Abstract
INS-VNTR (insulin-variable number of tandem repeats) and AIRE (autoimmune regulator) have been associated with the modulation of insulin gene expression in thymus, which is essential to induce either insulin tolerance or the development of insulin autoimmunity and type 1 diabetes. We sought to analyze whether each functional domain of AIRE is critical for the activation of INS-VNTR in human thymic epithelial cells. Twelve missense or nonsense mutations in AIRE and two chimeric AIRE constructs were generated. A luciferase reporter assay and a pulldown assay using biotinylated INS-class I VNTR probe were performed to examine the transactivation and binding activities of WT, mutant, and chimeric AIREs on the INS-VNTR promoter. Confocal microscopy analysis was performed for WT or mutant AIRE cellular localization. We found that all of the AIRE mutations resulted in loss of transcriptional activation of INS-VNTR except mutant P252L. Using WT/mutant AIRE heterozygous forms to modulate the INS-VNTR target revealed five mutations (R257X, G228W, C311fsX376, L397fsX478, and R433fsX502) that functioned in a dominant negative fashion. The LXXLL-3 motif is identified for the first time to be essential for DNA binding to INS-VNTR, whereas the intact PHD1, PHD2, LXXLL-3, and LXXLL-4 motifs were important for successful transcriptional activation. AIRE nuclear localization in the human thymic epithelial cell line was disrupted by mutations in the homogenously staining region domain and the R257X mutation in the PHD1 domain. This study supports the notion that AIRE mutation could specifically affect human insulin gene expression in thymic epithelial cells through INS-VNTR and subsequently induce either insulin tolerance or autoimmunity.
Keywords: gene regulation, insulin, protein domain, transcription, type 1 diabetes, AIRE, VNTR, insulin autoimmunity, thymus
Introduction
Type 1 diabetes (T1D)2 is defined by absolute loss of insulin secretion due to the autoimmune destruction of insulin-producing β cells in the pancreas. There are a few target auto-antigens identified in β cell autoimmune destruction with insulin and pro-insulin auto-antibodies detected in 23 and 34% of T1D patients, respectively (1, 2). The autoimmune polyendocrinopathy type 1 syndrome (APS-1 also known as APECED) is an autosomal recessive, monogenic form of human autoimmunity that is characterized by autoimmune destruction of multiple endocrine organs and defective cell-mediated immunity (3, 4).
In 1997 an autoimmune regulator (AIRE) gene, which underlies APS-1, was identified on chromosome 21q22.3 using a positional cloning strategy (5, 6). The specific domains that the AIRE protein contains include (i) the N terminus of a homogenously staining region (HSR) domain and/or caspase recruitment domain (CARD), which is believed to be involved in dimerization and/or caspase recruitment (7, 8), (ii) a nuclear localization signal, (iii) a SAND (Sp100, AIRE-1, NucP41/75, DEAF-1) domain, which is thought to be involved in DNA binding (9), and (iv), two plant homeodomain (PHD) type zinc fingers (10, 11) and four LXXLL motifs (12). The AIRE protein has been shown to activate hundreds of tissue-restricted antigen (TRA) expression in medullary thymic epithelial cells. The expression of TRA modulates the induction of self-antigen recognizing T-cells for negative selection, thereby inducing tolerance of the TRAs (13–15). Insulin is one of those TRAs specific for T1D that is differentially expressed in the thymus and is modulated by an insulin-variable number of tandem repeats (INS-VNTR) and AIRE (16). The absence of insulin in the thymus induces insulin autoimmunity and the risk of developing T1D (17). The AIRE knock-out animal model supported that AIRE controls autoimmunity by regulating TRA expression in medullary thymic epithelial cells (18). In this study we analyzed AIRE functional domains using a T1D-specific target, INS-VNTR, which contains a unique polymorphic tandem repeat sequence with the insulin basal promoter. The combination of AIRE mutation and the diabetic risk INS-VNTR haplotype could predispose individuals to insulin autoimmunity. The mutations in AIRE that we selected span the different functional domains so that we could investigate the contribution of each domain in the AIRE/INS-VNTR interaction and its transcription function. The dominant negative activity was assessed because the APS-1 mutations are mostly autosomal recessive except for the G228W mutant that was reported as a dominant negative (19). Four additional AIRE mutants behaved in a dominant negative fashion as assessed by INS-VNTR target transcription. The significance of the current study is to identify the AIRE functional domain for the transcriptional activation, binding of the INS-VNTR, and the cellular translocation in thymic epithelial cells.
Experimental Procedures
AIRE Mutant Constructs and Generation of Chimeric AIREs
All AIRE mutant plasmids were prepared from the backbone plasmid of wild-type (WT) AIREs and generated using a site-direct mutagenesis kit (Stratagene, La Jolla, CA), verified by DNA sequencing, and protein expression. The primer pairs containing each individual mutation used are listed in Table 1. Two chimeric AIREs were constructed using primers that would amplify AIRE amino acids: 1–257, 397–433, and 397–545 (Table 2). The chimeric AIREs were R257-LXXLL-3 (amino acids 1–257) and 397–433 and R257-LXXLL-3-PHD2 (amino acids 1–257 and 397–545). The entire class I-VNTR and insulin basal promoter were synthesized as a biotin-INS-class I-VNTR probe for pulldown experiments. The biotin probe was constructed by using biotinylated primers (biotin-5′-TCACACGGAAGAATGAGGTC and biotin-5′-TTTGCAGCCTGTCCTGGAGG) to amplify class I-VNTR (20). An unrelated biotin-probe was generated using biotinylated primers (biotin-5′-AGGCTATGAAGAGATACGCC and biotin-5′-CCTTGTCAATCAAGGCGTTG) for negative control. Ad-AIRE viral vector was generated in a previous study (16).
TABLE 1.
Primers for AIRE mutagenesis
F, forward; R, reverse. Nucleotide change is denoted in bold and underlined. Deletions are denoted with a down arrow (↓).
