Abstract
Agonist-evoked endocytosis of G protein-coupled receptors has been extensively studied. The mechanisms by which agonists stimulate mobilization and plasma membrane translocation of G protein-coupled receptors from intracellular stores are unexplored. Protease-activated receptor-2 (PAR2) traffics to lysosomes, and sustained protease signaling requires mobilization and plasma membrane trafficking of PAR2 from Golgi stores. We evaluated the contribution of protein kinase D (PKD) and Gβγ to this process. In HEK293 and KNRK cells, the PAR2 agonists trypsin and 2-furoyl-LIGRLO-NH2 activated PKD in the Golgi apparatus, where PKD regulates protein trafficking. PAR2 activation induced translocation of Gβγ, a PKD activator, to the Golgi apparatus, determined by bioluminescence resonance energy transfer between Gγ-Venus and giantin-Rluc8. Inhibitors of PKD (CRT0066101) and Gβγ (gallein) prevented PAR2-stimulated activation of PKD. CRT0066101, PKD1 siRNA, and gallein all inhibited recovery of PAR2-evoked Ca2+ signaling. PAR2 with a photoconvertible Kaede tag was expressed in KNRK cells to examine receptor translocation from the Golgi apparatus to the plasma membrane. Irradiation of the Golgi region (405 nm) induced green-red photo-conversion of PAR2-Kaede. Trypsin depleted PAR2-Kaede from the Golgi apparatus and repleted PAR2-Kaede at the plasma membrane. CRT0066101 inhibited PAR2-Kaede translocation to the plasma membrane. CRT0066101 also inhibited sustained protease signaling to colonocytes and nociceptive neurons that naturally express PAR2 and mediate protease-evoked inflammation and nociception. Our results reveal a major role for PKD and Gβγ in agonist-evoked mobilization of intracellular PAR2 stores that is required for sustained signaling by extracellular proteases.
Keywords: 7-helix receptor, Golgi, protein kinase D (PKD), protein trafficking (Golgi), receptor desensitization
Introduction
The ability of cells to respond to extracellular signals depends, to a large degree, on the levels of receptors at the plasma membrane. The cell-surface expression of receptors is a balance between their removal by endocytosis and the insertion of receptors that may arise from recycling pathways or intracellular stores. In many instances, these trafficking events are regulated by receptor agonists.
For G protein-coupled receptors (GPCRs),4 the largest family of cell-surface signaling proteins, the mechanisms of agonist-evoked endocytosis have been thoroughly studied. Upon activation, many GPCRs become phosphorylated by G protein receptor kinases, which enhance receptor affinity for β-arrestins (1). β-Arrestins uncouple GPCRs from heterotrimeric G proteins and thereby desensitize signaling. β-Arrestins also couple receptors to adaptor protein 2 and clathrin, and thereby mediate dynamin- and clathrin-dependent endocytosis of receptors. These general processes mediate endocytosis of many GPCRs, including the neurokinin 1 receptor (2), μ-opioid receptor (3), and protease-activated receptor-2 (PAR2) (4). Other receptors internalize by clathrin-independent mechanisms, such as those involving caveolin. For example, endothelin promotes dynamin-dependent budding of caveolae enriched in the endothelin B receptor in endothelial cells (5). By depleting receptors from the cell surface, GPCR endocytosis may attenuate cellular responsiveness to extracellular signals. Endocytosis may also contribute to signal transduction, because certain GPCRs can continue to signal from endosomes by β-arrestin- and G protein-mediated mechanisms (6–10).
The mechanisms responsible for the replenishment of the plasma membrane with receptors, which is necessary for sustained signaling by extracellular agonists, are less well understood. For some receptors, for example the substance P neurokinin 1 receptor, the calcitonin gene-related peptide receptor, and the μ-opioid receptor, recycling of internalized receptors is the main pathway for replenishing the plasma membrane. For these receptors, ligand dissociation and degradation in acidified early endosomes, and receptor dissociation from β-arrestins, are key events controlling recycling (11–15). Other receptors traffic to degradation pathways, and here the synthesis of new receptors or the mobilization of intracellular pools of fresh receptors is required for replenishment of the plasma membrane. For example, PAR2 and δ-opioid receptors traffic to lysosomes, and the mobilization of new receptors serves to repopulate the plasma membrane and maintain signaling by extracellular agonists (16, 17). However, the mechanisms that mediate GPCR mobilization from intracellular stores and translocation to the plasma membrane are not fully understood, and how they may be regulated by GPCR agonists requires further study.
Herein, we investigate the mechanisms of mobilization and membrane translocation of PAR2 from the Golgi apparatus. PAR2 is a receptor for diverse serine and cysteine proteases, including trypsin, tryptase, kallikreins, cathepsin S, and elastase (18–25). Proteases such as trypsin and tryptase that cleave PAR2 at Arg36↓Ser37 reveal a tethered ligand domain (37SLIGKV, human PAR2) that binds to and activates the cleaved receptor (18–20). This irreversible mechanism of proteolytic activation dictates that a cleaved receptor cannot be reactivated by a protease. Moreover, trypsin-activated PAR2 undergoes β-arrestin-, clathrin-, and dynamin-dependent endocytosis (4, 7, 16) and ubiquitin-mediated trafficking to lysosomes (26). Because the mobilization of intracellular stores of intact receptors is necessary for sustained signaling by extracellular proteases (16), PAR2 is an ideal candidate for analysis of the mechanisms of GPCR mobilization and plasma membrane translocation.
We examined the role of protein kinase D (PKD) and Gβγ in protease-evoked mobilization of PAR2 from the Golgi apparatus, which is required for recovery of protease signaling (16). The three PKD isoforms (PKD1–3) are serine/threonine kinases belonging to the Ca2+-calmodulin-dependent family of protein kinases. Many receptor tyrosine kinases and GPCRs, including PAR2 (27), can activate PKDs by multiple mechanisms (28). A predominant mechanism involves phospholipase Cβ-mediated hydrolysis of phosphatidylinositol 4,5-bisphosphate to generate diacylglycerol (DAG) at the plasma membrane. DAG recruits PKD to the plasma membrane and activates protein kinase C (PKC), which phosphorylates PKD at Ser738/Ser742 (human PKD1) and thereby enables autophosphorylation at Ser916, leading to PKD activation. DAG can also recruit a pool of PKD to the trans-Golgi network (TGN) (29). Gβγ subunits can translocate from the plasma membrane to the TGN, where both Gβγ and PKC activate PKD (30–34). PKDs regulate diverse cellular processes, including cell proliferation, differentiation, apoptosis, and migration (35). In view of the importance of PKD in fission of transport carriers (36) and protein sorting in the TGN (37), we evaluated whether Gβγ and PKD control TGN mobilization of PAR2 and recovery of cell-surface protease signaling. Our results show that PAR2 activation at the cell surface triggers Gβγ translocation to the Golgi apparatus and subsequent activation of PKD. PKD controls TGN to plasma membrane translocation of PAR2, which is required for sustained signaling by extracellular proteases.
Experimental Procedures
Animals
The Animal Ethics Committee of Monash University and Queen's University approved the procedures using mice. C57BL/6 mice (8–12 weeks, male) were studied. Mice were maintained under temperature-controlled (22 ± 4 °C) and light-controlled (12-h light/dark cycle) conditions with free access to food and water.
Agonists, Inhibitors, and Antibodies
Porcine pancreatic trypsin was from Sigma. 2-Furoyl-LIGRLO-NH2 was from Bachem (Torrance, CA). CRT0066101 was from Tocris Bioscience (Ellisville, MO). Other inhibitors were from Sigma. Rabbit antibodies to phosphorylated PKD (p-PKD) (Ser916, catalog no. 2051) and total PKD (catalog no. 2052) were from Cell Signaling Technologies (Danvers, MA). Rat anti-HA11 was from Roche Applied Science. Sheep anti-TGN-38 was from Novus Biologicals (Littleton, CO). Donkey anti-rabbit IgG conjugated to IRDye800® was from LI-COR Biosciences (Lincoln, NE). Donkey anti-rabbit, anti-rat, and anti-sheep IgG conjugated to Alexa568, Alexa488, or Alexa 647 were from Invitrogen.
Cell Lines
Human embryonic kidney (HEK) 293 cells and rat sarcoma virus transformed kidney epithelial (KNRK) cells were maintained in DMEM with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin (5% CO2, 37 °C). HEK293 and KNRK cells expressing human PAR2 with N-terminal FLAG and C-terminal HA11 epitopes have been described (26). Non-transformed human colonic epithelial NCM460 cells were maintained in Ham's F-12 medium containing FBS (20%), insulin (10 mg/ml), transferrin (5.5 mg/ml), selenium A (6.7 μg/ml), hydrocortisone (0.4 μg/ml), penicillin and streptomycin (100 units/ml), and GlutaMAX (0.58 μg/ml) (5% CO2, 37 °C) (38).
Photoconvertible PAR2-Kaede
cDNA encoding Kaede flanked by 5′ NotI and 3′ XhoI restriction sites was generated using Phusion high fidelity PCR (New England Biolabs, Ipswich, MA) using the primers sense, ata aga atg cgg ccg ctc gtg agt ctg att aaa ccag, and antisense, ccg ctc gag tta ctt gac gtt gtc cggc. Products were digested with NotI and XhoI and ligated into the pcDNA5-FRT (Invitrogen) using the NotI and XhoI sites. cDNA encoding human PAR2 flanked with 5′ BamHI and 3′ NotI has been described (39). PAR2 and pcDNA5-FRT Kaede cDNA were digested with BamHI and NotI, and PAR2 was ligated into pcDNA5-FRT Kaede using the BamHI and NotI sites. KNRK-FLPN cells were maintained in DMEM, 10% FBS, and Zeocin (100 μg/ml). Cells were transfected with 1 μg of pcDNA5-FRT PAR2-Kaede and 5 μg of the Flp-recombinase pOG44 (Invitrogen) with a DNA/polyethyleneimine ratio of 1:6. Cells expressing PAR2-Kaede were selected in DMEM, 10% FBS containing 200 μg/ml HygroGold (Invitrogen). KNRK-PAR2-Kaede cells were sorted for Kaede fluorescence using a Beckman Coulter MoFlo Astrios (Lane Cove, Australia) cell sorter with doublet exclusion gate, and the top 1% of PAR2-Kaede-expressing cells were re-populated and used for experiments.
