Abstract
The m.3243A > G mutation is the most prevalent, disease-causing mitochondrial DNA (mtDNA) mutation. In a national cohort study of 48 families harbouring the m.3243A > G mutation, we identified three families in which the mutation appeared to occur sporadically within these families. In this report we describe these three families. Based on detailed mtDNA analysis of three different tissues using two different quantitative pyrosequencing assays with sensitivity to a level of 1% mutated mtDNA, we conclude that the m.3243A > G mutation has arisen de novo in each of these families. The symptomatic carriers presented with a variety of symptoms frequently observed in patients harbouring the m.3243A > G mutation. A more severe phenotype is seen in the de novo families compared to recent cohort studies, which might be due to reporting bias.
The observation that de novo m.3243A > G mutations exist is of relevance for both diagnostic investigations and genetic counselling. Firstly, even where there is no significant (maternal) family history in patients with stroke-like episodes, diabetes and deafness or other unexplained organ dysfunction, the m.3243A > G mutation should be screened as a possible cause of the disease. Second, analysis of maternally-related family members is highly recommended to provide reliable counselling for these families, given that the m.3243A > G mutation may have arisen de novo.
Abbreviations: mtDNA, mitochondrial DNA; MELAS, mitochondrial myopathy, encephalopathy, lactate acidosis and stroke-like episodes; MIDD, maternally inherited diabetes and deafness; MERRF, myoclonic epilepsy with ragged-red fibres
Keywords: Inheritance; m.3243A > G mutation; Mitochondrial myopathy, encephalopathy, lactate acidosis and stroke-like episodes (MELAS); Maternally inherited diabetes and deafness (MIDD); Genetic counselling
Highlights
-
•
De novo m.3243A > G mutations are more frequent than previously reported.
-
•
Even in absence of a family history, the. m.3243A > G mutation should be considered.
-
•
Testing maternally-related family members is needed to provide reliable counselling.
1. Introduction
In recent years, a large body of data concerning the inheritance of mitochondrial disorders has been published [1], [2], [3]. Knowledge relating to the mode of inheritance has great importance for both the diagnosis and counselling of patients. Due to the involvement of the nuclear and mitochondrial genomes, mitochondrial diseases can be transmitted in a Mendelian manner, in the case of a nuclear aetiology, be maternally transmitted in the case of primary mitochondrial DNA (mtDNA) defects or they may occur sporadically [4].
In the case of single, large-scale mtDNA deletions such as those observed in patients with the Kearns-Sayre Syndrome, Chronic Progressive External Ophthalmoplegia or Pearson's Syndrome, occurrence is usually sporadic and recurrence in the offspring of female carriers is limited to 4% of the patients [5]. Our study focuses on the m.3243A > G MTTL1 gene mutation, first described by Goto, Nonaka and Horai [6] as a cause of the mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes (MELAS) syndrome but which also causes maternally-inherited diabetes and deafness (MIDD) [7]. Other clinical phenotypes include cardiac, ocular, gastrointestinal and renal involvement. The m.3243A > G mutation is the most prevalent, multi-system disease-causing mitochondrial DNA mutation, with a reported mutation prevalence of between 7.59 and 236 per 100,000 [8], [9], [10].
A considerable number of case series and small cohorts have been published describing the phenotypic expression of the m.3243A > G mutation [11], [12], [13], [14], [15], [16], [17]. In contrast to what is seen for other mtDNA mutations, the sporadic occurrence of the m.3243A > G mutation, with an expressed phenotype, is rare with only four de novo cases published to date [18], [19], [20], [21] (see Table 1).
Table 1.
An overview of all de novo reports of the m.3243A > G mutation.
