Abstract
Background:
NGAL is one of the most promising AKI biomarkers in cardiac surgery. However, the best timing to dose it and the reference values are still matter of discussion.
Aim of the Study:
We performed a uNGAL perioperative time course, to better understand its perioperative kinetics and its role in AKI diagnosis.
Setting of the Study:
San Raffaele University Hospital, cardiac surgery department.
Material and Methods:
We enrolled in this prospective observational study 19 patients undergoing cardiac surgery with cardiopulmonary bypass (CPB). Based on preoperative characteristics, they were divided in low-risk and high-risk patients. uNGAL measurements were collected at pre-defined times before, during, and up to 24 hours after surgery.
Statistical Analysis:
Data were analysed by use of SAS 1999-2001 program or IBM SPSS Statistics.
Results:
In low-risk patients, uNGAL had the highest value immediately after general anesthesia induction (basal dosage: uNGAL: 12.20ng×ml-1, IQR 14.00). It later decreased significantly (3.40 ng×ml-1, IQR 4.80; P = 0.006) during CPB, and finally return to its original value 24 hours after surgery. In high-risk patients, uNGAL increased immediately after surgery; it had the highest value on ICU arrival (38,20 ng×ml-1; IQR 133,10) and remained high for several hours. A difference in uNGAL levels between the two groups was already observed at the end of surgery, but it became statistically significant on ICU arrival (P = 0.002).
Conclusion:
This study helps to better understand the different kinetics of this new biomarker in low-risk and high-risk cardiac patients.
Keywords: Acute kidney injury, Cardiac surgery, Neutrophil gelatinase-associated lipocalin
INTRODUCTION
Postoperative acute kidney injury (AKI) is a severe complication of cardiac surgery leading to longer hospital stay, morbidity and mortality.[1,2] The pathogenesis of AKI is multifactorial.[3] In this specific setting, AKI depends on heart and kidney interactions and often, could be defined as type 1 cardio-renal syndrome.[4] In 2004, the acute dialysis quality initiative working party published the risk, injury, failure, loss, end (RIFLE) criteria.[5] In 2005 Chertow et al.[6] demonstrated that small changes in serum creatinine were indicative of significant renal dysfunction. In 2007, the AKI network (AKIN) proposed a modified version of the RIFLE criteria: The AKIN classification.[7] A recent study suggests using RIFLE criteria in cardiac surgery because AKIN could lead in this specific setting to an over-diagnosis of AKI.[8] Despite all these classifications, serum creatinine is an insensitive and unreliable biomarker of AKI.[9] Delay in detection also means delayed intervention. Therefore, there is a need for new early AKI biomarkers.[10,11]
Neutrophil gelatinase-associated lipocalin (NGAL) is a protein of lipocalin family and is expressed by neutrophils and renal tubular cells,[12] its gene was found to be highly upregulated in mouse models of renal ischemia-reperfusion injury and its level correlates with the duration of ischemia.[13] NGAL can be measured both in plasma and urine samples. NGAL is the most promising among all emerging AKI biomarkers,[11] and it has been validated in cardiac surgery.[14,15] A meta-analysis by Haase et al.[16] also including noncardiac surgery, plasmatic and urine NGAL (uNGAL) seemed to give diagnostic and prognostic values for AKI. The role of NGAL, both plasmatic and urinary, in the early diagnosis of AKI in cardiac surgery is now well-recognized as a “renal biomarker.” Recently, elevated NGAL levels have been reported in a wider range of cardiovascular diseases, including both acute and chronic heart failure, coronary heart disease, and stroke.[17] Furthermore, a study of ours[18] enrolling 27 high-risk patients undergoing cardiac surgery with cardiopulmonary bypass (CPB), found uNGAL 24 h after surgery as a predictor of prolonged Intensive Care Unit (ICU) stay. Moreover, some studies suggest that NGAL plays a direct role in vascular remodeling, plaque instability, atherosclerosis, and myocardium remodeling.[19,20] Collectively, these findings provide a new the potential role of NGAL as a biomarker for cardiovascular diseases.[17] However, the best timing to estimate it postoperatively and the reference values that should be used to detect the presence of renal dysfunction from normal function still need to be assessed. There are no studies set up with the aim to accurately evaluate the reference values of NGAL measurement in blood or urine specimens using large reference populations, stratified according to age, gender, and ethnicity.[21] To this aim, we performed a uNGAL time course with a small number of patients, to understand its perioperative kinetics and its potential role as a biomarker for AKI. This work is a pilot study in a small number of patients, to understand the best timing to investigate uNGAL in the perioperative period. The aim of our study is to evaluate how uNGAL levels change during cardiac surgery (setting where CPB and hemodilution may alter uNGAL concentration) and in the postoperative period.