Bioluminescence Resonance Energy Transfer (BRET) PAR2 Trafficking Assays
To study the trafficking of PAR2 between the plasma membrane and endosomal compartments, BRET was measured between PAR2 and proteins resident to plasma membrane and endosomal microdomains. Proteins resident in the plasma membrane (RIT, KRas, and HRas) or endosomal compartments (Rab5a, Rab7a, and Rab11) tagged with Venus were a gift from Nevin Lambert (Georgia Health Sciences University). PAR2-RLuc8 has been described (39). HEK293 cells were transfected with 1 μg of PAR2-RLuc8 and 4 μg of RIT-, KRas-, HRas-, Rab5a-, Rab7a-, or Rab11-Venus using polyethyleneimine. At 24 h post-transfection, cells were plated in poly-d-lysine-coated 96-well plates and cultured overnight. Cells were allowed to equilibrate in Hanks' balanced salt solution (HBSS) containing 0.05% w/v BSA at 37 °C, in the presence of 10 μg/ml cycloheximide to prevent de novo protein synthesis. BRET was measured using a LumiSTAR Omega Luminometer (BMG LabTech, Offenburg, Germany). Coelenterazine H (5 μm, Promega, Madison, WI) was added 10 min before BRET assays. BRET was measured before and after stimulation with porcine pancreatic trypsin (100 nm, 20 min). To study BRET at later times, trypsin-stimulated cells (100 nm, 20 min) were washed and incubated in trypsin-free medium for 2 h at 37 °C before BRET assays. BRET data were corrected by subtracting the BRET ratio of vehicle-treated cells expressing PAR2-RLuc8 alone.
BRET Gγ Translocation Assays
BRET was used to study Gγ translocation to the Golgi apparatus. HEK293 cells were transfected with PAR2 (0.9 μg), Gαq (1.33 μg), Gβ1, Gβ4, or Gβ5 (1.33 μg), Gγ2-Venus (2 μg), and giantin-RLuc8 (0.5 μg) as described above. BRET was measured before and after stimulation with trypsin (10−13–10−7 m).
PKD Western Blotting
HEK293 cells were seeded into 6-well dishes at a density of 600,000 cells/well and serum-starved overnight. Cells were incubated in HBSS with vehicle (control), trypsin (10 nm), or 2-furoyl-LIGRLO-NH2 (10 μm) for 0–5 min at 37 °C. Cells were lysed in RIPA buffer containing Halt proteases and phosphatase inhibitor mixture (ThermoFisher Scientific, Waltham, MA). Lysates (40 μg of protein) were fractionated by 10% SDS-PAGE, and proteins were transferred to a PVDF membrane. Membranes were incubated with rabbit antibodies against phosphorylated PKD (p-PKD) (Ser916) or total PKD (both 1:1,000, overnight, 4 °C). Membranes were washed and incubated with donkey anti-rabbit IgG conjugated to IRDye800® (1:10,000, 1 h, room temperature). Membranes were washed and imaged on a LI-COR Odyssey® infrared imager. Signals were quantified by densitometry (ImageJ).
Measurement of [Ca2+]i
HEK293, KNRK-PAR2-Kaede, or NCM460 cells were loaded with Fura-2/AM (1 μm, Invitrogen) in assay buffer (150 mm NaCl, 2.6 mm KCl, 0.1 mm CaCl2, 1.18 mm MgCl2, 10 mm d-glucose, 10 mm HEPES, pH 7.4) containing 4 mm probenecid and 0.5% BSA for 1 h at 37 °C (12). Fluorescence was measured at 340 and 380 nm excitation and 530 nm emission using a FlexStation III microplate reader (Molecular Devices, Sunnyvale, CA). To assess desensitization and recovery of Ca2+ signaling, HEK293 or NCM460 cells were incubated with vehicle (control), trypsin (10 nm), or 2-furoyl-LIGRLO-NH2 (10 μm) for 10 min, washed, and allowed to recover for 25, 90, or 120 min at 37 °C. Cells were then re-challenged with the same concentration of the agonists. PAR2 recovery was calculated as a percentage of the response to the vehicle control. To study the capacity of PAR2-Kaede to signal, KNRK-PAR2-Kaede cells were challenged with graded concentrations of trypsin (10−13–10−7 m), and maximal increases in the 340/380 ratio, which is proportional to [Ca2+]i, were measured.
Immunofluorescence
HEK293, HEK-FLAG-PAR2-HA, KNRK-FLAG-PAR2, and NCM460 cells were serum-starved overnight. In some studies, HEK293 cells were plated into 10-cm dishes at a density of 2,000,000 cells/dish and were transiently transfected with 4 μg of TGN38-YFP with a DNA/polyethyleneimine ratio of 1:6. After 48 h, cells were incubated in HBSS with vehicle or trypsin (10 nm) for 0–30 min at 37 °C and were fixed in 4% paraformaldehyde for 20 min at 4 °C. Cells were washed and incubated in blocking buffer (PBS containing 3% normal horse serum, 0.3% saponin) for 1 h at room temperature. Cells were incubated in blocking buffer with the following primary antibodies: rabbit anti-p-PKD (Ser916, 1:100), rat anti-HA11 (1:1,000), and sheep anti-TGN-38 (1:200) overnight at 4 °C. Cells were then washed and incubated with donkey anti-rabbit IgG conjugated to Alexa568, donkey anti-rat IgG conjugated to Alexa488, or donkey anti-sheep IgG conjugated to Alexa647 (1:400–1:1,000, 1–2 h, room temperature). Cells were washed and mounted on glass slides with Vectashield (Vector Laboratories, Burlingame, CA).
Confocal and Super-resolution Microscopy and Image Analysis
Cells were observed using a Leica TCS SP8 laser scanning confocal microscope (North Ryde, New South Wales, Australia) with a Leica HCX PL APO ×63 oil immersion objective (numerical aperture 1.4). For super-resolution microscopy, cells were observed using a Leica DMI6000 ground state depletion microscope with an HCX PL APO ×100 (NA 1.47) objective, a SuMo Stage, an Andor iXon 3 897 camera (Andor, Belfast, UK) and LAS AF software (version 3.2.0.9652, Leica). Images were assembled using Adobe Photoshop, and alterations in brightness and contrast were applied to the entire image and identically for control and treatment groups.
Live Cell Imaging
KNRK-PAR2-Kaede cells were maintained in a microincubator in HBSS, 10 mm HEPES at 37 °C. The perinuclear region of the cell was defined as the region of interest. The Kaede fluorophore was red/green photoconverted using the Leica FRAP wizard with the following settings: pre-photo conversion image at 400 Hz, UV laser at 15% power, and 5 passes at 400 Hz. Images were collected for 2 min; cells were challenged with trypsin (10 nm), and images were collected for a further 15 min. The images for control and experimental groups were collected under identical conditions. The intensity of PAR2-Kaede fluorescence at the plasma membrane and the perinuclear Golgi region was determined using FIJI software (National Institutes of Health). Intensity levels for Kaede green (an indication of the proportion of receptor expressed at the plasma membrane out of total receptor) were measured and expressed as a ratio of the signal at the plasma membrane to total Kaede green at 0 and 15 min. Intensity of Kaede red (an indication of the distribution of PAR2-Kaede that was photo-converted) was measured at both plasma membranes, the perinuclear region of the cell and total signal within the cell at 0 and 15 min. The ratios between different subcellular compartments versus total Kaede red signal were expressed.
Inhibitors
Cells were incubated with vehicle, brefeldin-A (10 μg/ml), CRT0066101 (100 nm), cycloheximide (10 μg/ml), or gallein (10 μm) for 1 h, and the inhibitors were included during incubation with agonists.
siRNA Transfection
OnTarget SMARTpool human PKD1 siRNA (L-005028-00-0005) or control non-targeting siRNA 5′-uuc ucc gaa cgu guc acgu-3′ was from Dharmacon (Lafayette, CO). HEK293 cells were transfected with 60 pmol of siRNA using RNAiMAX according to the manufacturer's instructions (Invitrogen). RNA was extracted using RNeasy (Qiagen, Hilden, Germany). PKD mRNA expression was determined using the TaqMan probes for human PKD1 (Hs00177037) and GAPDH (Hs03929097, Applied Biosystems, Scoresby Victoria, Australia). cDNAs were generated using the High Capacity cDNA reverse transcription kit (Applied Biosystems). The quantitative RT-PCR was analyzed using TaqMan gene expression master mix (Applied Biosystems) on a Bio-Rad CFX96 touch qPCR system. ΔΔCT values were analyzed with the CFX manager software (Bio-Rad).
Patch Clamp Studies of Nociceptors
Dorsal root ganglia (T9–T13) from C57BL/6 mice were dispersed and cultured overnight as described (23). Perforated patch clamp recording were made from small diameter neurons (<30 picofarad capacitance). Changes in excitability were quantified by measuring rheobase and number of action potential discharges at twice rheobase, as described (23). Cells were incubated for 10 min with trypsin (50 nm) or vehicle (control) and were then transferred to F-12 medium. Excitability was measured at 0, 30, or 60 min after washing. After 60 min, some neurons were exposed to a second trypsin challenge (50 nm, 10 min) and washed, and the excitability was immediately assessed. In some experiments, neurons were pre-incubated with CRT0066101 (10 nm) for 30 min before the first trypsin application.
Statistics
Data are presented as mean ± S.E. of triplicate observations from n >3 experiments. Differences were assessed using Student's t test for two comparisons and one- or two-way analyses of variance followed by Bonferroni or Student's-Newman-Keuls test for multiple comparisons. p < 0.05 was considered significant at the 95% confidence level.
Results
Trypsin Evoked PAR2 Trafficking between Plasma Membrane and Endosomal Microdomains
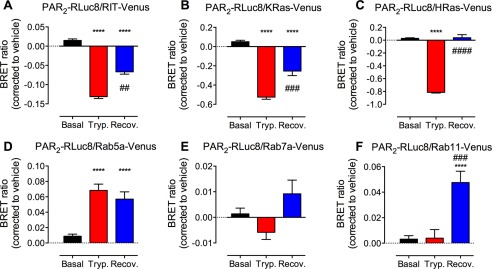
To quantitatively study PAR2 trafficking between microdomains of plasma and endosomal membranes, we used BRET to assess the proximity between PAR2 and proteins normally resident in the plasma membrane (RIT, KRas, and HRas) and in endosomes (Rab5a, Rab7a, and Rab11) of HEK cells. Cycloheximide was used to inhibit de novo PAR2 synthesis. Trypsin (100 nm, 20 min) induced a decrease in BRET between PAR2-RLuc8 and RIT-, KRas-, and HRas-Venus and a corresponding increase in BRET between PAR2-RLuc8 and Rab5a-Venus (Fig. 1, A–E). These results are consistent with PAR2 translocation from the plasma membrane to Rab5a-containing early endosomes. To examine the redistribution of PAR2 after recovery from trypsin stimulation, cells were exposed to trypsin (100 nm, 20 min), washed, and incubated in trypsin-free medium for 2 h. After recovery in trypsin-free medium, there was an increase in BRET between PAR2-RLuc8 and RIT-, KRas-, and HRas-Venus compared with 20 min, which suggests replenishment of the plasma membrane with stores of fresh receptors (Fig. 1, A–C). At 2 h after trypsin stimulation, there was a large increase in BRET between PAR2-RLuc8 and Rab11-Venus (Fig. 1F). Rab11 is localized to the TGN, post-Golgi vesicles, and recycling endosomes (40), and it regulates exocytic and recycling processes to direct proteins toward the cell surface (41). Thus, trypsin evokes PAR2 trafficking from the plasma membrane to early and then late endosomes (and lysosomes). The recovery of PAR2 at the cell surface coincides with translocation to Rab11-containing vesicles, which is consistent with mobilization of TGN stores of PAR2. There were no significant changes in PAR2-RLuc8 and Rab7a-Venus BRET, although it is well established that PAR2 traffics to the lysosomal pathway (16).
