE_METABOLISM | −1.95 | =0.036 | −2.17 | =0.002 |
FIGURE 5.

NRP-V7-reactive CD8+ T cells have lower rates of proliferation compared with phenotype+ CD8+ T cells. Cell proliferation was measured by flow cytometry analysis ex vivo by Ki67 incorporation in NPR-V7+ or phenotype+ splenocytes from 11- and 12-week-old NOD mice. Ki67+ T cells was lower in the NRP-V7-reactive versus phenotype+ cells. Each symbol represents cells from an individual mouse (**, p = 0.009, Mann-Whitney).
There was also increased expression of genes associated with oxidative phosphorylation pathways in the phenotype+ cells (Table 1). To confirm the functional differences, we cultured phenotype+ and NRP-V7-reactive T cells with oligomycin, which blocks ATP synthase used in oxidative phosphorylation but not aerobic glycolysis (30). The level of cellular proliferation was greater at the start of the experiments in the phenotype+ cells without oligomycin versus tetramer+ cells (Fig. 6a), as predicted from the gene array analysis and similar to the data in Fig. 5. Oligomycin reduced the frequency of proliferating phenotype+ cells but had little effect on proliferation of the NRP-V7-reactive cells (Fig. 6, a and b, p < 0.05). In addition, we found that the uptake of 2-NBDG was significantly greater in the NRP-V7-reactive versus phenotype+ (p < 0.05) or cells without the same phenotype (Fig. 6c, p < 0.001).
FIGURE 6.
Metabolic responses of NRP-V7-reactive and phenotype+ T cells. a, splenocytes from 11-week-old NOD mice were first cultured overnight with anti-CD3 and IL-2 in media supplemented with 20 mm glucose, washed, and recultured with media supplemented with IL-2 for 48 h. Proliferation was analyzed by flow cytometry. The data shown (mean ± S.E.) are from a single experiment representative of two independent experiments each with 3 mice. b, the change in the proportion of proliferating tetramer+ or phenotype+ cells when 4 ng/ml of oligomycin was added is shown for individual mice (p = 0.027). c, splenocytes from 11-week and older NOD mice were cultured with 2-NBDG for 48 h, and the uptake of the 2-NBDG was analyzed in tetramer+ and phenotype+ cells by flow cytometry(p < 0.001, ANOVA). The data from individual mice are shown (*, p < 0.05; ***, p < 0.001).
Treatment with DG Reduces NRP-V7-reactive T Cells and Improves β Cell Granularity
To determine whether aerobic metabolism is crucial for survival and pathogenicity of NRP-V7-reactive cells, we treated NOD mice with 2DG for 6 days. We compared the frequency of tetramer+ and phenotype+ cells in the spleen and pancreatic lymph nodes from litter mates treated with saline for the same time period by flow cytometry. There was a reduction in the frequency of tetramer+ cells and a decrease in the ratio of tetramer+:phenotype+ T cells in the mice treated with 2DG in the lymph nodes (p = 0.009, Mann-Whitney) (Fig. 7a).
FIGURE 7.
2DG treatment reduces NRP-V7-reactive T cells. 13-Week-old NOD mice were treated with 2DG or left untreated for 6 days. The mice were then sacrificed, and the islets were hand-picked and dispersed into single cells. The frequency of NRP-V7-reactive and phenotype+, but NRP-V7-tetramer− cells in the pancreatic lymph nodes (a, c, and d) and spleen (b), was measured by flow cytometry. There was a decrease in the ratio of NRP-V7-reactive:phenotype+ T cells at both locations (*, p < 0.05 in pancreatic LN, Mann-Whitney). Representative staining of the tetramer+ cells in the pancreatic lymph nodes in mice treated with 2DG (c) or matched control mice (d) are shown.
