Abstract
As innovative technology continues to be developed and is implemented into the realm of cardiac surgery, surgical teams, cardiothoracic anesthesiologists, and health centers are constantly looking for methods to improve patient outcomes and satisfaction. One of the more recent developments in cardiac surgical practice is minimally invasive robotic surgery. Its use has been documented in numerous publications, and its use has proliferated significantly over the past 15 years. The anesthesiology team must continue to develop and perfect special techniques to manage these patients perioperatively including lung isolation techniques and transesophageal echocardiography (TEE). This review article of recent scientific data and personal experience serves to explain some of the challenges, which the anesthetic team must manage, including patient and procedural factors, complications from one-lung ventilation (OLV) including hypoxia and hypercapnia, capnothorax, percutaneous cannulation for cardiopulmonary bypass, TEE guidance, as well as methods of intraoperative monitoring and analgesia. As existing minimally invasive techniques are perfected, and newer innovations are demonstrated, it is imperative that the cardiothoracic anesthesiologist must improve and maintain skills to guide these patients safely through the robotic procedure.
Keywords: Cardiac surgery, Davinci, One-lung ventilation, Totally endoscopic coronary artery bypass, Transesophageal echocardiography, Robotic surgery, Minimally invasive cardiac surgery
INTRODUCTION
Traditional cardiac surgery, involving the performance of a median sternotomy to access the mediastinum and coronary structures, has been intensively reviewed in the literature and as technological advances in surgical practice continue to evolve. The use of robotic assisted devices to aid cardiac surgery took place when clinicians developed thoracoscopic techniques for take-down and anastomosis of the left internal mammary artery to anterior distal targets. The first totally endoscopic coronary artery bypass (TECAB) procedure using robotic assistance on the arrested heart was performed in 1998 by Dr. Loulmet using the daVinci Surgical System (Intuitive Surgical, Sunnyvale, CA, USA).[1] As the experience with the robotic system has grown, advances have enabled robotic assisted techniques to be developed for coronary revascularization, as well as mitral valve disease.[2] Totally endoscopic mitral valve surgery with a chest incision <1.5 cm using robotic assistance and femoral arterial and venous access for cardiopulmonary bypass (CPB) has been performed successfully. Robotic atrial septal defect repair and endoscopic treatment of atrial fibrillation have been performed.[3] The use of robotic techniques has expanded to pericardial procedures and placement of epicardial pacemaker leads.[4] Studies using laboratory animals have suggested that with further refinement, robotic approaches can be developed for the endoscopic repair of certain congenital lesions.[5]
ROBOTIC INSTRUMENTATION
The daVinci Surgical System (Intuitive Surgical, Sunnyvale, CA, USA) is the only commercially available surgical robot system consisting of a surgeon console, patient cart and video system [Figure 1]. The surgeon is seated away from the operating room table, providing him with a three dimensional view of the surgical field, including depth of field, magnification and high resolution [Figure 2]. The surgical instruments are attached to the robotic arms, which are inserted into the patient's thorax through 8 mm ports [Figure 3]. Master controls on the console enable the surgeon to manipulate the surgical instruments, translating the surgeon's hand and wrist movements into precise and scaled actions.[6] These instrument arms provide 7° of movement; a motion similar to the human wrist, but with even greater precision, dexterity, and nonexistent tremors [Figure 4]. Foot pedals allow precise camera control so that the surgeon can instantly zoom in and out to alter the view of the surgical field.
Figure 1.
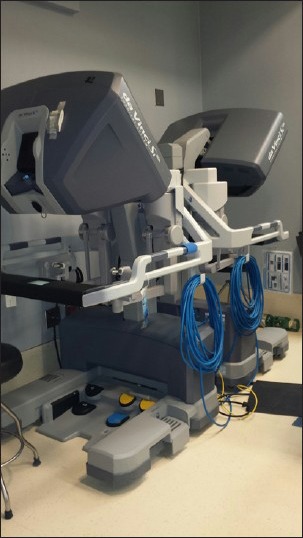
The da Vinci Surgical dual console can be used for both training and collaboration (Intuitive Surgical, Sunnyvale, CA, USA)
Figure 2.
Operative suite showing the location of the cardiac surgeon at remote da Vinci console
Figure 3.
Photograph of the 8 mm ports on patient's thorax used for insertion of the surgical instruments
Figure 4.
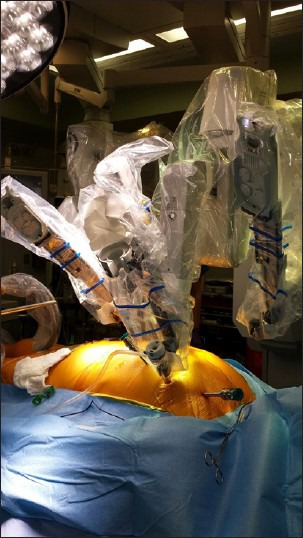
da Vinci surgical robotic arms, which provide 7° of movement with a motion similar to the human wrist, but with even greater precision, dexterity, and nonexistent tremors
METHODS
Minimally invasive cardiac surgery refers to the size of the incision, the avoidance of sternotomy and the avoidance of CPB. Robotic heart surgery is just one method of a minimally invasive technique and can be performed on both the arrested and beating heart.
Arrested heart using CPB provides optimal conditions for the surgeon and is the preferred method in most institutions. However, not all patients are candidates for this method due to either patient anatomy (tortuous femoral vessels, severe atherosclerotic aortic disease), hemodynamic instability or ventricular fibrillation (VF).
POTENTIAL BENEFITS FROM ROBOTIC APPROACH
Early robot usage was limited by poor visualization, and difficulty with hand-eye coordination. Experience with TECAB has demonstrated that performing cardiac procedures through a small incision with robotic assistance is a safe procedure in select individuals and produces excellent graft patency.[7] The use of port technology, along with a high definition optics and fine motor control of the robotic system has been touted as a way to reduce invasiveness, increase safety and improve outcomes for properly selected patients.
The use of bilateral internal mammary grafts for coronary revascularization significantly improves hospital outcomes as well as late survival in patients with coronary disease.[8] Studies have shown that patients who have had total arterial coronary revascularization suffer fewer late cardiac events and reoperations.[9] The use of robotic techniques facilitates the takedown of both mammary grafts, without putting the patient at risk for poor wound healing and deep sternal wound infection. This approach may prove a better solution for diabetic patients, patients with renal dysfunction, and reoperations.