FIGURE 1.
Trypsin-induced PAR2 endocytosis and recovery. HEK293 cells expressing PAR2-RLuc8 and RIT (A), KRas (B), HRas (C), Rab5a (D), Rab7a (E), or Rab11 (F) tagged with Venus were stimulated with trypsin (100 nm, 20 min). Cells were washed and recovered in trypsin-free medium for 2 h. BRET was measured after trypsin (Tryp.) and after recovery (Recov.). ****, p < 0.0001 compared with basal; ##, p < 0.01; ###, p < 0.001; ####, p < 0.0001 compare with trypsin, n = 3 experiments.
PAR2 Activates PKD in the Golgi Apparatus
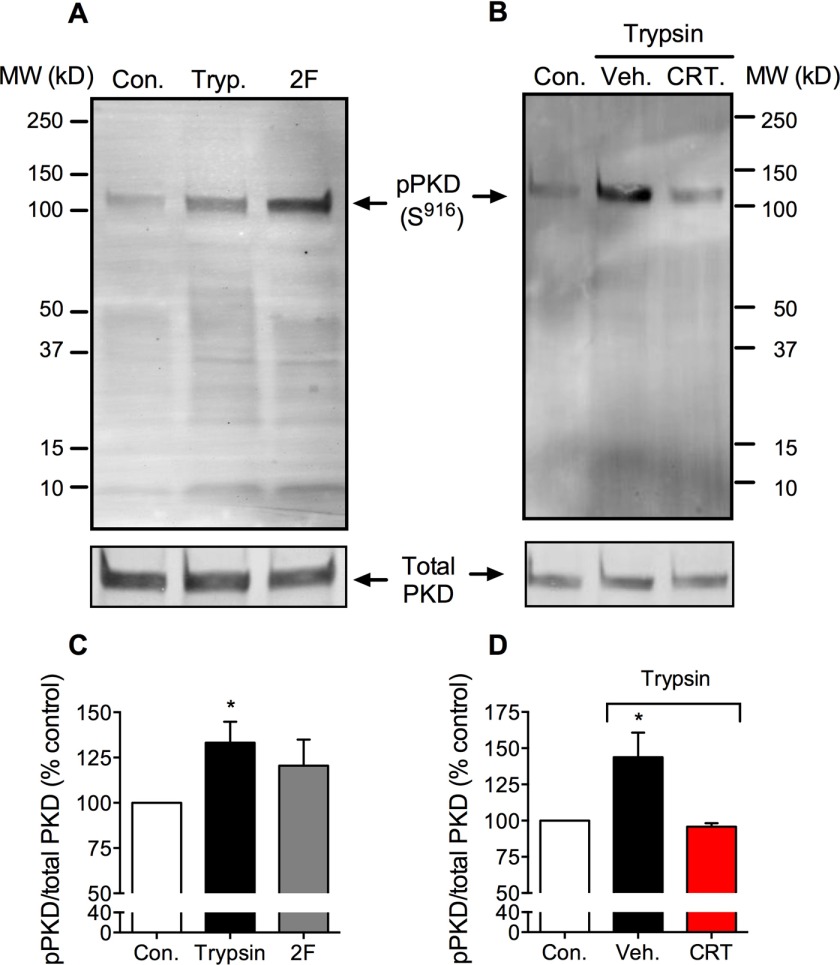
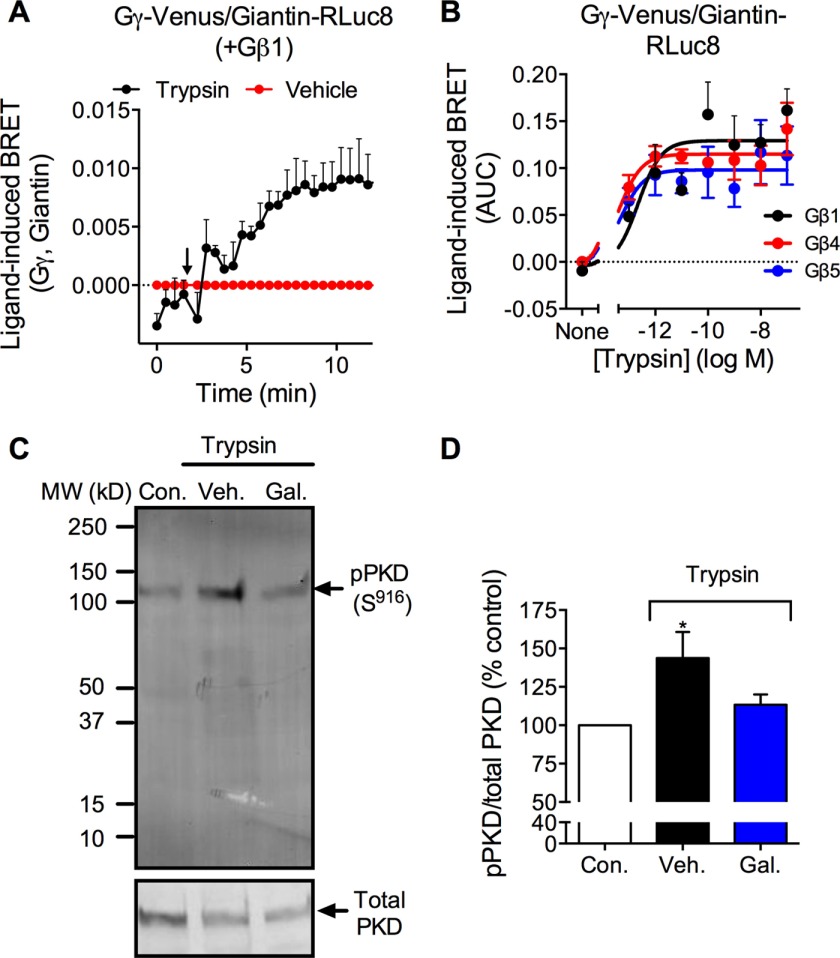
Activation of PAR2 by peptides that mimic the tethered ligand domain leads to rapid PKD phosphorylation and redistribution within the subcellular compartments (27). To investigate whether PAR2-stimulating proteases can also activate PKD, we incubated HEK293 cells, which naturally express PAR2, with trypsin (10 nm) for 5 min, and we assessed PKD auto-phosphorylation (Ser916) by Western blotting. Trypsin increased PKD phosphorylation (Fig. 2, A and C). The PKD inhibitor CRT0066101 (100 nm) abolished trypsin-induced PKD phosphorylation (Fig. 2, B and D). Consistent with our previous report (27), a PAR2-selective analogue of the tethered ligand peptide, 2-furoyl-LIGRLO-NH2 (10 μm, 5 min), also induced PKD phosphorylation to a similar degree as trypsin (Fig. 2, A and C), although this change did not reach significance. Thus, trypsin and a PAR2-selective agonist can activate PKD in HEK293 cells.
FIGURE 2.
PAR2-induced activation of PKD. Western blots for Ser916 phospho-PKD (p-PKD) and total PKD in HEK293 cell are shown. Cells were unstimulated (control, Con.) or were stimulated with trypsin (Tryp., 10 nm) or 2-furoyl-LIGRLO-NH2 (2F, 10 μm) for 5 min. Cells were preincubated with CRT0066101 (CRT) or vehicle (Veh.). A and B, representative blots. C and D, quantified results. *, p < 0.05, n = 3 experiments.
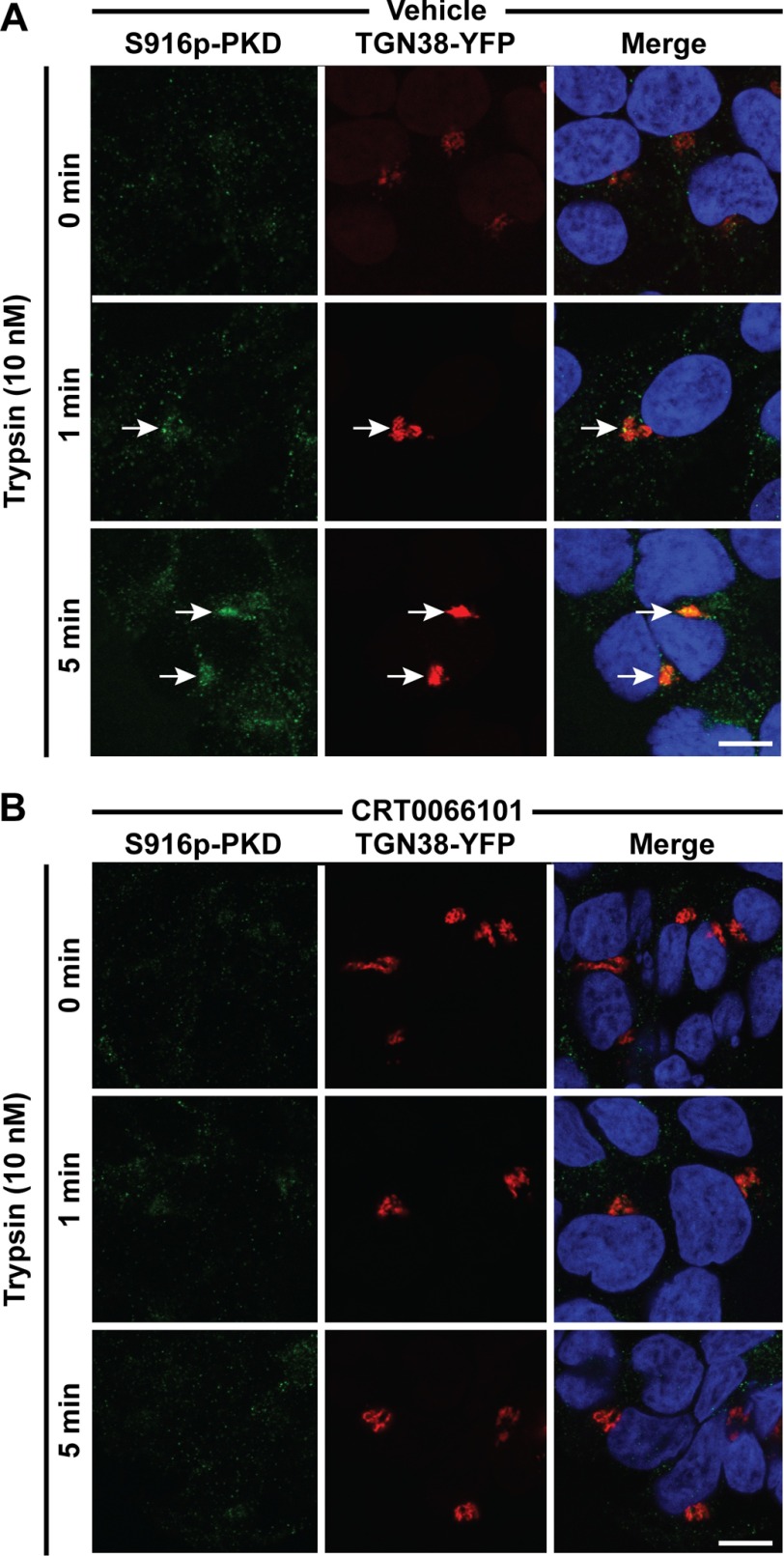
To determine the localization of activated PKD, we incubated HEK293 cells expressing the trans-Golgi marker TGN38-YFP with trypsin and localized phospho-(Ser916) PKD by immunofluorescence and confocal microscopy. In unstimulated cells, phospho-PKD was undetectable. Trypsin (10 nm) induced detectable PKD phosphorylation within 1 min and robust PKD phosphorylation within 5 min (Fig. 3A). Phosphorylated PKD was colocalized with TGN38-YFP (Fig. 3A, arrows). Pretreatment of cells with CRT0066101 prevented trypsin-evoked phosphorylation of PKD in the TGN (Fig. 3B).