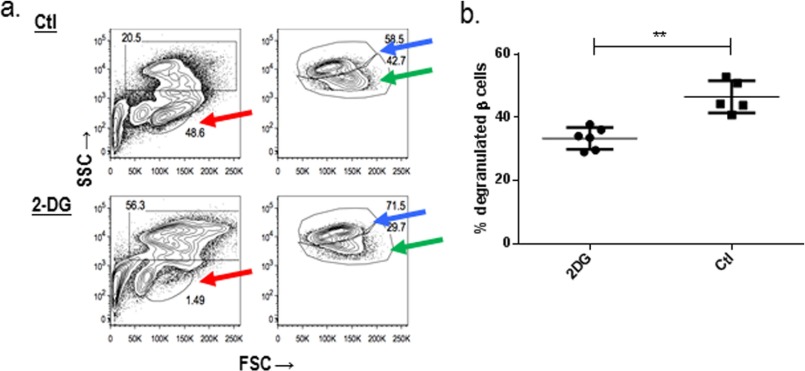
To test whether this intervention affected β cells and islet infiltrates, we studied the effects of the 2DG treatment on the granularity of β cells by flow cytometry and islet-infiltrating lymphocytes. We previously showed that there are degranulated β cells in the islets of NOD mice with new onset disease that can recover with successful immune therapy (24). β cells from dissociated hand-picked islets were identified by staining with FluroZn and TMRE, and the granularity of the cells was assessed by side scatter. Degranulated β cells were found in the islets of 10–12-week-old NOD (Fig. 8a and Ref. 24). Treatment with 2DG reduced the islet infiltrating lymphocytes (red arrows; Fig. 8a) and improved the granularity of the β cells compared with the control mice (Fig. 8, a, blue and green arrows, b, p = 0.004, Mann-Whitney test). The effects of the 2DG were not due to direct effects of the drug on β cells because we did not find changes in β cells in NOD/scidγc−/− mice that do not have immune cells (not shown). These results indicate that the 2DG-reduced cellular infiltrates into the islets and improved β cell function, consistent with its effects on decreasing the survival of diabetogenic T cells.
FIGURE 8.
2DG treatment reduces intraislet lymphocytes and improves β cell granularity. The islets from 13-week old NOD mice were dispersed and stained with FluoroZn to identify insulin granules and TMRE (a). Islet infiltrating lymphocytes are identified by red arrows and were reduced in frequency with 2DG treatment. Normal (blue arrows) and degranulated (green arrows) β cells were detected by side scatter (SSC) (numbers refer to the percent of the total Zn+TMRE+ cells). Results from a single experiment are representative of two experiments with three mice in each group. FSC, forward-scattered light. b, the frequency of degranulated β cells (of the total Zn+TMRE+ cells) is shown. Each symbol represents an individual mouse. **, p = 0.004, Mann-Whitney. The percentage of degranulated β cells was reduced by treatment with 2DG.
Discussion
Studies of the past two decades have identified diabetes antigen-specific T cells that can cause diabetes in NOD mice, and T cells in human studies have similar specificities (3, 6, 12–15). Most investigations of regulation of these cells have focused on immunologic ligands that initiate and regulate their activation, but little is known about the characteristics of the cells or the factors that lead to their pathogenicity. To address this, we studied diabetes antigen-specific CD8+ T cells that have previously been shown to mediate autoimmune diabetes in NOD mice (13). Our studies show that the diabetogenic cells can be identified at 10 weeks in the secondary lymphoid structures; their appearance corresponds to deterioration in glucose tolerance without overt hyperglycemia (24). The expression of activation markers (CD44 and CD69) and Tbet and Eomes is consistent with a memory Tc1 phenotype. CD8 T cells reactive with another diabetes antigen (insulin L9 peptide) showed a similar phenotype to the NRP-V7-reactive cells. The NRP-V7-reactive cells also shared some phenotypic features with LCMV-reactive cells including high expression of PD1, which with FOXO1, is thought to create a positive feedback pathway to desensitize cells to antigen but support their survival (18). A gene array analysis showed lower expression of genes associated with pathways of cellular proliferation and oxidative phosphorylation in the NRP-V7-reactive T cells compared with CD8+ T cells that were not diabetes antigen-specific or LCMV-reactive CD8+ T cells. Moreover, when we blocked glucose aerobic glucose metabolism in vivo with 2DG, there was reduced frequency of the NRP-V7-reactive cells and increased β cell granularity in vivo. They suggest an unrecognized relationship between nutrient availability and selection and expansion of diabetogenic T cells and suggest how elevated glucose may contribute to accelerated β cell killing.
