Abstract
Introduction:
Electroconvulsive therapy (ECT) is an effective treatment for many mental disorders, especially severe and persistent depression, bipolar disorder, and schizophrenia. The aim of this study is to compare the effect of dexmedetomidine and alfentanil on agitation, satisfaction, seizure duration, and patients hemodynamic after ECT.
Materials and Methods:
In a three phase crossover randomized clinical trial, 75 patients aged between 18 and 50 years and candidate for ECT were enrolled and assigned into three groups (25 patients in each group). All patients, respectively, took premedication of dexmedetomidine, alfentanil, or saline in three consecutive phases. Patients received 0.5 μg/kg dexmedetomidine, 10 μg/kg alfentanil or normal saline intravenously, 10 min before induction. Finally, seizure and recovery duration, satisfaction and agitation score, and hemodynamic parameters were evaluated.
Results:
There was no significant difference about seizure duration, agitation score, and hemodynamic parameters between groups but recovery duration was significantly lower in the control group than dexmedetomidine (P = 0.016) and alfentanil group (P = 0.0001). Patients’ satisfaction was significantly higher in intervention groups (alfentanil and dexmedetomidine groups) (P = 0.0001).
Conclusion:
Given the equal effects of alfentanil and dexmedetomidine, it seems that choosing one of these two drugs for premedication of patients undergoing ECT is appropriate. Drug choice is influenced by numerous factors such as accessibility of each drug and the dominance of anesthesiologist and psychiatrist.
Keywords: Alfentanil, Dexmedetomidine, Electroconvulsive therapy
INTRODUCTION
Psychiatric illnesses are one of the important health problems due to its dysfunction and burden of diseases in developing countries[1] and related to many functional disorders and economical cost in health.[1,2,3,4] Electroconvulsive therapy (ECT) is an effective and well-established treatment for a variety of psychiatric illnesses[4] such as patients with persistent and severe depression who have not responded to pharmacotherapy.[5,6] Nevertheless, all the ECT procedures were conducted under the full anesthesia and after muscular sleazy.[5] However, the ECT procedure is related with some acute hyperdynamic responses and variation in seizure and recovery time,[5] emergence agitation, hyperdynamic response, and lessened satisfaction in some patients after ECT.[6]
There are many studies that evaluated the effect of anesthesia drugs on outcomes after ECT including speed of recovery from disorientation,[7] agitation,[6,8] satisfaction,[9,10,11] hemodynamic responses, seizure time, and duration of recovery.[3,5,9,10,11,12,13] In addition, the effectiveness of anesthesia drugs such as dexmedetomidine,[5,6,7,8] propofol and thiopental[14] ketamine,[15] remifentanil to propofol,[16] remifentanil versus methohexital[17] is evaluated for anesthesia before ECT.
Nevertheless, we did not find any study that compared the effect of dexmedetomidine versus alfentanil effects in anesthesia before ECT. Dexmedetomidine is a robust α-2 receptor and helpful in decreasing of stress-induced sympathoadrenal responses and improve intraoperative hemodynamic stability during anesthesia procedures. In addition, it is reported that dexmedetomidine is effective in ECT without any serious side effects.[6] Because the hyperdynamic response to ECT is associated with an acute increase in the concentrations of epinephrine and norepinephrine in plasma, the effectiveness of dexmedetomidine comparing to alfentanil hypothesized in modifying the ECT-evoked hyperdynamic response, agitation, and satisfaction of patients. In addition, since based on our search was not found a specific research regarding to comparing the effect of alfentanil and dexmedetomidine, the current clinical trial was designed to assess and compare the effect of these drugs on the acute hyperdynamic response, agitation, seizure activity time, and duration of recovery in patients undergoing ECT.