FIGURE 3.
Trypsin-induced activation of PKD in the Golgi apparatus. HEK293 cells expressing TGN38-YFP were stimulated with trypsin (10 nm, 0, 1, or 5 min). Ser916 phospho-PKD was detected by immunofluorescence and confocal microscopy. A, vehicle control. B, CRT0066101-treated cells. Scale, 10 μm.
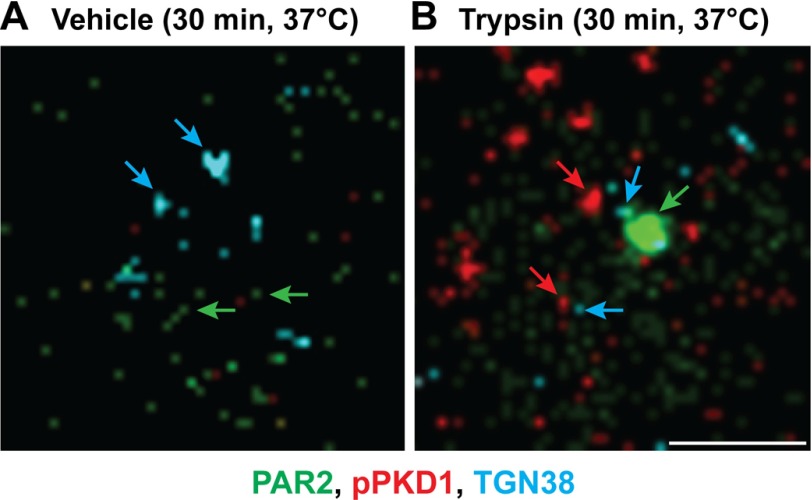
We have previously shown that PAR2 is localized in prominent Golgi stores as well as in the plasma membrane (16). To examine the colocalization of PAR2 and activated PKD within the Golgi apparatus, we used immunofluorescence and super-resolution microscopy. In unstimulated HEK-FLAG-PAR2-HA cells, immunoreactive PAR2 was detected in close proximity to immunoreactive TGN38, and phospho-PKD was undetectable (Fig. 4A). In trypsin (10 nm, 30 min)-treated cells, PAR2, phospho-PKD, and TGN38 were closely associated (Fig. 4B). Our results indicate that proteolytic activation of cell-surface PAR2 results in a rapid activation of PKD within the TGN in close proximity to stores of intact PAR2.
FIGURE 4.
Trypsin-induced localization of phospho-PKD in proximity to PAR2 in the Golgi apparatus. HEK-FLAG-PAR2-HA cells were incubated with vehicle (A) or trypsin (10 nm) (B) for 30 min at 37 °C. Cells were fixed, and Ser916 phospho-PKD (pPKD, red), PAR2 (green) and TGN38 (blue) were localized by immunofluorescence and super-resolution microscopy. Representative images from >3 experiments are shown. Scale, 1 μm.
PKD Mediates Recovery of PAR2 Signaling at the Cell Surface
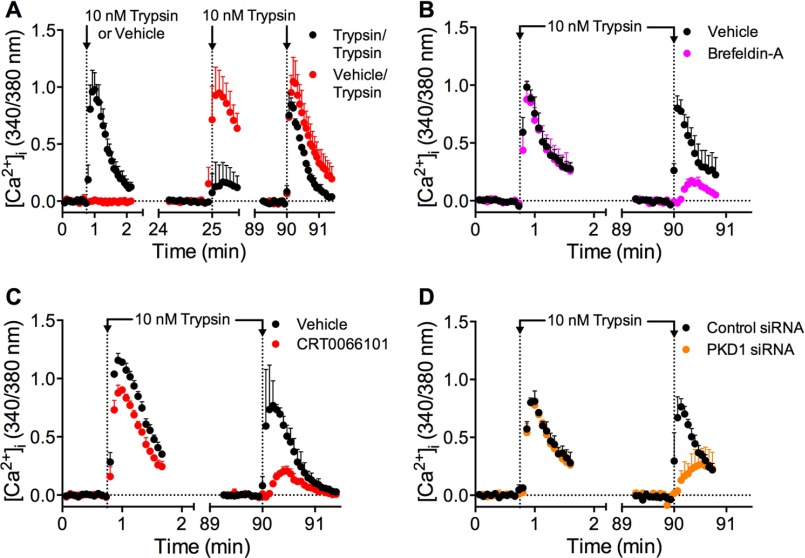
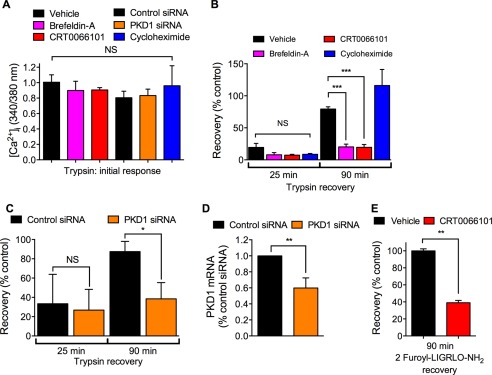
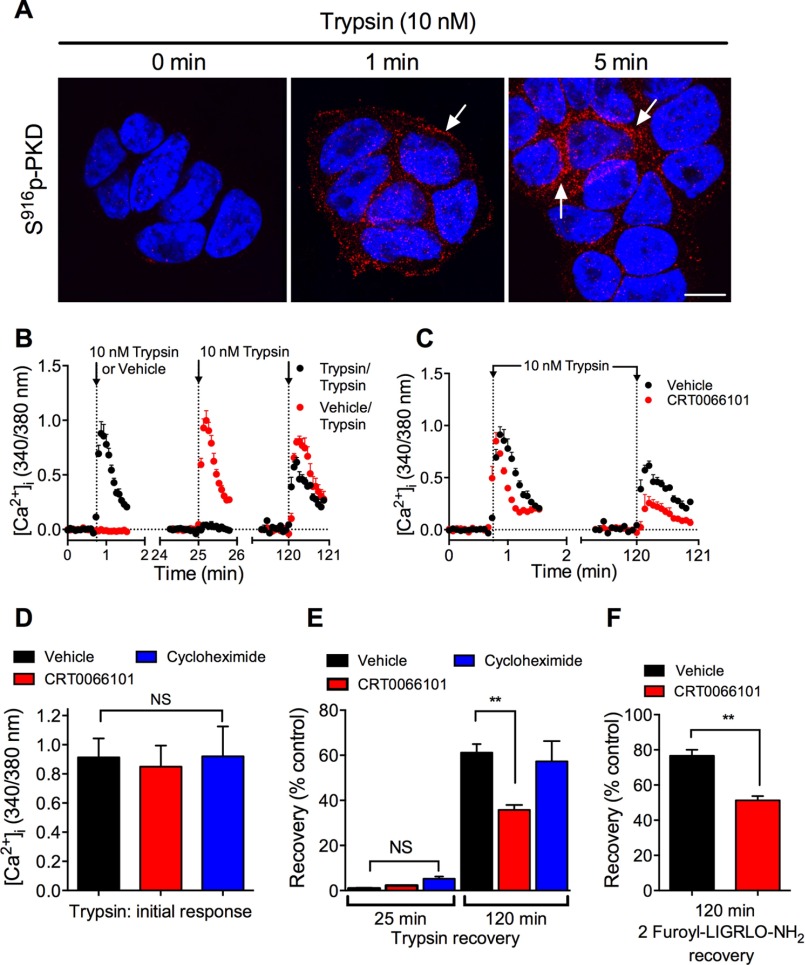
PKD activation in the TGN regulates protein trafficking from Golgi to plasma membranes (36, 37). We have previously shown that mobilization of the Golgi stores of PAR2 is necessary for recovery of protease-evoked signaling (16). To investigate the contribution of PKD to sustained PAR2 signaling, we examined desensitization and recovery of trypsin-evoked Ca2+ signaling in HEK293 cells. Cells were incubated with vehicle (control) or with trypsin (10 nm, 10 min) to desensitize PAR2, washed, and then challenged with trypsin (10 nm) 25 or 90 min later to evaluate recovery of Ca2+ signaling. Compared with vehicle control, trypsin-evoked Ca2+ signaling remained strongly desensitized at 25 min (∼20% recovery) but had mostly recovered after 90 min (∼80% recovery) (Figs. 5A and 6, A and B). Brefeldin A is a lactone antibiotic that disrupts Golgi organization (42). Brefeldin A (10 μg/ml) did not affect the initial Ca2+ response to trypsin or the extent of desensitization at 25 min, but it strongly inhibited recovery at 90 min (Figs. 5B and 6, A and B; ∼20% recovery). These data suggest that an intact Golgi apparatus is necessary for PAR2 recovery, which requires re-population of the plasma membrane with intact receptors. The PKD inhibitor CRT0066101 did not affect the initial response to trypsin or desensitization, but it inhibited recovery at 90 min to a similar degree as brefeldin A (Figs. 5B and 6, A and B; ∼20% recovery). To confirm the involvement of PKD in recovery, we treated cells with PKD1 siRNA. PKD1 siRNA also did not affect the initial response to trypsin or desensitization, but it inhibited recovery at 90 min (Figs. 5D and 6, A and C; ∼40% recovery). This degree of inhibition was achieved by <50% reduction in PKD1 mRNA expression, assessed by quantitative RT-PCR (Fig. 6D). Incubation of cells with the PAR2-selective agonist 2-furoyl-LIGRLO-NH2 (10 μm, 10 min) desensitized Ca2+ signaling at 25 min, which re-sensitized after 90 min. CRT0066101 did not affect the initial response to 2-furoyl-LIGRLO-NH2 but inhibited recovery of Ca2+ signaling at 90 min (Fig. 6E). Cycloheximide (10 μg/ml) had no effect on initial or recovered Ca2+ response (Fig. 6, A and B).
