Abstract
Objective:
Acute kidney injury (AKI) is a commonly encountered postoperative complication after cardiac surgery especially in high risk patients. AKI though seen more commonly after conventional on pump coronary artery bypass surgery (CCABG), is not uncommon after off pump coronary bypass surgery (OPCAB). Various biomarkers have shown promise over last one decade as an early marker for predicting AKI postoperatively. NGAL is one such biomarker whose concentration is increased in urine after any nephrotoxic and ischemic insult. The objective of this study was to assess the role of urine NGAL in predicting AKI after OPCAB in patients with increased risk of developing AKI.
Design:
A prospective cohort study.
Setting:
A clinical study in a multi specialty hospital.
Participants:
Eighty patients.
Materials and Methods:
study was approved by the hospital research ethics committee. 80 patients posted for OPCAB with an increased risk of developing AKI defined as having a Cleveland Clinic Foundation Acute renal failure scoring System score of ≥6 were included in the study. Patients with coronary angiography (CAG) within 48 hrs prior to surgery, pre-existing AKI, preoperative renal replacement therapy (RRT) and CKD stage 5 were excluded. Urine NGAL level before the start of surgery baseline and at 4 hrs post surgery were done. Renal function tests were assessed on the day of surgery (4 hrs post surgery) and on the next three days.
Result:
Seven patients developed AKI as defined by acute kidney infection network (AKIN) and risk injury failure loss end stage (RIFLE) criteria for AKI. NGAL value at 4 hrs in patients who developed AKI was significantly higher than in those patients who did not develop AKI (P < 0.05).
Conclusion:
urine NGAL is an early biomarker of acute kidney injury in patients undergoing OPCAB surgeries. However, large multicentre studies may be needed to confirm it.
Keywords: Acute kidney injury, Neutrophil gelatinase associated lipocalin, Off pump coronary artery bypass
INTRODUCTION
Acute kidney injury (AKI) is not uncommon after cardiac surgery and is associated with increased morbidity and mortality. Up to one-third of the patients undergoing cardiac surgery can develop some form of AKI, and 1–5% of these patients may go on to develop a severe form of kidney injury, requiring dialysis.[1,2,3]
There is increasing evidence that AKI is associated with increased risk of both short-term and long-term risk of death, as well as the risk of progressive renal failure. Even a subtle increase in serum creatinine levels resulting from a mild AKI can increase the risk of 30 days mortality to 3-fold, and larger serum creatinine increases are associated with a more than 18-fold elevation.[4,5]
There are a number of preoperative risk factors which are associated with increased likelihood of developing AKI after cardiac surgery. Prominent among those include female gender, reduced left ventricular function, history of congestive heart failure, diabetes requiring insulin, preoperative use of an intra-aortic balloon pump (IABP), chronic obstructive pulmonary disease (COPD), the need for emergent surgery, and an elevated preoperative serum creatinine. Valve replacement surgeries have higher incidence of AKI as compared to coronary artery bypass graft (CABG) surgery.[6,7,8,9,10]
Another controversial risk factor is cardiopulmonary bypass, i.e., conventional CABG (CCABG) versus off-pump CABG (OPCAB). Most of the studies have shown that OPCAB is associated with lower incidence of AKI postoperatively as compared to CCABG. Patients undergoing OPCAB have a significantly lesser increase in inflammatory markers as compared to those who undergo CCABG. Nigwekar et al. reported a significant reduction in overall AKI (odds ratio [OR] 0.57, 95% confidence interval [CI] 0.43–0.76) and AKI-requiring renal replacement therapy (RRT) (OR 0.55, 95% CI 0.43–0.71) in OPCAB cases compared from CCABG.[11,12] A meta-analysis of 22 randomized controlled trials suggested that OPCAB performed in a relatively heterogeneous patient population with coronary artery disease was associated with a reduction in the incidence of AKI but had no effect on dialysis requirement or all-cause mortality.[13]
Serum creatinine is a degradation product of muscle cells and represents a surrogate for the efficiency of glomerular filtration. However, it has a poor predictive accuracy for kidney injury, particularly in the early stages of AKI.[14]
Neutrophil gelatinase-associated lipocalin (NGAL) is 25 kDa iron-transporting glycoprotein which accumulates in the kidney tubules and urine after nephrotoxic and ischemic insults. Human NGAL was originally isolated from the supernatant of activated neutrophils. It has been proposed as an early, sensitive, noninvasive biomarker for AKI.[15,16,17,18,19,20,21] We conducted a pilot study to assess the role of NGAL in predicting AKI in patients undergoing OPCAB, who were at increased risk of developing AKI.