Our analysis of autoantigen-specific CD8+ T cells is similar to previous reports by Trudeau et al. (25) and Chee et al. (31). Those investigators described the phenotype of the NRP-V7-reactive cells as effector memory T cells. In our analysis we have included the phenotype of insulin-reactive cells, which like the findings of Trudeau et al. (25), we find at a much lower frequency than NRP-V7-reactive cells. We have also differentiated the autoantigen-reactive T cells from those reactive with viral antigen (gp33) as well as the phenotype+ CD8+ T cells. The phenotype that we identified has differences from those described for human autoantigen-reactive cells. Skowera et al. (32) found that IGRP-reactive T cells in patients with T1D include naïve and effector cells. There was variability in CD45RO and CCR7 expression among these cells. In view of our recent observations that the time of maximal effector T cell function may be before the onset of hyperglycemia, the phenotype of the diabetes antigen-specific cells may change after diagnosis (33). Although it appears that there are differences in the phenotypes of IGRP-reactive T cells in humans and NOD mice, a prospective study of the phenotype of human diabetogenic T cells has not been reported.
The transcriptional features of murine or human autoantigen-reactive T cells have not previously been described and were not reported by Chee et al. (31). Our analysis showed that gene expression in the diabetogenic CD8+ cells is more consistent with MPEC than the LCMV-reactive or phenotype+ cells that were a mixture of cells but more closely terminally differentiated SLEC. MPEC have a less terminally differentiated phenotype, may self-renew, or may remain resting memory cells for long periods of time, which may account for the durability of the NRP-V7-reactive cells (34–37). Although the factors that lead to the differentiation of the NRP-V7-reactive cells into MPECs are not known, this observation may account for the prolonged kinetics of disease progression in contrast to the rapid occurrence of viral antigen responses in which the antigen specific cells show a SLEC phenotype.
The differences between the populations of CD8+ cells have identified features that may be important in designing therapies. The differential (lower) expression of genes associated with oxidative phosphorylation distinguish the NRP-V7 cells from conventional MPECs. To carry out their function, effector T cells use metabolic pathways that are distinct from memory cells (30, 38–40). Memory T cells generally rely on mitochondrial oxidative phosphorylation involving fatty acid oxidation for their longevity. The processes that cause the switch in cellular metabolism are not clear, but the microenvironment and nutrient availability may be important factors (38). Changes in metabolic substrates (i.e. glucose) in diabetes presents a unique situation as it changes as a result of the effector function. In this way, there may be a reciprocal relationship between effector activity and the consequences of that activity that modulate the immune response, the differentiation of effector cells, and use of metabolic pathways. The utilization and dependence of the diabetogenic T cells on aerobic glycolysis is also consistent with their ability to produce IFNγ and other cytolytic cytokines (38). In keeping with the array data we found an increase in the expression on Myc (1.35-fold, p = 0.06) and a decreased expression of malic enzyme 2 (Me2) (0.75-fold, p = 0.03) compared with phenotype+ cells. The transcription factor Myc has been found to control metabolic reprogramming upon T cell activation (41). The differences that we found in cell cycle gene expression were unexpected as effector T cells utilizing aerobic glycolysis would be expected to show higher rates of proliferation (38–40). Rather than an exclusive dependence on a single metabolic pathway, it is most likely that the NRP-V7-reactive and phenotype+ cells use both oxidative phosphorylation and aerobic glycolysis but to varying degrees and perhaps with differences between individual mice. Consistent with this notion is the variability in the time to diabetes within these inbred mice housed under identical conditions. Other metabolic pathways may also be utilized (38).