METHODS
After ethical committee approval and informed written consent, this prospective observational study enrolled 19 patients undergoing cardiac surgery with CPB. According to the preoperative evaluation, the sample was so divided into: Eight consecutive low-risk patients undergoing mitral valve repair and eleven high-risk patients undergoing complex and combined cardiac surgery. This division was useful to better evaluate uNGAL kinetics: In this way we considered the two extremity scenarios of patients undergoing cardiac surgery. The sample size was small because we expected to find a big difference in perioperative uNGAL kinetics between the two groups.
Low-risk patients
Inclusion criteria were: Age >18, logistic EUROSCORE <3, good preoperative ventricular function (ejection fraction >55%; end-diastolic diameter <60 mm, systolic pulmonary artery pressure <30 mmHg), no other co-pathologies except for essential hypertension in good pharmacological control, mitral valve repair in median sternotomy. Exclusion criteria were: Mitral valve repair in right thoracotomy, previous cardiac surgery, preoperative serum creatinine >1.5 mg/dL, intra-operative or postoperative need for inotropes, vasopressors, blood transfusions or mechanical circulatory support devices, and emergent surgery.
High-risk patients
Inclusion criteria were: Age >18, logistic EUROSCORE >5, complex and combined cardiac surgery, poor ventricular function (ejection fraction <40% and/or end diastolic diameter >60 mm and/or systolic pulmonary artery pressure >45 mmHg), and intra-operative and postoperative need for pharmacological and/or mechanical circulatory support. Exclusion criteria were: Preoperative serum creatinine >1.5 mg/dL, previous cardiac surgery and emergent surgery.
All patients underwent preoperative anesthetic evaluation, routine blood tests, resting electrocardiogram, chest X-ray and transesophageal echocardiography, this in order to evaluate cardiac function and valvular disease, also during surgery. Preoperative therapy was administered until the day of surgery. General anesthesia was induced with fentanyl (4–6 mcg/kg), propofol (1–2 mg/kg) and rocuronium 0.6 mg/kg. Anesthesia was maintained with sevoflurane and additional doses of fentanyl and rocuronium when required. All patients underwent median sternotomy. Standard CPB technique and hyperkalemic cardioplegia were used. During CPB, moderate hemodilution (hematocrit 24% to 28%) with mild systemic hypothermia (32–34°C) were utilized. The extra-corporeal device consisted of a centrifugal pump. The circuit priming consisted of 1500 mL Ringer's lactate containing 0.5 g/kg mannitol 18%, 0.5 g tranexamic acid, 5000 UI of heparin. Pump flows were adjusted to maintain a flow equal to or >4.5 L/min. An intra-operative infusion of tranexamic acid (1 g over 20 min followed by 400 mg/h) was used. Protamine was administered after separation from CPB and attaining satisfactory hemodynamics. Fluids and diuretics were infused to the patients to maintain urinary output above 1 mL/kg/h and inotropes were administered to ensure a mean arterial pressure (MAP) of 70 mmHg. Standard monitoring was used. When needed pulmonary artery pressure, wedge pressure and cardiac output were monitored with pulmonary artery catheter. After surgery, all patients were transferred to an ICU. AKI was defined according to the R of RIFLE criterion. Indications for renal replacement therapy (RRT), were onset of uremic complications, persistent oliguria/anuria or refractory fluid overload, hyperkalemia (plasma potassium concentration >6.5 meq/L) and metabolic acidosis (pH <7.1) refractory to medical therapy. The RRT was performed with continuous veno-venous hemofiltration. The decision to transfer the patient from the ICU to the main ward was based on the following criteria: Pulse oxymetry >94% at an FiO2 <0.5 by facemask, adequate cardiac stability with no hemodynamically significant arrhythmias, chest tube drainage <50 mL/h, urine output >0.5 mL/kg/h, no intravenous inotropic or vasopressor agent in excess of dopamine 5 mcg/kg/min. Criteria for hospital discharge were hemodynamic and cardiac rhythm stability, presence of clean and dry incisions, afebrile, normal bowel movement, and independent ambulation and feeding. Serum creatinine was measured preoperatively, at ICU arrival, 24 h later and every 24 h until the patient was in ICU. The uNGAL, hematocrit, serum sodium and MAP were measured at the following time points: Prior to CPB (baseline); during CPB; from end of CPB to end of surgery; at ICU arrival; 4 h after ICU arrival and 24 h after ICU arrival. For each patient, urine was drawn, processed and frozen until the end of enrolment. All intra-operative and postoperative data were collected.