MATERIALS AND METHODS
This was a prospective cohort study, conducted at our tertiary care hospital after taking permission from the institutional review board. Eighty patients posted for OPCAB with an increased risk of developing AKI, defined as having a Cleveland Clinic Foundation acute renal failure scoring system score of ≥6 were included in the study. Venous blood samples were collected on the day of admission for routine investigations including renal function tests. A baseline urine NGAL level before the start of surgery was assessed and another urine NGAL level was assessed at 4 h postsurgery. Renal function tests were assessed on the day of surgery (4 h postsurgery) and on the next 3 days.
All the patients underwent OPCAB using a standard technique as per the institution protocol. All patients were premedicated with lorazepam 2 mg and pantaprazole 40 mg orally a night before and on the morning of surgery. Anesthesia was induced with thiopentone sodium 2–4 mg/kg, fentanyl 4–5 μg/kg and midazolam 0.03–0.04 mg/kg intravenously. Neuromuscular Blockade was achieved with vecuronium bromide (0.1 mg/kg) and following tracheal intubation; anesthesia was maintained with isoflurane and intermittent doses of fentanyl, midazolam, and vecuronium. A median sternotomy was performed and heparinization was done to keep activated clotting time >300 s. Heparin sulfate was reversed with protamine after the completion of revascularization. Patients were shifted to Intensive Care Unit (ICU) immediately after the surgery and were managed as per protocol.
Inclusion criteria
Age group: 18–85 years
Patients with increased risk of AKI postoperatively, defined as having a Cleveland Clinic Foundation acute renal failure scoring system score of ≥6
Patients with severe left ventricular dysfunction (left ventricular ejection fraction [LVEF] ≤30%)
Patients giving valid written informed consent
Patients planned for elective OPCAB.
Exclusion criteria
Preoperative hematocrit <25%
Leukocyte-rich blood transfusion <30 days before surgery
Coronary angiography within 48 h before surgery
Preexisting AKI
Preoperative RRT
Chronic kidney disease stage 5
Postrenal transplant.
Patients scheduled for OPCAB were examined a day before the procedure, and all the baseline investigations were noted. Demographic data (age, sex, height, and weight), preoperative risk factors and perioperative surgical and anesthetic management were recorded for all the patients. Urine sample for baseline NGAL levels was taken before the start of surgery. Patients were shifted to ICU after surgery, and another urine sample for NGAL was taken 4 h postsurgery. Renal function tests were assessed on the day of surgery at 4 h postsurgery and for the next 3 days.
Diagnostic criteria for defining AKI was taken from Acute kidney injury network (AKIN/RIFLE) criteria, i.e., an AKI with a Rapid time course (<48 h), reduction of kidney function with an absolute increase in serum creatinine of ≥0.3 mg/dl (≥26.4 μmol/l) or a percentage increase in serum creatinine of ≥50% and a reduction in urine output, defined as <0.5 ml/kg/h for more than 6 h.
Statistical methods
All the values are expressed as in terms of means and standard deviation. Qualitative/categorical data are expressed in terms of absolute number and percentages. All statistical tests were two-tailed. Mann–Whitney U-test was used for comparison and a P < 0.05 was considered statistically significant. Statistical analysis was performed in SPSS version 18.0 (IBM, SPSS Statistics, Bangalore, India).
RESULTS
Eighty patients who were planned to undergo OPCAB at our center were enrolled in this prospective study. Baseline demographics are shown in Table 1. Mean age of the patients in our study was 64.18 ± 8.88 years with 90% male patients and 10% of patients being female. All the patients had a preoperative LVEF of ≤30%. All the patients had IABP inserted at least 1 day before the surgery. In addition to that, all the patients in the study were diabetics. Five patients had a history of COPD and four surgeries in the study were redo-surgeries.
Table 1.