| Report: author (year) | Gender and age at onset | Probands clinical sign & symptoms | Heteroplasmy levels | Number of tested maternal family members and tested tissues | Special characteristics |
|---|---|---|---|---|---|
| Yamamoto (1995) | m, 21 years | MELAS syndrome; SLE, mild deafness, weakness, | Muscle: 89% | 3; all studied in muscle. | Mother was the de novo patient experiencing MIDD. (muscle 79%, blood 10%) |
| Blood: 36% | |||||
| Campos et al. (1996) | m, 2 years | MELAS/MERRF overlap syndrome; epilepsy, SLE, weakness, psychomotor delay | Muscle: 70% | 4; all studied in blood, 1 studied in muscle, 1 studied in hair | |
| Blood: 30% | |||||
| Ko et al. (2001) | m, 5 years | MELAS syndrome; epilepsy, SLE, ataxia, blurred speech, paralytic ileus, | Muscle: 54% | 6; all studied in blood, hair and buccal saliva | Mother was the de novo patient experiencing mild deafness, (blood 11%, hair 27%, buccal saliva 32%); the younger brother was a dormant carrier. (blood 65%, hair 79%, buccal saliva 70%) |
| Blood: 56% | |||||
| Hair: 70% | |||||
| Buccal saliva: 64% | |||||
| Maassen et al. (2002) | f, 8 years | MIDD; bilateral deafness, diabetes, hypertension, proteinuria | Blood: 18% | 4; all studied in blood and buccal saliva | |
| Buccal saliva: 55% | |||||
| Patient 1 (this study) | m, 34 years | MELAS syndrome; aphasia, encephalopathy, deafness, SLE, epilepsy, myopathy. | Muscle: 82% | 16; all studied in blood, UEC and buccal saliva | |
| Blood: 23% | |||||
| UEC: 63% | |||||
| Buccal saliva: 40 | |||||
| Patient 2 (this study) | m, 1 year | Transient hypotonia, ataxia, ptosis and ophthalmoplegia, motor retardation. Improvement to normal at age 8. | Muscle: 23% | 6; all studied in blood, UEC and buccal saliva | Mother was the de novo patient, without clinical symptoms. (UEC 6%, Muscle 5%, undetectable in blood and buccal saliva) |
| UEC: 38% | |||||
| Buccal saliva: 27% | |||||
| Patient 3 (this study | m, 1 day | Foetal distress, transient tachypneu of the neonate, transient left ventricular hypertrophy. | Muscle: 12% | 3; all studied in blood, UEC and buccal saliva | A sibling died post vaccination at age 4 months. No mutation load was found in muscle. |
| Blood: 16% | |||||
| UEC: 20% | |||||
| Buccal saliva:16% |
m = male, f = female, SLE = stroke-like episodes, UEC = urinary epithelial cells.
In our own cohort of Dutch m.3243A > G mutation carriers, we identified three families where the m.3243A > G mutation appears to have arisen de novo. The purpose of this paper is to document these families, describing the probands' presentation in detail and highlighting the family members in whom the m.3243A > G mutation was not detected. Maternal inheritance in a pedigree is a trigger for clinicians to consider the presence of an mtDNA mutation but even if there is no significant family history in the maternal line, the m.3243A > G mutation should be considered in patients with symptoms consistent with the MIDD or MELAS syndromes.
2. Methods
All patients participated in the m.3243A > G mutation cohort study at the Radboud Center for Mitochondrial Medicine [22]. 135 patients from 48 families were included in this study. The study was approved by the ethics committee of the Nijmegen–Arnhem region. Written informed consent according to the Helsinki agreement was obtained from all parents and patients ≥ 12 years.
Heteroplasmy levels were determined in urinary epithelial cells, blood and buccal cells of all participants using Pyrosequencing™ technology (Pyrosequencing, Uppsala, Sweden) as described before by Lowik, Hol, Steenbergen, Wetzels and van den Heuvel [23]. The pyrosequencing assay of the m.3243A > G mutation in the mtDNA (Genbank accession# NC_012920.1) has a precision of 1.5%, the precision relates to the correlation between independent measurements of the same sample. A sensitivity of 4.5% has been quoted for this technique, as determined by serial dilution of an m.3243A > G positive sample in a sample containing wild type mtDNA, and by determining the background signal + 3 × SD in a panel of 43 control samples.
The tissue samples of patient II-3 of family 3 were collected within 2 h post-mortem. Samples were snap frozen using liquid nitrogen and stored at − 80 °C. DNA was extracted using a Genomic DNA Purification Kit (Gentra, Mineapolis, USA), following the manufacturer's procedures.
In the families in which a de novo m.3243A > G mutation was suspected, heteroplasmy levels in urinary epithelial cells from first degree family members were measured using a second pyrosequencing assay with a sensitivity calculated at 1% as previously described by Alston et al, [24].
The data of this study were submitted to the MITOMAP database (www.mitomap.org).