| Site of mutation | Mutagenesis primers |
|---|---|
| L28P | F, 5′-GACAGCGCCTTCCCACCGCTGCACGCGCTGGC-3′ |
| R, 5′-GCCAGCGCGTGCAGCGGTGGGAAGGCGCTGTC-3′ | |
| L29P | F, 5′-CAGCGCCTTCCCACTGCCGCACGCGCTGGCTGACC-3′ |
| R, 5′-GGTCAGCCAGCGCGTGCGGCAGTGGGAAGGCGCTG-3′ | |
| W78R | F, 5′-CCACAGCCATCCTGGACTTCAGGAGGGTGCTGTTCAAGGAC-3′ |
| R, 5′-GTCCTTGAACAGCACCCTCCTGAAGTCCAGGATGGCTGTGG-3′ | |
| L93R | F, 5′-GCGCTATGGCCGGCGGCAGCCCATCCTGG-3′ |
| R, 5′-CCAGGATGGGCTGCCGCCGGCCATAGCGC-3′ | |
| G228W | F, 5′-GTGCATCCAGGTTGGTTGGGAGTTCTACACTCCC-3′ |
| R, 5′-GGGAGTGTAGAACTCCCAACCAACCTGGATGCAC-3′ | |
| P252L | F, 5′-CAGCAGCAGTGGCCTGAAGCCTCTGGTTCG-3′ |
| R, 5′-CGAACCAGAGGCTTCAGGCCACTGCTGCTG-3′ | |
| R257X | F, 5′-CCGAAGCCTCTGGTTTGAGCCAAGGGAGCCC-3′ |
| R, 5′-GGGCTCCCTTGGCTCAAACCAGAGGCTTCGG-3′ | |
| C311Y | F, 5′-GGGAGCTCATCTGCTATGACGGCTGCCCT-3′ |
| R, 5′-AGGGCAGCCGTCATAGCAGATGAGCTCCC-3′ | |
| C311fsX376 | F, 5′-GGGAGCTCATCTGC↓GTGACGGCTGCCCT-3′ |
| R, 5′-AGGGCAGCCGTCAC↓GCAGATGAGCTCCC-3′ | |
| P326Q | F, 5′-GCCTGCCTGTCCCATCCGCTCCGGGAGATC-3′ |
| R, 5′-GATCTCCCGGAGCGGATGGGACAGGCAGGC-3′ | |
| L397fsX478 | F, 5′-CGACTCTTGTCTACAAGCAC↓TGCCGGCTCCGGCCTTCTGCAG-3′ |
| R, 5′-CTGCAGAAGGCCGGAGCCGGCA↓GTGCTTGTAGACAAGAGTCG-3′ | |
| R433fsX502 | F, 5′-CCTGGTGCGCGTTGCACGGGTGTGCGGAGATGGT-3′ |
| R, 5′-ACCATCTCCGCACACCCGTGCAACGCGCACCAGG-3′ |
TABLE 2.
Primers for Chimeric AIRE
E1, HindIII; E2, EcoRI; E3, ApaI.
| Amino acid sequence | Primers with restriction enzyme sites |
|---|---|
| 1–257 | Forward ( E1), 5′-GATCAAGCTTCGGCCGCCACTGTGC-3′ |
| Reverse (E2), 5′-GATCGAATTCGGTCGGGCCACTGCTGCTG-3′ | |
| 397–433 | Forward (E2), 5′-GATCGAATTCGACCTGCCGGCTCCGCC-3′ |
| Reverse (E3), 5′-GATCGGGCCCGATTACTAGCGCAACGCGCACCAG-3′ | |
| 397–545 | Forward, (E2) 5′-GATCGAATTCGACCTGCCGGCTCCGCC-3′ |
| Reverse (E3): 5′-GATCGGGCCCTCAGGAGGGGAAGGGGGC-3′ |
Luciferase Reporter Assay
The human thymic epithelial cell line (hTEC) derived from cortical epithelium (21) was cultured in RPMI 1640 medium with 1× penicillin/streptomycin and 10% FBS. Transfection was performed using Lipofectamine 2000 (Life Technologies). INS-VNTR-luciferase vectors were generated in a previous study (16). For the transfection assay, equal moles of promoter equivalent were used (∼50 ng). Luciferase activities were normalized with a co-transfected TK-Renilla plasmid (10 ng) (Promega, Madison, WI). AIRE cDNA (100 ng) was transfected because the endogenous AIRE protein is not detected in the hTEC cell line (22). For the heterozygous expression of WT and mutant AIRE proteins, equal amount of expression vectors were introduced (50 ng each). Luciferase activities were measured using the Dual-Glo luciferase assay kit (Promega) from four to six experiments.
Western Blot Analysis
After transfection of HEK-293 cells with WT, mutant, or chimeric AIRE, samples were collected and separated on 10% SDS-PAGE gel and probed with a goat anti-AIRE antibody (Abcam, Cambridge, MA). In addition, a second rabbit antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was used to detect the AIRE proteins that had been pulled down using the biotin-INS-class I-VNTR probe. Signals were visualized using a chemiluminescence kit (Pierce). The BCA assay was performed for equal protein loading. Anti-actin was used as control. In the pulldown experiment, HEK-293 cells were transfected with WT, mutant, or chimeric AIRE for 72 h. Cell lysate (200 μg) was incubated with biotin-INS-class I-VNTR probe or unrelated probe (300 ng) overnight at 4 °C. The protein/DNA probe mixture was incubated with streptavidin-agarose (Thermo Scientific) for 1 h at room temperature and washed 4 times in pulldown buffer. The DNA-protein complex was subjected to SDS-PAGE. AIRE protein was detected by Western blot analysis using rabbit anti-AIRE antibody.
Confocal Microscopy Analysis
hTECs were seeded on coverslips and transfected with WT or mutant AIRE. After 72 h the cells were fixed with 4% formaldehyde and stained with rabbit anti-AIRE followed by Alexa Fluor 488-conjugated goat anti-rabbit IgG and DAPI. The coverslips were mounted and viewed using an inverted Zeiss LSM 510 microscope. Zeiss LSM and Axiovision software was used to analyze the images.
Statistical Analysis
All values were corrected and expressed relative to a control group. Results were presented as the mean ± S.D. Statistical analysis was assessed by one-way analysis of variance comparison of multiple groups using the Tukey-Kramer test with differences at a p value < 0.05 considered as significant.
Results
Construction of 12 AIRE Mutants Derived from APS-1 Patients
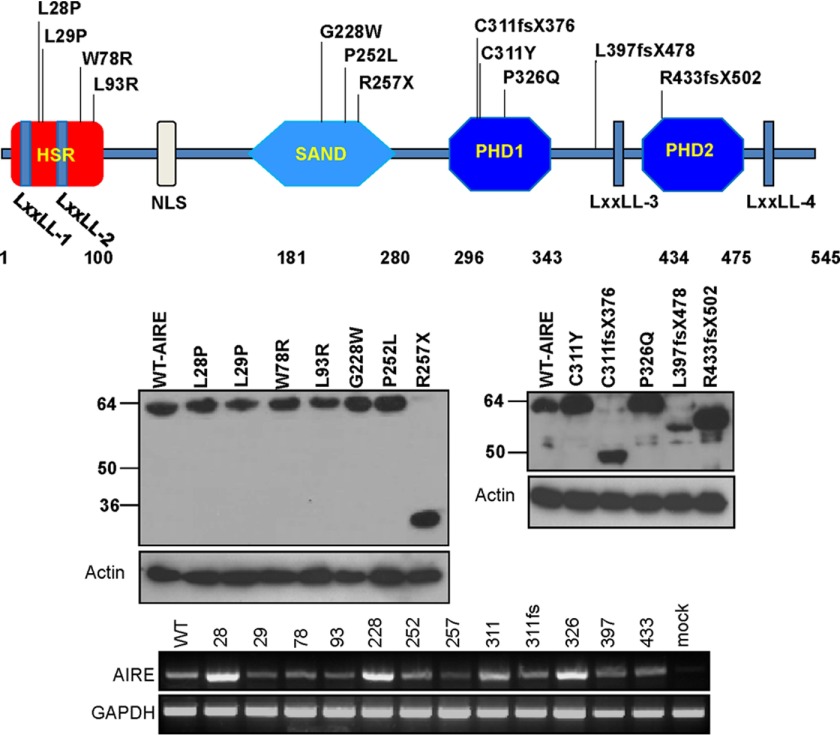
We selected 12 of the AIRE gene mutations spanning different functional domains to study which domain of AIRE is essential for INS-VNTR target activation (Fig. 1). The mutants were constructed using a site-directed mutagenesis kit and confirmed by DNA sequence analysis and protein expression. In Fig. 1, eight of the point mutations (L28P, L29P, W78R, L93R, G228W, P252L, C311Y, and P326Q) harbor a single amino acid substitution with identical protein molecular weight as shown by Western blot analysis (23–25). Four other mutations, R257X, C311fsX376, L397fsX478, and R433fsX502, introduce nonsense frameshift and premature stop codons that resulted in truncations of the AIRE protein and thus a smaller molecular weight (9, 26). The most prevalent mutation, R257X, is the common Finnish APECED mutation found in 83% of the Finnish APECED chromosomes (9). The other regions more susceptible to mutations are located in exons 2 and 10 (27). The transfected AIRE mRNA levels are shown using RT-PCR.
