Abstract
Aims:
The aim of the study was to measure airway patency objectively during dexmedetomidine sedation under radiographic guidance in spontaneously breathing pediatric patients scheduled for cardiac catheterization procedures.
Subjects and Methods:
Thirty-five patients in the age group 5–10 years scheduled for cardiac catheterization procedures were enrolled. All study patients were given loading dose of dexmedetomidine at 1 μg/kg/min for 10 min and then maintenance dose of 1.5 μg/kg/h. Radiographic airway patency was assessed at the start of infusion (0 min) and after 30 min. Antero-posterior (AP) diameters were measured manually at the nasopharyngeal and retroglossal levels. Dynamic change in airway between inspiration and expiration was considered a measure of airway collapsibility. Patients were monitored for hemodynamics, recovery time and complications.
Statistical Analysis:
Student paired t-test was used for data analysis. P < 0.05 was considered significant.
Results:
Minimum and maximum AP diameters were compared at 0 and 30 min. Nasopharyngeal level showed significant reduction in the minimum (6.27 ± 1.09 vs. 4.26 ± 1.03, P < 0.0001) and maximum (6.51 ± 1.14 vs. 5.99 ± 1.03, P < 0.0001) diameters. Similarly retroglossal level showed significant reduction in the minimum (6.98 ± 1.09 vs. 5.27 ± 1.15, P < 0.0001) and maximum (7.49 ± 1.22 vs. 6.92 ± 1.12, P < 0.0003) diameters. The degree of collapsibility was greater at 30 min than baseline (P < 0.0001). There was a significant decrease in heart rate (P < 0.0001), and the average recovery time was 39.86 ± 12.22 min.
Conclusion:
Even though airway patency was maintained in all children sedated with dexmedetomidine, there were significant reductions in the upper airway dimensions measured, so all precautions to manage the airway failure should be taken.
Keywords: Airway, dexmedetomidine, radiographic assessment
INTRODUCTION
The Pediatric airways, unlike adult, are anatomically different. The small caliber airway makes pediatric patients more susceptible to airway collapse secondary to anesthetic agents used for procedural sedation. In earlier days, it was thought that infant tongue was relatively large compared to the rest of oral cavity causing airway obstruction. But later studies have shown that the contribution of tongue to airway obstruction was minor, and much of the collapse occurred at the level of nasopharynx and at epiglottic level.[1] Various anesthetic agents were used for procedural sedation in cardiac catheterization lab and studies were published in search of the ideal agent that would maintain adequate sedation without airway compromise and with the least effect on hemodynamics.[2,3] Numerous clinical trials have demonstrated sedation, anxiolytic and analgesic effects of dexmedetomidine,[4,5,6] and it is increasingly preferred as a sedative of choice for various pediatric procedures.
Dexmedetomidine is a potent alpha (α) α2 -adrenoceptor agonist of the imidazole group with a high ratio of specificity. The activity ratio of α2: α1 is 1620:1. It has rapid α half-life of ~7 min, elimination (β) half-life of ~2–3 h with a steady state volume of distribution of ~118 L and clearance of ~15 ml/kg/min. It is a bio transformed in the liver by Uridine 5’-diphospho-glucuronosyl transferase-glucuronyl transferase and cytochrome P450 into inactive metabolites of methyl and glucoronide conjugates. A small portion of the drug is excreted by the kidneys. It decreases the central sympathetic outflow resulting in decreased heart rate and blood pressure. When administered through intravenous route, 93% of the drug is protein bound. The bioavailability of dexmedetomidine varies depending on the route of administration. Its 16% for oral, 65% for intra nasal and 104% for intramuscular routes.[7,8]
Although advantages of dexmedetomidine in maintaining airway have been clinically studied, the degree of its effect on the upper airways by manual measurement has not been examined in detail. Documentation of its effects is critical to its safe use in patients at risk and equips us to manage any adverse event.
The aim of our study was to measure airway patency with dexmedetomidine sedation under radiographic guidance[9] in spontaneously breathing pediatric patients scheduled for cardiac catheterization procedures.