2.1. Family reports
2.1.1. Family 1
The proband (III-2, Fig. 1a) presented with aphasia and encephalopathy at the age of 34 years having experienced difficulty in speaking for a few months with a history of hearing impairment since age 5. During this episode of encephalopathy he spoke in short sentences and was unable to respond to complex commands. There were no abnormalities in the evaluation of cranial nerves, reflexes, motor skills or sensation. MRI imaging showed abnormal signal intensities in both cortical and sub-cortical areas of the left parieto-temporal lobe. MRI-spectroscopy demonstrated elevated levels of lactic acid in the affected areas. A muscle biopsy was performed showing diminished ATP production and decreased complex I activity (see Table 2). Unfortunately no histopathological data are available. m.3243A > G mutation screening was performed, revealing a high level of m.3243A > G mutation load (82%) in muscle, confirming the diagnosis of m.3243A > G related mitochondrial disease. Assessment of m.3243A > G mutation levels in leucocytes, urinary epithelial cells and buccal cells were 23%, 63% and 40%, respectively. During follow-up, the patient developed epilepsy at the age of 35 years and diastolic dysfunction on echocardiography. He was myopathic and unable to perform normal work. Lactate levels in blood were elevated to an average level of 4.0 mmol/L (reference < 2.2 mmol/L). An extensive family study was performed. Family members with symptoms that could be consistent with the m.3243A > G mutation include a cousin (III-6) and her baby child (IV-6) who experienced severe congenital hearing impairment, and the proband's mother (II-3) who reported non-insulin dependent diabetes and mild hearing impairment at the age of 71 years. A total of 16 family members were tested for the presence of the m.3243A > G mutation, but this was not detected in any other family member apart from the proband (Fig. 1a). The underlying cause of the congenital hearing loss reported by (III-6) and (IV-6) was investigated by whole exome sequencing, and a nuclear-encoded mutation was identified which is likely to be pathogenic.
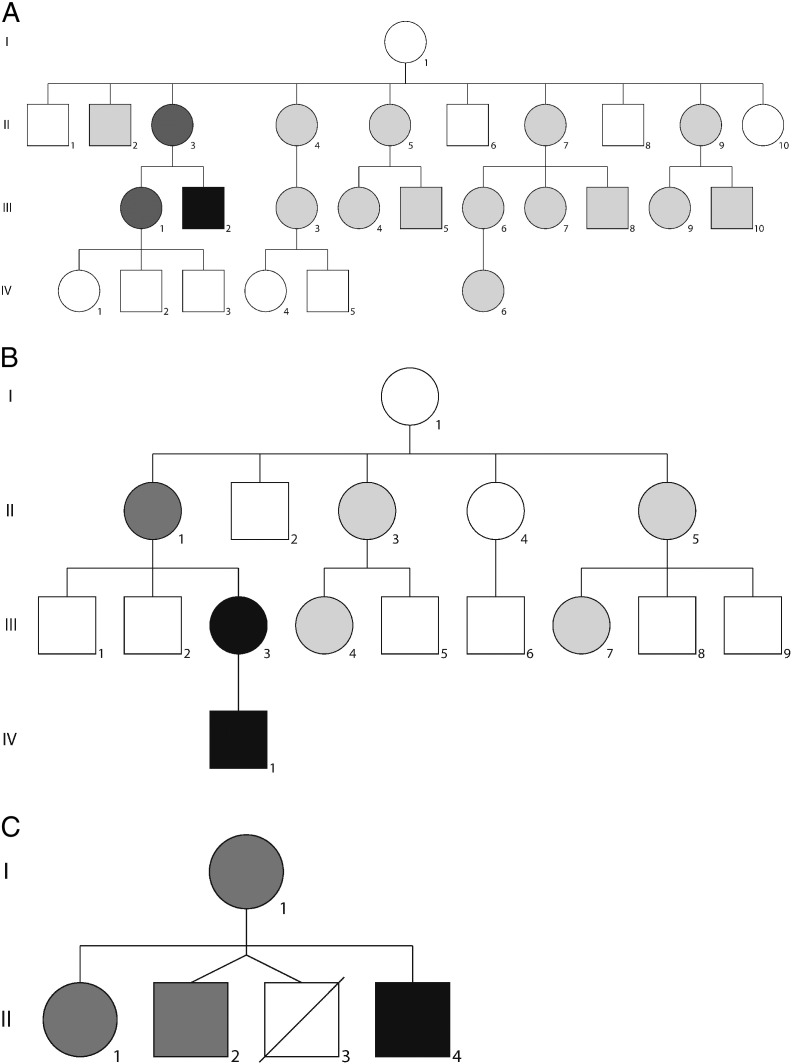
Fig. 1.