When compared to the traditional median sternotomy approach, robotic cardiac surgery has demonstrated a reduction in postoperative pain, with decreased postoperative analgesia requirement. Robotic surgical techniques allow for a smaller incision with minimal scarring, sparing the patient from a median sternotomy and painful sternal retraction.[10,11] Avoidance of median sternotomy and painful sternal retraction may lead to more stability of the bony thorax, allowing for more activity in the postoperative period. A shorter recovery and quicker return to daily and professional activities may be responsible for the improved physical health and positive outlook on life.[12]
ANESTHETIC IMPLICATIONS
Preoperative evaluation
Appropriate patient selection is important in reducing the risk of perioperative complications with robotic heart surgery. Endoscopic surgery is more difficult in smaller patients with insufficient thoracic space (<3 cm) or patients with body mass index of greater than 35 kg/m2.[13] Studies have shown that TECAB grafting is beneficial in morbidly obese patients who have a greater risk for deep sternal wound infection.[14]
Anatomical issues that hinder port placement, limit robotic arm movements or reduce the already limited view within the thorax will increase the risk for surgical error and compromise patient safety. Patients with prior thoracic surgery, external beam radiation to the chest or a history of thoracic trauma with chest tube insertion may make the endoscopic procedure technically difficult. Various other anatomic issues such as an enlarged or rotated heart are also important considerations and may place the patient at unnecessary risk.
If percutaneous cannulation is planned for CPB, it is prudent to evaluate the vasculature for adequacy of flow, diameter, tortuosity and the presence of atheromatous plaque.[15]
Assessment of peripheral pulses, and computed tomography angiogram of the chest, abdomen and pelvis will provide a detailed assessment of the thoracic and abdominal aorta as well as iliac and femoral vessels. The presence of even mild atherosclerotic disease was associated with intra-aortic CPB perfusion device related difficulties, including intra-balloon migration and endoaortic balloon rupture.
During totally endoscopic robotic procedures with an arrested heart, an endoaortic balloon catheter is inserted percutaneously into the ascending aorta, and the balloon is inflated to occlude the ascending aorta.[16] The heart is then arrested following administration of cardioplegia solution. A perioperative transesophageal echocardiography (TEE) should be performed to measure the size of the ascending aorta and confirm that it is <3.8 cm to facilitate occlusion of the ascending aorta during administration of anterograde cardioplegia and minimize the risk of migration.[17]
Evaluation of the carotid arteries should be performed to stratify the patient's risk for stroke in the perioperative period. There has been an increased risk of vascular complications and stroke reported with peripheral arterial cannulation that has not been observed in cohort studies that utilized central access cannulation techniques.[18]
Robot assisted coronary artery bypass grafting requires continuous one-lung ventilation (OLV) and carbon dioxide insufflation, which may put patient at risk for hypoxemia, hypercarbia, and barotrauma. Patients with a history of chronic obstructive pulmonary disease (COPD), restrictive or infiltrative diseases of the lung, empyema, pleural effusions, or pulmonary hypertension should be considered for additional pulmonary testing, including pulmonary function tests to determine lung capacity and whether an alternative approach may be better tolerated. Patients with mild COPD should be optimized with a course of bronchodilators and potentially steroids prior to endoscopic heart surgery. Patients who smoke should be encouraged to stop at least 2 weeks prior to surgery. It has been demonstrated that patients with resting hypercarbia (>50 mmHg), hypoxia (PO2 <65 mmHg on room air), significantly lower forced vital capacity and forced expiratory volume in 1 s are unable to tolerate OLV and thus should not be considered for endoscopic procedures.[19,20]
Patients with unstable angina or recent myocardial infarction can have greater myocardial irritability and dysfunction in the setting of prolonged OLV.[21] Predictors of significant hypoxemia have been demonstrated in patients with high pulmonary vascular resistance and low ventricular ejection fraction during internal mammary takedown.[22] There are a few isolated reports of patients with severely impaired left ventricular function successfully undergoing TECAB with satisfactory results.[23] Patients with multivessel disease may still have an incomplete revascularization after robotic heart surgery and still require further percutaneous intervention.
For those patients who are deemed candidates for robotic heart surgery, other coronary factors may alter the surgical plan. Intramyocardial left anterior descending artery, small target vessels or even heavily calcified vessel will require the use of CPB or arrested heart techniques.
INTRAOPERATIVE ISSUES
Anesthetic management
A general anesthetic technique should be selected that takes into account the patient's history and comorbidities. An anesthetic with intravenous (IV) induction, maintenance with inhaled anesthetic agents, intermittent opioids and muscle relaxant is recommended. Muscle relaxation is especially important to prevent patient movement while the robot is docked to avoid accidental perforation of the myocardium, great vessels or other structures when the robot arms are engaged.[24]
Room layout
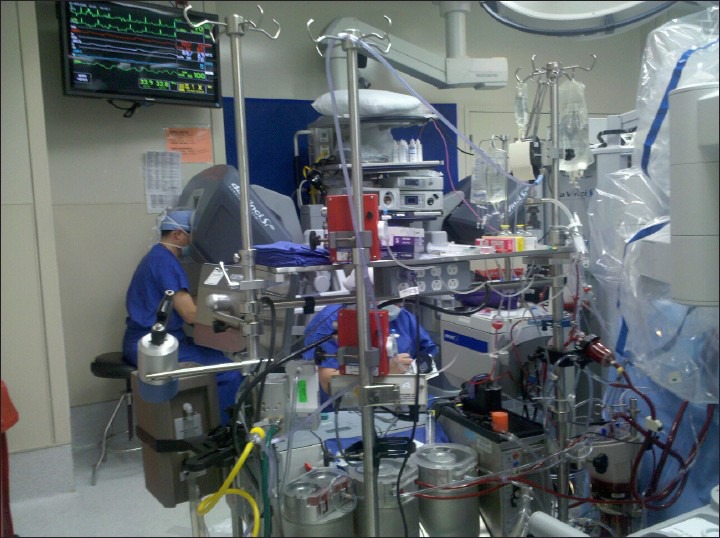
The amount of equipment utilized in robotic procedures can negatively impinge on the cardiac anesthesiologist's working space [Figure 5]. The anesthesia machine must be positioned away from operating room table, so that robot does not hit it when brought in from the side [Figure 6]. The surgical drapes and IV poles should be moved as cephalad as possible to provide adequate clearance for the robotic arms. Far reaching robotic arms can often obstruct the patient monitors and limit access to the head of the patient bed. The endotracheal tube should be angled cephalad and posteriorly, so as to prevent any dislodgement or unintentional tracheal extubation by a swinging arm of the robot.[24,25] Additional care should be taken to secure the endotracheal tube at several points, as access to the patient's chest, and airway is nearly impossible after the robot has docked at the patient's bedside. TEE machine and probe should be set up near the head of the bed for continuous monitoring. Vigilance must be employed to ensure that no equipment is positioned in a way to place pressure on the patient's head or face.
Figure 5.
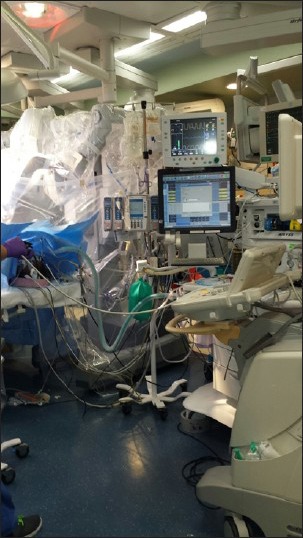
Crowded anesthesia workspace with anesthesia machine, monitors, and transesophageal echocardiography machine
Figure 6.