SUBJECTS AND METHODS
After getting institutional ethical committee approval and informed written consent from the subjects’ parents, 35 children in the age group of 5–10 years scheduled for elective cardiac catheterization procedures were enrolled in the study. Only children who were calm and co-operated for baseline lateral airway images were included. Children with a history of obstructive sleep apnea, respiratory tract infection and cyanotic congenital heart disease were excluded from the study. None of the study subjects received any premedication. All the patients had intravenous cannula inserted the day before. All the children had 5% EMLA™ (Eutectic Mixture of Local Anesthetic) cream applied to the groin for catheter insertion, 1 h prior to the procedure as per our study protocol. The subjects’ head and neck were placed in silicone head ring gel pad with the cervical spine in a neutral position and secured with a nonadhesive tape. Artificial airways of any kind were not used. Supplemental oxygen was provided with facemask at 6 L/min throughout the procedure. All patients were monitored for heart rate, oxygen saturation and noninvasive blood pressure. Sedation score was monitored with modified Ramsay sedation scale.[10,11] A score of >5 was targeted throughout the procedure. All study subjects received a loading dose of dexmedetomidine at 1 μg/kg over 10 min and then maintenance dose of 1.5 μg/kg/h. If the subject moved, a supplemental bolus of 0.1 μg/kg dexmedetomidine was given.[12] If the subject moved a second time, they were excluded from the study. Similarly, the study was terminated if the subjects’ heart rate decreased more than 20% from the baseline, oxygen saturation decreased below 90% or if the child became apneic.
Lateral views of airway were obtained with Philips Allura Xper FD10, Software version SW R8.1 (Philips Medical Systems NL BV, Veenpluis 4-6, Netherlands) before drug administration (0 min) and at 30 min after starting the infusion. All images were reviewed, and manual measurements were made by an independent observer who was not involved in the study. Each measurement was taken after proper calibration [Figure 1]. Manually, minimum and maximum airway antero-posterior (AP) diameters were measured at the level of nasopharynx (the shortest obliquely oriented line between soft palate and posterior pharyngeal wall) and at retroglossal level (the shortest distance between posterior aspect of the tongue, behind the epiglottis and posterior pharyngeal wall). Dynamic change in airway diameter between inspiration and expiration was considered a measure of airway collapsibility.[13] In the postanesthesia care unit (PACU), patients were monitored for the time taken to recover after stopping the infusion. Recovery time was defined as the time taken for the sedation score to return to <3, in the PACU.
Figure 1.
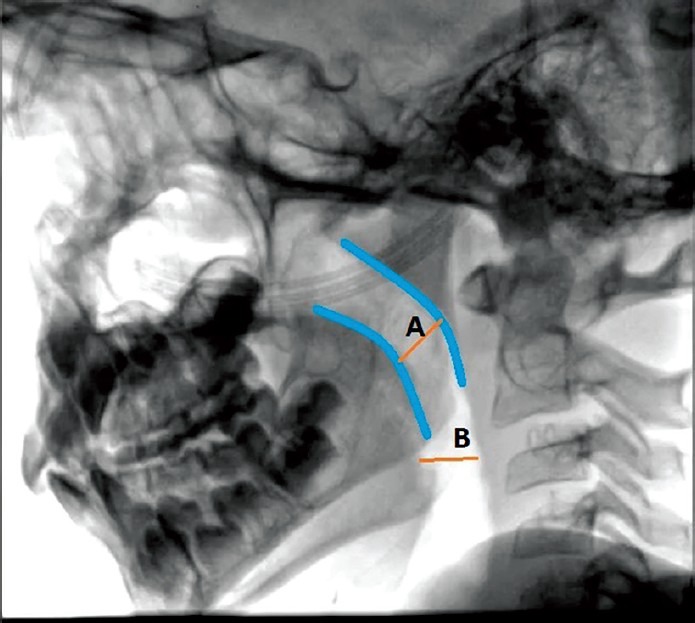
Lateral view of Airway to demonstrate measurement of AP diameter at nasopharyngeal and retroglossal level
All manual measurements and data were saved in excel database. The aggregated data were imported and analyzed with MedCalc statistical software version 13.0 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2014). Student paired t-test was used to compare the minimum and maximum airway diameters at 0 and 30 min at nasopharyngeal and retroglossal levels. The data were reported as mean ± standard deviation. P < 0.05 was considered statistically significant. To detect a 20% change in the airway dimension from baseline after the sedation, using a two tailed t-test of comparison of proportions, with power of 80% and a significance level of 5%, a sample size of 26 will be sufficient. The number has been increased to 35 considering the predicted drop outs from the study limb to be around 30% (effect of increasing depth of dexmedetomidine anesthesia on upper airway morphology in children. Pediatric Anesthesia 2010; 20:506–515).