a–c Pedigrees of the three patients with a de novo mutation. Fathers are not indicated in the pedigrees. The patients with a detectable m.3243A > G mutation load are indicated as black squares and circles. The patients that were tested using pyrosequencing as earlier described by Lowik et al. (2005) are indicated in light grey shading; the patients that were additionally tested using the more sensitive pyrosequencing assay described by Alston et al. (2011) are indicated with darker grey shading.
a: Family 1; the proband is indicated as individual III-2. Family members in whom the mutation was undetectable in urinary epithelial cells, leucocytes and buccal cells are; II-2, II-3, II-4, II-5, II-7, II-9, III-1, III-3, III-4, III-5, III-6, III-7, III-8, III-9, III-10, IV-6. No further family members were available to participate in the study. Family members II-3 and III-1, mother and sister of the proband respectively, were screened for m.3243 A > G heteroplasmy in urinary epithelial cells using the more sensitive pyrosequencing assay.
b: Family 2; the proband is indicated as patient IV-1 however individual III-3 (the proband's asymptomatic mother) is considered to be the de novo case following carrier testing. The m.3243A > G mutation was undetectable in urinary epithelial cells, leucocytes and buccal cells of relatives denoted II-1, II-3, II-5, III-4 and III-7. The other family members did not participate in the study. Heteroplasmy was tested in urinary epithelial cells from individual II-1, the proband's mother, using the more sensitive pyrosequencing assay.
c: Family 3; the proband is indicated as patient II-4. Tested family members in whom the mutation was undetectable in urinary epithelial cells, leucocytes and buccal cells are; I-1, II-1 and II-2. In all three family members, m.3243A > G mutation load in urinary epithelial cells was tested using the more sensitive pyrosequencing assay. Patient II-3 died at the age of 4 months. Post mortem muscle and liver biopsies were screened and no evidence of the m.3243A > G mutation was reported.
Table 2.
Muscle biopsy results.
| Patient | Heteroplasmy % | ATPa (normal range) | %b | Complex Ic (normal range) | %b | Complex IVc (normal range) | %b | Histopathology |
|---|---|---|---|---|---|---|---|---|
| Proband family 1 (III-2) | 82% | 5.8 (42.1–81.2) | 13.8% | 30 (100–401) | 30% | 809 (810–3120) | 99.9% | n.a. |
| Proband family 2 (IV-1) | 23% | 24.4 (42.1–81.2) | 60.0% | 49 (70–251) | 70% | 1269 (810–3120) | 157% | Normal |
| Mother of proband family 2 (III-3) | 5% | 13.8 (15.4–30.2) | 90.0% | 53 (47–154) | 113% | 1172 (470–1842) | 249% | Normal |
| Proband family 3 (II-4) | 12% | 10.4 (42.1–81.2) | 24.7% | 52 (70–251) | 74.2% | 759 (810–3120) | 93.7% | Normal |
ATP metabolism (nmol/h.mUCS).
percentage of lower limit of normal range.
Complex activity (mU/UCS); na = not available.
2.1.2. Family 2
The proband (IV-1, Fig. 1b) presented at the age of almost 2 years, with neurological deterioration following a viral infection resulting in hypotonia, axial ataxia, ptosis and ophthalmoplegia. He had elevated lactate levels (5.5 mmol/L, reference < 2.2 mmol/L) and abnormal signal intensities in the parieto-occipital region on MRI. His neurological symptoms improved after normalization and increase of his calorific intake. A muscle biopsy was performed, demonstrating diminished ATP production, decreased complex I activity but no histopathological abnormalities (Table 2). Genetic testing revealed the m.3243A > G mutation to be present at a level of 23% heteroplasmy in skeletal muscle. Motor skill development was somewhat slow, requiring physiotherapy but by the age of years he attended mainstream school, and had normal motor skills and strength. Heteroplasmy levels in urinary epithelial cells and buccal cells were 38% and 27%, respectively. Six family members were assessed; they were all in good health. Heteroplasmy analysis showed a low level (6% mutation load) of m.3243A > G mutation in the urinary epithelial cells from the mother (III-3), whereas the mutation was undetectable in her blood and buccal cells. The patient's mother (III-3) underwent muscle biopsy which showed a slightly diminished ATP production, normal complex activities and no histopathological abnormalities suggestive of mitochondrial disease; heteroplasmy analysis showed a 5% m.3243A > G mutation load in the muscle (see Table 2). Five further asymptomatic maternally-related individuals including grandmother underwent genetic testing in urinary epithelial cells and did not harbour the m.3243A > G mutation (Fig. 1b). Patients III-1 and III-2 did not consent to genetic testing, their case history did not suggest any mitochondrial symptoms.