A curious finding from our studies is the dramatic and continued increase in the frequency of the NRP-V7-specific T cells beginning at 10 weeks with only modest changes after diagnosis of diabetes and elimination of β cell antigens. This time period corresponds to early deterioration of glucose tolerance associated with reduction in β cell mass, which leads to increased availability of glucose (34). Certainly, antigen is required for activation of the T cells initially, but glucose may foster maintenance and expansion in a “feed forward” mechanism. Our results with 2DG treatment and the differential effects of oligomycin, which inhibits oxidative phosphorylation, are consistent with this interpretation. They suggest that inhibiting glucose utilization and aerobic glycolysis can preferentially affect the diabetogenic T cells, which we showed in vivo. There are little data on metabolic pathways used by autoreactive T cells, but other observations support an important role for metabolic factors in promoting diabetogenesis (42). Delmastro-Greenwood et al. (43, 44) postulated that reduced aerobic glycolysis may contribute to immune cell quiescence and reduced diabetogenic potential in NOD mice.
In summary, we have shown differences in gene expression of diabetogenic T cells compared with other cells that share their phenotype or are viral antigen-reactive. Our studies suggest mechanisms that may be used to prevent the expansion or even eliminate these pathologic cells.
Author Contributions
J. W. G. designed and performed the studies and wrote the manuscript. A. S., J. S., S. D., M. M. S., and J. R. performed the studies and wrote the manuscript. K. C. H., S. M. K., M. U., and S. H. K. analyzed data and wrote the manuscript.
Acknowledgment
We thank Lesley Devine for assistance with preparation of tetramers and flow analysis.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 DK057846, U01 AI02011, and UC4 DK104205. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- T1D
- type 1 diabetes
- LCMV
- lymphocytic choriomeningitis virus
- 2DG
- 2-deoxyglucose
- FDR
- false discovery rate
- ANOVA
- analysis of variance
- LN
- lymph node
- SLEC
- short-lived effector cell
- TMRE
- tetramethylrhodamine ethyl ester
- MPEC
- memory precursor effector cells
- 2-NBDG
- 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose
- IGRP
- islet-specific glucose-6-phosphatase catalytic subunit-related protein.
References
- 1. Herold K. C., Vignali D. A., Cooke A., and Bluestone J. A. (2013) Type 1 diabetes: translating mechanistic observations into effective clinical outcomes. Nat. Rev. Immunol. 13, 243–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Han B., Serra P., Yamanouchi J., Amrani A., Elliott J. F., Dickie P., Dilorenzo T. P., and Santamaria P. (2005) Developmental control of CD8 T cell-avidity maturation in autoimmune diabetes. J. Clin. Invest. 115, 1879–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lieberman S. M., Evans A. M., Han B., Takaki T., Vinnitskaya Y., Caldwell J. A., Serreze D. V., Shabanowitz J., Hunt D. F., Nathenson S. G., Santamaria P., and DiLorenzo T. P. (2003) Identification of the beta cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc. Natl. Acad. Sci. U.S.A. 100, 8384–8388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lieberman S. M., Takaki T., Han B., Santamaria P., Serreze D. V., and DiLorenzo T. P. (2004) Individual nonobese diabetic mice exhibit unique patterns of CD8+ T cell reactivity to three islet antigens, including the newly identified widely expressed dystrophia myotonica kinase. J. Immunol. 173, 6727–6734 [DOI] [PubMed] [Google Scholar]
- 5. Santamaria P. (2003) Kinetic evolution of a diabetogenic CD8+ T cell response. Ann. N.Y. Acad. Sci. 1005, 88–97 [DOI] [PubMed] [Google Scholar]