RESULTS
All the 35 patients, who had enrolled, completed the study [Table 1]. All the patients achieved a sedation score of >5 throughout the procedure. Six patients required rescue medication at the time of device insertion. The various procedures performed were for patent ductus arteriosus (26%), atrial septal defect (37%), ventricular septal defect (17%), coarctation of aorta (9%), renal stenosis and cath angio studies (11%).
Table 1.
Comparison of Hemodynamics, Saturation and Airway diameter at 0 and 30 minutes
| Dimension | Mean±SD | 95% CI | P | |
|---|---|---|---|---|
| 0 min | 30 min | |||
| HR (beats/min) | 119.94±13.22 | 102.17±12.64 | −23.94 to −11.60 | 0.0001 |
| SBP (mmHg) | 116.49±6.73 | 114.29±6.74 | −5.41 to 1.01 | 0.18 |
| DBP (mmHg) | 62.37±6.91 | 60.26±5.66 | −5.13 to 0.899 | 0.17 |
| Oxygen saturation (%) | 99.7±0.63 | 99.66±0.64 | −0.33 to 0.27 | 0.84 |
| Retroglossal | ||||
| AP diameter (mm) | ||||
| Minimum | 6.98±1.097 | 5.27±1.15 | −1.97 to −1.46 | 0.0001 |
| Maximum | 7.49±1.22 | 6.92±1.12 | −0.86 to −0.28 | 0.0003 |
| Collapsibility | 0.502±0.72 | 1.64±0.57 | 0.89 to 1.39 | 0.0001 |
| Nasopharyngeal | ||||
| AP diameter (mm) | ||||
| Minimum | 6.27±1.09 | 4.26±1.03 | −2.22 to −1.81 | 0.0001 |
| Maximum | 6.51±1.14 | 5.99±1.03 | −0.66 to −0.36 | 0.0001 |
| Collapsibility | 0.23±0.33 | 1.74±0.70 | 1.27 to 1.74 | 0.0001 |
HR: Heart rate, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, SD: Standard deviation, CI: Confidence interval, AP: Anteroposterior
The average age of the study subjects was 7.69 ± 1.91 years, and the average height of the study subjects was 115.11 ± 6.3 centimeter (cm) with a maximum of 126 cm and a minimum of 102 cm. The average weight was 17.31 kg ± 3.8 kg with a maximum weight of 26 kg and minimum of 12 kg. The body mass index of the study subjects was of 12.95 ± 1.92.
There was a significant reduction in heart rate at 30 min (102.17 ± 12.64) compared to baseline (119.94 ± 13.22) which was statistically significant (P = 0.0001). The systolic and diastolic blood pressures were also reduced at 30 min compared to baseline values, but were not statistically significant (P = 0.18 and P = 0.17). There was no difference in saturation observed [Figure 2].
Figure 2.