MATERIALS AND METHODS
A three phase crossover randomized clinical trial was conducted on patients who were a candidate for ECT in Arak Amirkabir Hospital. Sample size formula used based on type one error 0.05 and study power equal 0.90 and 25 eligible subjects selected for each three group. Therefore, 75 eligible criteria with inclusion criteria were assigned to three groups (25 patients in each group) after taken informed consent. Seventy-five patients with American Society of Anesthesiologists (ASA II) that were candidate for ECT according to psychologist diagnosis enrolled for the study. Then, based on inclusion and exclusion criteria selected for participating in randomized controlled trial and informed consent were taken from all eligible participants. Inclusion criteria were as follows: Affecting to ASA II, should be between 18 and 50 years old, should not be pregnant women, should be without the history of cardiovascular disease and no history of using beta-receptor blocker drugs or narcotic usage, and should not be sensitive to understudy drugs (alfentanil or dexmedetomidine). Moreover, patients who need to treatment due to severe hemodynamic instability, patients with duration of tonic–clonic seizure lower than 25 s, patients that need to more than 60% energy for induction of tonic–clonic seizure and patients that were not satisfied cooperation in the study were excluded.
All patients were visited by anesthesiologist and were assigned randomly to one of three groups after data collection regarding to demographic characteristics, vital signs, and arterial saturation oxygen percent. Block randomization method was used in the random allocation and multiple crossover design applied in this study. In group A, dexmedetomidine given as 0.5 μg/kg 10 min before induction. In group B, alfentanil was used as 10 μg/kg 10 min before induction. Moreover, in group C, saline injected as 10 ml. To blind the patients of study the volume of injected drug in A and B groups increased to 10 ml. Each intervention changed among groups after a washing phase equal at least 48 h. The framework of the study was as Figure 1.
Figure 1.
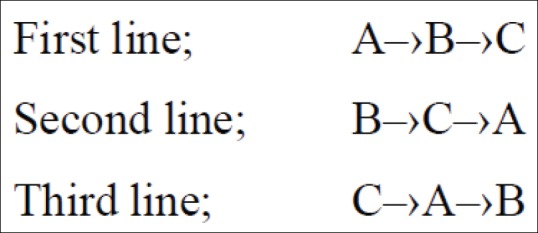
The sequences of intervention in three phase crossover trial of dexmedetomidine (A), alfentanil (B), and saline (C). First line; A‒›B‒›C, Second line; B‒›C‒›A, Third line; C‒›A‒›B
After premedication, anesthesia was induced with thiopental (2–3 mg/kg) for all patients and for muscular sleazy and then succinylcholine 0.5 mg/kg intravenous (IV) was administered. Moreover, atropine injected for all patients for prophylactic effect in bradycardia after using succinylcholine. Ventilation conducted for all patients in three groups by 100% oxygen. After full anesthesia in patients, electrical shock applied by 30–60% energy according to psychologist diagnosis in bilateral of temporal site. Seizure time and recovery duration, arterial blood pressure mean, heart pulse rate, and the percent of arterial saturation oxygen recorded by monitoring at 5th, 15th min, and after recovery. Recovery duration defined as from the end of succinylcholine injection to conducting orders by the patients. The interventions have been changes among groups after washing phase as bellow diagram.
Agitation score of all patients was recorded after full recovery. Scoring conducted in a Likert scale as 1 = sleepy, 2 = awake and peaceful, 3 = irritable and noisy, 4 = disconsolate noisy, and 5 = severe blenched or willing to wake up from bed or sitting on the bed and shrieking.[18] In addition, the satisfaction of patients measured according to another scale that presented 1 = happy and peaceful, 2 = without rumble and not bad satisfaction, 3 = having no acquiescence or moderate satisfaction, and 4 = patient unsatisfaction and shows that patients do not like any same treatment.[19] Seizure score and the mean of agitation and satisfaction scores measured by a blind psychologist regarding to treatments of groups.
All the studied patients took informed consent, and the Ethical Committee of Arak University of Medical Sciences approved the study protocol. In addition, patients that did not included or excluded from the study due to our criteria were referred to the clinic for suitable treatment.
Data were analyzed by PASW Statistics ver. 18 (IBM Co., Armonk, NY, USA) and descriptive statistics including mean and percent was used. Moreover, t-test, analysis of variance (ANOVA), Chi-square, and repeated measurement test were used in inferential analyses.
RESULTS
Generally, 25 patients studied in this study that 15 (60%) were female. The mean age of male and females was 37.5 ± 10.8 and 39.6 ± 12.5 years, respectively (P = 0.465). Baseline hemodynamic parameters including arterial blood pressure mean, heart pulse rate, and percent of arterial saturation oxygen were not statistically among three groups. The values of these factors are presented at baseline column of Table 1.
Table 1.