2.1.3. Family 3
The proband (II-4, Fig. 1c) was born at term with a birth weight of 4750 g (> 97th centile). He was delivered by an emergency caesarean section due to foetal distress and required short time respiratory support because of transient tachypnea of the neonate. He had hypoglycaemia that was treated with glucose infusions and elevated lactate (pH 7.32, reference 7. 34–7.45; lactic acid 4.2 mmol/L, reference < 2.2 mmol/L) Left ventricular hypertrophy was seen on echocardiogram with no hemodynamic consequence. Muscle biopsy was performed and showed a decrease of complex I and complex IV activities, histopathological assays were normal (Table 2); mtDNA analysis revealed the m.3243A > G mutation at a heteroplasmy level of 12% in muscle. Following a rapid recovery, the patient was discharged from the hospital. At age five months, a second echocardiogram showed a normal heart with no signs of hypertrophy or cardiomyopathy. Quantitative mutation analysis of urinary epithelial cells, leucocytes and buccal cells showed m.3243A > G heteroplasmy levels of 16%, 20% and 16%, respectively. At six years of age the patient had no medical problems, in particular no cardiomyopathy, fatigue or muscle weakness. DNA samples from his mother and siblings showed no detectable levels of the m.3243A > G mutation in urinary epithelial cells, leucocytes and buccal cells (Fig. 1c). One of his siblings (II-3) died at four months of age from dehydration following campylobacter gastro-enteritis complicated by metabolic acidosis and liver failure. Post-mortem muscle and liver biopsies showed a diffuse steatosis with no further abnormalities on either conventional and electron microscopy nor following further biochemical and molecular genetic testing; the m.3243A > G mutation was absent in these post-mortem tissues.
3. Discussion
Genetic counselling in mitochondrial diseases caused by an mtDNA mutation is complicated. With this report we provide additional information regarding the inheritance of the m.3243A > G mutation.
There are only four previous reports of a sporadically occurring m.3243A > G mutation, it is therefore of great significance that we describe these new patients who most likely have de novo mutations. We included 48 m.3243A > G mutation-positive families in our cohort study; this is a follow-up study to our previously published national inventory [22]. 37 of these families demonstrated maternal inheritance of the m.3243A > G mutation, whilst three families (8% of our cohort families) had a likely de novo occurrence of the m.3243A > G mutation, no data were available concerning the family members in the remaining seven families.
The clinical presentation of patients with a de novo m.3243A > G mutation includes MELAS, MELAS/MERFF overlap, MIDD and cardiomyopathy. In recent cohort studies there is a predominance of milder phenotypes, such as MIDD, amongst carriers of the m.3243A > G mutation [16], [22]. Interestingly, across all cases reported to date, a MELAS phenotype was seen in the proband of five out of eight families with a de novo appearance of the m.3243A > G mutation (including the probands of families 1 and 2 from this study) [18], [19], [20]. One proband had a MIDD phenotype [21], and one proband had an isolated cardiomyopathy (proband of family 3 of this study). There seems to be a tendency towards a more severe phenotype in the index patients of the families with a de novo appearance of the m.3243A > G mutation, this could however also be the result of a bias in recognition of the phenotype, whereas a mildly affected individual would never be subjected to mitochondrial DNA sequencing and these mild cases of de novo m.3243A > G mutation may therefore be never found. In two of the families with a de novo m.3243A > G mutation, the proband's mother had either MIDD or an isolated deafness phenotype [18], [20]. In fact, these mothers are the individuals in whom the de novo mutational event occurred, and their genetic diagnosis was only made following the identification of the m.3243A > G mutation in their more severely affected children.
An isolated incidence of an mtDNA mutation within a family can have one of three causes, as stated by Maassen et al. [21]; 1.) other family members in the maternal lineage harbour the mutation, but below the detection limit, 2.) there is no biological relationship between the proband and the other family members tested, 3.) a de novo mutational event has occurred23. We investigated the cause of the isolated m.3243A > G mutation in the three families described in this report using these three possibilities.