FIGURE 5.
PKD-mediated recovery of trypsin-evoked Ca2+ signaling. HEK293 cells were challenged with vehicle or trypsin (10 nm, 10 min), washed, and challenged again with trypsin (10 nm) 25 or 90 min later. [Ca2+]i was measured. A, time course showing desensitization at 25 min and recovery at 90 min. B–D, effects of brefeldin-A (B), CRT0066101 (C), or control siRNA or PKD1 siRNA (D) on recovery at 90 min. n = 3 experiments.
FIGURE 6.
PKD-mediated recovery of trypsin-evoked Ca2+ signaling. A–C and E, HEK293 cells were preincubated with vehicle, brefeldin-A, CRT0066101, or cycloheximide or were transfected with control siRNA or PKD1-siRNA. Cells were challenged with vehicle, trypsin (10 nm, 10 min), or 2-furoyl-LIGRLO-NH2 (10 μm, 10 min), washed, and challenged again with trypsin (10 nm) or 2-furoyl-LIGRLO-NH2 (10 μm) 25 or 90 min later. [Ca2+]i was measured. A, initial response to trypsin. B, recovery of trypsin responses at 25 and 90 min. C, effects of control siRNA or PKD1 siRNA on recovery of trypsin responses at 25 and 90 min. D, PKD expression at 24 h after transfection with control siRNA or PKD1 siRNA. E, recovery of 2-furoyl-LIGRLO-NH2 response at 90 min. *, p < 0.05; **, p < 0.01; ***, p < 0.0001, NS, not significant, n = 3 experiments.
Our results suggest that an intact Golgi apparatus and activation of PKD are necessary for recovery of PAR2 Ca2+ signaling in HEK293 cells. De novo protein synthesis is not required for recovery of protease signaling in the short term.
PKD Mediates PAR2 Translocation from the Golgi Apparatus to the Plasma Membrane
Our results suggest that PKD activity and Golgi stores of PAR2 are required for recovery of PAR2 signaling. Because PKD controls protein sorting within the Golgi apparatus, we investigated whether PKD regulates Golgi to plasma membrane translocation of PAR2.
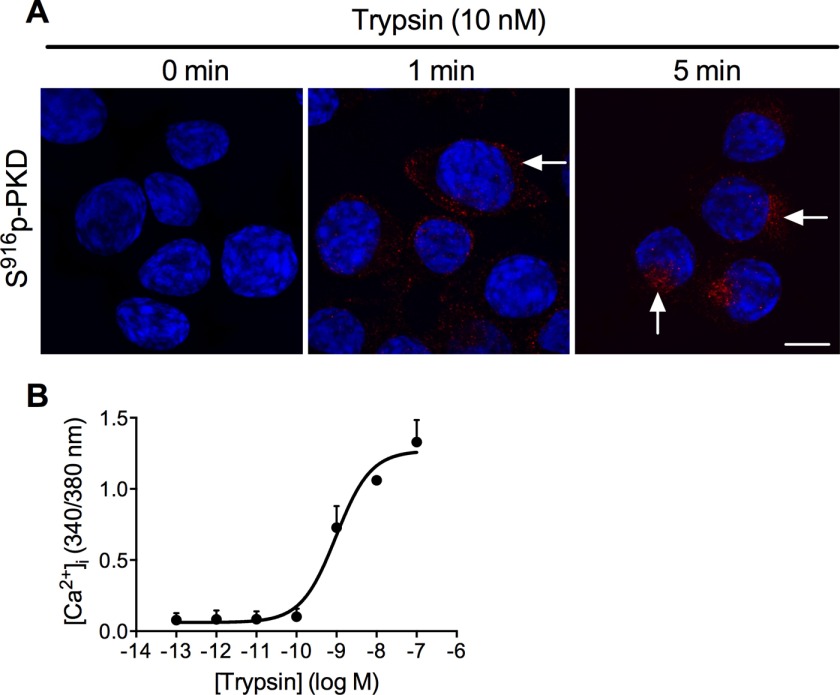
We expressed photoconvertible PAR2-Kaede in KNRK cells. KNRK-PAR2 cells have been used extensively to study PAR trafficking and signaling because untransfected KNRK cells express negligible levels of endogenous PAR2 (18). We have previously shown that recovery of PAR2-mediated Ca2+ signaling in KNRK-PAR2 cells requires mobilization of Golgi stores of intact PAR2 (16). In unstimulated KNRK-PAR2-Kaede cells, phospho-PKD was not detected by immunofluorescence and confocal microscopy (Fig. 7A). Trypsin (10 nm) increased phospho-PKD levels within 1 min, and by 5 min there was prominent phospho-PKD localization in a perinuclear region, consistent with activation within the Golgi apparatus. Trypsin caused a concentration-dependent increase in [Ca2+]i in KNRK-PAR2-Kaede cells (EC50 ∼1 nm) (Fig. 7B). These results suggest that PAR2-Kaede retains functional activity.
FIGURE 7.
Trypsin-induced activation of PKD and PAR2 in KNRK-PAR2-Kaede cells. A, localization of Ser916 phospho-PKD in KNRK-PAR2-Kaede cells treated with trypsin (10 nm, 0, 1, and 5 min). Scale, 10 μm. B, trypsin-evoked changes in [Ca2+]i in KNRK-PAR2-Kaede cells. n = 3 experiments.
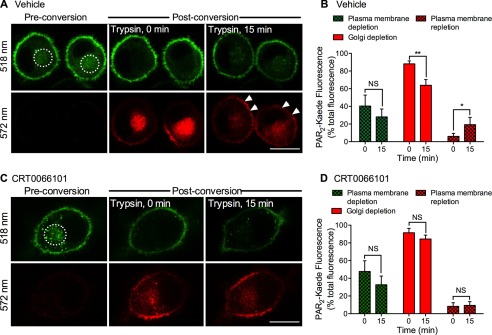
To specifically examine the translocation of intracellular pools of PAR2-Kaede to the plasma membrane, we green-red photoconverted the prominent perinuclear pool of PAR2-Kaede, which is likely to include Golgi stores of PAR2 in KNRK cells (16). By selecting a discrete region of interest, we were able to selectively photoconvert intracellular stores of PAR2-Kaede without affecting PAR2-Kaede at the plasma membrane (Fig. 8, A and C). We challenged cells with trypsin and observed cells over time to assess depletion of PAR2-Kaede red from intracellular stores and re-population of the plasma membrane with fresh PAR2-Kaede red. Trypsin (10 nm, 15 min) induced a depletion of intracellular (Golgi) PAR2-Kaede red and a concomitant increase in plasma membrane PAR2-Kaede red (Fig. 8, A and B). The reduction in plasma membrane PAR2-Kaede green reflects receptor endocytosis. CRT0066101 prevented trypsin-evoked intracellular depletion and plasma membrane repletion of PAR2-Kaede red (Fig. 8, C and D). These results suggest that PAR2 activation induces PKD-mediated Golgi to plasma membrane translocation of intact PAR2.
FIGURE 8.
PKD-mediated plasma membrane translocation of PAR2-Kaede. PAR2-Kaede in the vicinity of the Golgi apparatus of KNRK-PAR2-Kaede cells was green-red photoconverted using a confocal laser (illuminated region: dashed circle). Cells were incubated with trypsin (10 nm) for 0 or 15 min. PAR2-Kaede fluorescence was measured at 518 and 572 nm at the plasma membrane and in the vicinity of the Golgi apparatus at 0 and 15 min. A and C, representative images of cells treated with vehicle (A) or CRT0066101 (C). Scale, 10 μm. B and D, quantification of plasma membrane depletion of non-converted PAR2-Kaede (518 nm, green), Golgi depletion of converted PAR2-Kaede (572 nm, red), and plasma membrane repletion of PAR2-Kaede (572 nm, red). *, p < 0.05; **, p < 0.001, NS, not significant, n = 5 experiments, >45 cells analyzed per condition.
Gβγ Mediates PAR2-dependent Activation of PKD in the Golgi Apparatus
Gβγ subunits can activate PKD within the Golgi apparatus (32, 43). We hypothesized that PAR2 induces translocation of Gβγ to the Golgi apparatus, where Gβγ activates PKD and thereby controls mobilization and recovery of PAR2. We used BRET to assess translocation of Gγ to the Golgi compartment. Giantin is a resident Golgi protein that is anchored to the membrane of Golgi. It forms a complex with other Golgi proteins, such as p115 and Rab1, and regulates vesicle tethering and fusion processes in the Golgi apparatus (44). We used Giantin-RLuc8 as a localization marker to examine PAR2-induced translocation of Gβγ to the Golgi compartment. In HEK293 cells, trypsin increased BRET between Gγ-Venus and giantin-RLuc8 within 5 min (EC50 ∼10 nm) (Fig. 9, A and B). Trypsin-evoked Gγ-Venus/giantin-RLuc8 BRET was similar in cells expressing Gβ1, Gβ4, or Gβ5 (Fig. 9B). To investigate whether Gβγ activation mediates trypsin-induced PKD activation, we challenged HEK293 cells with trypsin (10 nm) for 5 min in the presence and absence of the Gβγ inhibitor gallein (10 μm). Gallein abolished trypsin-induced phosphorylation of PKD, assessed by Western blotting (Fig. 9, C and D). Thus, PAR2 activation promotes rapid translocation of Gβγ to the Golgi apparatus, where Gβγ can activate PKD.
FIGURE 9.
Gβγ translocation to the Golgi apparatus and activation of PKD. A and B, trypsin-induced BRET between Gγ-Venus and giatin-RLuc8 in HEK293 cells coexpressing Gβ1, Gβ4, or Gβ5. A, time course trypsin response. B, trypsin concentration-response, area under curve (AUC) over 15 min. n = 4–7 experiments. C and D, Western blot for Ser916 phospho-PKD and total PKD in HEK cells. Cells were unstimulated (control, Con.) or were stimulated with trypsin (10 nm) for 5 min. Some cells were preincubated with gallein (Gal) or vehicle (Veh.). C, representative blot. D, quantified results. *, p < 0.05, n = 3 experiments.