FIGURE 1.
Twelve AIRE mutants derived from APS-1 patients. The location of 12 mutations (above the chart) scattered around all four functional domains of AIRE protein was constructed in a mammalian expression vector. The chart displays each mutation that corresponds to the functional domain. The transfected and expressed WT and mutant AIREs in HEK-293 cells were shown by anti-AIRE Western blot analysis. Actin was shown as the loading control. The AIRE mRNA expression was shown by RT-PCR with GAPDH as an internal control.
Transcription Activation of INS-VNTR Target with AIRE or AIRE Mutants
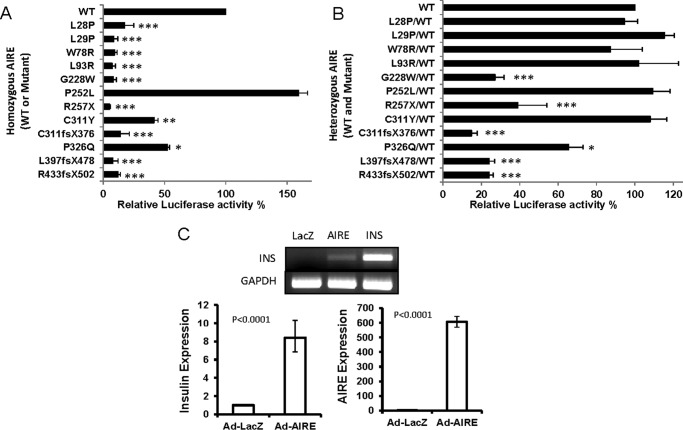
We measured the transcriptional activation of an INS-class III-VNTR-luciferase reporter using either WT or mutant AIRE. In Fig. 2A, the first four mutants (L28P, L29P, W78R, and L93R) located at the HSR domain displayed very little transcriptional activity. Because the HSR domain has been linked to the dimerization of AIRE (28), mutations in this domain may affect the formation of a functionally active complex for transcriptional regulation (7). The next three mutants (G228W, P252L, and R257X) are located at the SAND domain, which was previously suggested as the domain for DNA binding (9). Our reporter assay showed the G228W and R257X mutants completely lost their ability to activate the INS-class-III-VNTR, in contrast to the P252L mutant that has higher activity than WT-AIRE. The differences between these three mutations that are located in the same functional domain suggest that mutants G228W and R257X disrupt an essential function of AIRE, whereas the other mutant (P252L) retains or enhances its functionality. The mutants (C311Y and P326Q) lost ∼50% of the activity due to a single amino acid mutation. Although there is a decrease in efficiency of activation, the mutants partially retain their functionality. The other three mutants (C311fsX376, L397fsX478, and R433fsX502) contain a single amino acid mutation that resulted in a frameshift of the subsequent coding sequence and express truncated proteins. A 75–80% reduction in activity was measured. The 11 AIRE mutants show diminishing transcriptional activity of up to >90% of the WT activity except P252L, which shows higher activity than WT-AIRE (Fig. 2A).
FIGURE 2.
Transcriptional activities of INS-class III-VNTR-luciferase assay using WT or mutant AIRE in hTEC. A, each mutant or WT AIRE cDNA (as homozygous AIRE) was co-transfected with the INS-class III-VNTR reporter vector into hTEC cells. TK-Renilla was used as an internal control. The 12 mutants' transcriptional activities were normalized with TK-Renilla for transfection efficiency and calculated relative to the WT AIRE (as 100%). The relative activity versus WT AIRE showed high significance with a p value of <0.01 for P252L and P326Q and a p value of <0.001 for the remaining mutants. B, equal amount of each mutant mixed with WT AIRE (as heterozygous AIRE) were co-transfected with INS-class III-VNTR reporter vector as above and presented as calculated relative luciferase activity to WT/WT (as 100%). The p value shows: *, <0.05; **, <0.01: ***, <0.001. C, the hTECs were infected with either Ad-LacZ or Ad-AIRE or transfected with CMV-INS cDNA for 3 days. The AIRE and the endogenous human insulin gene expression were measured using RT-PCR and real time PCR.
We further tested the AIRE mutants co-transfected with WT-AIRE as the heterozygous form in regulating INS-class III-VNTR transcription. As shown in Fig. 2B, the first four HSR domain mutants when expressed with WT show no hindrance of WT-AIRE activation of INS-VNTR and have levels similar to that of WT/WT. This could be due to the inability of mutants L28P, L29P, W78R, and L93R to form a heterozygous dimer with WT-AIRE and consequently voiding the ability to hinder WT-AIRE functionality. Activation of INS-VNTR by C311Y/WT-AIRE is restored to the activity of WT/WT, indicating that this mutation and the mutants L28P, L29P, W78R, and L93R can function in an autosomal recessive fashion consistent with APS-1 patients. However, mutant 326 shows a 35% reduced activity, and the truncated AIREs R257X, C311fsX376, L397fsX478, and R433fsX502 show 75–80% reduced activities when mutant and WT form heterozygous complexes to activate the INS-VNTR. This drastic decrease of WT-AIRE activation suggests that these mutants and G228W may function in a dominant negative fashion like the G228W-mutant previously reported (19). Certain mutants do not exhibit blocking activity toward WT AIRE (such as L28P, L29P, W78R, L93R, P252L, C311Y, and P326Q) as they contain a relatively intact C-terminal portion of the AIRE, which is important for the INS-VNTR transcriptional activity. Four of the five dominant negative mutants (except G228W) have a truncated C terminus that lacks INS-VNTR transcriptional activity. Our study indicates that the integrity of the C terminus containing two PHD domains or at least the PHD2 domain and the last two LXXLL motifs is important for transcriptional activity.
AIRE Induces Endogenous Insulin Expression from hTEC
We further measured whether overexpression of AIRE in hTEC is capable of inducing the endogenous human insulin gene. First, we used transient transfection of AIRE and monitored insulin induction. RT-PCR analysis revealed the proper expression of WT or mutant AIRE transcripts; however, human insulin transcript was not induced (data not shown). Therefore, an adenoviral AIRE vector was used to infect hTEC versus Ad-LacZ negative control or transfection with a CMV-insulin cDNA as positive control (Fig. 2C). Overexpression of AIRE using an adenoviral vector is capable of inducing endogenous human insulin expression.