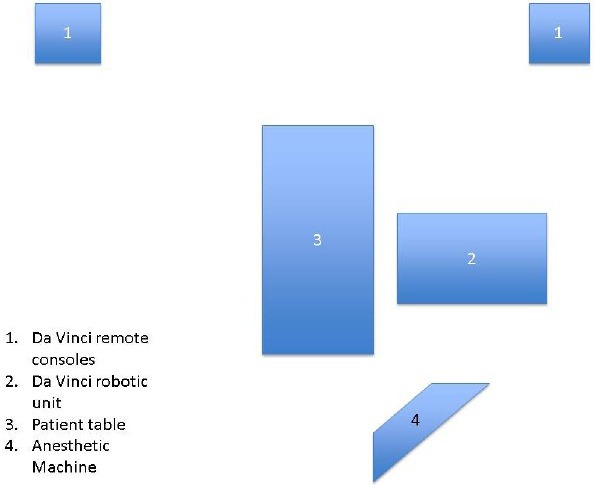
Diagram of operating room set up
All of the staffs need to be trained to be able to quickly detach and remove the robot from the patient in the event of a surgical emergency requiring median sternotomy. The patient should always be prepped and draped for emergency sternotomy if needed. Early reports suggest a conversion to an open sternotomy in greater than 50% of cases; however, with increased experience, this number has been reduced to the 10% range.[26]
Positioning of the patient
For most robotic cardiac surgeries, the patient will be placed initially in the supine position with pressure points adequately padded. For most robotic cardiac procedures, the need for exposure to the left hemithorax necessitates placement of a small roll underneath the patient's scapula. This maneuver will elevate the left hemidiaphragm allowing port placement in a triangular fashion.
The left arm may need to be posteriorly displaced at the side, maximizing the available surgical field. Excessive posterior displacement has been implicated in the occurrence of postoperative brachial plexopathies and should be avoided.[19] Patients are draped with the thorax, abdomen, bilateral groins, and one lower extremity exposed for potential surgical access.
MONITORING
Electrocardiogram monitoring
Anterior surgical incisions and exposure of the chest for potential conversion to sternotomy make the positioning of electrocardiogram (ECG) leads difficult in robotic heart procedures. Lateral ECG leads must be placed posteriorly and laterally to the midaxillary line to avoid port placement sites. However, this location may confound the interpretation of ischemia. The induction of capnothorax also changes the electrical axis and amplitude of the ECG, due to the increased distance between the chest wall and the heart.[27] Decreases in myocardial function due to ischemia can be detected from the ECG monitor, in conjunction with data from the TEE and pulmonary artery (PA) catheter (PA vent or Swan Ganz catheter).
Arterial pressure monitoring
Radial artery cannulation can be performed pre- or post-induction, depending on the patient's presentation and overall clinical status. The planned use of an endovascular occlusion device (endoaortic occlusion balloon clamp [EAOBC]) in the ascending aorta requires continuous simultaneous monitoring of arterial catheters in the bilateral upper extremities. If a left upper extremity arterial line is contraindicated, or if the left axillary artery is used as an arterial cannulation site by the surgeon, a femoral arterial line should be used.
Axillary artery cannulation has proven to be an acceptable method for robotic TECAB and is associated with less risk of retrograde cerebral embolization.[28] An arterial line on the same side as the cannula would reflect line pressure instead of arterial blood pressure when the cannula is in use and therefore has limited hemodynamic significance. The loss of a right sided arterial waveform may suggest that the endoaortic occlusion balloon catheter has migrated from the ascending aorta, obstructing the flow within the innominate artery. If a right upper extremity arterial line cannot be placed, alternative revascularization strategies such as beating-heart techniques should be considered. For beating-heart robotic procedures, a single radial arterial line can be utilized.
Cerebral oximetry
Cerebral oximetry (INVOS device, Somanetics Corporation, Troy, Michigan, USA) has been established as a noninvasive monitor of cerebral oxygenation in cardiac surgery. In robotic heart surgery, cerebral oximetry is helpful in monitoring proper placement of the inflated EAOBC and may be the first indication of a technical problem or physiological change in the patient, which could potentially lead to an adverse outcome.[29] Malposition of the EAOBC may cause a sudden decrease in the right-sided value, whereas a decline of the left-sided value may be due to occlusion of the left common carotid artery. A decline of >20% in bilateral readings, with appropriate EAOBC positioning, should prompt stepwise assessments and interventions to optimize oxygen delivery to the brain. Such interventions may include increasing oxygen delivery via an increase in systemic blood pressure, FiO2, or hematocrit, as well as a decrease in cerebral metabolism such as increasing anesthetic agent or decreasing systemic temperature.[30]
Transesophageal echocardiography
The use of minimally invasive robotic techniques provides opportunities for advanced training in TEE. TEE provides valuable information about baseline cardiac function and valvular abnormalities that may identify previously undetected contraindications to the robotic procedure, such as dilatation of the ascending aorta or severe aortic regurgitation.
Transesophageal echocardiography is important for the safe and accurate positioning of guidewires and cannulae for peripheral CPB during robotic heart surgery.[17] Using TEE, the femoral venous cannula guidewire should be seen in the right atrium and subsequent positioning of the cannula in the superior vena cava (SVC) (ME bicaval view). The femoral arterial guidewire should be visualized in the descending aorta prior to cannula placement (descending aortic Short axis view). The guidewire for the EAOBC should be seen in the descending aorta (descending aortic SAX view) prior to passing the cannula into the ascending aorta (ME aortic valve long axis view). TEE should also be used to continuously monitor the position of the inflated EAOBC during CPB to determine if migration of the catheter has occurred [Figure 7]. Using TEE, the descending aorta should be evaluated when commencing CPB to rule out aortic dissection (descending aortic SAX view). TEE has proven valuable during cannulation of the inferior vena cava (IVC) or SVC, as well as the ascending aorta, with only one aortic perforation noted under TEE guidance (0.78%).[31] This compares to the historical control rates of successful aortic cannulation using fluoroscopy as 92.86%, with a complication rate of 7.14%.[32]
Figure 7.