Gβγ Mediates Recovery of PAR2
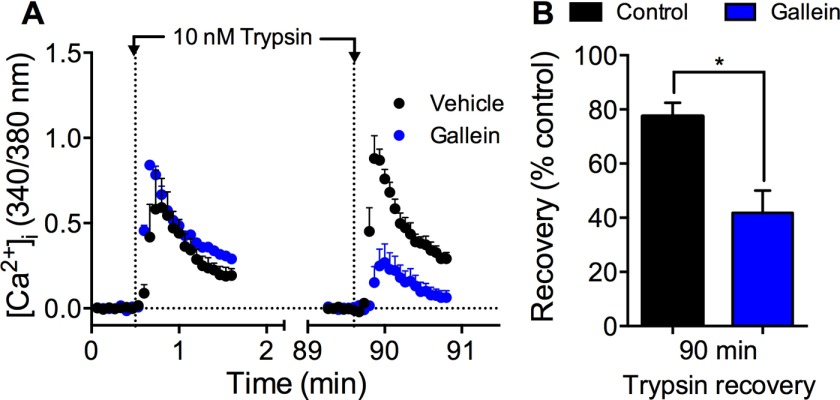
To evaluate the role of Gβγ in sustained protease signaling, we incubated HEK293 cells with gallein and examined recovery of trypsin-evoked Ca2+ signaling. Gallein did not affect the initial response to trypsin but inhibited PAR2 recovery after 90 min (Fig. 10, A and B; ∼40% recovery).
FIGURE 10.
Gβγ-mediated recovery of trypsin-evoked Ca2+ signaling. HEK293 cells were challenged with vehicle or trypsin (10 nm, 5 min), washed, and challenged again with trypsin (10 nm) 90 min later. [Ca2+]i was measured. A, effects of gallein or vehicle on kinetics. B, effects of gallein on recovery at 90 min. *, p < 0.05, n = 3 experiments.
PKD Mediates Recovery of PAR2 in Colonocytes
PAR2 is prominently expressed in colonocytes (45), where activation by digestive and proinflammatory proteases can regulate paracellular permeability (38), cyclooxygenase-2 activity (46), and cytokine production (47), and thereby contribute to inflammatory disease (48–51). To examine the importance of PKD for sustained protease signaling in a physiologically relevant cell type, we assessed protease-evoked PKD activation and PKD-mediated PAR2 recovery in NCM460 cells, a non-transformed human colonocyte cell line that endogenously expresses PAR2 (38, 50). Unstimulated NCM460 cells had low levels of phospho-PKD, assessed by immunofluorescence and confocal microscopy (Fig. 11A, 0 min). Trypsin (10 nm) increased phospho-PKD within vesicles throughout the cytosol within 1–5 min (Fig. 11A). Incubation with trypsin (10 nm, 10 min) strongly desensitized trypsin-stimulated Ca2+ signaling at 25 min, which was partially recovered by 120 min after washing (Fig. 11B). CRT0066101 did not affect the initial response to trypsin or the extent of desensitization, but it inhibited recovery at 120 min (Fig. 11, C–E). CRT0066101 also prevented recovery of 2-furoyl-LIGRLO-NH2-evoked Ca2+ signaling at 120 min (Fig. 11E). Similar to HEK293 cells, cycloheximide had no effect on initial or recovered Ca2+ responses to trypsin (Fig. 11, D and E). Thus, PAR2 signaling activates PKD in colonocytes, and PKD activity is necessary for recovery of Ca2+ signaling.
FIGURE 11.
Trypsin-induced activation of PKD and PKD-mediated recovery of trypsin-evoked Ca2+ signaling in colonocytes. A, localization of Ser916 phospho-PKD in NCM460 cells treated with trypsin (10 nm, 0, 1, and 5 min). Scale, 10 μm. B–E, NCM460 cells were preincubated with vehicle or CRT0066101. Cells were challenged with vehicle, trypsin (10 nm, 10 min), or 2-furoyl-LIGRLO-NH2 (10 μm, 10 min), washed, and challenged again with trypsin (10 nm) or 2-furoyl-LIGRLO-NH2 (10 μm) 25 or 120 min later. [Ca2+]i was measured. B, time course showing desensitization at 25 min and recovery at 120 min. C, effects of CRT0066101 on recovery of trypsin responses at 120 min. D, initial response to trypsin. E, recovery of trypsin responses at 25 and 120 min. F, recovery of 2-furoyl-LIGRLO-NH2 response at 120 min. **, p < 0.001. NS, not significant, n = 3 experiments.
PKD Mediates Sustained Protease-induced Excitability of Nociceptors
Proteases that activate PAR2 on nociceptive neurons induce the release of neuropeptides that mediate neurogenic inflammation and nociceptive transmission (52, 53). PAR2 activates PKD in nociceptors (27) but with unknown functional relevance. Because PAR2-activating proteases induce hyperexcitability of nociceptors (23, 24), we investigated whether PKD mediates sustained excitability to proteases. To examine excitability, we made patch clamp recordings from small diameter neurons of mouse dorsal root ganglia. We assessed excitability by measuring the rheobase (minimum current required to fire an action potential) and the frequency of action potential discharge at twice rheobase.
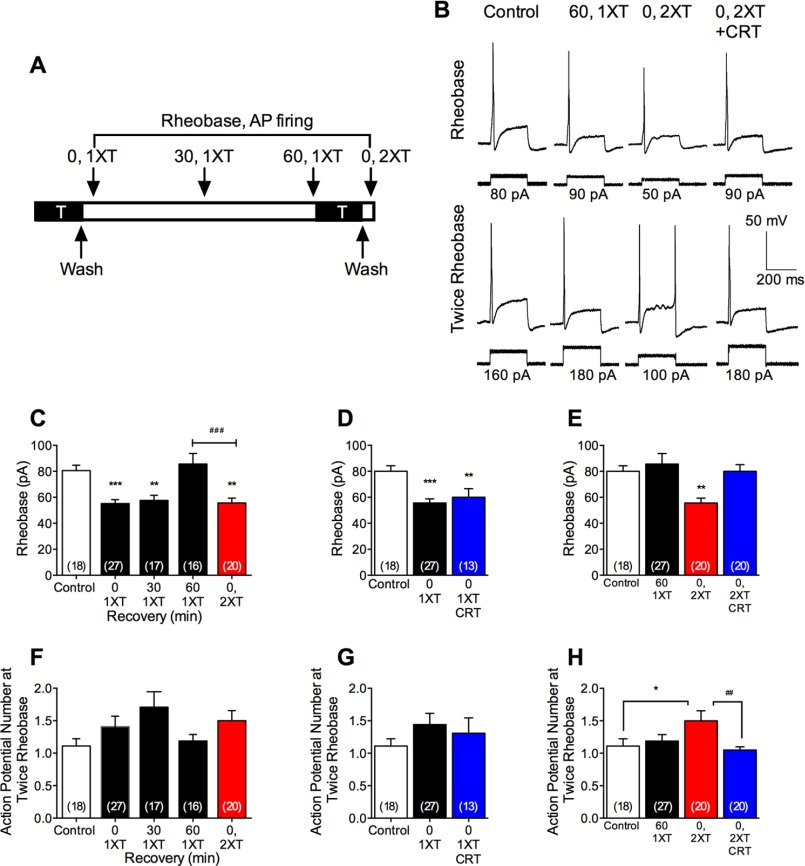
When neurons were exposed to trypsin (50 nm, 10 min), washed, and then immediately studied, there was a >30% decrease in rheobase and an increase in action potential discharge compared with vehicle-treated controls, indicative of hyperexcitability (Fig. 12, A–C and F). Hyperexcitability was maintained at 30 min after stimulation with trypsin and washing but declined to pre-stimulation levels after 60 min. To determine whether neurons had regained their capacity to respond to trypsin at this time, they were exposed again to trypsin (50 nm, 10 min), washed, and immediately studied. After a second trypsin challenge, the rheobase was decreased, and action potential discharge increased to a similar extent as observed immediately after a single trypsin exposure. These results are consistent with a full recovery of PAR2-mediated hyperexcitability within 60 min.
FIGURE 12.
PKD-mediated sustained neuronal hyperexcitability. A, dorsal root ganglion neurons were exposed to trypsin (T, 50 nm) for 10 min, washed, and incubated in trypsin-free medium. Excitability was assessed at 0 (0, 1XT), 30 (30, 1XT), or 60 (60, 1XT) min after first trypsin (0, 1XT). Some neurons were subjected to a second trypsin exposure 60 min after the first and were washed, and excitability was assessed (0, 2XT). B, representative action potential firing at rheobase and twice rheobase. C and D, pooled measurements of rheobase. F–H, pooled measurements of action potential firing at twice rheobase. *, p < 0.05; **, p < 0.001; ***, p < 0.0001 compared with control; ##, p < 0.001; ###, p < 0.0001. n denotes number of recorded neurons from studies of 16 mice.
To examine whether PKD mediates the recovery of trypsin-evoked hyperexcitability, neurons were preincubated with CRT0066101 (10 nm, 30 min and inclusion throughout). CRT0066101 did not affect neuronal responsiveness immediately after the first exposure to trypsin (Fig. 12, D and G). However, CRT0066101 abolished the recovery of hyperexcitability after the second trypsin exposure (Fig. 12, B, E, and H). Thus, PKD mediates the recovery of trypsin-evoked nociceptor hypersensitivity, suggesting a role of PKD in PAR2 mobilization from TGN stores in nociceptors.
Discussion
We report the identification of a mechanism by which activation of a GPCR at the cell surface triggers the rapid mobilization of TGN stores of fresh receptors, leading to replenishment of the plasma membrane with signaling-competent receptors. Our results show that proteolytic activation of PAR2 induces translocation of Gβγ subunits to the Golgi apparatus and Gβγ-mediated activation of PKD within the TGN. This process mediates the translocation of PAR2 from the TGN to the plasma membrane and permits recovery of cellular responsiveness to extracellular proteases. This mechanism may allow rapid recovery of cellular responsiveness to many GPCRs that traffic to lysosomes upon activation.
PAR2, Gβγ, and PKD Axis
Our results show that PAR2 activation causes rapid activation of a pool of PKD within the TGN. We observed in HEK293 cells that trypsin stimulated phosphorylation of PKD at the Ser916 autophosphorylation site within minutes, assessed by Western blotting. The PAR2-selective agonist 2-furoyl-LIGRLO-NH2 also stimulated phosphorylation of PKD, and the PKD inhibitor CRT0066101 prevented the response to trypsin, suggesting selectivity. Phospho-PKD was colocalized with TGN38-YFP, which indicates that activation of PAR2 at the cell surface can induce the rapid phosphorylation and activation of the TGN pool of PKD. Examination by immunofluorescence and super-resolution microscopy showed that trypsin promoted Ser916 phosphorylation of PKD in the immediate proximity of preformed stores of intact PAR2, detected with an antibody to an N-terminal HA that would be removed by proteolysis, within the TGN of HEK293 cells. We also observed that trypsin stimulated Ser916 phosphorylation of PKD in PAR2-expressing KNRK cells and in human NCM460 colonocytes, although we did not determine the subcellular location of activated PKD in these cells. We have previously shown that PAR2 also activates PKD in nociceptors (27). Thus, the capacity of PAR2 to activate PKD is observed in several cell types, including functionally relevant colonocytes.