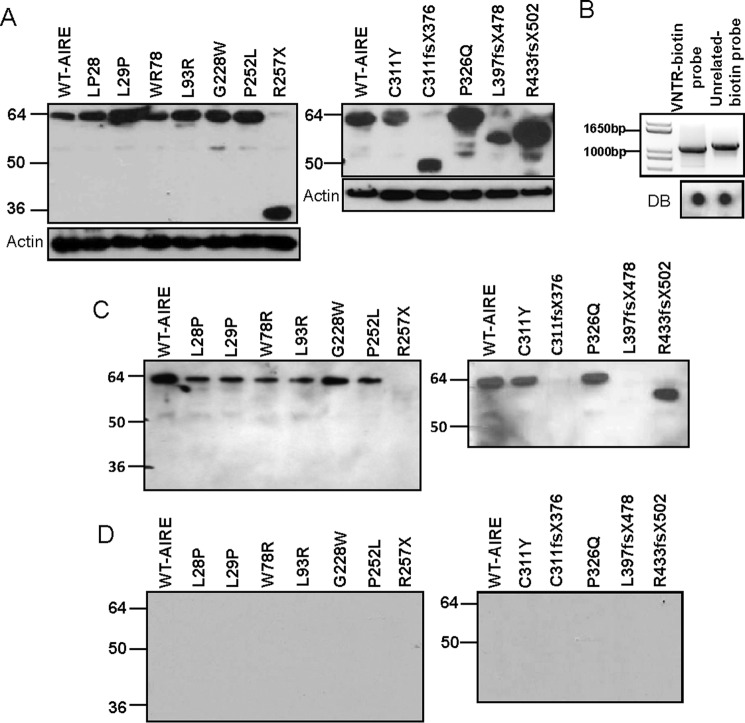
AIRE and Mutated AIRE Proteins That Retain the LXXLL-3 Motif Can Effectively Bind to the INS-VNTR Probe
As a transcription regulator, AIRE is capable of binding to DNA and interacting with common co-activators like CREB-binding protein (CBP) (29, 30). In our DNA pulldown assay, we showed that AIRE is capable of interacting with both the class I and III-VNTR insulin gene (16). In this study we examined mutations in different domains of AIRE that could affect the AIRE binding to a biotin-labeled DNA probe, thereby negating its ability to initiate INS-VNTR activation. For AIRE to effectively mediate the expression of insulin, it must first interact and bind with the INS-VNTR. Biotin-labeled primers spanning the human insulin gene that covers the 5′-class I VNTR and the insulin basal promoter region were designed along with a pair of unrelated biotin-labeled primers to produce a biotin-labeled control probe (Fig. 3B). Western blot analysis with an anti-AIRE antibody of AIRE-transfected cell lysate (Fig. 3A) and INS-VNTR probe (Fig. 3C) or unrelated control probe (Fig. 3D) with AIRE pulldown revealed that the AIRE protein binds to the DNA probe readily and not to the unrelated biotin probe. AIRE protein with point mutations in the HSR and PHD-1 domain L28P, L29P, W78R, L93R, C311Y, and P326Q are capable of binding the INS-VNTR DNA probe (Fig. 3C). The mutations G228W and P252L that cause mutations within the SAND domain do not impede the AIRE binding efficiency to the INS-VNTR probe, revealing that the G228W mutant does not disrupt AIRE binding affinity to INS-VNTR. Mutations R257X, C311fsX376, and L397fsX478 that cause truncations of the protein with loss of the LXXLL-3 motif are unable to bind to the INS-VNTR probe, whereas the R433fsX502 truncation, which retains the LXXLL-3, is able to bind supporting a possible role for LXXLL-3 motif in INS-VNTR binding.
FIGURE 3.
Biotin-labeled INS-VNTR probe pulldown assay. A, Western blot of WT or mutant AIRE transfected HEK-293 cell lysate with anti-AIRE or actin antibody shows AIRE protein expression in transfected cells. B, a biotin-labeled INS-VNTR probe and an unrelated probe were generated by PCR amplification. An anti-biotin dot blot (DB) of INS-VNTR and unrelated probes are shown underneath. C, anti-AIRE antibody Western blot analysis of biotin-INS-VNTR pulled-down complexes. The WT or mutant AIRE-transfected HEK-293 cell lysate was mixed with biotin-INS-class I-VNTR probe or biotin-unrelated probe separately at 4 °C overnight. The DNA-protein complex was pulled down by streptavidin beads, washed, and separated by SDS-PAGE. An anti-AIRE Western blot analysis revealed WT or mutant AIRE binding ability to the biotin-INS-class I-VNTR probe. D, anti-AIRE antibody Western blot analysis of unrelated biotin-probe pulldown.
Dominant Negative AIRE Mutants Do Not Affect INS-VNTR Target Binding
Heterozygous WT and R257X, C311fs, L397fs, or R433fs were co-transfected into HEK293 cells and subjected to biotin-labeled INS-VNTR or control probe pulldown assay (Fig. 4). R257X mutant lowers WT AIRE pulldown, whereas C311fs, L397fs, and R433fs mutants do not affect WT AIRE binding to the INS-VNTR target. In contrast, WT AIRE retained L397fs mutant binding activity to the INS-VNTR target probably by forming the protein complex with WT AIRE.
FIGURE 4.
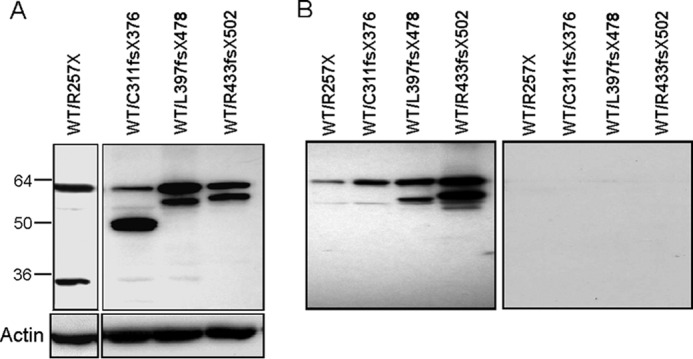
Dominant negative AIRE mutants do not affect WT AIRE binding. A, heterozygous WT and R257X, C311fs, L397fs, or R433fs were co-transfected into HEK293 cells and showed as Western blot analysis. B, the WT and mutant AIRE lysates were subjected to biotin-labeled INS-VNTR or control probe pulldown assay.