Repeated measurement in hemodynamic parameters in baseline, 5th, 15th min, and after study in three studied groups
| Hemodynamic parameters | Baseline | 5th min | 15th min | After study | P |
|---|---|---|---|---|---|
| MAP | |||||
| Dexmedetomidine | 90.48±6.5 | 86.12±7.7 | 98.6±6.8 | 93.96±6.9 | 0.436 |
| Alfentanil | 89.8±6.2 | 84.36±6.9 | 97.54±7.8 | 93.76±6.8 | |
| Control | 90.1±6.3 | 89.52±5.9 | 99.12±6.5 | 92.96±6.4 | |
| HR | |||||
| Dexmedetomidine | 87.20±9.3 | 81.9±8.7 | 95.52±6.7 | 93.28±7.4 | 0.823 |
| Alfentanil | 85.76±10 | 80.28±11.6 | 94.92±8.4 | 93.16±7.8 | |
| Control | 84.32±10.9 | 84.84±12.1 | 95.96±9.5 | 92.24±9.8 | |
| SO2 | |||||
| Dexmedetomidine | 98.96±0.2 | 98.93±0.3 | 97.44±1.4 | 99.1±0.1 | 0.882 |
| Alfentanil | 98.9±0.3 | 98.96±0.2 | 97.24±1.4 | 98.96±0.2 | |
| Control | 99.0±0.12 | 99.12±0.1 | 97.4±1.8 | 98.98±0.1 |
MAP: Mean arterial pressure, HR: Heart rate
There was not statistical significant in electroshock energy (P = 0.487) and seizure duration (P = 0.355) among groups as it presented in Table 2. Furthermore, there was a significant difference in recovery duration (P < 0.05) among groups. Post hoc test (Tukey) showed that the mean duration of recovery in the control group was lower than two intervention groups (P < 0.01) and there was no difference between alfentanil and dexmedetomidine groups regarding to recovery duration (P > 0.05).
Table 2.
Comparison of energy, seizure, and recovery duration among patient in three studied groups after intervention
| Variables | Dexmedetomidine | Alfentanil | Control | P |
|---|---|---|---|---|
| Energy (jowl) | 47.6±10.6 | 46.48±13.1 | 50.6±13.6 | 0.325 |
| Seizure duration (min) | 31.64±5.9 | 32.68±6.3 | 34.2±6.5 | 0.548 |
| Recovery duration (min) | 29.2±4.7 | 31.2±3.9 | 26.0±3.2 | 0.023 |
The outcome variables in our study were agitation and satisfaction rate of patients after ECT. Table 3 showed the mean difference among three groups regarding to agitation and satisfaction scores. According to the results, there was no significant difference among groups in agitation score (P > 0.05). Furthermore, a significant difference observed among groups in satisfaction score among three groups. Tukey test as a post hoc analysis showed that there was a significant difference between dexmedetomidine and control group (P < 0.001) and in alfentanil and control group too (P < 0.001). Nevertheless, the difference between dexmedetomidine and alfentanil was not significant (P = 0.482).
Table 3.
Comparison of agitation and satisfaction score among patient in three studied groups after intervention
| Variables | Dexmedetomidine | Alfentanil | Control | P |
|---|---|---|---|---|
| Agitation | 1.96±0.2 | 1.84±0.37 | 2.06±0.04 | 0.287 |
| Satisfaction score | 1.44±0.51 | 1.28±0.46 | 2.2±0.5 | 0.012 |
Hemodynamic parameters measured in 4 times in baseline, 5th, 15th min, and after study in all patients and repeated measurement test showed that [Table 1] the trends of changes were not significant in four measurements regarding mean arterial pressure (MAP), heart rate (HR), and SO2 (P > 0.05). In addition, ANOVA conducted to assess the difference among groups in each time and except in 5th min here was no significant difference among three studied groups. Tukey test in MAP analysis showed that there was a significant difference between alfentanil and control groups in 5th min after intervention (P = 0.027).