Cell-surface GPCRs have been proposed to activate PKD within the TGN by several mechanisms. They include translocation of specific Gβγ and Gα subunits to the TGN, activation of phospholipase Cβ, and formation of DAG (30–34, 43, 54). DAG can activate PKC and promote PKD translocation of TGN, thereby enhancing PKD activation (29). We used BRET to quantitatively assess PAR2-dependent translocation of Gβγ to the Golgi apparatus. Trypsin stimulated a rapid (minutes) and concentration-dependent increase in BRET between Gγ-Venus and giantin-RLuc8. These results suggest that PAR2 activation stimulates translocation of Gβγ subunits to the Golgi apparatus. Our results are consistent with other reports that agonists of GPCRs, including muscarinic M2 receptors, promote the rapid translocation of Gβγ from the plasma membrane to the Golgi apparatus (30, 33, 34). We found that the Gβγ inhibitor gallein prevented trypsin-stimulated activation of PKD, suggesting that Gβγ mediates PAR2-dependent activation of PKD within the Golgi TGN.
PKD-dependent Mobilization of PAR2 from the TGN
By using BRET to assess the proximity of PAR2 to proteins resident at the plasma membrane (RIT, KRas, and HRas) and endosomes (Rab5a, Rab7a, and Rab11), we found that trypsin resulted in removal of PAR2 from the cell surface and translocation to Rab5a-containing early endosomes. After recovery in trypsin-free medium, the increase in BRET between PAR2 and plasma membrane proteins coincided with a large increase in BRET between PAR2 and Rab11, which participates within post-Golgi trafficking and recycling processes (40, 41). These results suggest that trypsin activation results in PAR2 translocation to Rab11-positive vesicles that deliver stores of PAR2 from the TGN to the cell surface.
Our results show that PKD promotes mobilization of PAR2 from the TGN to the plasma membrane. To selectively examine the translocation of PAR2 from intracellular pools to the plasma membrane, we expressed photoconvertible PAR2-Kaede in KNRK cells. We found that trypsin activated PKD and mobilized intracellular Ca2+ with the expected potency in KNRK-PAR2-Kaede cells and that PAR2-Kaede was localized as expected to the plasma membrane and to intracellular stores, which probably represent the TGN. Thus, Kaede fusion did not appreciably alter PAR2 function or localization. Trypsin treatment resulted in the depletion of photoconverted PAR2-Kaede from intracellular stores and its repletion at the plasma membrane. The PKD inhibitor CRT0066101 prevented this redistribution. Pharmacological inhibitors of PKD and Gβγ, and PKD1 knockdown, all suppressed recovery of trypsin-evoked Ca2+ signaling. These results are consistent with the proposal that PAR2 activation at the plasma membrane triggers Gβγ-mediated activation of PKD within the TGN, which stimulates the replenishment of intact PAR2 at the plasma membrane. The involvement of PKD in TGN to plasma membrane trafficking and recovery of cellular responses to PAR2 agonists is consistent with the established role of PKD in regulating the fission of transport carriers from the TGN that are destined for the plasma membrane (36, 37). Although we did not define which isoform of PKD mediates the TGN mobilization of PAR2, all isoforms have been implicated in TGN vesicle fission (37).
Further studies are required to identify the precise Gβγ subunits that mediate PAR2-evoked activation of PKD in the TGN and to determine whether Gα subunits and PKC isoforms also participate in this process. Other processes can also regulate mobilization of PAR2 from the TGN, including p24A, a transmembrane protein of the Golgi apparatus that interacts with PAR2 and controls recovery (55). Whether PAR2-stimulated activation of PKD within the TGN selectively controls the mobilization of PAR2 or also regulates plasma membrane trafficking of other GPCRs or secreted proteins remains to be determined.
Sustained Protease Signaling
Our results suggest that PKD is necessary for sustained PAR2-dependent signaling by extracellular proteases. Although injury and inflammation result in the activation of multiple PAR2-activating proteases, cellular responses are efficiently attenuated by mechanisms that include β-arrestin-dependent desensitization and endocytosis and ubiquitin-dependent lysosomal degradation of PAR2 (4, 7, 16, 26). Sustained signaling by extracellular proteases requires replenishment of the plasma membrane by mobilization of the prominent pools of intact receptors in the TGN and synthesis of new receptors. Our results show that activation of PAR2 at the cell surface by the canonical agonist trypsin and by an analogue of the trypsin-exposed tethered ligand results in Gβγ- and PKD-mediated mobilization and plasma membrane translocation of TGN stores of intact PAR2. This mechanism is necessary for sustained signaling by extracellular proteases. We report that PKD mediates recovery of protease signaling in colonocytes, where PAR2 activation promotes inflammatory signaling and colitis and thereby contributes to inflammatory disease (38, 46–51). PKD activity is also required for sustained trypsin-evoked hyperexcitability of nociceptors, which is nociceptive in mice (53). Thus, PKD is likely to be required for protease- and PAR2-mediated inflammation and nociception, and therapies that disrupt this mechanism may attenuate inflammation and pain.
Proteases such as trypsin, which activate PAR2 by canonical mechanisms, induce coupling to Gαq and β-arrestins and receptor endocytosis and degradation. Other proteases, including cathepsin S and elastase, are biased agonists that cleave PAR2 at distinct sites to induce coupling to Gαs but not Gαq or β-arrestins (23–25). Cathepsin S- and elastase-activated PAR2 cannot be reactivated by proteolysis, but the cleaved receptors remain at the cell surface. Whether PKD also mediates sustained signaling by biased agonists of PAR2 remains to investigated.
Author Contributions
D. J., P. Z., N. N. J., T. L., D. P. P., M. G., H. R. Y., and M. C. designed and performed experiments. S. J. V. designed electrophysiological studies. N. W. B. conceived, designed, and coordinated the study and wrote the manuscript. All authors analyzed the results and approved the final version of the manuscript.
Acknowledgments
We thank Cameron Nowell for help with image analysis and Dr. Jane Murphy for generating PAR2-Kaede.
This work was supported by the National Health and Medical Research Council, The Australian Research Council, and Monash University. Research in the authors' laboratory was funded in part by Takeda Pharmaceuticals, Inc.
- GPCR
- G protein-coupled receptor
- PAR2
- protease-activated receptor-2
- DAG
- diacylglycerol
- TGN
- trans-Golgi network
- BRET
- bioluminescence resonance energy transfer
- HBSS
- Hanks' balanced salt solution.
References
- 1. Kang D. S., Tian X., and Benovic J. L. (2014) Role of β-arrestins and arrestin domain-containing proteins in G protein-coupled receptor trafficking. Curr. Opin. Cell Biol. 27, 63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McConalogue K., Déry O., Lovett M., Wong H., Walsh J. H., Grady E. F., and Bunnett N. W. (1999) Substance P-induced trafficking of β-arrestins. The role of β-arrestins in endocytosis of the neurokinin-1 receptor. J. Biol. Chem. 274, 16257–16268 [DOI] [PubMed] [Google Scholar]
- 3. Williams J. T., Ingram S. L., Henderson G., Chavkin C., von Zastrow M., Schulz S., Koch T., Evans C. J., and Christie M. J. (2013) Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol. Rev. 65, 223–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Déry O., Thoma M. S., Wong H., Grady E. F., and Bunnett N. W. (1999) Trafficking of proteinase-activated receptor-2 and β-arrestin-1 tagged with green fluorescent protein. β-Arrestin-dependent endocytosis of a proteinase receptor. J. Biol. Chem. 274, 18524–18535 [DOI] [PubMed] [Google Scholar]
- 5. Oh P., Horner T., Witkiewicz H., and Schnitzer J. E. (2012) Endothelin induces rapid, dynamin-mediated budding of endothelial caveolae rich in ET-B. J. Biol. Chem. 287, 17353–17362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DeFea K. A., Vaughn Z. D., O'Bryan E. M., Nishijima D., Déry O., and Bunnett N. W. (2000) The proliferative and antiapoptotic effects of substance P are facilitated by formation of a β-arrestin-dependent scaffolding complex. Proc. Natl. Acad. Sci. U.S.A. 97, 11086–11091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DeFea K. A., Zalevsky J., Thoma M. S., Déry O., Mullins R. D., and Bunnett N. W. (2000) β-Arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J. Cell Biol. 148, 1267–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Irannejad R., Tomshine J. C., Tomshine J. R., Chevalier M., Mahoney J. P., Steyaert J., Rasmussen S. G., Sunahara R. K., El-Samad H., Huang B., and von Zastrow M. (2013) Conformational biosensors reveal GPCR signalling from endosomes. Nature 495, 534–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Irannejad R., and von Zastrow M. (2014) GPCR signaling along the endocytic pathway. Curr. Opin. Cell Biol. 27, 109–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murphy J. E., Padilla B. E., Hasdemir B., Cottrell G. S., and Bunnett N. W. (2009) Endosomes: a legitimate platform for the signaling train. Proc. Natl. Acad. Sci. U.S.A. 106, 17615–17622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gupta A., Fujita W., Gomes I., Bobeck E., and Devi L. A. (2015) Endothelin-converting enzyme 2 differentially regulates opioid receptor activity. Br. J. Pharmacol. 172, 704–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jensen D. D., Halls M. L., Murphy J. E., Canals M., Cattaruzza F., Poole D. P., Lieu T., Koon H. W., Pothoulakis C., and Bunnett N. W. (2014) Endothelin-converting enzyme 1 and β-arrestins exert spatiotemporal control of substance P-induced inflammatory signals. J. Biol. Chem. 289, 20283–20294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Padilla B. E., Cottrell G. S., Roosterman D., Pikios S., Muller L., Steinhoff M., and Bunnett N. W. (2007) Endothelin-converting enzyme-1 regulates endosomal sorting of calcitonin receptor-like receptor and β-arrestins. J. Cell Biol. 179, 981–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pelayo J. C., Poole D. P., Steinhoff M., Cottrell G. S., and Bunnett N. W. (2011) Endothelin-converting enzyme-1 regulates trafficking and signalling of the neurokinin 1 receptor in endosomes of myenteric neurones. J. Physiol. 589, 5213–5230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roosterman D., Cottrell G. S., Padilla B. E., Muller L., Eckman C. B., Bunnett N. W., and Steinhoff M. (2007) Endothelin-converting enzyme 1 degrades neuropeptides in endosomes to control receptor recycling. Proc. Natl. Acad. Sci. U.S.A. 104, 11838–11843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Böhm S. K., Khitin L. M., Grady E. F., Aponte G., Payan D. G., and Bunnett N. W. (1996) Mechanisms of desensitization and resensitization of proteinase-activated receptor-2. J. Biol. Chem. 271, 22003–22016 [DOI] [PubMed] [Google Scholar]