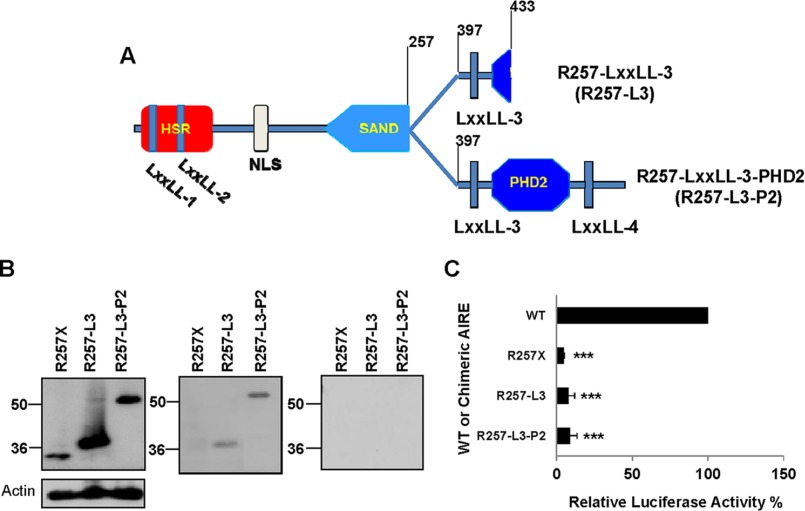
Chimeric AIRE Demonstrates That the LXXLL-3 Motif Is Essential for INS-VNTR Binding
From the binding study, all three AIRE mutants, R257X, C311fsX376, and L397fsX478, failed to bind to the biotin-labeled INS-VNTR DNA probe. Consistently, these mutants are truncated AIRE without the LXXLL-3 motif. To determine if the LXXLL-3 motif is actually important for AIRE and INS-VNTR binding, we constructed two chimeric AIRE proteins, R257-LXXLL-3 and R257-LXXLL-3-PHD2 (Fig. 5A). Testing the role of the LXXLL-3 motif in AIRE interaction with INS-VNTR, we performed either the INS-VNTR or control probe with the chimeric AIRE pulldown assay. Fig. 5B shows that chimeric AIRE R257-LXXLL-3 and R257-LXXLL-3-PHD2 are capable of binding to the INS-VNTR probe as they all contain the LXXLL-3 motif. The R257-LXXLL-3 is capable of binding the INS-VNTR without the amino acids 258–396 or 434–545, which contains the PHD1, PHD2, and LXXLL-4 motif. It appears that the LXXLL-3 motif (amino acids 398–433) is important for the DNA binding ability of AIRE to INS-VNTR target, whereas the other portion of the C-terminal end is not needed. Our data reveal for the first time that using the specific INS-VNTR target, the SAND domain has little to do with DNA binding; instead, the LXXLL-3 motif contributes to the INS-VNTR DNA binding.
FIGURE 5.
LXXLL-3 motif is essential for INS-VNTR binding, and PHD1, LXXLL-3, PHD2, and LXXLL-4 motifs are critical for transcriptional activation. A, chimeric AIRE R257-LXXLL-3 (R257-L3) composed of amino acids 1–257 and 397–433 and chimeric R257-LXXLL-3-PHD2 (R257-L3-P2) composed of amino acids 1–257 and 397–545. B, Western blot analysis of R257X mutant and the chimeric AIREs (R257X-L3 and R257-L3-P2) to confirm the expression of AIRE proteins (left). Biotin-labeled INS-class I-VNTR probe pulldown assay was performed to show whether the chimeric AIRE can restore binding activity (right). C, the WT, mutant, and chimeric AIREs are subjected to an assay for transcriptional activity of INS-class III-VNTR reporter in hTEC. Renilla was used as an internal control. Chimeric AIRE transcriptional activities were calculated relative to the WT AIRE (as 100%). The significance of mutant and chimeric AIRE versus WT AIRE has a p value of <0.001 (***).
Both PHD1 and PHD2 Domains Are Important for INS-VNTR Activation
In Fig. 2A, WT and mutant AIRE shows that the PHD1 and LXXLL-3 motif alone are not sufficient to maintain INS-VNTR transcriptional activation as the R433fsX502 mutation contain both motifs but lack the PHD2 domain and failed to activate INS-VNTR. The two chimeric AIREs (R257-LXXLL-3 and R257-LXXLL-3-PHD2; Fig. 5A) were also used to evaluate whether the LXXLL-3 motif and PHD2 domain could restore AIRE transcriptional activation of INS-class III-VNTR-luciferase in the absence of the PHD1 domain. Each chimeric AIRE was transfected into hTEC with INS-class III-VNTR-luciferase and compared with the transcriptional activation with WT-AIRE. Fig. 5C shows that the chimeric AIRE R257-LXXLL-3 and R257-LXXLL-3-PHD2 are incapable of initiating transactivation of the INS-VNTR. This result demonstrates that the addition of the LXXLL-3 motif and/or PHD2 domain in the absence of the PHD1 domain is unable to restore transcriptional activity to the INS-VNTR. The entire C-terminal end from amino acids 296 to the end, which expresses the PHD1, LXXLL-3, PHD2, and LXXLL-4, are important and work together to facilitate the INS-VNTR activation.
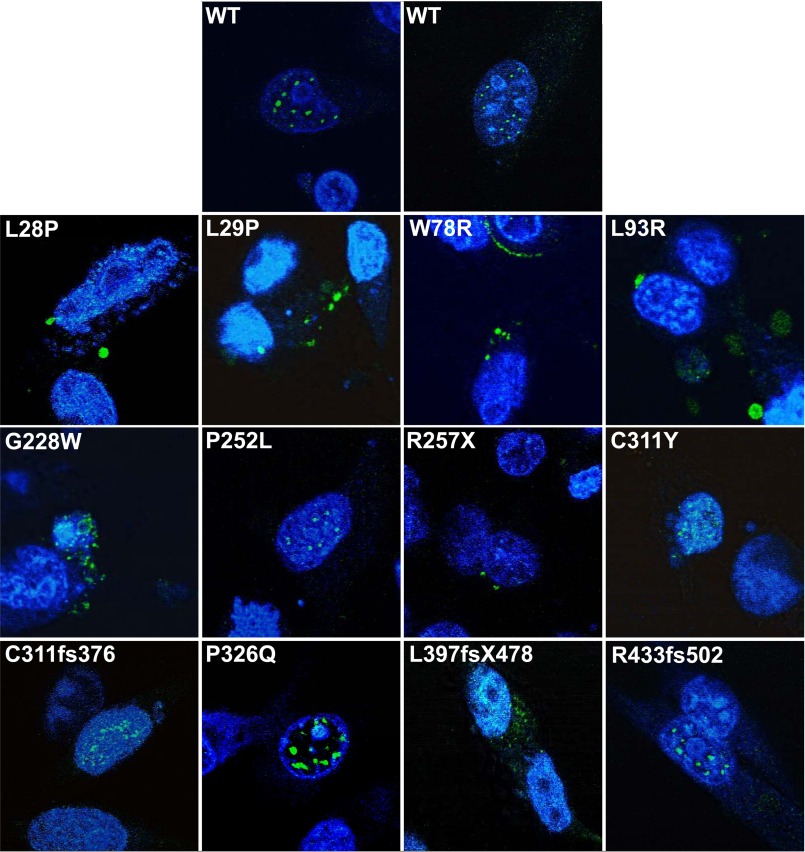
Mutation in Different Functional Domains Interferes with AIRE Cellular Localization and the Formation of Clusters in the Nucleus
AIRE transportation into the nucleus is important to its function as a transcription factor. If a mutation hinders the ability of AIRE to localize in the nucleus, it is then incapable of modulating gene expression of the INS-VNTR. Fig. 6 shows that the WT-AIRE protein localized in the nucleus of hTEC and in concentrated specks, forming nuclear bodies, but can also be found dispersed in the cytoplasm. Mutated AIRE protein with point mutations in the HSR domain (L28P, L29P, W78R, and L93R) are distributed throughout the cytoplasm with some presence in the nucleus but not found in the concentrated specks as WT-AIRE, which is consistent with HSR domain mutations causing drastic decreases in INS-VNTR activation. Our study showed cytoplasm distribution of L28P AIRE in clusters and was not as apparent as previously reported as totally diffuse in the cytoplasm and nucleus (31, 32). The discrepancy could be due to the cell type difference and/or fluorescence-tagged fusion L28P (7). Mutations in the SAND domain G228W and P252L did not prevent localization in the nucleus, whereas R257X only localized in the cytoplasm (Fig. 6). Mutant G228W had a more dispersed pattern in the nucleus with nuclear bodies in the cytoplasm, whereas P252L formed the concentrated specks in the nucleus similar to WT-AIRE and retained higher transcriptional activation activity. PHD1 mutations, C311Y, C311fsX376, and P326Q localized in the nucleus with a combination of nuclear bodies and a diffused pattern, whereas L397fsX478 had a more dispersed pattern throughout the nucleus and cytoplasm. PHD2 mutation R433fsX502 localized in the nucleus and cytoplasm. AIRE mutations in certain domains resulted in inhibition of AIRE nuclear localization or disruption of a concentrated nuclear body aggregate pattern, which may contribute to the inactivity on the INS-VNTR target gene. Pitkänen et al. (32) observed that leptomycin B inhibits the CRM-1-mediated nuclear export pathway causing increased nuclear localization of AIRE and the importance of N-terminal in the nuclear export. We performed experiments to evaluate the effect of leptomycin B on AIRE to regulate the INS-VNTR transcription. Even leptomycin B is effective in increasing AIRE nuclear localization; it is non-functional in activating INS-VNTR transcription with either WT or mutant AIRE (data not shown).