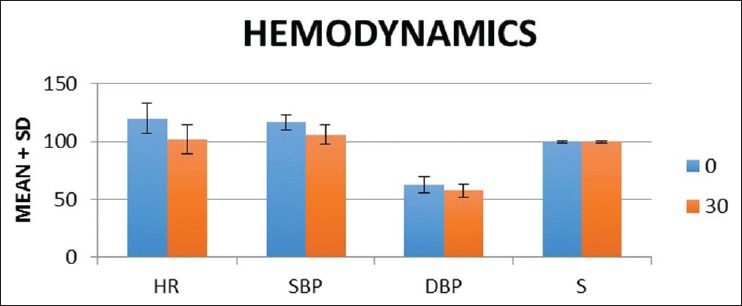
Haemodynamics parameters. HR: Heart rate, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, S: Saturation
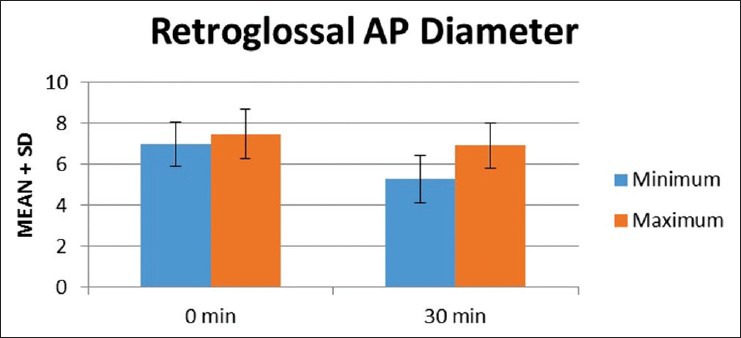
In retroglossal AP diameter [Figure 3] there was a reduction in minimum diameter at 30 min (5.27 mm ± 1.1 mm) compared to baseline (6.98 mm ± 1.097 mm). The difference was statistically significant (P = 0.0001). Similarly, there was also a reduction in maximum diameter at 30 min (6.92 mm ± 1.12 mm) compared to baseline (7.49 mm ± 1.22 mm) and was statistically significant (P = 0.0003). There was a significant increase in the degree of collapsibility at 30 min (1.64 mm ± 0.57 mm) compared to baseline (0.502 mm ± 0.72 mm) and was statistically significant (P = 0.0001).
Figure 3.
Mean airway diameter at nasopharyngeal level
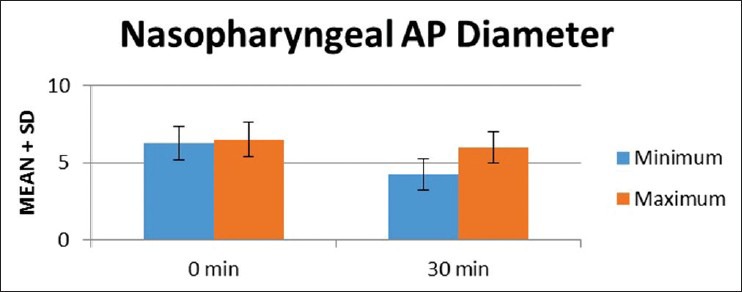
In nasopharyngeal AP diameter [Figure 4], there was a reduction in minimum diameter at 30 min (4.26 ± 1.03 mm) compared to baseline (6.27 ± 1.09 mm). The difference was statistically significant (P = 0.0001). Similarly there was reduction in maximum diameter at 30 min (5.99 ± 1.03 mm) compared to baseline (6.51 ± 1.14 mm) and was statistically significant (P = 0.0001). There was a significant increase in the degree of collapsibility at 30 min (1.74 ± 0.70 mm) compared to baseline (0.23 ± 0.33 mm) and it was statistically significant (P = 0.0001). The average recovery time from dexmedetomidine sedation after stopping the infusion was 39.86 ± 12.22 min with maximum being 70 min.
Figure 4.
Mean airway diameter at retroglossal level
DISCUSSION
More and more pediatric procedures are scheduled outside the operation room locations and various anesthetic agents like ketamine, propofol and dexmedetomidine are used for maintenance of anesthesia[14,15,16,17,18] Maintenance of airway and hemodynamics in such situations is a major concern even though practice guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures are available.[19,20]
Our study was prospective, single-blinded study designed to evaluate the effect of dexmedetomidine sedation on the airway patency in spontaneously breathing pediatric patients during cardiac catheterization procedures. There was a significant reduction in the minimum and maximum AP diameter measured at the retroglossal and nasopharyngeal levels with dexmedetomine anesthesia. Even though all our patients achieved the target level of sedation throughout the procedure, six patients required rescue dose of dexmedetomidine. There was also a significant reduction in the heart rate. The time to recovery after stopping the infusion was also prolonged. The results obtained in our study were comparable with that of other similar studies.