Patient demographics
| Study Parameter | Value |
|---|---|
| Age in years | 64.18±8.88 |
| Male, n (%) | 72 (90.0) |
| DM, n (%) | 80 (100.0) |
| HTN, n (%) | 64 (80.0) |
| IABP, n (%) | 80 (100.0) |
| COPD, n (%) | 5 (6.25) |
| Previous cardiac surgery, n (%) | 4 (5.0) |
SD: Standard deviation, DM: Diabetes mellitus, IABP: Intra-aortic balloon pump, COPD: Chronic obstructive pulmonary disease, HTN: Hypertension
Seven patients (8.75%) developed AKI as defined by AKIN and RIFLE criteria for AKI [Table 2]. Six patients who developed AKI had stage one AKI while one patient had stage 2 AKI. One patient had a very high value of NGAL both preoperatively and 4 h after the surgery, suggesting that the patient already had developed AKI before the surgery and was therefore excluded from the study. Mean baseline NGAL value in patients who developed AKI, although higher but was comparable with those who did not develop AKI (20.05 ng/ml vs. 15.14 ng/ml, respectively) [Table 3 and Figure 1]. NGAL value at 4 h in patients who developed AKI was 385.58 ng/ml, which was significantly higher as compared to patients who did not develop AKI (35.42 ng/ml, P < 0.05). Serum creatinine levels at baseline and at 4 h postoperatively were comparable in patients who develop AKI and those who did not develop AKI (P > 0.05). However, serum creatinine levels at 24, 48, and 72 h were significantly higher in patients who developed AKI as compared to those who did not develop AKI [Table 3 and Figure 2]. None of the patients who developed AKI required dialysis. There was no mortality in the 7 days postoperative period.
Table 2.
Distribution of acute kidney injury
| AKI | n (%) |
|---|---|
| Yes | 7 (8.75) |
| No | 73 (91.25) |
| Total | 80 (100.0) |
AKI: Acute kidney injury
Table 3.
Comparison of neutrophil gelatinase-associated lipocalin and serurm creatinine at various time intervals in acute kidney injury and no acute kidney injury patients
| Parameters | Mean±SD | P | |
|---|---|---|---|
| AKI (n=7) | No AKI (n=73) | ||
| NGAL (ng/ml) | |||
| Base line | 20.05±21.52 | 15.14±11.73 | 0.740 |
| At 4 h | 385.58±197.17 | 35.42±32.54 | 0.000* |
| Serum creatinine (mg %) | |||
| Preoperative | 1.23±0.14 | 1.07±0.18 | 0.062 |
| At 4 h | 1.21±0.25 | 1.07±0.19 | 0.074 |
| At 24 h | 1.71±0.50 | 1.03±0.15 | <0.0001* |
| At 48 h | 1.89±0.32 | 1.03±0.14 | <0.0001* |
| At 72 h | 2.13±0.42 | 1.05±0.14 | <0.0001* |
Mann–Whitney U-test, *P<0.05, statistically significant. NGAL: Neutrophil gelatinase-associated lipocalin, SD: Standard deviation, AKI: Acute kidney injury
Figure 1.
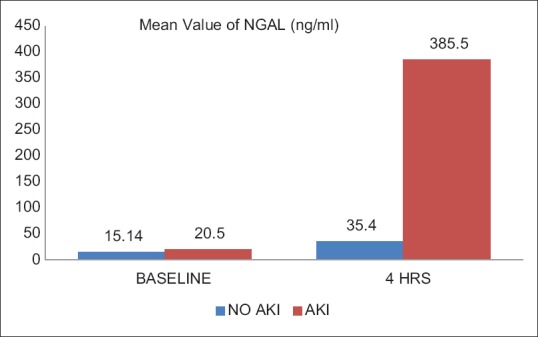
Mean value of urine neutrophil gelatinase-associated lipocalin in acute kidney injury and no acute kidney injury patients
Figure 2.
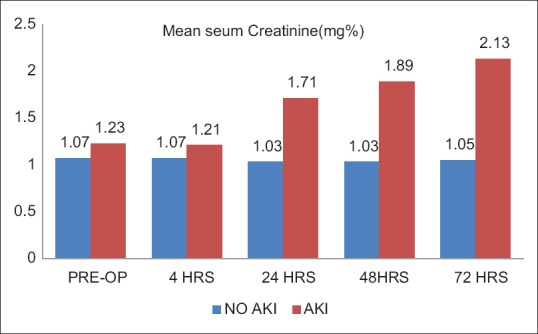
Mean value of serum creatinine in acute kidney injury and no acute kidney injury patients
DISCUSSION
Serum creatinine has long been used as a marker of renal function in spite of it being an unreliable and delayed indicator of renal injury. Serum creatinine in a setting of AKI may take several hours to days to reach a steady state. Furthermore, serum creatinine level is influenced by several nonrenal factors, such as age, gender, muscle mass, muscle metabolism, medications, and hydration status.