FIGURE 6.
Cellular localization and nuclear pattern study of mutated AIRE in hTEC. The WT or mutated AIRE proteins were expressed in hTEC by transient transfection for 2 days, fixed, treated with anti-AIRE antibody, and stained with Alexa Fluor 488 secondary antibody. The nucleus of the cell is counterstained with DAPI.
Discussion
AIRE and INS-VNTR play a role in the differential expression of the human insulin gene in thymus and subsequently dictates insulin tolerance or autoimmunity induction. Therefore, it is highly significant to dissect the mechanism by which they interact and function as a modulator of insulin gene in thymus. The structurally defined functional domains in AIRE are a concrete starting point to investigate what is essential in AIRE to regulate insulin expression. In this study mutations in each domain of AIRE drastically reduced transactivation activity of AIRE on the INS-VNTR promoter except for one point mutation (P252L) in the SAND domain. Heterozygous expression of WT and mutated AIRE caused a decrease in transactivation activity when expressed with mutations in the SAND, PHD1, and PHD2 domains but not in the HSR domain. We identified five dominant negative mutations in the SAND (G228W and R257X), PHD1 (C311fsX376 and L397fsX478), and PHD2 (R433fsX502) for the INS-VNTR activation. In APS-1, the G228W mutation functions in a dominant negative fashion whose loss of transcriptional activity was defined by a conformational change in AIRE (19). The G228W mutation was first reported as a dominant negative mutation and was found in an Italian kindred with APS-1, acting in a dominant fashion and strongly co-segregating with hypothyroid autoimmune thyroiditis (33). An animal study using a G228W knock-in mouse model has shown that this variant acted in a dominant negative manner to cause a unique autoimmune syndrome (34). A recent report has identified multiple cases and families with non-allelic mutations in AIRE with dominant inheritance (31). They studied the AIRE dominant negative mutations in the PHD1 domain of APS-1 patients. Interestingly, we have identified four other dominant negative mutants that are specific to the INS-VNTR target. The additional dominate negative mutations identified in this study in AIRE when acting on the INS-VNTR transcriptional activation are mostly due to the truncations in the C terminus and hints to its importance in transcriptional activation of INS-VNTR. The importance of the C terminus in transcriptional activation is not unexpected because the PHD2 domain is essential for AIRE transcriptional activation and is located at the end of the AIRE protein (35). However, our study identifies that the C terminus is also involved in INS-VNTR DNA binding. Mutations in AIRE that retain the LXXLL-3 motif are capable of interacting with the INS-class I-VNTR probe denoting it is essential for INS-VNTR binding. The SAND domain has been implicated as the region of DNA binding for AIRE, with the KNKA motif (amino acids 244–247) responsible for this interaction because of its similarity to the KNWK DNA modules in other SAND domains (9). In contrast, SAND domain-mediated binding has been found to be nonessential to the recruitment of AIRE to TRA genes in chromatin (36).
In our study we determined that the AIRE protein peptide located at amino acids 397–433 houses the LXXLL-3 motif and is essential for AIRE binding to the INS-VNTR promoter aside from other amino acid sequences that may contribute to its binding activity. The LXXLL motif was originally associated with protein-protein interactions that occur in cell signaling, cell adhesion, and regulation of transcription and translation (37). AIRE contains four LXXLL motifs that are positioned throughout the AIRE protein and the function of LXXLL-2 and LXXLL-4 has been described in different mechanisms that promote or regulate transcription (35). By utilizing chimeric AIREs, we were able to determine that the LXXLL-3 motif is essential for AIRE binding to the INS-VNTR promoter. To further analyze the functional domains important for the transcriptional activation, we compared four mutants with diminished transcriptional activity, R257X, C311fsX376, L397fsX478, and R433fsX502. R433fsX502 has minimal truncations mostly in the PHD2 and LXXLL-4 but still lacked transcriptional activity. Therefore, we generated another chimeric AIRE with the inclusion of LXXLL-3, PHD2, and LXXLL-4 (amino acid 397–545) to evaluate whether they can restore the binding as well as transcriptional activity. To our surprise, the AIRE peptide sequence (amino acid 397–545), although restoring the binding activity, did not restore the transcriptional activity, suggesting that the N-terminal portion of the AIRE including PHD1, LXXLL-3, PHD2, and LXXLL-4 is required for transcriptional activity on the INS-VNTR target. Proteins such as unmodified histone H3K4 (H3K4me0) and DNA-dependent protein kinase (DNA-PK) are needed for the AIRE recruitment and activity with TRA genes and have been shown to interact with the AIRE PHD1 domain (32, 38–40).
We have identified two critical components in AIRE that regulate insulin gene expression in thymic epithelial cells. One is the LXXLL-3 motif that is essential for DNA binding. The other is the C-terminal sequence (amino acid 296–545) critical for transcriptional activation of INS-VNTR. However, a group of mutants in the HSR domain (L28P, L29P, W78R, and L93R) contain both the LXXLL-3 motif and C-terminal sequence (amino acid 296–545) but still lack transcriptional activity. The N terminus of AIRE contains the HSR domain, two LXXLL motifs, and a nuclear localization sequence. The nuclear localization sequence is responsible for nuclear localization, but the HSR has a nuclear export signal that can function in the absence of the nuclear localization sequence. It is believed that the shuttling of AIRE between the nucleus and the cytoplasm may play a role in the regulatory mechanisms for AIRE function (32). Our cellular localization data revealed that mutations in the HSR domain disrupted AIRE localization and pattern formation in the nucleus of hTEC revealing an unambiguous reason for the drastic decrease of transcriptional activation of AIRE on the INS-VNTR. The AIRE ability to localize in the nucleus is important to its function as a transcription factor. If AIRE is unable to localize in the nucleus, the activation activity would be impaired. Other mutations, C311fsX376 and R433fsX502, although they retain a nuclear localization pattern still displayed a drastically decreased transcriptional activation probably due to the protein truncation of PHD1/2 domain and/or the loss of DNA binding capacity. The C311Y and P326Q point mutations have some disruptions of the nuclear body pattern formation, which could have contributed to the 59 and 48% decrease in transcriptional activation. The cellular localization is disrupted in mutants G228W, R257X, and L397fsX478, and the transcriptional activation was also diminished. Thus, all three components including the DNA binding region, transcriptional activation domain, and the nuclear localization pattern are important to maintain the AIRE function in modulation of the INS-VNTR target.