Data were analyzed using SAS 1999–2001 program (release 8.2 by SAS Institute Inc., Cary, NC, US) or IBM SPSS statistics (IBM Corp. Released 2011, version 20.0. Armonk, NY, US). Continuous measures are expressed as mean ± standard deviation or as median (25th–75th percentile). Categorical variables are reported as the number (per cent). Whenever normality assumption was not met, nonparametric tests were applied. To compare continuous variables among the risk groups we applied analysis of variance test, where normality assumption was met. Otherwise, we applied Kruskal–Wallis tests or Friedman test. Chi-square analysis or Fisher's test, when appropriate, were used to compare discrete variables. Mann-Whitney test was used to compare uNGAL levels among groups. Pearson's correlation test and linear regression were applied to evaluate the relations among continuous variables. Spearman's correlation test and Friedman test were applied for not normally distributed variables. To perform receiver operating characteristic curve analysis and comparison situation, task, action, result tool was used (http://protein.bio.puc.cl/star/home.php).[22]
RESULTS
Low-risk patients
Perioperative characteristics of low-risk patients undergoing mitral valve repair in median sternotomy are described in Table 1. Perioperative uNGAL, hematocrit, serum sodium and MAP time courses are described in Figures 1 and 2.
Table 1.
Perioperative characteristics of low-risk and high-risk patients
| Low risk patients | High risk patients | P | |
|---|---|---|---|
| Age (years) | 55±11.12 | 61.91±12.22 | NS |
| Sex, female (n/%) | 1/12.5 | 3/27.3 | NS |
| Logistic EUROSCORE (%) | 1.79±0.51 | 8.37±3.12 | <0.0001 |
| Preoperative ejection fraction (%) | 56.88±3.84 | 49±14.6 | NS |
| Preoperative MAP (mmHg) | 94.38±10.84 | 105.00±13.78 | NS |
| Diabetes mellitus (n/%) | 0/0 | 0/0 | NS |
| Atrial fibrillation (n/%) | 0/0 | 5/45.5 | 0.045 |
| Coronary artery disease (n/%) | 0/0 | 1/9 | NS |
| Previous ischemic stroke (n/%) | 0/0 | 1/9 | NS |
| Chronic obstructive pulmonary disease (n/%) | 0/0 | 3/27.3 | NS |
| Cardio-pulmonary bypass time (min) | 73.25±8.56 | 107.09±52.09 | 0.09 |
| Aortic cross-clamp time (min) | 57.13±11.28 | 72.64±46.51 | NS |
| Preoperative serum creatinine, (mg/dL) | 0.82±0.11 | 1.02±0.32 | NS |
| 24 h postoperative serum creatinine, (mg/dL) | 0.79±0.11 | 1.3±0.52 | NS |
| Preoperative hematocrit (%) | 40.14±3.96 | 38.95±4.63 | NS |
| 24 h postoperative hematocrit (%) | 38.19±2.99 | 35.46±4.51 | NS |
| Preoperative plasmatic sodium (mmol/L) | 139.37±1.99 | 139.58±2.21 | NS |
| 24 h postoperative plasmatic sodium (mmol/L) | 139.56±2.10 | 142.48±3.01 | NS |
MAP: Mean arterial pressure, NS: Not significant, EUROSCORE: European system for cardiac operative risk evaluation
Figure 1.