- 6. Coppieters K. T., Dotta F., Amirian N., Campbell P. D., Kay T. W., Atkinson M. A., Roep B. O., and von Herrath M. G. (2012) Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J. Exp. Med. 209, 51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mallone R., Martinuzzi E., Blancou P., Novelli G., Afonso G., Dolz M., Bruno G., Chaillous L., Chatenoud L., Bach J. M., and van Endert P. (2007) CD8+ T-cell responses identify beta-cell autoimmunity in human type 1 diabetes. Diabetes 56, 613–621 [DOI] [PubMed] [Google Scholar]
- 8. Martinuzzi E., Novelli G., Scotto M., Blancou P., Bach J. M., Chaillous L., Bruno G., Chatenoud L., van Endert P., and Mallone R. (2008) The frequency and immunodominance of islet-specific CD8+ T-cell responses change after type 1 diabetes diagnosis and treatment. Diabetes 57, 1312–1320 [DOI] [PubMed] [Google Scholar]
- 9. Serreze D. V., Marron M. P., and Dilorenzo T. P. (2007) “Humanized” HLA Transgenic NOD mice to identify pancreatic beta cell autoantigens of potential clinical relevance to type 1 diabetes. Ann. N.Y. Acad. Sci. 1103, 103–111 [DOI] [PubMed] [Google Scholar]
- 10. Standifer N. E., Ouyang Q., Panagiotopoulos C., Verchere C. B., Tan R., Greenbaum C. J., Pihoker C., and Nepom G. T. (2006) Identification of Novel HLA-A*0201-restricted epitopes in recent-onset type 1 diabetic subjects and antibody-positive relatives. Diabetes 55, 3061–3067 [DOI] [PubMed] [Google Scholar]
- 11. Unger W. W., Pearson T., Abreu J. R., Laban S., van der Slik A. R., der Kracht S. M., Kester M. G., Serreze D. V., Shultz L. D., Griffioen M., Drijfhout J. W., Greiner D. L., and Roep B. O. (2012) Islet-specific CTL cloned from a type 1 diabetes patient cause beta-cell destruction after engraftment into HLA-A2 transgenic NOD/scid/IL2RG null mice. PLoS ONE 7, e49213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Amrani A., Verdaguer J., Serra P., Tafuro S., Tan R., and Santamaria P. (2000) Progression of autoimmune diabetes driven by avidity maturation of a T-cell population. Nature 406, 739–742 [DOI] [PubMed] [Google Scholar]
- 13. Krishnamurthy B., Mariana L., Gellert S. A., Colman P. G., Harrison L. C., Lew A. M., Santamaria P., Thomas H. E., and Kay T. W. (2008) Autoimmunity to both proinsulin and IGRP is required for diabetes in nonobese diabetic 8.3 TCR transgenic mice. J. Immunol. 180, 4458–4464 [DOI] [PubMed] [Google Scholar]
- 14. Jarchum I., Nichol L., Trucco M., Santamaria P., and DiLorenzo T. P. (2008) Identification of novel IGRP epitopes targeted in type 1 diabetes patients. Clin Immunol 127, 359–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Samanta D., Mukherjee G., Ramagopal U. A., Chaparro R. J., Nathenson S. G., DiLorenzo T. P., and Almo S. C. (2011) Structural and functional characterization of a single-chain peptide-MHC molecule that modulates both naive and activated CD8+ T cells. Proc. Natl. Acad. Sci. U.S.A. 108, 13682–13687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moore A., Grimm J., Han B., and Santamaria P. (2004) Tracking the recruitment of diabetogenic CD8+ T-cells to the pancreas in real time. Diabetes 53, 1459–1466 [DOI] [PubMed] [Google Scholar]
- 17. Blattman J. N., Grayson J. M., Wherry E. J., Kaech S. M., Smith K. A., and Ahmed R. (2003) Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat. Med. 9, 540–547 [DOI] [PubMed] [Google Scholar]
- 18. Staron M. M., Gray S. M., Marshall H. D., Parish I. A., Chen J. H., Perry C. J., Cui G., Li M. O., and Kaech S. M. (2014) The transcription factor FoxO1 sustains expression of the inhibitory receptor PD-1 and survival of antiviral CD8(+) T cells during chronic infection. Immunity 41, 802–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Du P., Kibbe W. A., and Lin S. M. (2008) lumi: a pipeline for processing Illumina microarray. Bioinformatics 24, 1547–1548 [DOI] [PubMed] [Google Scholar]