Author Contributions
A. E. S. researched the data, wrote the manuscript, and contributed to discussions. C. C. researched the data and reviewed and edited the manuscript. M. B. B. reviewed and edited the manuscript and contributed to discussions. M. S. L. designed the experiments, edited the manuscript, and contributed to discussions.
Acknowledgment
We acknowledge the contribution of Dr. C. Q. Cai (previous postdoctoral fellow) for making some of the mutant AIRE constructs.
This work was supported by American Diabetes Association Research Grant ADA-1-13-BS-101 (to M. S. L.) and in part by the Research Institute for Children, Children's Hospital, New Orleans, LA. Part of the data was presented as a poster in the 74th Scientific Sessions of the American Diabetes Association annual meeting, June 13–17, 2014 in San Francisco. The authors declare that they have no conflicts of interest with the contents of this article.
- T1D
- type 1 diabetes
- APS-1 (APECED)
- autoimmune polyendocrinopathy type 1 syndrome
- AIRE
- autoimmune regulator
- HSR
- homogenously staining region
- PHD
- plant homeodomain
- TRA
- tissue-restricted antigen
- hTEC
- human thymic epithelial cell line
- SAND
- Sp100, AIRE-1, NucP41/75, DEAF-1.
References
- 1. Palmer J. P., Asplin C. M., Clemons P., Lyen K., Tatpati O., Raghu P. K., and Paquette T. L. (1983) Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science 222, 1337–1339 [DOI] [PubMed] [Google Scholar]
- 2. Kuglin B., Gries F. A., and Kolb H. (1988) Evidence of IgG autoantibodies against human proinsulin in patients with IDDM before insulin treatment. Diabetes 37, 130–132 [DOI] [PubMed] [Google Scholar]
- 3. Ahonen P. (1985) Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED): autosomal recessive inheritance. Clin. Genet. 27, 535–542 [DOI] [PubMed] [Google Scholar]
- 4. Ahonen P., Myllärniemi S., Sipilä I., and Perheentupa J. (1990) Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N. Engl. J. Med. 322, 1829–1836 [DOI] [PubMed] [Google Scholar]
- 5. Finnish-German APECED Consortium (1997) An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains: The Finnish-German APECED Consortium Atuoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy. Nat. Genet. 17, 399–403 [DOI] [PubMed] [Google Scholar]
- 6. Nagamine K., Peterson P., Scott H. S., Kudoh J., Minoshima S., Heino M., Krohn K. J., Lalioti M. D., Mullis P. E., Antonarakis S. E., Kawasaki K., Asakawa S., and Ito F. (1997) Positional cloning of the APECED gene. Nat. Genet. 17, 393–398 [DOI] [PubMed] [Google Scholar]
- 7. Halonen M., Kangas H., Rüppell T., Ilmarinen T., Ollila J., Kolmer M., Vihinen M., Palvimo J., Saarela J., Ulmanen I., and Eskelin P. (2004) APECED-causing mutations in AIRE reveal the functional domains of the protein. Hum. Mutat. 23, 245–257 [DOI] [PubMed] [Google Scholar]
- 8. Ferguson B. J., Alexander C., Rossi S. W., Liiv I., Rebane A., Worth C. L., Wong J., Laan M., Peterson P., Jenkinson E. J., Anderson G., Scott H. S., Cooke A., and Rich T. (2008) Aire's CARD revealed, a new structure for central toleerance provikes transcriptional plasticity. J. Biol. Chem. 283, 1723–1731 [DOI] [PubMed] [Google Scholar]
- 9. Björses P., Halonen M., Palvimo J. J., Kolmer M., Aaltonen J., Ellonen P., Perheentupa J., Ulmanen I., and Peltonen L. (2000) Mutations in the AIRE gene: effects on subcellular location and transactivation function of the autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy protein. Am. J. Hum. Genet. 66, 378–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bottomley M. J., Stier G., Pennacchini D., Legube G., Simon B., Akhtar A., Sattler M., and Musco G. (2005) NMR structure of the first PHD ginger of autoimmune regulator protein (AIRE1). Insights into autoimmune polyendocrinopathy-candidiasis-ectodermal dynstrophy (APECED) disease. J. Biol. Chem. 280, 11505–11512 [DOI] [PubMed] [Google Scholar]
- 11. Uchida D., Hatakeyama S., Matsushima A., Han H., Ishido S., Hotta H., Kudoh J., Shimizu N., Doucas V., Nakayama K. I., Kuroda N., and Matsumoto M. (2004) AIRE functions as an E3 ubiquitin ligase. J. Exp. Med. 199, 167–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heery D. M., Kalkhoven E., Hoare S., and Parker M. G. (1997) A signature motif in transciptional co-activators mediates binding to nuclear receptors. Nature 387, 733–736 [DOI] [PubMed] [Google Scholar]
- 13. Derbinski J., Gäbler J., Brors B., Tierling S., Jonnakuty S., Hergenhahn M., Peltonen L., Walter J., and Kyewski B. (2005) Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J. Exp. Med. 202, 33–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kyewski B., and Peterson P. (2010) Aire, master of many trades. Cell 140, 24–26 [DOI] [PubMed] [Google Scholar]
- 15. Peterson P., Org T., and Rebane A. (2008) Transcriptional regulation by AIRE: molecular mechanisms of central tolerance. Nat. Rev. Immunol. 8, 948–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cai C. Q., Zhang T., Breslin M. B., Giraud M., and Lan M. S. (2011) Both polymorphic variable number of tandem repeats and autoimmune regulator modulate differential expression of insulin in human thymic epithelial cells. Diabetes 60, 336–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fan Y., Rudert W. A., Grupillo M., He J., Sisino G., and Trucco M. (2009) Thymus-specific deletion of insulin induces autoimmune diabetes. EMBO J. 28, 2812–2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anderson M. S., Venanzi E. S., Klein L., Chen Z., Berzins S. P., Turley S. J., von Boehmer H., Bronson R., Dierich A., Benoist C., and Mathis D. (2002) Projection of an immunological self shadow within the thymus by the aire protein. Science 298, 1395–1401 [DOI] [PubMed] [Google Scholar]
- 19. Ilmarinen T., Eskelin P., Halonen M., Rüppell T., Kilpikari R., Torres G. D., Kangas H., and Ulmanen I. (2005) Functional analysis of SAND mutations in AIRE supports dominant inheritance of the G228W mutation. Hum. Mutat. 26, 322–331 [DOI] [PubMed] [Google Scholar]
- 20. Awata T., Kawasaki E., Ikegami H., Kobayashi T., Maruyama T., Nakanishi K., Shimada A., Iizuka H., Kurihara S., Osaki M., Uga M., Kawabata Y., Tanaka S., Kanazawa Y., and Katayama S. (2007) Insulin gene/IDDM2 locus in Japanese type 1 diabetes: contribution of class I alleles and influence of class I subdivision in susceptibility to type 1 diabetes. J. Clin. Endocrinol. Metab 92, 1791–1795 [DOI] [PubMed] [Google Scholar]