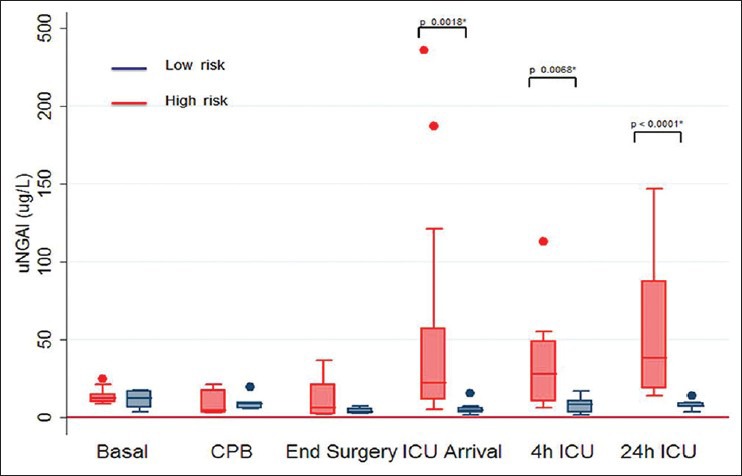
Urine neutrophil gelatinase-associated lipocalin time course in low-risk and high-risk patients (*difference between groups)
Figure 2.
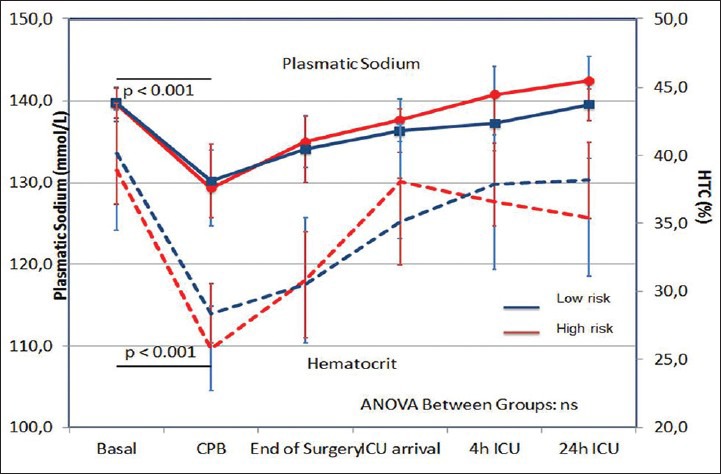
Hemodilution in low-risk and high-risk patients
In these patients uNGAL had the highest value at baseline (12.2; IQR 5.7-17.2 ng/mL, then decreased during CPB reaching the lowest level at the end of surgery (3.4; IQR 2-5-6.0 ng/mL, P = 0,006). The decrease in uNGAL during CPB could be due to concomitant hemodilution (hematocrit [HCT] from 40.14% ±1.54 to 28.38% ±1.03; P < 0.0001). For the same reason, serum Na+ decreased from 139.74 mmol/L ± 0.75 to 134.06 mmol/L ± 1.34 (P < 0.0001) during CPB and returned to baseline values 24 h after surgery. On ICU arrival uNGAL, serum Na and HCT returned respectively to their baseline values (uNGAL: 7.2; IQR 6.8-9.6; P value not significant vs. baseline). MAP had a little decrease immediately after general anesthesia induction, then remained stable with a little increase at the end of surgery. No patient needed inotropes or vasopressors and no patient developed AKI according to RIFLE criteria.
High-risk patients
Perioperative characteristics and perioperative uNGAL kinetics are described in Table 1 and Figure 1. The uNGAL started to decrease immediately after CPB (lowest value 4.8 ng/ml, IQR 3.2–17.6; P = 0.077 vs. baseline), but, in contrast to low-risk group, increased from end of surgery to the ICU arrival, reaching the highest value at 24 h after ICU arrival (38.2 ng/ml; IQR 18.7–87.5; P = 0.04 vs. baseline; P < 0.0001 vs. end of surgery). As in low-risk patients, HCT decreased from 38.94% ±1.32 to 25.82% ±0.88; P < 0.0001 and serum Na+ decreased from 139.58 mmol/L ± 0.64 to 135.00 mmol/L ± 1.14; (P < 0.0001) during CPB. No difference was registered in HCT and plasmatic sodium levels between low-risk and high-risk patient at any time. The MAP decreased significantly in high-risk patients after general anesthesia induction as compared with low-risk group (MAP [mmHg] 73.8 ± 6.03 vs. 78.8 ± 3.23; P >012). Four patients from the high-risk group developed AKI and two of them died. The first one developed severe AKI, which required RRT for 15 days, then died on the 20th postoperative day from septic shock. The highest uNGAL level in this patient was 147 ng/ml at 24 h after ICU arrival, whereas the highest serum creatinine level was on the 4th postoperative day (6.03 mg/dL). The second one developed severe AKI with preserved diuresis on the 2nd postoperative day, (maximum serum creatinine 5.04 mg/dL on the 6th postoperative day) and died on the 16th postoperative day from severe cardiogenic shock refractory to pharmacological and mechanical circulatory support with intra-aortic balloon pump and veno-arterous extra-corporeal membrane oxygenator. In this case the highest uNGAL level was reached 4 h after ICU arrival (112.8 ng/mL) [Table 2]. The other two patients developed milder postoperative AKI with preserved diuresis and did not require RRT. In both cases uNGAL elevation was earlier than the rise in serum creatinine level (uNGAL 484 ng/mL and 48.9 ng/mL at 4 h after ICU arrival.