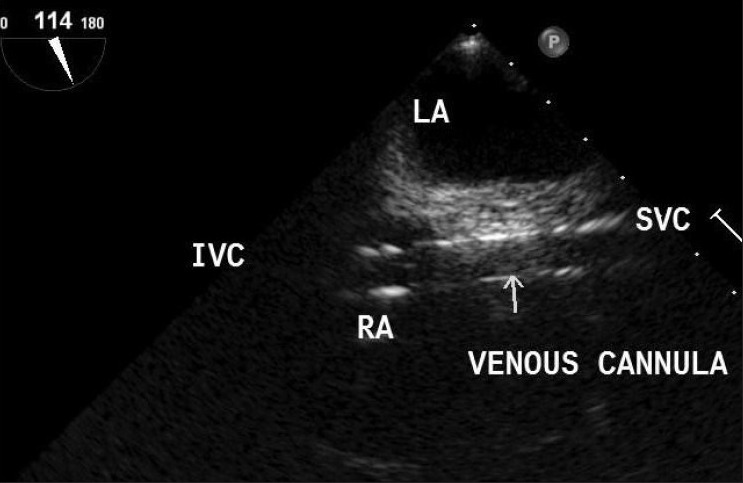
Transesophageal echocardiography bicaval view showing venous cannula passing into superior vena cava from inferior vena cava
Transesophageal echocardiography is vital for the conduct and safety of TECAB procedures since it can provide early detection of rare, but catastrophic, complications of remote-access perfusion, including aortic dissection or SVC injury during cannulation. TEE is also valuable in detecting reversible segmental wall motion abnormalities. Significant right ventricular dysfunction has been reported in patients undergoing TECAB prior to myocardial revascularization.[33] Increased intrathoracic pressure from the capnothorax may cause mediastinal shift, decreased venous return and subsequent compression of the right ventricle leading to segmental wall abnormalities.
In the future, addition, real-time three dimensional echocardiography may further provide adequate imaging and anatomic detail to act as an even better guide for surgical procedures.[34] Further technological development is needed to minimize the transducer size and optimize the spatial resolution for the clinical setting.
Temperature management
Temperature monitoring should take place in both the bladder and a central site (nasopharynx/esophageal). For arrested heart robotic procedures, systemic cooling on CPB will provide myocardial and cerebral protection. Beating heart robotic procedures will require relative maintenance of systemic temperature. Systemic hypothermia can result from patient exposure, prolonged surgery, and CO2 insufflation. Forced air warming should be employed to prevent intraoperative hypothermia, which can delay emergence and cause increased oxygen consumption postoperatively.
Ventricular fibrillation
Management of VF is challenging in robotic heart surgery since internal defibrillation is not possible, and chest compressions are difficult to perform. External defibrillating patches must be placed on the patient, avoiding the operative side of the chest and allowing the chest to be prepped for emergent conversion to sternotomy.[35] This configuration of the defibrillation pads may not be optimal for conduction across the cardiac axis, and defibrillation may be less effective. Capnothorax also may insulate the heart from the defibrillation current. Prior to attempting external defibrillation, alternative strategies including amiodarone and lidocaine should be administered. To increase its effectiveness, CO2 insufflation should be stopped with the capnothorax evacuated, and two-lung ventilation resumed before attempting defibrillation.[36]
ONE-LUNG VENTILATION
Utilization of thoracoscopic ports for robotic heart surgery requires the initiation of OLV for adequate visualization of cardiac structures. Unfortunately, a DLT needs to be replaced at the end of the surgical procedure, which may prove challenging in cases of a difficult airway or swelling of the pharynx.[37] The use of an airway exchange catheter, in conjunction with direct or video laryngoscopy, may provide a safe alternative to the extubation-reintubation technique.
Patients may experience arterial oxygen desaturation as a result of OLV and subsequent hypoxic pulmonary vasoconstriction. The initiation of OLV can decrease PaO2 values by 51%. This may require the use of higher concentrations of inhaled oxygen throughout the procedure.[25,38] The application of continuous positive airway pressure to the atelectatic lung has been demonstrated to improve oxygenation. However, the intrapleural pressure associated with capnothorax may reduce its effect in these cases. Positive end expiratory pressure can be applied to the ventilated lung. If the patient is unable to tolerate OLV, a plan must be in place to convert to an open procedure.
CAPNOTHORAX
Insufflation of the left hemithorax with carbon dioxide is performed during robotic cardiac surgical procedures for adequate exposure of the heart and great vessels, as well as preventing smoke formation during cautery usage. CO2 insufflation pressures between 5 and 10 mmHg optimizes visualization of cardiac structures, but may cause an increase in intrathoracic pressure, and a decreased venous return.[24] Higher insufflation pressures of 10 mmHg to 15 mmHg has been associated with a decline in cardiac index and the associated decrease in both mean arterial pressure and mixed venous oxygen values.[39] This decline in cardiac index and mean arterial pressure and mixed venous oxygen values is more pronounced in patients with reduced ventricular function.[40]
Significant increased arterial pCO2 can cause coronary artery vasoconstriction. Hemodynamic compromise can be alleviated with the use of fluids, transfusion and judicious use of inotropes and vasopressors.[41] With significant hypotension, insufflation pressures may need to be decreased. In patients with poor left ventricular function, an intraaortic balloon pump may even be indicated.
Increasing minute ventilation to compensate for the rise in PaCO2 may be difficult during OLV.
An 18G venous cannula in the pleural space can be used to measure pleural pressure and even act as a vent for excess CO2, avoiding tension pneumothorax/capnothorax. In some instances, a return to two-lung ventilation may be warranted. The placement of a nasogastric or orogastric tube can relieve gastric distention and prevent the rise of airway or intrapleural pressures. Continued vigilance and excellent communication between the anesthesiologist and surgeon is essential to deal with the continued hypoxia and progressive hypercarbia with CO2 insufflation. In some instances, a conversion to an open procedure is needed if OLV is not tolerated. Intrapleural pressures must constantly be monitored, and a pressure relief system should be working to prevent the development of a tension capnothorax, which can cause significant hemodynamic collapse.[38,40,42]
PERCUTANEOUS CANNULATION FOR BYPASS
In cases requiring CPB, a transfemoral approach for cannulation is most commonly employed due to its ease of placement and cosmesis. However, the axillary artery can also be used for arterial perfusion in patients with significant atherosclerotic disease of the aorta or peripheral arterial disease.[28]
Arterial access
Caution must be used when passing the arterial cannula through the descending aorta and aortic arch as atheromatous plaque can become dislodged and embolize distally. TEE is essential for evaluation of the distal descending aorta for atherosclerotic disease and determination of the degree of plague burden prior to cannulaton.
Venous access
Transesophageal echocardiography guides femoral venous cannulation, guide wire placement and final positioning from the IVC into the SVC [Figure 7]. Care must be taken when passing the venous cannula in the perihepatic region to avoid venous injury. Any loss of volume on bypass, particularly if persistent, should raise suspicion of retroperitoneal bleeding. Sometimes, the transfemoral venous cannula may not be adequate to completely drain the heart and cannulation of the SVC may be needed.
Pulmonary artery vent
For percutaneous CPB, the anesthesiologist may also place a PA vent for venting the PA. This catheter is inserted in a manner similar to a Swan Ganz catheter [Figure 8]. Since the PA vent catheter is not heparin coated, a 5000-unit dose of heparin is administered intravenously prior to its insertion, and it is it is important to remember to withdraw the PA vent after protamine administration., The PA vent allows passive venting of the PA at approximately 50 ml/min.
Figure 8.