- 20. Gentleman R. C., Carey V. J., Bates D. M., Bolstad B., Dettling M., Dudoit S., Ellis B., Gautier L., Ge Y., Gentry J., Hornik K., Hothorn T., Huber W., Iacus S., Irizarry R., Leisch F., Li C., Maechler M., Rossini A. J., Sawitzki G., Smith C., Smyth G., Tierney L., Yang J. Y., and Zhang J. (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5, R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mootha V. K., Lindgren C. M., Eriksson K. F., Subramanian A., Sihag S., Lehar J., Puigserver P., Carlsson E., Ridderstråle M., Laurila E., Houstis N., Daly M. J., Patterson N., Mesirov J. P., Golub T. R., Tamayo P., Spiegelman B., Lander E. S., Hirschhorn J. N., Altshuler D., and Groop L. C. (2003) PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273 [DOI] [PubMed] [Google Scholar]
- 22. Katsuta H., Aguayo-Mazzucato C., Katsuta R., Akashi T., Hollister-Lock J., Sharma A. J., Bonner-Weir S., and Weir G. C. (2012) Subpopulations of GFP-marked mouse pancreatic beta cells differ in size, granularity, and insulin secretion. Endocrinology 153, 5180–5187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Akirav E. M., Lebastchi J., Galvan E. M., Henegariu O., Akirav M., Ablamunits V., Lizardi P. M., and Herold K. C. (2011) Detection of beta cell death in diabetes using differentially methylated circulating DNA. Proc. Natl. Acad. Sci. U.S.A. 108, 19018–19023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sherry N. A., Kushner J. A., Glandt M., Kitamura T., Brillantes A. M., and Herold K. C. (2006) Effects of autoimmunity and immune therapy on beta-cell turnover in type 1 diabetes. Diabetes 55, 3238–3245 [DOI] [PubMed] [Google Scholar]
- 25. Trudeau J. D., Kelly-Smith C., Verchere C. B., Elliott J. F., Dutz J. P., Finegood D. T., Santamaria P., and Tan R. (2003) Prediction of spontaneous autoimmune diabetes in NOD mice by quantification of autoreactive T cells in peripheral blood. J. Clin. Invest. 111, 217–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jackson S. R., Berrien-Elliott M., Yuan J., Hsueh E. C., and Teague R. M. (2014) Neuropilin-1 expression is induced on tolerant self-reactive CD8+ T cells but is dispensable for the tolerant phenotype. PLoS ONE 9, e110707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Campos-Mora M., Morales R. A., Pérez F., Gajardo T., Campos J., Catalan D., Aguillón J. C., and Pino-Lagos K. (2015) Neuropilin-1+ regulatory T cells promote skin allograft survival and modulate effector CD4+ T cells phenotypic signature. Immunol. Cell Biol. 93, 113–119 [DOI] [PubMed] [Google Scholar]
- 28. Wu Q., Gardiner G. J., Berry E., Wagner S. R., Lu T., Clay B. S., Moore T. V., Ferreira C. M., Williams J. W., Luster A. D., Medoff B. D., Cannon J. L., Sperling A. I., and Shilling R. A. (2013) ICOS-expressing lymphocytes promote resolution of CD8-mediated lung injury in a mouse model of lung rejection. PLoS ONE 8, e72955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cui W., Joshi N. S., Liu Y., Meng H., Kleinstein S. H., and Kaech S. M. (2014) TLR4 ligands lipopolysaccharide and monophosphoryl lipid a differentially regulate effector and memory CD8+ T cell differentiation. J. Immunol. 192, 4221–4232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chang C. H., Curtis J. D., Maggi L. B. Jr., Faubert B., Villarino A. V., O'Sullivan D., Huang S. C., van der Windt G. J., Blagih J., Qiu J., Weber J. D., Pearce E. J., Jones R. G., and Pearce E. L. (2013) Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chee J., Ko H. J., Skowera A., Jhala G., Catterall T., Graham K. L., Sutherland R. M., Thomas H. E., Lew A. M., Peakman M., Kay T. W., and Krishnamurthy B. (2014) Effector-memory T cells develop in islets and report islet pathology in type 1 diabetes. J. Immunol. 192, 572–580 [DOI] [PubMed] [Google Scholar]