DISCUSSION
Our results showed that the satisfaction score in dexmedetomidine and alfentanil groups was statistically higher than control group. Besides, the recovery duration was significantly lower in the control group. Nevertheless, did not observe a significant difference among groups regarding to agitation and hemodynamic parameters including MAP, HR, and SO2. These results are as the same of other studies. Mizrak et al.[6] study showed that premedication with low-dose IV dexmedetomidine, 0.5 μg/kg could be useful in patients who had experienced previously and had been resistant to treatment before ECT in agitation after ECT without creating any adverse effects.[6] Moreover, that study showed that the mean of convulsive activity duration was longer in group dexmedetomidine than in control group that received saline.[6]
Begec et al.[5] study also showed that IV dose of dexmedetomidine (1 μg/kg) over 10 min before anesthesia induction could be effective in preventing the acute hyperdynamic responses to ECT without any changes in seizure activity duration and recovery time. In our study, dexmedetomidine was not different by control group regarding to hemodynamic parameters. In addition, the recovery time in patients that administrated with dexmedetomidine was significantly higher. These differences may be justifiable due to lower than dose of dexmedetomidine (half of Begec study) in our study.
In Fu and White study, dexmedetomidine 0.5–1 μg used as co-anesthesia drug and could not create a significant difference with control group regarding to arterial blood pressure mean and pulse rate.[20] Similar results to our and Flu study observed in Mizrak et al. study.[6] Furthermore, in another study, remifentanil (100 μg) was effective on the incidence of acute hemodynamic responses of ECT after anesthesia with methohexital without any change in recovery and seizure duration.[12] Although the mechanism of ECT is unknown but the seizure duration is an index for effectiveness of ECT and seizure time more than 25 s is an indicator for sufficiently of ECT.[19] The most anesthesia drugs with short effect that using in ECT decreasing the seizure time or other complication of psychiatric disorders based on dosage in depressive patients.[4,13,14,18,21,22,23] Therefore, narcotics such as alfentanil used for increasing of seizure time beside anesthesia drugs synthetic or separately.[10,24,25] Moreover, the effect of dexmedetomidine on seizure is evaluated in different studies.[5,25,26] Flu study showed that dexmedetomidine increase seizure time.[20] In Mizrak et al. study, dexmedetomidine increased the seizure time of patients without changes in recovery duration and satisfaction of patients.[6]
Alfentanil effect on seizure time was assessed in some studies. Akcaboy et al. study showed that using alfentanil 10 μg/kg was prepared the effective anesthesia and increased the seizure time comparing to propofol.[10] In addition, similar results achieved in Nguyen et al. study with same dose of alfentanil in patients that candidate for ECT without consequences of narcotics.[27] In addition, other studies evaluated the effect of remifentanil and methohexital separately or mixed and founded that these interventions are effective on seizure time comparing to control groups.[12,16,17,25]
Since ECT applies in patients with psychological disorder frequently, drugs that using in anesthesia should be have short recovery time. Our results in the same of Fu and White study[20] showed that recovery duration was longer in intervention group. However, different results obtained in Akcaboy et al. study and recovery duration was similar in alfentanil and remifentanil groups and lower than control group.[10] Nevertheless, in Begec et al. and Mizrak et al.[5,6] did not observe a significant difference between intervention and control group regarding to recovery duration. These paradoxes are due to difference of drug dosage and using succinylcholine.
The seizure time of patients in current study assessed by observation of tonic-clonic activities in patient's organs and did not use from electroencephalogram. This monitoring is a limitation due to the central seizure recorded by electroencephalogram may be longer than tonic-clonic activities.[28] Therefore, since recording seizure time by electroencephalogram could achieve precious data of patients regarding to electrophysiological seizure activities, it is suggested that electroencephalogram method used in future studies. Moreover, side effect consequences of interventions could be comparing.