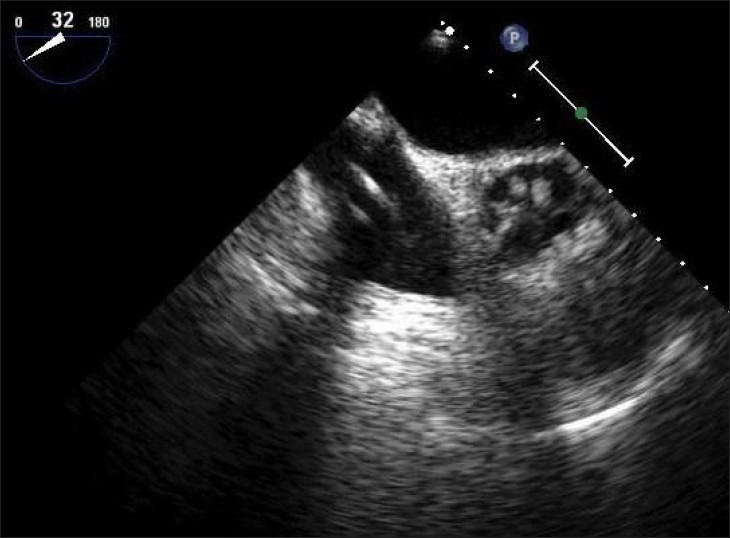
Transesophageal echocardiography image showing pulmonary artery vent passing from the right atrium into the pulmonary artery
Coronary sinus catheter
A coronary sinus catheter is placed by the anesthesiologist in the right internal jugular vein. Its position is confirmed by TEE guidance [Figure 9]. On inflating the coronary sinus catheter balloon, the pressure waveform will change from the appearance of a right atrial trace to the right ventricular trace. A 100 units/kg dose of heparin is recommended before coronary sinus manipulation to avoid coronary sinus thrombosis. Retrograde cardioplegia provides myocardial protection during a prolonged cross-clamp, multivessel coronary bypass grafting, tight stenoses or aortic regurgitation.
Figure 9.
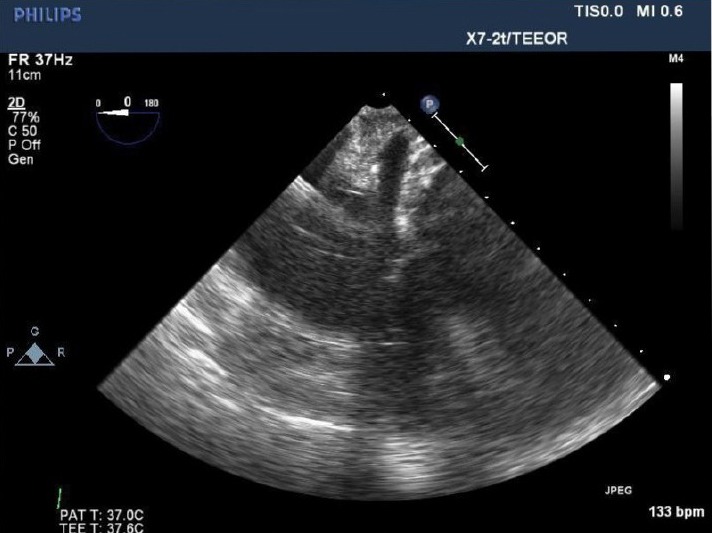
Visualization of the coronary sinus is critical for safe passage and positioning of retrograde coronary sinus catheter. Transesophageal echocardiography is the most commonly used modality, however fluoroscopy can also be used effectively
Endoaortic occlusion balloon clamp
The EAOBC is a balloon-tipped catheter, which is inserted through the femoral artery and positioned in the ascending aorta. TEE is used to guide and continuously assess the position of the aortic endoclamp, measure aortic root diameters, aortic distensibility and aortic wall appearance prior to and after aortic endoclamp, as well as guide weaning from CPB.[43] The endoclamp balloon must not occlude the coronary ostia or migrate distally to occlude the innominate artery, which can lead to obstruction of the innominate artery, resulting in cerebral hypoperfusion and neurological injury. Occasionally the balloon may migrate proximally obstructing the coronary arteries, causing myocardial dysfunction and major complications.[44] Use of the endoaortic balloon catheter should be avoided in heavily atherosclerotic aorta for fear of dislodgement and embolization of plaque.
Cardiopulmonary bypass is initiated with monitoring of right radial arterial pressure, aortic root pressure and with a vacuum assisted or kinetic assisted venous drainage. The endoaortic balloon is inflated to 250–300 mmHg pressure after its position is confirmed by TEE. The reported incidence of aortic dissection with the EAOBC is about 3/1000 patients.[45]
OFF PUMP PROCEDURES
Recent data have shown that off pump heart surgery has benefits in terms of reducing systemic inflammation and lowering the risks of hemodilution.[46] The International Society for Minimally Invasive Cardiothoracic Surgery reported that off pump bypass can reduce perioperative morbidity, reduce neurocognitive dysfunction and further reduce hospital length of stay.[47] Off pump procedures are further recommended in patients with severe aortic atherosclerotic disease, cirrhosis, or renal insufficiency to reduce the risk of morbidity and mortality.
TRANSFUSION RISK
Patients undergoing robotic cardiac surgery have relatively high rates of transfusion when compared to other surgical procedures.[48,49,50] A number of factors including low preoperative hematocrit, length of CPB, and use of antiplatelet agents are associated with increased blood transfusion requirements. The use of antiplatelet agents in the preoperative period also increases the likelihood of platelet transfusion intraoperatively. Additionally, though prospective data are scarce, it has been suggested by Woo in a single center observational study that the use of robotic technology in mitral valve repair, when compared to a traditional sternotomy approach, led to fewer packed red blood cell transfusions (5.0 vs. 2.8 units) and a shorter mean length of hospitalization (10.6 vs. 7.1 days).[48] The decision to transfuse must be made based on patient specific indications with an understanding of the risks involved.
ANALGESIA
The use of robotic technology has enabled a small incision and reduced the need for a formal sternotomy. Postoperative analgesia includes IV opioid analgesics and other approaches to provide analgesia and allow for early emergence and extubation. Intercostal nerve blocks are an inexpensive and relatively safe method of short-term analgesia. Newer technology involving preprogrammed ambulatory pumps, may allow patients to continue local anesthetic infiltration for several days postoperatively. Thoracic epidural analgesia has been used to control pain postoperatively.