Table 2.
Serum creatinine's time course in low-risk and high-risk patients (mean, SD, minimum and maximum)
| n | Mean (mg/dL) | SD | Minimum | Maximum | |
|---|---|---|---|---|---|
| Creatinine baseline | |||||
| Low-risk group | 8 | 0.95 | 0.12 | 0.63 | 1.00 |
| High-risk group | 11 | 1.00 | 0.30 | 0.64 | 1.80 |
| Total | 19 | 0.98 | 0.24 | 0.63 | 1.80 |
| Creatinine ICU arrival | |||||
| Low-risk group | 8 | 1.00 | 0.00 | 1.00 | 1.00 |
| High-risk group | 11 | 0.94 | 0.22 | 0.66 | 1.49 |
| Total | 19 | 0.96 | 0.17 | 0.66 | 1.49 |
| Creatinine 24 h | |||||
| Low-risk group | 8 | 1.00 | 0 E-7 | 1.00 | 1.00 |
| High-risk group | 11 | 1.24 | 0.46 | 0.57 | 2.00 |
| Total | 19 | 1.14 | 0.37 | 0.57 | 2.00 |
SD: Standard deviation, ICU: Intensive care unit
Comparative analysis
The only differences detected between the two groups were those related to the underlying diseases of the patients. No difference was found in aortic cross-clamp time or total extra-corporeal circulation time. In both groups significant hemodilution (P < 0.0001) occurred during CBP. The magnitude of this hemodilution was similar between low-risk and high-risk patients, and no difference were registered in HCT levels at any time [Figure 2]. Comparing the uNGAL time course in low-risk versus high-risk patients, a different behavior was observed. Initially, a reduction in uNGAL concentration was observed from anesthesia induction to CBP in low-risk as well as high-risk patients. Later, while, in low-risk patients, uNGAL values slowly returned to baseline, the uNGAL values in high-risk patients recorded gradually increasing uNGAL values starting as early as the end of the CBP. The difference between the two subgroups was observed from the end of CBP, and it became statistical significant at ICU arrival (p 0.0018) and remained significant for all the later tests during ICU stay (P = 0.0068 and < 0.0001) [Figure 1]. AKI occurred four times in “high-risk” patients, but only two of them reached a pathological value of uNGAL based on our laboratory range (>133 ng/mL).[23,24] One patient, from this same group, reached uNGAL level of 187.4 ng/mL but didn’t develop acute renal damage. If we consider the value of uNGAL higher than the laboratory range as pathological, we could conclude that this marker was not able to discriminate “low-risk” versus “ high-risk” patients (P > 0.1). With regards to renal outcome, uNGAL showed a borderline ability to predict postoperative AKI (P = 0.097). “If a change in uNGAL level is compared to the baseline level, a significant association was observed for both AKI and group association. In particular, if a double or triple value of uNGAL compared to baseline is observed, it was possible to start to describe a better correlation with either the correct identification of high-risk patient (P value Fisher exact test 0.054) or the patients with the renal outcome (P value Fisher exact test 0.005). Nevertheless, when we considered as pathological a triplication of uNGAL value compared to baseline, a better correction with AKI predictive power was observed (P = 0.009); however the identification of low-risk versus high-risk patients was less accurate (P = 0.08).”
In our study, we show that in addition to looking at the absolute value of uNGAL (pathological/normal), it is also useful to evaluate the performance of the baseline value.
DISCUSSION
This study adds information regarding uNGAL kinetics: These data will help to understand the kinetics of this new biomarker in low-risk and high-risk patients. Preoperative and intra-operative characteristics of the two groups of patients were similar, except for logistic EUROSCORE, but uNGAL time course was different. In low-risk patients the uNGAL value remains almost unchanged for the duration of the surgery and after, while in high-risk patients the uNGAL value starts to increase at the end of CPB and reaches the maximum in 24 h.