Mahmoud et al.,[13] in their study on the effect of increasing depth of dexmedetomidine anesthesia on upper airway morphology in children, have shown a similar reduction in the airway dimensions. However, in their study, no significant reduction in heart rate was noted. The cause for the significant reduction in heart rate in our study can be attributed to the loading dose of dexmedetomidine used.
Evans et al.,[21] in their study on the effect of increasing depth of propofol anesthesia on upper airway configuration in children, showed reduction in airway cross-sectional area, AP and transverse diameters, but baseline measurements were not made. In our study even though we measured baseline values, cross-sectional area or transverse diameters were not measured.
Machata et al.,[22] in their work on upper airway size and configuration during propofol-based sedation for magnetic resonance imaging, studied a series of 138 infants and children. However, they compared airway between different age groups, and more than one anesthetic agent was used. In our study neither premedication nor anesthetic agents other than dexmedetomidine were used.
Mahmoud et al.,[23] in their retrospective study comparing the dexmedetomidine with propofol for magnetic resonance imaging sleep studies in children, concluded that heart rate reduction was significant with dexemedetomidine and the need for artificial airway or manoeuvre was less with dexmedetomidine. Similarly, none of our study patients required any artificial airway or had clinically significant airway compromise.
The limitations in our study were that only AP diameter was measured. Cross-sectional area or transverse diameter was not measured. The quality of our images was inferior compared to that of magnetic resonance imaging or computed tomography. Apart from the above, our study subjects were a small subset of the population, and hence the results obtained were not representative of the general population.
Even though clinically significant airway compromise was not noted in any children sedated with dexmedetomidine, there were significant reductions in the measured upper airway dimension. Its use as a sole anesthetic agent required administration of high dose for maintenance of anesthesia that was not frequently accompanied with unfavorable decrease in heart rate and prolonged recovery time. It may find its application in noninvasive procedures like computed tomography angiography where apart from sedation, a certain degree of decrease in heart rate is favored.
CONCLUSION
Dexmedetomidine does has a favorable safety profile with respect to maintenance of airway; however, its use in high-risk group requires all precautions to be taken to manage airway failure and hemodynamics.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Litman RS, Fiadjoe JE. The pediatric airway. In: Cote CJ, Lerman J, editors. A Practice of Anesthesia for Infants and Children. 5th ed. Philadelphia, PA: Saunders, Elsevier Inc; 2013. pp. 237–76. [Google Scholar]
- 2.Munro HM, Tirotta CF, Felix DE, Lagueruela RG, Madril DR, Zahn EM, et al. Initial experience with dexmedetomidine for diagnostic and interventional cardiac catheterization in children. Paediatr Anaesth. 2007;17:109–12. doi: 10.1111/j.1460-9592.2006.02031.x. [DOI] [PubMed] [Google Scholar]
- 3.Tosun Z, Akin A, Guler G, Esmaoglu A, Boyaci A. Dexmedetomidine-ketamine and propofol-ketamine combinations for anesthesia in spontaneously breathing pediatric patients undergoing cardiac catheterization. J Cardiothorac Vasc Anesth. 2006;20:515–9. doi: 10.1053/j.jvca.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Tobias JD, Berkenbosch JW. Initial experience with dexmedetomidine in paediatric-aged patients. Paediatr Anaesth. 2002;12:171–5. doi: 10.1046/j.1460-9592.2002.00805.x. [DOI] [PubMed] [Google Scholar]