Availability of early biomarkers in predicting AKI in cardiac surgery may enable the physicians to perform timely interventions to prevent further deterioration of kidney functions. A number of biomarkers have recently been investigated as possible biomarkers for early and accurate identification of AKI. These include plasma biomarkers such as NGAL and cystatin C and urine biomarkers such as NGAL, interleukin-18, and kidney injury molecule-1.[22,23,24]
NGAL is one such novel biomarker, which is primarily a marker of renal tubular injury There is a marked up-regulation of NGAL mRNA in the thick ascending limb of Henle's and the collecting ducts,[25] with resultant synthesis of NGAL protein in the distal nephron and secretion into the urine where it comprises the major fraction of urinary NGAL.
We found a significant relationship between a rise in urine NGAL levels at 4 h postoperatively to the occurrence of AKI, although it was not clear whether the degree of rise in NGAL levels was associated with more severe form of AKI or not. This was because of the reason that six out of seven patients in our study who developed AKI had only stage one AKI. Furthermore, the rise in urine NGAL levels at 4 h in these patients was not uniform. One patient who developed AKI had 100-fold rise in urine NGAL levels at 4 h while the other six had a lesser degree of elevation of urine NGAL at 4 h. Six patients who developed AKI had a normal serum creatinine value at 4 h postoperatively. Serum creatinine levels rose only at 24 h or more in all these six patients. However, urine NGAL levels at 4 h were significantly high in the patients who developed AKI suggesting that urine NGAL rises much earlier than serum creatinine levels and is an early biomarker of AKI. One patient who developed AKI had high urine NGAL levels at 4 h as well as raised serum creatinine levels at 4 h postoperatively.
Since none of the patient in our study who developed AKI had grade 3 AKI and neither of the patient had dialysis requirement, the diagnostic utility of NGAL may be better assessed by determining its predictive utility for clinical outcomes such as mortality or progression to dialysis-dependent renal failure.
One of the strengths of our study was that all the patients who were selected in the study had a high risk for developing AKI postoperatively. Various studies and scoring systems for predicting AKI in patients undergoing cardiac surgery have shown that patients with poor left ventricular function and diabetes mellitus are at increased risk of developing AKI after cardiac surgery. The effect of diabetes mellitus on postoperative renal dysfunction may be the result of renal parenchymal diseases, such as glomerulonephritis or glomerulosclerosis. Renal artery stenosis in diabetic patients may further compromise renal function. In addition, perioperative IABP is an independent predictor of perioperative AKI and in-hospital mortality. Wijeysundera et al. also demonstrated that the preoperative placement of an IABP significantly increased the risk for AKI in a heterogeneous group of cardiac surgical patients.[26]
Our study is in agreement with other similar studies which have found that NGAL is an early biomarker of AKI. Bennett et al. in a prospective uncontrolled cohort study concluded that NGAL predicts AKI, mortality, and morbidity after pediatric cardiac surgery.[27] Several other studies have reported NGAL as a sensitive and specific predictor of AKI after cardiac surgery.[28,29]
It also supports the fact that OPCAB may have a protective effect as far as postoperative renal dysfunction is concerned as compared to CCABG, as only 8.75% of patients had AKI in our study which is consistent with the findings in other studies which showed that risk of AKI was much more in CCABG.[30] Loef et al. found significantly less changes in microalbuminuria, free hemoglobin, fractional excretion of sodium, and free water clearance as well as N-acetyl-D-glucosaminidase as a marker for tubular function and damage, respectively, in patients undergoing OPCAB as compared with CCABG patients. Patients with concomitant diseases or risk factors seem to benefit from the beating heart approach as compared to CCABG.[31,32]
Limitations
One of the important limitations of our study is that only a small study group (n = 80) was included in the study. Large randomized prospective trials are needed to validate the diagnostic utility of urine NGAL in predicting AKI.
Another limitation of the study is that the urine NGAL level was assessed at 4 h postoperatively and not after that, which is a relatively short period of observation. However, earlier studies have indicated that AKI and a rise in urine NGAL levels occur mostly within this period.