CONCLUSION
In our study, using dexmedetomidine and alfentanil were effective on satisfaction of patients who were candidate for ECT. Dexmedetomidine and alfentanil were not preferable regarding to seizure time or recovery duration. Therefore, regarding to equivalency effect of dexmedetomidine and alfentanil in ECT, it seems that there was no difference between two interventions. Nevertheless, alfentanil is more accessible in Iran, and the authors suggest that drug could be select based on accessibility of dexmedetomidine and alfentanil, and psychologist and anesthesiologist prefer as well as patient condition.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
REFERENCES
- 1.Bayati A, Beigi M, Salehi M. Depression prevalence and related factors in Iranian students. Pak J Biol Sci. 2009;12:1371–5. doi: 10.3923/pjbs.2009.1371.1375. [DOI] [PubMed] [Google Scholar]
- 2.Bilgen AE, Bozkurt Zincir S, Zincir S, Ozdemir B, Ak M, Aydemir E, et al. Effects of electroconvulsive therapy on serum levels of brain-derived neurotrophic factor and nerve growth factor in treatment resistant major depression. Brain Res Bull. 2014;104:82–7. doi: 10.1016/j.brainresbull.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Ren J, Li H, Palaniyappan L, Liu H, Wang J, Li C, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: A systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:181–9. doi: 10.1016/j.pnpbp.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Aksay SS, Bumb JM, Janke C, Biemann R, Borucki K, Lederbogen F, et al. Serum lipid profile changes after successful treatment with electroconvulsive therapy in major depression: A prospective pilot trial. J Affect Disord. 2016;189:85–8. doi: 10.1016/j.jad.2015.09.037. [DOI] [PubMed] [Google Scholar]
- 5.Begec Z, Toprak HI, Demirbilek S, Erdil F, Onal D, Ersoy MO. Dexmedetomidine blunts acute hyperdynamic responses to electroconvulsive therapy without altering seizure duration. Acta Anaesthesiol Scand. 2008;52:302–6. doi: 10.1111/j.1399-6576.2007.01462.x. [DOI] [PubMed] [Google Scholar]
- 6.Mizrak A, Koruk S, Ganidagli S, Bulut M, Oner U. Premedication with dexmedetomidine and midazolam attenuates agitation after electroconvulsive therapy. J Anesth. 2009;23:6–10. doi: 10.1007/s00540-008-0695-2. [DOI] [PubMed] [Google Scholar]
- 7.Magne Bjølseth T, Engedal K, Šaltyte Benth J, Bergsholm P, Strømnes Dybedal G, Lødøen Gaarden T, et al. Speed of recovery from disorientation may predict the treatment outcome of electroconvulsive therapy (ECT) in elderly patients with major depression. J Affect Disord. 2016;190:178–86. doi: 10.1016/j.jad.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Haq AU, Espinoza R, Chen S. Use of dexmedetomidine for prevention of post-ictal agitation after electroconvulsive therapy in the elderly versus the young. Am J Geriatr Psychiatry. 2014;22(3 Suppl):S76. [Google Scholar]
- 9.Kayser S, Bewernick BH, Hurlemann R, Soehle M, Schlaepfer TE. Comparable seizure characteristics in magnetic seizure therapy and electroconvulsive therapy for major depression. Eur Neuropsychopharmacol. 2013;23:1541–50. doi: 10.1016/j.euroneuro.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Akcaboy ZN, Akcaboy EY, Yigitbasl B, Bayam G, Dikmen B, Gogus N, et al. Effects of remifentanil and alfentanil on seizure duration, stimulus amplitudes and recovery parameters during ECT. Acta Anaesthesiol Scand. 2005;49:1068–71. doi: 10.1111/j.1399-6576.2005.00766.x. [DOI] [PubMed] [Google Scholar]
- 11.Cano M, Cardoner N, Urretavizcaya M, Martínez-Zalacaín I, Goldberg X, Via E, et al. Modulation of limbic and prefrontal connectivity by electroconvulsive therapy in treatment-resistant depression: A preliminary study. Brain Stimul. 2016;9:65–71. doi: 10.1016/j.brs.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Recart A, Rawal S, White PF, Byerly S, Thornton L. The effect of remifentanil on seizure duration and acute hemodynamic responses to electroconvulsive therapy. Anesth Analg. 2003;96:1047–50. doi: 10.1213/01.ANE.0000054002.65040.B3. [DOI] [PubMed] [Google Scholar]
- 13.Bjølseth TM, Engedal K, Benth JŠ, Dybedal GS, Gaarden TL, Tanum L. Clinical efficacy of formula-based bifrontal versus right unilateral electroconvulsive therapy (ECT) in the treatment of major depression among elderly patients: A pragmatic, randomized, assessor-blinded, controlled trial. J Affect Disord. 2015;175:8–17. doi: 10.1016/j.jad.2014.12.054. [DOI] [PubMed] [Google Scholar]