- 5.Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77:1134–42. doi: 10.1097/00000542-199212000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–94. doi: 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Mason KP, Lerman J. Review article: Dexmedetomidine in children: Current knowledge and future applications. Anesth Analg. 2011;113:1129–42. doi: 10.1213/ANE.0b013e31822b8629. [DOI] [PubMed] [Google Scholar]
- 8.Tobias JD. Dexmedetomidine: Applications in pediatric critical care and pediatric anesthesiology. Pediatr Crit Care Med. 2007;8:115–31. doi: 10.1097/01.PCC.0000257100.31779.41. [DOI] [PubMed] [Google Scholar]
- 9.Fink BR. Roentgenographic studies of the oropharyngeal airway. Anesthesiology. 1957;18:711–8. doi: 10.1097/00000542-195709000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–9. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrawal D, Feldman HA, Krauss B, Waltzman ML. Bispectral index monitoring quantifies depth of sedation during emergency department procedural sedation and analgesia in children. Ann Emerg Med. 2004;43:247–55. doi: 10.1016/s0196-0644(03)00721-2. [DOI] [PubMed] [Google Scholar]
- 12.Mason KP, Zurakowski D, Zgleszewski SE, Robson CD, Carrier M, Hickey PR, et al. High dose dexmedetomidine as the sole sedative for pediatric MRI. Paediatr Anaesth. 2008;18:403–11. doi: 10.1111/j.1460-9592.2008.02468.x. [DOI] [PubMed] [Google Scholar]
- 13.Mahmoud M, Radhakrishman R, Gunter J, Sadhasivam S, Schapiro A, McAuliffe J, et al. Effect of increasing depth of dexmedetomidine anesthesia on upper airway morphology in children. Paediatr Anaesth. 2010;20:506–15. doi: 10.1111/j.1460-9592.2010.03311.x. [DOI] [PubMed] [Google Scholar]
- 14.Tobias JD, Berkenbosch JW. Sedation during mechanical ventilation in infants and children: Dexmedetomidine versus midazolam. South Med J. 2004;97:451–5. doi: 10.1097/00007611-200405000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Tobias JD, Gupta P, Naguib A, Yates AR. Dexmedetomidine: Applications for the pediatric patient with congenital heart disease. Pediatr Cardiol. 2011;32:1075–87. doi: 10.1007/s00246-011-0092-8. [DOI] [PubMed] [Google Scholar]
- 16.Eastwood PR, Platt PR, Shepherd K, Maddison K, Hillman DR. Collapsibility of the upper airway at different concentrations of propofol anesthesia. Anesthesiology. 2005;103:470–7. doi: 10.1097/00000542-200509000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Heard CM, Joshi P, Johnson K. Dexmedetomidine for pediatric MRI sedation: A review of a series of cases. Paediatr Anaesth. 2007;17:888–92. doi: 10.1111/j.1460-9592.2007.02272.x. [DOI] [PubMed] [Google Scholar]
- 18.Litman RS, Weissend EE, Shrier DA, Ward DS. Morphologic changes in the upper airway of children during awakening from propofol administration. Anesthesiology. 2002;96:607–11. doi: 10.1097/00000542-200203000-00016. [DOI] [PubMed] [Google Scholar]
- 19.American Academy of Pediatrics, American Academy of Pediatric Dentistry, Coté CJ, Wilson S, Work Group on Sedation. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: An update. Pediatrics. 2006;118:2587–602. doi: 10.1542/peds.2006-2780. [DOI] [PubMed] [Google Scholar]
- 20.American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004–17. doi: 10.1097/00000542-200204000-00031. [DOI] [PubMed] [Google Scholar]
- 21.Evans RG, Crawford MW, Noseworthy MD, Yoo SJ. Effect of increasing depth of propofol anesthesia on upper airway configuration in children. Anesthesiology. 2003;99:596–602. doi: 10.1097/00000542-200309000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Machata AM, Kabon B, Willschke H, Prayer D, Marhofer P. Upper airway size and configuration during propofol-based sedation for magnetic resonance imaging: An analysis of 138 infants and children. Paediatr Anaesth. 2010;20:994–1000. doi: 10.1111/j.1460-9592.2010.03419.x. [DOI] [PubMed] [Google Scholar]
- 23.Mahmoud M, Gunter J, Donnelly LF, Wang Y, Nick TG, Sadhasivam S. A comparison of dexmedetomidine with propofol for magnetic resonance imaging sleep studies in children. Anesth Analg. 2009;109:745–53. doi: 10.1213/ane.0b013e3181adc506. [DOI] [PubMed] [Google Scholar]