Another study from our group[24] in low-risk cardiac surgical patients made a perioperative time course for N-terminal-pro-brain natriuretic peptide (NT-proBNP), catecholamines and endogenous ouabain (EO), which is a hormone secreted from the adrenal gland with renal and hemodynamic effects. EO started to increase during CPB, peaked 4 h after surgery and then decreased. Recently, our group demonstrated that high circulating levels of this hormone are associated with a worse prognosis and a greater development of acute renal complications, even in those patients classified as “low-risk” by a clinical point of view.[25] Adrenaline and noradrenaline levels increased immediately after CBP. NT-proBNP increased 4 h after surgery. Comparing biomarkers, uNGAL seems to increase earlier in low-risk cardiac surgical patients.
In our study, four high-risk patients developed postoperative AKI, but only two of them reached a pathological uNGAL value according to our laboratory range. Tuladhar et al.[26] measured uNGAL in 50 patients undergoing cardiac surgery with CPB and find the values measured 2 h after CPB related to renal injury. Wagener et al.[15] studied 426 patients undergoing cardiac surgery and found a rise of urinary NGAL (uNGAL) values immediately after cardiac surgery, but showed limited accuracy for predicting AKI. Comparing our results with Tuladhar[26] and Wagener's study,[15] in our patients’ uNGAL had lower levels. In Tuladhar's study uNGAL increased 2 h after the end of CPB, compared to baseline levels, but they did not measure uNGAL level after the end of surgery. In Wagener's work, as in our patients, uNGAL remained high for 24 h and the peak level of uNGAL occurred immediately at the end of surgery. In our study, we considered a change in uNGAL levels compared with baseline in each patient. A doubling of uNGAL was related to the identification of a high-risk patient (P = 0.005; RR 6.54, CI 95% 1.03–41.8), while the triplication was associated to AKI (P = 0.009). This observation may suggest the usefulness of considering the time course of uNGAL, and not only its absolute value, in the AKI diagnosis. Considering the relation between preoperative and postoperative uNGAL values could be more important in AKI diagnosis than the absolute postoperative value. This, in our opinion, is the most important finding of our work and could have an impact on our clinical practice, leading to measurement of uNGAL also in the preoperative period. NGAL is not only an AKI biomarker, but also has a role in cardiovascular diseases: It is highly expressed in the failing heart and atherosclerotic plaques.[17] This finding might explain why perioperative doubling of this biomarker could help to identify high-risk patients.
The major limitations of our study are a small number of patients and the specific setting of cardiac surgery. Moreover, in order to consider separately high-risk and low-risk patients, we included patients undergoing different cardiac surgery procedures: However, the patients share the presence of CPB, which is an important determinant in postoperative AKI. Further larger studies are needed to ascertain the best timing of estimating uNGAL in cardiac surgery to diagnose postoperative AKI.
In conclusion, our study could help to understand perioperative uNGAL kinetics in cardiac surgery patients and may differentiate high-risk and low-risk patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bove T, Calabrò MG, Landoni G, Aletti G, Marino G, Crescenzi G, et al. The incidence and risk of acute renal failure after cardiac surgery. J Cardiothorac Vasc Anesth. 2004;18:442–5. doi: 10.1053/j.jvca.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 2.Mariscalco G, Lorusso R, Dominici C, Renzulli A, Sala A. Acute kidney injury: A relevant complication after cardiac surgery. Ann Thorac Surg. 2011;92:1539–47. doi: 10.1016/j.athoracsur.2011.04.123. [DOI] [PubMed] [Google Scholar]
- 3.Rosner MH, Portilla D, Okusa MD. Cardiac surgery as a cause of acute kidney injury: Pathogenesis and potential therapies. J Intensive Care Med. 2008;23:3–18. doi: 10.1177/0885066607309998. [DOI] [PubMed] [Google Scholar]