To investigate the possibility that the mutation is present below the sensitivity of the assay, we used two different pyrosequencing techniques as described previously [23], [24]. The pyrosequencing assay described by Lowik et al. has a sensitivity of 4.5% whereas the assay described by Alston et al. has a higher sensitivity (1.0%). We tested three different tissues for each available family member, urinary epithelial cells, leucocytes and buccal cells. No muscle was available of the family members (except for patient II-3 of family 3), but previous studies have shown a good correlation between the m.3243A > G mutation load in urinary epithelial cells and muscle biopsy [25], [26]. With sensitivities of 4.5% and 1.0% respectively for the two pyrosequencing assays, the probability of multiple false-negative results are highly unlikely, it is therefore unlikely that other family members in the maternal lineage also carry the mutation below the detection limit. The family members were also clinically assessed using the NMDAS for symptoms that could be related to the m.3243A > G mutation. From a total of 23 adult relatives, two had hearing problems (including patient III-6 from family 1), two had diabetes mellitus (one insulin dependent), three reported constipation, two relatives reported psychiatric complaints and two reported visual abnormalities. No relatives reported ataxia or myopathy. The results from the screening of non-invasive familial samples suggest that the m.3243A > G mutation is unlikely to be present below the detection threshold in our proposed de novo m.3243A > G families.
In order to investigate the second possibility of non-kinship as the cause of isolated m.3243A > G occurrence, we obtained confirmation of kinship from the mothers within the families. Paternity testing was not performed given that paternity is not relevant in establishing the inheritance of a mitochondrial DNA mutation. Having excluded kinship and sensitivity issues, we conclude that there is most likely a de novo appearance of the m.3243 A > G mutation in our three families.
The m.3243A > G mutation has previously been detected in patients with different haplotypes [8], [27], indicating that de novo appearance of the m.3243A > G mutation has occurred frequently in the past. Although the mechanism behind de novo appearance of the m.3243A > G mutation is unknown, it is speculated that the mutational event is likely to have occurred during oogenesis (in the mother's embryonic development) or during early embryonic development of the proband [19]. These hypotheses are supported by our results given that each proband harbours the mutation in tissues that originate from each of the three primary germ cell layers.
This report detailing a further three cases harbouring a de novo m.3243A > G mutation highlights the importance of screening for the m.3243A > G mutation even if there is no significant (maternal) family history in a patient with MELAS or MIDD symptoms. Moreover, a genetic diagnosis of an m.3243A > G mutation in an isolated patient does not necessarily mean that others in the maternal lineage should automatically be presumed to harbour the mutation also. Despite the vast majority (> 90%) of m.3243A > G mutation cases being maternally inherited, a thorough family investigation should always be performed.
Conflict of interest
Paul de Laat received research support from the Stichting Energy4all.
Prof. Smeitink is the founder and CEO of Khondrion and is funded by the Netherlands Organization for Scientific Research and by ongoing Marie-Curie and Eurostars grants and grants of Stichting Energy4All and the Mitochondrial Medicine Foundation, all not related to the current study.
Mirian C.H. Janssen, Charlotte L Alston, Robert W Taylor and Richard J.T. Rodenburg declare that they have no conflict of interest.
This study was not industry sponsored.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients, or their legal guardians for being included in the study.
Details of the contributions of individual authors
Paul de Laat: manuscript preparation, patient collection, sample collection, analyses, study design.
Mirian C.H. Janssen: manuscript preparation, study design, patient collection.
Charlotte L Alston: heteroplasmy percentage determination, manuscript correction.
Robert W Taylor: heteroplasmy percentage determination, manuscript correction.
Richard J.T. Rodenburg: heteroplasmy percentage determination, manuscript correction.
Jan. A.M. Smeitink: manuscript correction, study design.
Transparency document
Transparency document.
Acknowledgements
Part of this work was supported by the Stichting Energy4All. Jan. Smeitink is CEO of Khondrion.
Footnotes
The Transparency document associated with this article can be found, in the online version.