There has been no increased incidence of epidural hematoma demonstrated, though a theoretical risk exists, particularly in cases that require full heparinization.[51] Paravertebral blocks, performed in the mid thoracic region, have been demonstrated as an effective method to control postoperative pain. This technique can decrease the risk of epidural hematoma while avoiding the hemodynamic perturbations associated the sympathectomy seen with neuraxial techniques.[52,53,54]
FUTURE DIRECTION
Minimally invasive heart surgery has grown in popularity due to the ability to achieve decreased pain, reduce hospital stays, and quicker recoveries. Since the daVinci robot (intuitive Surgical, Inc., USA) won federal approval in 2000, more than 860 have been installed in hospitals worldwide.[55] Significant data has emerged that confirms its safety and efficacy in a range of surgical procedures.[56]
Technological advances have flourished and expanded the indications for robotic cardiac surgery beyond coronary artery disease and mitral valve surgery. In the future, there is likely to be a greater request from patients seeking cardiac surgical options with reduced trauma that enable a faster return to baseline and improved quality of life. Despite the potential advantages, cardiac surgeons have been slow to abandon the median sternotomy and fully adopt robotic heart surgical techniques. A clear benefit to the robotic approach over other conventional methods has yet to be completely demonstrated.[57]
Operative times are considerably longer than for open procedures. A well-documented learning curve has been established. However with further simulation training, improved time efficiency can be achieved.[58]
Robotic cardiac surgery is also touted to be more expensive than other surgical alternatives.[59] As the surgical team becomes more familiar with the robotic technology, operational costs will decrease, and the benefits of robotic surgery may justify the initial investment.
Recently there has been an increase in the number of adverse side effects reported from the use of the surgical robot systems including organ and blood vessel punctures, surgical burns and death.[60] As a result, the Food and Drug Administration has prompted a survey with surgeons about their experience with this technology. However, widespread adoption of robotic cardiac surgical technique will not occur until long-term graft patency rates and outcome data have been demonstrated.
CONCLUSION
In order to properly care for cardiac patients undergoing robotic heart procedures, it is important for the cardiac anesthesiologists to be familiar with the various robotic systems, understand their potential uses, recognize their possible complications and develop an anesthetic plan to ensure safe patient care. With innovations in perfusion techniques, as well as specialized surgical instruments and robotic technology, expertise in TEE, and mastery of OLV techniques will be essential for every cardiac anesthesiologist to accomplish. Future direction will also center on team oriented approaches to patient management as well as efforts to reduce the potential costs.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Loulmet D, Carpentier A, d’Attellis N, Berrebi A, Cardon C, Ponzio O, et al. Endoscopic coronary artery bypass grafting with the aid of robotic assisted instruments. J Thorac Cardiovasc Surg. 1999;118:4–10. doi: 10.1016/S0022-5223(99)70133-9. [DOI] [PubMed] [Google Scholar]
- 2.Glower DD. Surgical approaches to mitral regurgitation. J Am Coll Cardiol. 2012;60:1315–22. doi: 10.1016/j.jacc.2011.11.081. [DOI] [PubMed] [Google Scholar]
- 3.Argenziano M, Williams MR. Robotic atrial septal defect repair and endoscopic treatment of atrial fibrillation. Semin Thorac Cardiovasc Surg. 2003;15:130–40. [PubMed] [Google Scholar]
- 4.DeRose JJ, Ashton RC, Belsley S, Swistel DG, Vloka M, Ehlert F, et al. Robotically assisted left ventricular epicardial lead implantation for biventricular pacing. J Am Coll Cardiol. 2003;41:1414–9. doi: 10.1016/s0735-1097(03)00252-3. [DOI] [PubMed] [Google Scholar]
- 5.Malhotra SP, Le D, Thelitz S, Hanley FL, Riemer RK, Suleman S, et al. Robotic-assisted endoscopic thoracic aortic anastomosis in juvenile lambs. Heart Surg Forum. 2002;6:38–42. doi: 10.1532/hsf.879. [DOI] [PubMed] [Google Scholar]
- 6.Lehr EJ, Zimrin D, Vesely M, Odonkor P, Griffith B, Bonatti J. Axillary-coronary sequential vein graft for total endoscopic triple coronary artery bypass. Ann Thorac Surg. 2010;90:e79–81. doi: 10.1016/j.athoracsur.2010.08.051. [DOI] [PubMed] [Google Scholar]
- 7.Gao C, Yang M, Wu Y, Wang G, Xiao C, Zhao Y, et al. Early and midterm results of totally endoscopic coronary artery bypass grafting on the beating heart. J Thorac Cardiovasc Surg. 2011;142:843–9. doi: 10.1016/j.jtcvs.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 8.Puskas JD, Sadiq A, Vassiliades TA, Kilgo PD, Lattouf OM. Bilateral internal thoracic artery grafting is associated with significantly improved long-term survival, even among diabetic patients. Ann Thorac Surg. 2012;94:710–5. doi: 10.1016/j.athoracsur.2012.03.082. [DOI] [PubMed] [Google Scholar]
- 9.Tatoulis J. Total arterial coronary revascularization-patient selection, stenoses, conduits, targets. Ann Cardiothorac Surg. 2013;2:499–506. doi: 10.3978/j.issn.2225-319X.2013.07.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bucerius J, Metz S, Walther T, Falk V, Doll N, Noack F, et al. Endoscopic internal thoracic artery dissection leads to significant reduction of pain after minimally invasive direct coronary artery bypass graft surgery. Ann Thorac Surg. 2002;73:1180–4. doi: 10.1016/s0003-4975(02)03385-4. [DOI] [PubMed] [Google Scholar]
- 11.Walther T, Falk V, Metz S, Diegeler A, Battellini R, Autschbach R, et al. Pain and quality of life after minimally invasive versus conventional cardiac surgery. Ann Thorac Surg. 1999;67:1643–7. doi: 10.1016/s0003-4975(99)00284-2. [DOI] [PubMed] [Google Scholar]
- 12.Bonaros N, Schachner T, Wiedemann D, Oehlinger A, Ruetzler E, Feuchtner G, et al. Quality of life improvement after robotically assisted coronary artery bypass grafting. Cardiology. 2009;114:59–66. doi: 10.1159/000212115. [DOI] [PubMed] [Google Scholar]