- 4.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–39. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 5.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative workgroup. Acute renal failure-definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–70. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 7.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Englberger L, Suri RM, Li Z, Casey ET, Daly RC, Dearani JA, et al. Clinical accuracy of RIFLE and Acute Kidney Injury Network (AKIN) criteria for acute kidney injury in patients undergoing cardiac surgery. Crit Care. 2011;15:R16. doi: 10.1186/cc9960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellomo R. Defining, quantifying, and classifying acute renal failure. Crit Care Clin. 2005;21:223–37. doi: 10.1016/j.ccc.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Parikh CR, Edelstein CL, Devarajan P, Cantley L. Biomarkers of acute kidney injury: Early diagnosis, pathogenesis, and recovery. J Investig Med. 2007;55:333–40. doi: 10.2310/6650.2007.00015. [DOI] [PubMed] [Google Scholar]
- 11.Coca SG, Yalavarthy R, Concato J, Parikh CR. Biomarkers for the diagnosis and risk stratification of acute kidney injury: A systematic review. Kidney Int. 2008;73:1008–16. doi: 10.1038/sj.ki.5002729. [DOI] [PubMed] [Google Scholar]
- 12.Flower DR. The lipocalin protein family: Structure and function. Biochem J. 1996;318(Pt 1):1–14. doi: 10.1042/bj3180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–43. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 14.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–8. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 15.Wagener G, Gubitosa G, Wang S, Borregaard N, Kim M, Lee HT. Urinary neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery. Am J Kidney Dis. 2008;52:425–33. doi: 10.1053/j.ajkd.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 16.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A NGAL Meta-analysis Investigator Group. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–24. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 17.Cruz DN, Gaiao S, Maisel A, Ronco C, Devarajan P. Neutrophil gelatinase-associated lipocalin as a biomarker of cardiovascular disease: A systematic review. Clin Chem Lab Med. 2012;50:1533–45. doi: 10.1515/cclm-2012-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bignami E, Frati E, Ceriotti F, Daverio R, Silvetti S, Landoni G, et al. Urinary neutrophil gelatinase-associated lipocalin as an early predictor of prolonged intensive care unit stay after cardiac surgery. Ann Card Anaesth. 2012;15:13–7. doi: 10.4103/0971-9784.91470. [DOI] [PubMed] [Google Scholar]
- 19.Yndestad A, Landrø L, Ueland T, Dahl CP, Flo TH, Vinge LE, et al. Increased systemic and myocardial expression of neutrophil gelatinase-associated lipocalin in clinical and experimental heart failure. Eur Heart J. 2009;30:1229–36. doi: 10.1093/eurheartj/ehp088. [DOI] [PubMed] [Google Scholar]
- 20.Daniels LB, Barrett-Connor E, Clopton P, Laughlin GA, Ix JH, Maisel AS. Plasma neutrophil gelatinase-associated lipocalin is independently associated with cardiovascular disease and mortality in community-dwelling older adults: The Rancho Bernardo Study. J Am Coll Cardiol. 2012;59:1101–9. doi: 10.1016/j.jacc.2011.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clerico A, Galli C, Fortunato A, Ronco C. Neutrophil gelatinase-associated lipocalin (NGAL) as biomarker of acute kidney injury: A review of the laboratory characteristics and clinical evidences. Clin Chem Lab Med. 2012;50:1505–17. doi: 10.1515/cclm-2011-0814. [DOI] [PubMed] [Google Scholar]
- 22.Vergara IA, Norambuena T, Ferrada E, Slater AW, Melo F. StAR: A simple tool for the statistical comparison of ROC curves. BMC Bioinformatics. 2008;9:265. doi: 10.1186/1471-2105-9-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cullen MR, Murray PT, Fitzgibbon MC. Establishment of a reference interval for urinary neutrophil gelatinase-associated lipocalin. Ann Clin Biochem. 2012;49:190–3. doi: 10.1258/acb.2011.011105. [DOI] [PubMed] [Google Scholar]
- 24.Bignami E, Casamassima N, Frati E, Messaggio E, Corno L, Zangrillo A, et al. Endogenous ouabain changes rapidly during cardiac pulmonary bypass. J Steroids Horm Sci. 2011;3:2–6. [Google Scholar]
- 25.Bignami E, Casamassima N, Frati E, Lanzani C, Corno L, Alfieri O, et al. Preoperative endogenous ouabain predicts acute kidney injury in cardiac surgery patients. Crit Care Med. 2013;41:744–55. doi: 10.1097/CCM.0b013e3182741599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tuladhar SM, Püntmann VO, Soni M, Punjabi PP, Bogle RG. Rapid detection of acute kidney injury by plasma and urinary neutrophil gelatinase-associated lipocalin after cardiopulmonary bypass. J Cardiovasc Pharmacol. 2009;53:261–6. doi: 10.1097/FJC.0b013e31819d6139. [DOI] [PubMed] [Google Scholar]