References
- 1.Koopman W.J., Willems P.H., Smeitink J.A. Monogenic mitochondrial disorders. N. Engl. J. Med. 2012;366:1132–1141. doi: 10.1056/NEJMra1012478. [DOI] [PubMed] [Google Scholar]
- 2.Cree L.M., Samuels D.C., Chinnery P.F. The inheritance of pathogenic mitochondrial DNA mutations. Biochim. Biophys. Acta. 2009;1792:1097–1102. doi: 10.1016/j.bbadis.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Laat P., Koene S., van de Heuvel L.P.W.J., Rodenburg R.J.T., Janssen M.C.H., Smeitink J.A.M. Inheritance of the m.3243A > G mutation. JIMD Rep. 2013;8:47–50. doi: 10.1007/8904_2012_159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz M., Vissing J. No evidence for paternal inheritance of mtDNA in patients with sporadic mtDNA mutations. J. Neurol. Sci. 2004;218:99–101. doi: 10.1016/j.jns.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Chinnery P.F., DiMauro S., Shanske S., Schon E.A., Zeviani M., Mariotti C., Carrara F., Lombes A., Laforet P., Ogier H., Jaksch M., Lochmuller H., Horvath R., Deschauer M., Thorburn D.R., Bindoff L.A., Poulton J., Taylor R.W., Matthews J.N., Turnbull D.M. Risk of developing a mitochondrial DNA deletion disorder. Lancet. 2004;364:592–596. doi: 10.1016/S0140-6736(04)16851-7. [DOI] [PubMed] [Google Scholar]
- 6.Goto Y., Nonaka I., Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990;348:651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- 7.van den Ouweland J.M., Lemkes H.H., Ruitenbeek W., Sandkuijl L.A., de Vijlder M.F., Struyvenberg P.A., van de Kamp J.J., Maassen J.A. Mutation in mitochondrial tRNA(Leu)(UUR) gene in a large pedigree with maternally transmitted type II diabetes mellitus and deafness. Nat. Genet. 1992;1:368–371. doi: 10.1038/ng0892-368. [DOI] [PubMed] [Google Scholar]
- 8.Majamaa K., Moilanen J.S., Uimonen S., Remes A.M., Salmela P.I., Karppa M., Majamaa-Voltti K.A., Rusanen H., Sorri M., Peuhkurinen K.J., Hassinen I.E. Epidemiology of A3243G, the mutation for mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes: prevalence of the mutation in an adult population. Am. J. Hum. Genet. 1998;63:447–454. doi: 10.1086/301959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manwaring N., Jones M.M., Wang J.J., Rochtchina E., Howard C., Mitchell P., Sue C.M. Population prevalence of the MELAS A3243G mutation. Mitochondrion. 2007;7:230–233. doi: 10.1016/j.mito.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Chinnery P.F., Johnson M.A., Wardell T.M., Singh-Kler R., Hayes C., Brown D.T., Taylor R.W., Bindoff L.A., Turnbull D.M. The epidemiology of pathogenic mitochondrial DNA mutations. Ann. Neurol. 2000;48:188–193. [PubMed] [Google Scholar]
- 11.Mancuso M., Orsucci D., Angelini C., Bertini E., Carelli V., Comi G.P., Donati A., Minetti C., Moggio M., Mongini T., Servidei S., Tonin P., Toscano A., Uziel G., Bruno C., Ienco E.C., Filosto M., Lamperti C., Catteruccia M., Moroni I., Musumeci O., Pegoraro E., Ronchi D., Santorelli F.M., Sauchelli D., Scarpelli M., Sciacco M., Valentino M.L., Vercelli L., Zeviani M., Siciliano G. The m.3243A > G mitochondrial DNA mutation and related phenotypes. A matter of gender? J. Neurol. 2013 doi: 10.1007/s00415-013-7225-3. [DOI] [PubMed] [Google Scholar]
- 12.Majamaa-Voltti K.A., Winqvist S., Remes A.M., Tolonen U., Pyhtinen J., Uimonen S., Karppa M., Sorri M., Peuhkurinen K., Majamaa K. A 3-year clinical follow-up of adult patients with 3243A > G in mitochondrial DNA. Neurology. 2006;66:1470–1475. doi: 10.1212/01.wnl.0000216136.61640.79. [DOI] [PubMed] [Google Scholar]
- 13.Finsterer J. A 3-year clinical follow-up of adult patients with 3243A > G in mitochondrial DNA. Neurology. 2007;68:163–164. doi: 10.1212/01.wnl.0000254820.44992.6b. [DOI] [PubMed] [Google Scholar]
- 14.Uusimaa J., Moilanen J.S., Vainionpaa L., Tapanainen P., Lindholm P., Nuutinen M., Lopponen T., Maki-Torkko E., Rantala H., Majamaa K. Prevalence, segregation, and phenotype of the mitochondrial DNA 3243A > G mutation in children. Ann. Neurol. 2007;62:278–287. doi: 10.1002/ana.21196. [DOI] [PubMed] [Google Scholar]