- 13.Seco M, Edelman JJ, Yan TD, Wilson MK, Bannon PG, Vallely MP. Systematic review of robotic-assisted, totally endoscopic coronary artery bypass grafting. Ann Cardiothorac Surg. 2013;2:408–18. doi: 10.3978/j.issn.2225-319X.2013.07.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rehman A, Garcia J, Deshpande S, Odonkor P, Reicher B, Vesely M, et al. Totally endoscopic coronary artery bypass grafting is feasible in morbidly obese patients. Heart Surg Forum. 2009;12:E134–6. doi: 10.1532/HSF98.20091038. [DOI] [PubMed] [Google Scholar]
- 15.Feuchtner GM, Schachner T, Bonaros N, Smekal A, Mallouhi A, Friedrich GJ, et al. Evaluation of ascending aortic atherosclerosis with 16-multidetector computed tomography is useful before total endoscopic coronary bypass surgery. Heart Surg Forum. 2006;9:E754–8. doi: 10.1532/HSF98.20051103. [DOI] [PubMed] [Google Scholar]
- 16.Anzai T, Iino Y, Kumeno T, Yozu R. Feasibility study of a direct endo-aortic clamp balloon. ASAIO J. 2007;53:136–9. doi: 10.1097/MAT.0b013e31802e1dc9. [DOI] [PubMed] [Google Scholar]
- 17.Deshpande SP, Lehr E, Odonkor P, Bonatti JO, Kalangie M, Zimrin DA, et al. Anesthetic management of robotically assisted totally endoscopic coronary artery bypass surgery (TECAB) J Cardiothorac Vasc Anesth. 2013;27:586–99. doi: 10.1053/j.jvca.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Iribarne A, Russo MJ, Easterwood R, Hong KN, Yang J, Cheema FH, et al. Minimally invasive versus sternotomy approach for mitral valve surgery: A propensity analysis. Ann Thorac Surg. 2010;90:1471–7. doi: 10.1016/j.athoracsur.2010.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murkin JM, Ganapathy S. Anesthesia for robotic heart surgery: An overview. Heart Surg Forum. 2001;4:311–4. [PubMed] [Google Scholar]
- 20.Lee JD, Srivastava M, Bonatti J. History and current status of robotic totally endoscopic coronary artery bypass. Circ J. 2012;76:2058–65. doi: 10.1253/circj.cj-12-0981. [DOI] [PubMed] [Google Scholar]
- 21.Srivastava S, Gadasalli S, Agusala M, Kolluru R, Barrera R, Quismundo S, et al. Beating heart totally endoscopic coronary artery bypass. Ann Thorac Surg. 2010;89:1873–9. doi: 10.1016/j.athoracsur.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 22.Liu TJ, Shih MS, Lee WL, Wang KY, Liu CN, Hung CJ, et al. Hypoxemia during one-lung ventilation for robot-assisted coronary artery bypass graft surgery. Ann Thorac Surg. 2013;96:127–32. doi: 10.1016/j.athoracsur.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Rehman A, Garcia J, Deshpande S, Fitzpatrick M, Odonkor P, Zimrin D, et al. Poor left ventricular function is not a contraindication for robotic totally endoscopic coronary artery bypass grafting. Heart Surg Forum. 2009;12:E152–4. doi: 10.1532/HSF98.20091048. [DOI] [PubMed] [Google Scholar]
- 24.Chauhan S, Sukesan S. Anesthesia for robotic cardiac surgery: An amalgam of technology and skill. Ann Card Anaesth. 2010;13:169–75. doi: 10.4103/0971-9784.62947. [DOI] [PubMed] [Google Scholar]
- 25.D’Attellis N, Loulmet D, Carpentier A, Berrebi A, Cardon C, Severac-Bastide R, et al. Robotic-assisted cardiac surgery: Anesthetic and postoperative considerations. J Cardiothorac Vasc Anesth. 2002;16:397–400. doi: 10.1053/jcan.2002.125154. [DOI] [PubMed] [Google Scholar]
- 26.de Cannière D, Wimmer-Greinecker G, Cichon R, Gulielmos V, Van Praet F, Seshadri-Kreaden U, et al. Feasibility, safety, and efficacy of totally endoscopic coronary artery bypass grafting: Multicenter European experience. J Thorac Cardiovasc Surg. 2007;134:710–6. doi: 10.1016/j.jtcvs.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 27.Damiano RJ, Jr, Ehrman WJ, Ducko CT, Tabaie HA, Stephenson ER, Jr, Kingsley CP, et al. Initial United States clinical trial of robotically assisted endoscopic coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2000;119:77–82. doi: 10.1016/s0022-5223(00)70220-0. [DOI] [PubMed] [Google Scholar]
- 28.Bonatti J, Garcia J, Rehman A, Odonkor P, Haque R, Zimrin D, et al. On-pump beating-heart with axillary artery perfusion: A solution for robotic totally endoscopic coronary artery bypass grafting? Heart Surg Forum. 2009;12:E131–3. doi: 10.1532/HSF98.20091036. [DOI] [PubMed] [Google Scholar]
- 29.Denault A, Deschamps A, Murkin JM. A proposed algorithm for the intraoperative use of cerebral near-infrared spectroscopy. Semin Cardiothorac Vasc Anesth. 2007;11:274–81. doi: 10.1177/1089253207311685. [DOI] [PubMed] [Google Scholar]
- 30.Murkin JM, Adams SJ, Novick RJ, Quantz M, Bainbridge D, Iglesias I, et al. Monitoring brain oxygen saturation during coronary bypass surgery: A randomized, prospective study. Anesth Analg. 2007;104:51–8. doi: 10.1213/01.ane.0000246814.29362.f4. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Gao CQ, Wang G, Wang JL. Transesophageal echocardiography guided cannulation for peripheral cardiopulmonary bypass during robotic cardiac surgery. Chin Med J (Engl) 2012;125:3236–9. [PubMed] [Google Scholar]
- 32.Reichenspurner H, Gulielmos V, Wunderlich J, Dangel M, Wagner FM, Pompili MF, et al. Port-Access coronary artery bypass grafting with the use of cardiopulmonary bypass and cardioplegic arrest. Ann Thorac Surg. 1998;65:413–9. doi: 10.1016/s0003-4975(97)01154-5. [DOI] [PubMed] [Google Scholar]
- 33.Mierdl S, Byhahn C, Lischke V, Aybek T, Wimmer-Greinecker G, Dogan S, et al. Segmental myocardial wall motion during minimally invasive coronary artery bypass grafting using open and endoscopic surgical techniques. Anesth Analg. 2005;100:306–14. doi: 10.1213/01.ANE.0000143565.18784.54. [DOI] [PubMed] [Google Scholar]
- 34.Suematsu Y, Kiaii B, Bainbridge DT, del Nido PJ, Novick RJ. Robotic-assisted closure of atrial septal defect under real-time three-dimensional echo guide: In vitro study. Eur J Cardiothorac Surg. 2007;32:573–6. doi: 10.1016/j.ejcts.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 35.Ganapathy S. Anaesthesia for minimally invasive cardiac surgery. Best Pract Res Clin Anaesthesiol. 2002;16:63–80. doi: 10.1053/bean.2001.0208. [DOI] [PubMed] [Google Scholar]
- 36.Hatton KW, Kilinski LC, Ramaiah C, Schell RM. Multiple failed external defibrillation attempts during robot-assisted internal mammary harvest for myocardial revascularization. Anesth Analg. 2006;103:1113–4. doi: 10.1213/01.ane.0000239242.69740.93. [DOI] [PubMed] [Google Scholar]
- 37.Kiaii B, Bainbridge D, Fernandes P. Surgical, anesthetic, perfusion-related advances in minimal access surgery. Semin Cardiothorac Vasc Anesth. 2007;11:282–7. doi: 10.1177/1089253207311160. [DOI] [PubMed] [Google Scholar]