- 14.Martínez-Amorós E, Gálvez Ortiz V, Porter Moli M, Llorens Capdevila M, Cerrillo Albaigés E, Garcia-Parés G, et al. Propofol and thiopental as anesthetic agents in electroconvulsive therapy: A retrospective study in major depression. Rev Psiquiatr Salud Ment. 2014;7:42–7. doi: 10.1016/j.rpsm.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Ghasemi M, Kazemi MH, Yoosefi A, Ghasemi A, Paragomi P, Amini H, et al. Rapid antidepressant effects of repeated doses of ketamine compared with electroconvulsive therapy in hospitalized patients with major depressive disorder. Psychiatry Res. 2014;215:355–61. doi: 10.1016/j.psychres.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Porter R, Booth D, Gray H, Frampton C. Effects of the addition of remifentanil to propofol anesthesia on seizure length and postictal suppression index in electroconvulsive therapy. J ECT. 2008;24:203–7. doi: 10.1097/YCT.0b013e3181662ca0. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan PM, Sinz EH, Gunel E, Kofke WA. A retrospective comparison of remifentanil versus methohexital for anesthesia in electroconvulsive therapy. J ECT. 2004;20:219–24. doi: 10.1097/00124509-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Kyeremanteng C, MacKay JC, James JS, Kent P, Cayer C, Anisman H, et al. Effects of electroconvulsive seizures on depression-related behavior, memory and neurochemical changes in Wistar and Wistar-Kyoto rats. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:170–8. doi: 10.1016/j.pnpbp.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 19.2nd edition. Washington: American Psychiatric Publishing; 2008. American Psychiatric Association. The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Privileging (A Task Force Report of the American Psychiatric Association) [Google Scholar]
- 20.Fu W, White PF. Dexmedetomidine failed to block the acute hyperdynamic response to electroconvulsive therapy. Anesthesiology. 1999;90:422–4. doi: 10.1097/00000542-199902000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Rapinesi C, Kotzalidis GD, Curto M, Serata D, Ferri VR, Scatena P, et al. Electroconvulsive therapy improves clinical manifestations of treatment-resistant depression without changing serum BDNF levels. Psychiatry Res. 2015;227:171–8. doi: 10.1016/j.psychres.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Ota M, Noda T, Sato N, Okazaki M, Ishikawa M, Hattori K, et al. Effect of electroconvulsive therapy on gray matter volume in major depressive disorder. J Affect Disord. 2015;186:186–91. doi: 10.1016/j.jad.2015.06.051. [DOI] [PubMed] [Google Scholar]
- 23.Narayanaswamy JC, Viswanath B, Reddy PV, Kumar KR, Thirthalli J, Gangadhar BN. Efficacy of ECT in bipolar and unipolar depression in a real life hospital setting. Asian J Psychiatr. 2014;8:43–6. doi: 10.1016/j.ajp.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Andersen FA, Arsland D, Holst-Larsen H. Effects of combined methohexitone-remifentanil anaesthesia in electroconvulsive therapy. Acta Anaesthesiol Scand. 2001;45:830–3. doi: 10.1034/j.1399-6576.2001.045007830.x. [DOI] [PubMed] [Google Scholar]
- 25.Tosun Z, Akin A, Guler G, Esmaoglu A, Boyaci A. Dexmedetomidine-ketamine and propofol-ketamine combinations for anesthesia in spontaneously breathing pediatric patients undergoing cardiac catheterization. J Cardiothorac Vasc Anesth. 2006;20:515–9. doi: 10.1053/j.jvca.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka K, Oda Y, Funao T, Takahashi R, Hamaoka N, Asada A. Dexmedetomidine decreases the convulsive potency of bupivacaine and levobupivacaine in rats: Involvement of alpha2-adrenoceptor for controlling convulsions. Anesth Analg. 2005;100:687–96. doi: 10.1213/01.ANE.0000144420.87770.FE. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen TT, Chhibber AK, Lustik SJ, Kolano JW, Dillon PJ, Guttmacher LB. Effect of methohexitone and propofol with or without alfentanil on seizure duration and recovery in electroconvulsive therapy. Br J Anaesth. 1997;79:801–3. doi: 10.1093/bja/79.6.801. [DOI] [PubMed] [Google Scholar]
- 28.Blumenfeld H, Varghese GI, Purcaro MJ, Motelow JE, Enev M, McNally KA, et al. Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain. 2009;132(Pt 4):999–1012. doi: 10.1093/brain/awp028. [DOI] [PMC free article] [PubMed] [Google Scholar]