- 15.Ma Y., Fang F., Cao Y., Yang Y., Zou L., Zhang Y., Wang S., Zhu S., Xu Y., Pei P., Qi Y. Clinical features of mitochondrial DNA m.3243A > G mutation in 47 Chinese families. J. Neurol. Sci. 2010;291:17–21. doi: 10.1016/j.jns.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Nesbitt V., Pitceathly R.D., Turnbull D.M., Taylor R.W., Sweeney M.G., Mudanohwo E.E., Rahman S., Hanna M.G., McFarland R. The UK MRC mitochondrial disease patient cohort study: clinical phenotypes associated with the m.3243A > G mutation--implications for diagnosis and management. J. Neurol. Neurosurg. Psychiatry. 2013 doi: 10.1136/jnnp-2012-303528. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann P., Engelstad K., Wei Y., Kulikova R., Oskoui M., Battista V., Koenigsberger D.Y., Pascual J.M., Sano M., Hirano M., DiMauro S., Shungu D.C., Mao X., De Vivo D.C. Protean phenotypic features of the A3243G mitochondrial DNA mutation. Arch. Neurol. 2009;66:85–91. doi: 10.1001/archneurol.2008.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campos Y., Martin M.A., Lorenzo G., Aparicio M., Cabello A., Arenas J. Sporadic MERRF/MELAS overlap syndrome associated with the 3243 tRNA(Leu(UUR)) mutation of mitochondrial DNA. Muscle Nerve. 1996;19:187–190. doi: 10.1002/(SICI)1097-4598(199602)19:2<187::AID-MUS10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto M. Did de novo MELAS common mitochondrial DNA point mutation (mtDNA 3243, A → G transition) occur in the mother of a proband of a Japanese MELAS pedigree? J. Neurol. Sci. 1996;135:81–84. doi: 10.1016/0022-510x(95)00272-4. [DOI] [PubMed] [Google Scholar]
- 20.Ko C.H., Lam C.W., Tse P.W., Kong C.K., Chan A.K., Wong L.J. De novo mutation in the mitochondrial tRNALeu(UUR) gene (A3243G) with rapid segregation resulting in MELAS in the offspring. J. Paediatr. Child Health. 2001;37:87–90. doi: 10.1046/j.1440-1754.2001.00611.x. [DOI] [PubMed] [Google Scholar]
- 21.Maassen J.A., Biberoglu S., t Hart L.M., Bakker E., de Knijff P. A case of a de novo A3243G mutation in mitochondrial DNA in a patient with diabetes and deafness. Arch. Physiol. Biochem. 2002;110:186–188. doi: 10.1076/apab.110.3.186.8294. [DOI] [PubMed] [Google Scholar]
- 22.de Laat P., Koene S., van de Heuvel L.P.W.J., Rodenburg R.J.T., Janssen M.C.H., Smeitink J.A.M. Clinical features and heteroplasmy in blood, urine and saliva in 34 Dutch families carrying the m.3243A > G mutation. J. Inherit. Metab. Dis. 2012;35:1059–1069. doi: 10.1007/s10545-012-9465-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowik M.M., Hol F.A., Steenbergen E.J., Wetzels J.F., van den Heuvel L.P. Mitochondrial tRNALeu(UUR) mutation in a patient with steroid-resistant nephrotic syndrome and focal segmental glomerulosclerosis. Nephrol. Dial. Transplant. 2005;20:336–341. doi: 10.1093/ndt/gfh546. [DOI] [PubMed] [Google Scholar]
- 24.Alston C.L., He L., Morris A.A., Hughes I., de Goede C., Turnbull D.M., McFarland R., Taylor R.W. Maternally inherited mitochondrial DNA disease in consanguineous families. Eur. J. Hum. Genet. 2011;19:1226–1229. doi: 10.1038/ejhg.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonnell M.T., Schaefer A.M., Blakely E.L., McFarland R., Chinnery P.F., Turnbull D.M., Taylor R.W. Noninvasive diagnosis of the 3243A > G mitochondrial DNA mutation using urinary epithelial cells. Eur. J. Hum. Genet. 2004;12:778–781. doi: 10.1038/sj.ejhg.5201216. [DOI] [PubMed] [Google Scholar]
- 26.Frederiksen A.L., Andersen P.H., Kyvik K.O., Jeppesen T.D., Vissing J., Schwartz M. Tissue specific distribution of the 3243A → G mtDNA mutation. J. Med. Genet. 2006;43:671–677. doi: 10.1136/jmg.2005.039339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elliott H.R., Samuels D.C., Eden J.A., Relton C.L., Chinnery P.F. Pathogenic mitochondrial DNA mutations are common in the general population. Am. J. Hum. Genet. 2008;83:254–260. doi: 10.1016/j.ajhg.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.