Although all the subjects in the study had normal serum creatinine measurements before surgery, an estimation of glomerular filtration rate to document normal kidney function was not made.
Role of perioperative diuretics in influencing the urine NGAL levels was not considered in the study. Patients without AKI, who received diuretics, may have a marked diuresis thereby diluting urinary NGAL, whereas patients with evolving AKI may have a blunted response to diuretics with less urinary dilution, thus enhancing the discriminant ability of urinary NGAL for AKI.
CONCLUSION
In our study, we found a definite relationship between a rising urine NGAL level and AKI after OPCAB. Further larger studies are required to confirm the potential role and limitations of NGAL as an AKI biomarker.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Himanshu Baweja: Senior Manager, Clinical Pharmacology, Medanta - The Medicity, Gurgaon, Haryana
Manish Singh: Bio-Statistician, Medanta - The Medicity, Gurgaon, Haryana.
REFERENCES
- 1.Conlon PJ, Stafford-Smith M, White WD, Newman MF, King S, Winn MP, et al. Acute renal failure following cardiac surgery. Nephrol Dial Transplant. 1999;14:1158–62. doi: 10.1093/ndt/14.5.1158. [DOI] [PubMed] [Google Scholar]
- 2.Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT. Renal dysfunction after myocardial revascularization: Risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med. 1998;128:194–203. doi: 10.7326/0003-4819-128-3-199802010-00005. [DOI] [PubMed] [Google Scholar]
- 3.Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1:19–32. doi: 10.2215/CJN.00240605. [DOI] [PubMed] [Google Scholar]
- 4.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: A prospective cohort study. J Am Soc Nephrol. 2004;15:1597–605. doi: 10.1097/01.asn.0000130340.93930.dd. [DOI] [PubMed] [Google Scholar]
- 5.Thakar CV, Worley S, Arrigain S, Yared JP, Paganini EP. Influence of renal dysfunction on mortality after cardiac surgery: Modifying effect of preoperative renal function. Kidney Int. 2005;67:1112–9. doi: 10.1111/j.1523-1755.2005.00177.x. [DOI] [PubMed] [Google Scholar]
- 6.Mehta RH, Grab JD, O’Brien SM, Bridges CR, Gammie JS, Haan CK, et al. Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation. 2006;114:2208–16. doi: 10.1161/CIRCULATIONAHA.106.635573. [DOI] [PubMed] [Google Scholar]
- 7.Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16:162–8. doi: 10.1681/ASN.2004040331. [DOI] [PubMed] [Google Scholar]
- 8.Tuttle KR, Worrall NK, Dahlstrom LR, Nandagopal R, Kausz AT, Davis CL. Predictors of ARF after cardiac surgical procedures. Am J Kidney Dis. 2003;41:76–83. doi: 10.1053/ajkd.2003.50025. [DOI] [PubMed] [Google Scholar]
- 9.Demirjian S, Schold JD, Navia J, Mastracci TM, Paganini EP, Yared JP, et al. Predictive models for acute kidney injury following cardiac surgery. Am J Kidney Dis. 2012;59:382–9. doi: 10.1053/j.ajkd.2011.10.046. [DOI] [PubMed] [Google Scholar]
- 10.Grayson AD, Khater M, Jackson M, Fox MA. Valvular heart operation is an independent risk factor for acute renal failure. Ann Thorac Surg. 2003;75:1829–35. doi: 10.1016/s0003-4975(03)00166-8. [DOI] [PubMed] [Google Scholar]
- 11.Garg AX, Devereaux PJ, Yusuf S, Cuerden MS, Parikh CR, Coca SG, et al. Coronary artery bypass grafting surgery off- or on-pump revascularisation study (CORONARY): Kidney substudy analytic protocol of an international randomised controlled trial. BMJ Open. 2012;2:e001080. doi: 10.1136/bmjopen-2012-001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nigwekar SU, Kandula P, Hix JK, Thakar CV. Off-pump coronary artery bypass surgery and acute kidney injury: A meta-analysis of randomized and observational studies. Am J Kidney Dis. 2009;54:413–23. doi: 10.1053/j.ajkd.2009.01.267. [DOI] [PubMed] [Google Scholar]