- 32. Skowera A., Ladell K., McLaren J. E., Dolton G., Matthews K. K., Gostick E., Kronenberg-Versteeg D., Eichmann M., Knight R. R., Heck S., Powrie J., Bingley P. J., Dayan C. M., Miles J. J., Sewell A. K., Price D. A., and Peakman M. (2015) Beta-cell-specific CD8 T cell phenotype in type 1 diabetes reflects chronic autoantigen exposure. Diabetes 64, 916–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Herold K. C., Usmani-Brown S., Ghazi T., Lebastchi J., Beam C. A., Bellin M. D., Ledizet M., Sosenko J. M., Krischer J. P., and Palmer J. P. (2015) Beta cell death and dysfunction during type 1 diabetes development in at-risk individuals. J. Clin. Invest. 125, 1163–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Intlekofer A. M., Takemoto N., Wherry E. J., Longworth S. A., Northrup J. T., Palanivel V. R., Mullen A. C., Gasink C. R., Kaech S. M., Miller J. D., Gapin L., Ryan K., Russ A. P., Lindsten T., Orange J. S., Goldrath A. W., Ahmed R., and Reiner S. L. (2005) Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol 6, 1236–1244 [DOI] [PubMed] [Google Scholar]
- 35. Joshi N. S., and Kaech S. M. (2008) Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J. Immunol. 180, 1309–1315 [DOI] [PubMed] [Google Scholar]
- 36. Kaech S. M., and Cui W. (2012) Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 12, 749–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van der Windt G. J., O'Sullivan D., Everts B., Huang S. C., Buck M. D., Curtis J. D., Chang C. H., Smith A. M., Ai T., Faubert B., Jones R. G., Pearce E. J., and Pearce E. L. (2013) CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proc. Natl. Acad. Sci. U.S.A. 110, 14336–14341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pearce E. L., and Pearce E. J. (2013) Metabolic pathways in immune cell activation and quiescence. Immunity 38, 633–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pearce E. L., Poffenberger M. C., Chang C. H., and Jones R. G. (2013) Fueling immunity: insights into metabolism and lymphocyte function. Science 342, 1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van der Windt G. J., Everts B., Chang C. H., Curtis J. D., Freitas T. C., Amiel E., Pearce E. J., and Pearce E. L. (2012) Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36, 68–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang R., Dillon C. P., Shi L. Z., Milasta S., Carter R., Finkelstein D., McCormick L. L., Fitzgerald P., Chi H., Munger J., and Green D. R. (2011) The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35, 871–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oresic M., Simell S., Sysi-Aho M., Näntö-Salonen K., Seppänen-Laakso T., Parikka V., Katajamaa M., Hekkala A., Mattila I., Keskinen P., Yetukuri L., Reinikainen A., Lähde J., Suortti T., Hakalax J., Simell T., Hyöty H., Veijola R., Ilonen J., Lahesmaa R., Knip M., and Simell O. (2008) Dysregulation of lipid and amino acid metabolism precedes islet autoimmunity in children who later progress to type 1 diabetes. J. Exp. Med. 205, 2975–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Delmastro-Greenwood M. M., Tse H. M., and Piganelli J. D. (2014) Effects of metalloporphyrins on reducing inflammation and autoimmunity. Antioxid. Redox Signal. 20, 2465–2477 [DOI] [PubMed] [Google Scholar]
- 44. Delmastro-Greenwood M. M., Votyakova T., Goetzman E., Marre M. L., Previte D. M., Tovmasyan A., Batinic-Haberle I., Trucco M. M., and Piganelli J. D. (2013) Mn porphyrin regulation of aerobic glycolysis: implications on the activation of diabetogenic immune cells. Antioxid. Redox Signal. 19, 1902–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]