- 38.Brock H, Rieger R, Gabriel C, Pölz W, Moosbauer W, Necek S. Haemodynamic changes during thoracoscopic surgery the effects of one-lung ventilation compared with carbon dioxide insufflation. Anaesthesia. 2000;55:10–6. doi: 10.1046/j.1365-2044.2000.01123.x. [DOI] [PubMed] [Google Scholar]
- 39.Dogan S, Aybek T, Mierdl S, Wimmer-Greinecker G. Totally endoscopic coronary artery bypass grafting on the arrested heart is a prerequisite for successful totally endoscopic beating heart coronary revascularisation. Interact Cardiovasc Thorac Surg. 2002;1:30–4. doi: 10.1016/s1569-9293(02)00008-7. [DOI] [PubMed] [Google Scholar]
- 40.Ohtsuka T, Imanaka K, Endoh M, Kohno T, Nakajima J, Kotsuka Y, et al. Hemodynamic effects of carbon dioxide insufflation under single-lung ventilation during thoracoscopy. Ann Thorac Surg. 1999;68:29–32. doi: 10.1016/s0003-4975(99)00319-7. [DOI] [PubMed] [Google Scholar]
- 41.Wang G, Gao C, Zhou Q, Chen T. Anesthesia management for robotically assisted endoscopic coronary artery bypass grafting on beating heart. Innovations (Phila) 2010;5:291–4. doi: 10.1097/IMI.0b013e3181ed20ca. [DOI] [PubMed] [Google Scholar]
- 42.Byhahn C, Mierdl S, Meininger D, Wimmer-Greinecker G, Matheis G, Westphal K. Hemodynamics and gas exchange during carbon dioxide insufflation for totally endoscopic coronary artery bypass grafting. Ann Thorac Surg. 2001;71:1496–501. doi: 10.1016/s0003-4975(01)02428-6. [DOI] [PubMed] [Google Scholar]
- 43.Falk V, Walther T, Diegeler A, Wendler R, Autschbach R, van Son JA, et al. Echocardiographic monitoring of minimally invasive mitral valve surgery using an endoaortic clamp. J Heart Valve Dis. 1996;5:630–7. [PubMed] [Google Scholar]
- 44.Mohr FW, Falk V, Diegeler A, Walther T, van Son JA, Autschbach R. Minimally invasive port-access mitral valve surgery. J Thorac Cardiovasc Surg. 1998;115:567–74. doi: 10.1016/S0022-5223(98)70320-4. [DOI] [PubMed] [Google Scholar]
- 45.Modi P, Rodriguez E, Hargrove WC, 3rd, Hassan A, Szeto WY, Chitwood WR., Jr Minimally invasive video-assisted mitral valve surgery: A 12-year, 2-center experience in 1178 patients. J Thorac Cardiovasc Surg. 2009;137:1481–7. doi: 10.1016/j.jtcvs.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 46.Iribarne A, Easterwood R, Chan EY, Yang J, Soni L, Russo MJ, et al. The golden age of minimally invasive cardiothoracic surgery: Current and future perspectives. Future Cardiol. 2011;7:333–46. doi: 10.2217/fca.11.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Puskas J, Cheng D, Knight J, Angelini G, Decannier D, Diegeler A, et al. Off-pump versus conventional coronary artery bypass grafting: A Meta-analysis and consensus statement from the 2004 ISMICS consensus conference. Innovations (Phila) 2005;1:3–27. doi: 10.1097/01243895-200512000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Woo YJ, Nacke EA. Robotic minimally invasive mitral valve reconstruction yields less blood product transfusion and shorter length of stay. Surgery. 2006;140:263–7. doi: 10.1016/j.surg.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Bonatti J, Schachner T, Wiedemann D, Weidinger F, Kolbitsch C, Knotzer H, et al. Factors influencing blood transfusion requirements in robotic totally endoscopic coronary artery bypass grafting on the arrested heart. Eur J Cardiothorac Surg. 2011;39:262–7. doi: 10.1016/j.ejcts.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 50.Puskas JD, Williams WH, Duke PG, Staples JR, Glas KE, Marshall JJ, et al. Off-pump coronary artery bypass grafting provides complete revascularization with reduced myocardial injury, transfusion requirements, and length of stay: A prospective randomized comparison of two hundred unselected patients undergoing off-pump versus conventional coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:797–808. doi: 10.1067/mtc.2003.324. [DOI] [PubMed] [Google Scholar]
- 51.Mehta Y, Swaminathan M, Mishra Y, Trehan N. A comparative evaluation of intrapleural and thoracic epidural analgesia for postoperative pain relief after minimally invasive direct coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 1998;12:162–5. doi: 10.1016/s1053-0770(98)90324-x. [DOI] [PubMed] [Google Scholar]
- 52.Mehta Y, Arora D, Sharma KK, Mishra Y, Wasir H, Trehan N. Comparison of continuous thoracic epidural and paravertebral block for postoperative analgesia after robotic-assisted coronary artery bypass surgery. Ann Card Anaesth. 2008;11:91–6. doi: 10.4103/0971-9784.41576. [DOI] [PubMed] [Google Scholar]
- 53.Dhole S, Mehta Y, Saxena H, Juneja R, Trehan N. Comparison of continuous thoracic epidural and paravertebral blocks for postoperative analgesia after minimally invasive direct coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2001;15:288–92. doi: 10.1053/jcan.2001.23271. [DOI] [PubMed] [Google Scholar]
- 54.Ganapathy S, Murkin JM, Boyd DW, Dobkowski W, Morgan J. Continuous percutaneous paravertebral block for minimally invasive cardiac surgery. J Cardiothorac Vasc Anesth. 1999;13:594–6. doi: 10.1016/s1053-0770(99)90015-0. [DOI] [PubMed] [Google Scholar]
- 55.Ryals J. Pioneer in Robotic Cardiac Surgery Completes Milestone. 2008. [Last accessed on 2008 Jun 23]. Available from: https://www.vidanthealth.com/vidant/newsroomdetail.aspx?id=5202 .
- 56.Athanasiou T, Ashrafian H, Rowland SP, Casula R. Robotic cardiac surgery: Advanced minimally invasive technology hindered by barriers to adoption. Future Cardiol. 2011;7:511–22. doi: 10.2217/fca.11.40. [DOI] [PubMed] [Google Scholar]
- 57.Sellke FW, Chu LM, Cohn WE. Current state of surgical myocardial revascularization. Circ J. 2010;74:1031–7. doi: 10.1253/circj.cj-10-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chitwood WR., Jr Robotic cardiac surgery by 2031. Tex Heart Inst J. 2011;38:691–3. [PMC free article] [PubMed] [Google Scholar]
- 59.Morgan JA, Thornton BA, Peacock JC, Hollingsworth KW, Smith CR, Oz MC, et al. Does robotic technology make minimally invasive cardiac surgery too expensive? A hospital cost analysis of robotic and conventional techniques. J Card Surg. 2005;20:246–51. doi: 10.1111/j.1540-8191.2005.200385.x. [DOI] [PubMed] [Google Scholar]
- 60.Lowes R. FDA Investigates Robotic Surgery System after Adverse Event Spike. [Last retrieved on 2013 Apr 30]. Available from: http://www.medscape.com/viewarticle/803339 .