- 21. Fernández E., Vicente A., Zapata A., Brera B., Lozano J. J., Martínez C., and Toribio M. L. (1994) Establishment and characterization of cloned human thymic epithelial cell lines. Analysis of adhesion molecule expression and cytokine production. Blood 83, 3245–3254 [PubMed] [Google Scholar]
- 22. Giraud M., Taubert R., Vandiedonck C., Ke X., Lévi-Strauss M., Pagani F., Baralle F. E., Eymard B., Tranchant C., Gajdos P., Vincent A., Willcox N., Beeson D., Kyewski B., and Garchon H. J. (2007) An IRF8-binding promoter variant and AIRE control CHRNA1 promiscuous expression in thymus. Nature 448, 934–937 [DOI] [PubMed] [Google Scholar]
- 23. Cihakova D., Trebusak K., Heino M., Fadeyev V., Tiulpakov A., Battelino T., Tar A., Halász Z., Blümel P., Tawfik S., Krohn K., Lebl J., and Peterson P. (2001) Novel AIRE mutations and P450 cytochrome autoantibodies in Central and Eastern European patients with APECED. Hum. Mutat. 18, 225–232 [DOI] [PubMed] [Google Scholar]
- 24. Heino M., Scott H. S., Chen Q., Peterson P., Mäebpää U., Papasavvas M. P., Mittaz L., Barras C., Rossier C., Chrousos G. P., Stratakis C. A., Nagamine K., Kudoh J., Shimizu N., Maclaren N., Antonarakis S. E., and Krohn K. (1999) Mutation analyses of North American APS-1 patients. Hum. Mutat. 13, 69–74 [DOI] [PubMed] [Google Scholar]
- 25. Ward L., Paquette J., Seidman E., Huot C., Alvarez F., Crock P., Delvin E., Kämpe O., and Deal C. (1999) Severe autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy in an adolescent girl with a novel AIRE mutation: response to immunosuppressive therapy. J. Clin. Endocrinol. Metab 84, 844–852 [DOI] [PubMed] [Google Scholar]
- 26. Wang C. Y., Davoodi-Semiromi A., Huang W., Connor E., Shi J. D., and She J. X. (1998) Characterization of mutations in patients with autoimmune polyglandular syndrome type 1 (APS1) Hum. Genet. 103, 681–685 [DOI] [PubMed] [Google Scholar]
- 27. Heino M., Peterson P., Kudoh J., Shimizu N., Antonarakis S. E., Scott H. S., and Krohn K. (2001) APECED mutations in the autoimmune regulator (AIRE) gene. Hum. Mutat. 18, 205–211 [DOI] [PubMed] [Google Scholar]
- 28. meloni A., Fiorillo E., Corda D., Perniola R., Cao A., and Rosatelli M. C. (2005) Two novel mutations of the AIRE protein affecting its homodimerization properties. Hum. Mutat. 25, 319–326 [DOI] [PubMed] [Google Scholar]
- 29. Pitkänen J., Doucas V., Sternsdorf T., Nakajima T., Aratani S., Jensen K., Will H., Vähämurto P., Ollila J., Vihinen M., Scott H. S., Antonarakis S. E., Kudoh J., Shimizu N., Krohn K., and Peterson P. (2000) The autoimmune regulator protein has transcriptional transactivating properties and interacts with the common coactivator CREB-binding protein. J. Biol. Chem. 275, 16802–16809 [DOI] [PubMed] [Google Scholar]
- 30. Kumar P. G., Laloraya M., Wang C. Y., Ruan Q. G., Davoodi-Semiromi A., Kao K. J., and She J. X. (2001) The autoimmune regulator (AIRE) is a DNA-binding protein. J. Biol. Chem. 276, 41357–41364 [DOI] [PubMed] [Google Scholar]
- 31. Oftedal B. E., Hellesen A., Erichsen M. M., Bratland E., Vardi A., Perheentupa J., Kemp E. H., Fiskerstrand T., Viken M. K., Weetman A. P., Fleishman S. J., Banka S., Newman W. G., Sewell W. A., Sozaeva L. S., et al. (2015) Dominant Mutations in the autoimmune regulator AIRE are associated with common organ-specific autoimmune diseases. Immunity 42, 1185–1196 [DOI] [PubMed] [Google Scholar]
- 32. Pitkänen J., Vähämurto P., Krohn K., and Peterson P. (2001) Subcellular localization of the autoimmune regulator protein. characterization of nuclear targeting and transcriptional activation domain. J. Biol. Chem. 276, 19597–19602 [DOI] [PubMed] [Google Scholar]
- 33. Cetani F., Barbesino G., Borsari S., Pardi E., Cianferotti L., Pinchera A., and Marcocci C. (2001) A novel mutation of the autoimmune regulator gene in an Italian kindred with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy, acting in a dominant fashion and strongly cosegregating with hypothyroid autoimmune thyroiditis. J. Clin. Endocrinol. Metab. 86, 4747–4752 [DOI] [PubMed] [Google Scholar]
- 34. Su M. A., Giang K., Zumer K., Jiang H., Oven I., Rinn J. L., Devoss J. J., Johannes K. P., Lu W., Gardner J., Chang A., Bubulya P., Chang H. Y., Peterlin B. M., and Anderson M. S. (2008) Mechanisms of an autoimmunity syndrome in mice caused by a dominant mutation in Aire. J. Clin. Invest. 118, 1712–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meloni A., Incani F., Corda D., Cao A., and Rosatelli M. C. (2008) Role of PHD fingers and COOH-terminal 30 amino acids in AIRE transactivation activity. Mol. Immunol. 45, 805–809 [DOI] [PubMed] [Google Scholar]
- 36. Žumer K., Low A. K., Jiang H., Saksela K., and Peterlin B. M. (2012) Unmodified histone H3K4 and DNA-dependent protein kinase recruit autoimmune regulator to target genes. Mol. Cell. Biol. 32, 1354–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Plevin M. J., Mills M. M., and Ikura M. (2005) The LXXLL motif: a multifunctional binding sequence in transcriptional regulation. Trends Biochem. Sci. 30, 66–69 [DOI] [PubMed] [Google Scholar]
- 38. Koh A. S., Kuo A. J., Park S. Y., Cheung P., Abramson J., Bua D., Carney D., Shoelson S. E., Gozani O., Kingston R. E., Benoist C., and Mathis D. (2008) Aire employs a histone-binding module to mediate immunological tolerance, linking chromatin regulation with organ-specific autoimmunity. Proc. Natl. Acad. Sci. U.S.A. 105, 15878–15883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Org T., Chignola F., Hetényi C., Gaetani M., Rebane A., Liiv I., Maran U., Mollica L., Bottomley M. J., Musco G., and Peterson P. (2008) The autoimmune regulator PHD finger binds to non-methylated histone H3K4 to activate gene expression. EMBO reports 9, 370–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liiv I., Rebane A., Org T., Saare M., Maslovskaja J., Kisand K., Juronen E., Valmu L., Bottomley M. J., Kalkkinen N., and Peterson P. (2008) DNA-PK contributes to the phosphorylation of AIRE: importance in transcriptional activity. Biochim. Biophys Acta 1783, 74–83 [DOI] [PMC free article] [PubMed] [Google Scholar]