- 13.Seabra VF, Alobaidi S, Balk EM, Poon AH, Jaber BL. Off-pump coronary artery bypass surgery and acute kidney injury: A meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol. 2010;5:1734–44. doi: 10.2215/CJN.02800310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waikar SS, Betensky RA, Bonventre JV. Creatinine as the gold standard for kidney injury biomarker studies? Nephrol Dial Transplant. 2009;24:3263–5. doi: 10.1093/ndt/gfp428. [DOI] [PubMed] [Google Scholar]
- 15.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–43. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 16.Mishra J, Mori K, Ma Q, Kelly C, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin: A novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol. 2004;24:307–15. doi: 10.1159/000078452. [DOI] [PubMed] [Google Scholar]
- 17.Mishra J, Mori K, Ma Q, Kelly C, Yang J, Mitsnefes M, et al. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2004;15:3073–82. doi: 10.1097/01.ASN.0000145013.44578.45. [DOI] [PubMed] [Google Scholar]
- 18.Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115:610–21. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devarajan P. Emerging biomarkers of acute kidney injury. Contrib Nephrol. 2007;156:203–12. doi: 10.1159/000102085. [DOI] [PubMed] [Google Scholar]
- 20.Mishra J, Ma Q, Kelly C, Mitsnefes M, Mori K, Barasch J, et al. Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatr Nephrol. 2006;21:856–63. doi: 10.1007/s00467-006-0055-0. [DOI] [PubMed] [Google Scholar]
- 21.Sargentini V, Mariani P, D’Alessandro M, Pistolesi V, Lauretta MP, Pacini F, et al. Assessment of NGAL as an early biomarker of acute kidney injury in adult cardiac surgery patients. J Biol Regul Homeost Agents. 2012;26:485–93. [PubMed] [Google Scholar]
- 22.Siew ED, Ware LB, Ikizler TA. Biological markers of acute kidney injury. J Am Soc Nephrol. 2011;22:810–20. doi: 10.1681/ASN.2010080796. [DOI] [PubMed] [Google Scholar]
- 23.de Geus HR, Betjes MG, Bakker J. Biomarkers for the prediction of acute kidney injury: A narrative review on current status and future challenges. Clin Kidney J. 2012;5:102–8. doi: 10.1093/ckj/sfs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xin C, Yulong X, Yu C, Changchun C, Feng Z, Xinwei M. Urine neutrophil gelatinase-associated lipocalin and interleukin-18 predict acute kidney injury after cardiac surgery. Ren Fail. 2008;30:904–13. doi: 10.1080/08860220802359089. [DOI] [PubMed] [Google Scholar]
- 25.Kuwabara T, Mori K, Mukoyama M, Kasahara M, Yokoi H, Saito Y, et al. Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int. 2009;75:285–94. doi: 10.1038/ki.2008.499. [DOI] [PubMed] [Google Scholar]
- 26.Wijeysundera DN, Karkouti K, Dupuis JY, Rao V, Chan CT, Granton JT, et al. Derivation and validation of a simplified predictive index for renal replacement therapy after cardiac surgery. JAMA. 2007;297:1801–9. doi: 10.1001/jama.297.16.1801. [DOI] [PubMed] [Google Scholar]
- 27.Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: A prospective study. Clin J Am Soc Nephrol. 2008;3:665–73. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krawczeski CD, Goldstein SL, Woo JG, Wang Y, Piyaphanee N, Ma Q, et al. Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. J Am Coll Cardiol. 2011;58:2301–9. doi: 10.1016/j.jacc.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–8. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 30.Celik JB, Gormus N, Topal A, Okesli S, Solak H. Effect of off-pump and on-pump coronary artery bypass grafting on renal function. Ren Fail. 2005;27:183–8. [PubMed] [Google Scholar]
- 31.Bucerius J, Gummert JF, Walther T, Schmitt DV, Doll N, Falk V, et al. On-pump versus off-pump coronary artery bypass grafting: Impact on postoperative renal failure requiring renal replacement therapy. Ann Thorac Surg. 2004;77:1250–6. doi: 10.1016/S0003-4975(03)01346-8. [DOI] [PubMed] [Google Scholar]
- 32.Loef BG, Epema AH, Navis G, Ebels T, van Oeveren W, Henning RH. Off-pump coronary revascularization attenuates transient renal damage compared with on-pump coronary revascularization. Chest. 2002;121:1190–4. doi: 10.1378/chest.121.4.1190. [DOI] [PubMed] [Google Scholar]