- 17. Poole D. P., Pelayo J. C., Scherrer G., Evans C. J., Kieffer B. L., and Bunnett N. W. (2011) Localization and regulation of fluorescently labeled δ opioid receptor, expressed in enteric neurons of mice. Gastroenterology 141, 982–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bohm S. K., Kong W., Bromme D., Smeekens S. P., Anderson D. C., Connolly A., Kahn M., Nelken N. A., Coughlin S. R., Payan D. G., and Bunnett N. W. (1996) Molecular cloning, expression and potential functions of the human proteinase-activated receptor-2. Biochem. J. 314, 1009–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Corvera C. U., Déry O., McConalogue K., Böhm S. K., Khitin L. M., Caughey G. H., Payan D. G., and Bunnett N. W. (1997) Mast cell tryptase regulates rat colonic myocytes through proteinase-activated receptor 2. J. Clin. Invest. 100, 1383–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cottrell G. S., Amadesi S., Grady E. F., and Bunnett N. W. (2004) Trypsin IV, a novel agonist of protease-activated receptors 2 and 4. J. Biol. Chem. 279, 13532–13539 [DOI] [PubMed] [Google Scholar]
- 21. Oikonomopoulou K., Hansen K. K., Saifeddine M., Tea I., Blaber M., Blaber S. I., Scarisbrick I., Andrade-Gordon P., Cottrell G. S., Bunnett N. W., Diamandis E. P., and Hollenberg M. D. (2006) Proteinase-activated receptors, targets for kallikrein signaling. J. Biol. Chem. 281, 32095–32112 [DOI] [PubMed] [Google Scholar]
- 22. Ossovskaya V. S., and Bunnett N. W. (2004) Protease-activated receptors: contribution to physiology and disease. Physiol. Rev. 84, 579–621 [DOI] [PubMed] [Google Scholar]
- 23. Zhao P., Lieu T., Barlow N., Metcalf M., Veldhuis N. A., Jensen D. D., Kocan M., Sostegni S., Haerteis S., Baraznenok V., Henderson I., Lindström E., Guerrero-Alba R., Valdez-Morales E. E., Liedtke W., et al. (2014) Cathepsin S causes inflammatory pain via biased agonism of PAR2 and TRPV4. J. Biol. Chem. 289, 27215–27234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao P., Lieu T., Barlow N., Sostegni S., Haerteis S., Korbmacher C., Liedtke W., Jimenez-Vargas N. N., Vanner S. J., and Bunnett N. W. (2015) Neutrophil elastase activates protease-activated receptor-2 (PAR2) and transient receptor potential vanilloid 4 (TRPV4) to cause inflammation and pain. J. Biol. Chem. 290, 13875–13887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhao P., Metcalf M., and Bunnett N. W. (2014) Biased signaling of protease-activated receptors. Front. Endocrinol. 5, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jacob C., Cottrell G. S., Gehringer D., Schmidlin F., Grady E. F., and Bunnett N. W. (2005) c-Cbl mediates ubiquitination, degradation, and down-regulation of human protease-activated receptor 2. J. Biol. Chem. 280, 16076–16087 [DOI] [PubMed] [Google Scholar]
- 27. Amadesi S., Grant A. D., Cottrell G. S., Vaksman N., Poole D. P., Rozengurt E., and Bunnett N. W. (2009) Protein kinase D isoforms are expressed in rat and mouse primary sensory neurons and are activated by agonists of protease-activated receptor 2. J. Comp. Neurol. 516, 141–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Steinberg S. F. (2012) Regulation of protein kinase D1 activity. Mol. Pharmacol. 81, 284–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baron C. L., and Malhotra V. (2002) Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science 295, 325–328 [DOI] [PubMed] [Google Scholar]
- 30. Akgoz M., Kalyanaraman V., and Gautam N. (2004) Receptor-mediated reversible translocation of the G protein βγ complex from the plasma membrane to the Golgi complex. J. Biol. Chem. 279, 51541–51544 [DOI] [PubMed] [Google Scholar]
- 31. Díaz Añel A. M., and Malhotra V. (2005) PKCη is required for β1γ2/β3γ2- and PKD-mediated transport to the cell surface and the organization of the Golgi apparatus. J. Cell Biol. 169, 83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jamora C., Yamanouye N., Van Lint J., Laudenslager J., Vandenheede J. R., Faulkner D. J., and Malhotra V. (1999) Gβγ-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell 98, 59–68 [DOI] [PubMed] [Google Scholar]
- 33. Saini D. K., Kalyanaraman V., Chisari M., and Gautam N. (2007) A family of G protein βγ subunits translocate reversibly from the plasma membrane to endomembranes on receptor activation. J. Biol. Chem. 282, 24099–24108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saini D. K., Karunarathne W. K., Angaswamy N., Saini D., Cho J. H., Kalyanaraman V., and Gautam N. (2010) Regulation of Golgi structure and secretion by receptor-induced G protein βγ complex translocation. Proc. Natl. Acad. Sci. U.S.A. 107, 11417–11422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rozengurt E. (2011) Protein kinase D signaling: multiple biological functions in health and disease. Physiology 26, 23–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liljedahl M., Maeda Y., Colanzi A., Ayala I., Van Lint J., and Malhotra V. (2001) Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell 104, 409–420 [DOI] [PubMed] [Google Scholar]
- 37. Yeaman C., Ayala M. I., Wright J. R., Bard F., Bossard C., Ang A., Maeda Y., Seufferlein T., Mellman I., Nelson W. J., and Malhotra V. (2004) Protein kinase D regulates basolateral membrane protein exit from trans-Golgi network. Nat. Cell Biol. 6, 106–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jacob C., Yang P. C., Darmoul D., Amadesi S., Saito T., Cottrell G. S., Coelho A. M., Singh P., Grady E. F., Perdue M., and Bunnett N. W. (2005) Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and β-arrestins. J. Biol. Chem. 280, 31936–31948 [DOI] [PubMed] [Google Scholar]
- 39. Jensen D. D., Godfrey C. B., Niklas C., Canals M., Kocan M., Poole D. P., Murphy J. E., Alemi F., Cottrell G. S., Korbmacher C., Lambert N. A., Bunnett N. W., and Corvera C. U. (2013) The bile acid receptor TGR5 does not interact with β-arrestins or traffic to endosomes but transmits sustained signals from plasma membrane rafts. J. Biol. Chem. 288, 22942–22960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ullrich O., Reinsch S., Urbé S., Zerial M., and Parton R. G. (1996) Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 135, 913–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Welz T., Wellbourne-Wood J., and Kerkhoff E. (2014) Orchestration of cell surface proteins by Rab11. Trends Cell Biol. 24, 407–415 [DOI] [PubMed] [Google Scholar]
- 42. Dinter A., and Berger E. G. (1998) Golgi-disturbing agents. Histochem. Cell Biol. 109, 571–590 [DOI] [PubMed] [Google Scholar]
- 43. Díaz Añel A. M. (2007) Phospholipase C β3 is a key component in the Gβγ/PKCη/PKD-mediated regulation of trans-Golgi network to plasma membrane transport. Biochem. J. 406, 157–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Koreishi M., Gniadek T. J., Yu S., Masuda J., Honjo Y., and Satoh A. (2013) The golgin tether giantin regulates the secretory pathway by controlling stack organization within Golgi apparatus. PLoS ONE 8, e59821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kong W., McConalogue K., Khitin L. M., Hollenberg M. D., Payan D. G., Böhm S. K., and Bunnett N. W. (1997) Luminal trypsin may regulate enterocytes through proteinase-activated receptor 2. Proc. Natl. Acad. Sci. U.S.A. 94, 8884–8889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang H., Wen S., Bunnett N. W., Leduc R., Hollenberg M. D., and MacNaughton W. K. (2008) Proteinase-activated receptor-2 induces cyclooxygenase-2 expression through β-catenin and cyclic AMP-response element-binding protein. J. Biol. Chem. 283, 809–815 [DOI] [PubMed] [Google Scholar]
- 47. Wang H., Moreau F., Hirota C. L., and MacNaughton W. K. (2010) Proteinase-activated receptors induce interleukin-8 expression by intestinal epithelial cells through ERK/RSK90 activation and histone acetylation. FASEB J. 24, 1971–1980 [DOI] [PubMed] [Google Scholar]
- 48. Cenac N., Coelho A. M., Nguyen C., Compton S., Andrade-Gordon P., MacNaughton W. K., Wallace J. L., Hollenberg M. D., Bunnett N. W., Garcia-Villar R., Bueno L., and Vergnolle N. (2002) Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2. Am. J. Pathol. 161, 1903–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cenac N., Garcia-Villar R., Ferrier L., Larauche M., Vergnolle N., Bunnett N. W., Coelho A. M., Fioramonti J., and Bueno L. (2003) Proteinase-activated receptor-2-induced colonic inflammation in mice: possible involvement of afferent neurons, nitric oxide, and paracellular permeability. J. Immunol. 170, 4296–4300 [DOI] [PubMed] [Google Scholar]
- 50. Cottrell G. S., Amadesi S., Pikios S., Camerer E., Willardsen J. A., Murphy B. R., Caughey G. H., Wolters P. J., Coughlin S. R., Peterson A., Knecht W., Pothoulakis C., Bunnett N. W., and Grady E. F. (2007) Protease-activated receptor 2, dipeptidyl peptidase I, and proteases mediate Clostridium difficile toxin A enteritis. Gastroenterology 132, 2422–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hansen K. K., Sherman P. M., Cellars L., Andrade-Gordon P., Pan Z., Baruch A., Wallace J. L., Hollenberg M. D., and Vergnolle N. (2005) A major role for proteolytic activity and proteinase-activated receptor-2 in the pathogenesis of infectious colitis. Proc. Natl. Acad. Sci. U.S.A. 102, 8363–8368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Steinhoff M., Vergnolle N., Young S. H., Tognetto M., Amadesi S., Ennes H. S., Trevisani M., Hollenberg M. D., Wallace J. L., Caughey G. H., Mitchell S. E., Williams L. M., Geppetti P., Mayer E. A., and Bunnett N. W. (2000) Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat. Med. 6, 151–158 [DOI] [PubMed] [Google Scholar]
- 53. Vergnolle N., Bunnett N. W., Sharkey K. A., Brussee V., Compton S. J., Grady E. F., Cirino G., Gerard N., Basbaum A. I., Andrade-Gordon P., Hollenberg M. D., and Wallace J. L. (2001) Proteinase-activated receptor-2 and hyperalgesia: A novel pain pathway. Nat. Med. 7, 821–826 [DOI] [PubMed] [Google Scholar]
- 54. Coria A. S., Masseroni M. L., and Díaz Añel A. M. (2014) Regulation of PKD1-mediated Golgi to cell surface trafficking by Gαq subunits. Biol. Cell 106, 30–43 [DOI] [PubMed] [Google Scholar]
- 55. Luo W., Wang Y., and Reiser G. (2007) p24A, a type I transmembrane protein, controls ARF1-dependent re-sensitization of protease-activated receptor-2 by influence on receptor trafficking. J. Biol. Chem. 282, 30246–30255 [DOI] [PubMed] [Google Scholar]