Abstract
Tocopherols (vitamin E) are lipophilic antioxidants synthesized by all plants and are particularly abundant in seeds. Despite cloning of the complete suite of tocopherol biosynthetic enzymes and successful engineering of the tocopherol content and composition of Arabidopsis thaliana leaves and seeds, the functions of tocopherols in plants have remained elusive. To address this issue, we have isolated and characterized two VITAMIN E loci (VTE1 and VTE2) in Arabidopsis that when mutated result in tocopherol deficiency in all tissues. vte1 disrupts tocopherol cyclase activity and accumulates a redox-active biosynthetic intermediate, whereas vte2 disrupts homogentisate phytyl transferase activity and does not accumulate pathway intermediates. Mutations at either locus cause significantly reduced seed longevity compared with the wild type, indicating a critical role for tocopherols in maintaining viability during quiescence. However, only vte2 mutants exhibited severe seedling growth defects during germination and contained levels of lipid hydroperoxides and hydroxy fatty acids elevated up to 4- and 100-fold, respectively, relative to the wild type. These data demonstrate that a primary function of tocopherols in plants is to limit nonenzymatic lipid oxidation during seed storage, germination, and early seedling development. The vte mutant phenotypes also explain the strong selection for retention of tocopherol biosynthesis during the evolution of seed-bearing plants.
INTRODUCTION
Tocopherols (vitamin E) are lipophilic antioxidants synthesized by all plants and some algae and cyanobacteria. The four types of tocopherols synthesized (α, β, γ, and δ) differ only in the number and positions of methyl substituents on the chromanol ring. All tocopherols are amphipathic molecules, with the hydrophobic tail associating with membrane lipids and the polar head groups remaining at the membrane surface. In plants, tocopherols are synthesized and localized in plastids (Soll et al., 1980, 1985; Lichtenthaler et al., 1981; Fryer, 1992; Kruk and Strzalka, 1995; Arango and Heise, 1998) and accumulate to varying degrees in all tissues, with seed generally containing the highest levels (Sheppard et al., 1993). All enzymes of the tocopherol biosynthetic pathway have been cloned and characterized in the past several years and exhibit a remarkable degree of evolutionary conservation between plants and cyanobacteria (Shintani and DellaPenna, 1998; Collakova and DellaPenna, 2001; Porfirova et al., 2002; Shintani et al., 2002; Cahoon et al., 2003; Cheng et al., 2003; Sattler et al., 2003). The only exception is 2-methyl-6-phytyl-1,4-benzoquinol/2-methyl-6-solanyl-1,4-benzoquinol methyl transferase, which is encoded by evolutionarily unrelated protein families in cyanobacteria and plants (Shintani et al., 2002; Cheng et al., 2003). These cloned pathway enzymes have been used in various transgenic approaches to successfully modify the amount and types of tocopherols that accumulate in leaves and seeds (Shintani and DellaPenna, 1998; Savidge et al., 2002; Tsegaye et al., 2002; Cahoon et al., 2003; Collakova and DellaPenna, 2003a).
Although tocopherols are only synthesized by photosynthetic organisms, little is known concerning their functions in plants. Instead, because of their essential role in human nutrition as vitamin E, our understanding of tocopherol chemistry and function has been derived primarily from studies in artificial membranes and animal systems (Liebler, 1998; Azzi et al., 2000). Although the biological activities of tocopherols will likely differ in plants and animals because of fundamental differences in their biology, the chemical properties of tocopherols are likely to be the same between the two kingdoms. In vitro studies in animals and artificial membranes have shown that tocopherols interact with the polyunsaturated acyl groups of lipids, stabilize membranes, and scavenge and quench various reactive oxygen species (ROS) and lipid soluble byproducts of oxidative stress (Brigelius-Flohe and Traber, 1999; Wang and Quinn, 2000). Singlet oxygen quenching by tocopherols is highly efficient, and it is estimated that a single α-tocopherol molecule can neutralize up to 120 singlet oxygen molecules in vitro before being degraded (Fukuzawa et al., 1982). Because of their chromanol ring structure, tocopherols are capable of donating a single electron to form the resonance-stabilized tocopheroxyl radical (Liebler, 1993; KamalEldin and Appelqvist, 1996), which contrasts with other phenolic antioxidants, such as hydroxyquinones, that must donate two electrons to attain a stable structure (Liebler and Burr, 2000). Tocopheroxyl radicals can also donate a second electron, resulting in opening of the chromanol ring to form the corresponding tocopherol quinone and other oxidized derivatives, which can also participate in electron transfer reactions (Liebler, 1998; Wang and Quinn, 2000).
Tocopherols also function as recyclable chain reaction terminators of polyunsaturated fatty acid (PUFA) radicals generated by lipid oxidation (Girotti, 1998). Tocopherols scavenge lipid peroxy radicals and yield a tocopheroxyl radical that can be recycled back to the corresponding tocopherol by reacting with ascorbate or other antioxidants (Liebler, 1993). This property allows each tocopherol molecule to participate in many lipid peroxidation chain-breaking events before being degraded. Finally, recent studies in animal systems have also demonstrated other nonantioxidant functions of tocopherols related to the modulation of signal transduction pathways and transcription (Brigelius-Flohe and Traber, 1999; Sen et al., 2000; Chan et al., 2001; Ricciarelli et al., 2001; Yamauchi et al., 2001; Clement et al., 2002; Nobata et al., 2002). Whether some or all of the functions identified for tocopherols in animal systems also occur in plants remains to be determined.
Tocopherols are a major lipid soluble antioxidant present in the PUFA-enriched membranes of chloroplasts and are proposed to be an essential component of the plastid antioxidant network. ROS generated as byproducts of photosynthesis and metabolism are potential sources of lipid peroxidation in plant cells, and though direct experimental evidence is lacking, tocopherols are assumed to function similarly to animals in limiting ROS damage to plant lipids. Tocopherol levels increase in photosynthetic plant tissues in response to a variety of abiotic stresses (Munne-Bosch and Alegre, 2002), and this is often cited as circumstantial evidence for a protective role. However, the engineering of several-fold increases in Arabidopsis thaliana leaf tocopherol levels did not alter chlorophyll and carotenoid losses during high light stress in comparison with the wild type (Collakova and DellaPenna, 2003b). Mutations disrupting the tocopherol cyclase enzyme in plants (the VITAMIN E 1 [VTE1] locus in Arabidopsis and the SUCROSE EXPORT DEFECTIVE 1 locus in maize [Zea mays]) result in tocopherol-deficient plants that accumulate the pathway intermediate 2,3-dimethyl-5-phytyl-1,4-benzoquinone (DMPBQ) (Porfirova et al., 2002; Sattler et al., 2003). Despite this tocopherol deficiency, mature vte1 plants are phenotypically indistinguishable from the wild type and exhibit only a minor decrease in photosynthetic efficiency after 5 d of high light treatment (Porfirova et al., 2002), whereas sxd1 plants have defective plasmodesmata between the bundle sheath cells and the vascular parenchyma that results in a defect in sucrose export from leaves (Provencher et al., 2001). These data suggest that tocopherol functions in photosynthetic tissues may be varied and species dependent. Alternatively, the presence of DMPBQ in tocopherol cyclase mutants may interfere with normal cellular processes in a species-specific manner.
In addition to photosynthetic tissues, seeds also accumulate tocopherols, often to the highest level of any plant tissue. Seeds often contain the highest concentration of lipids of any plant tissue (Mansfield and Briarty, 1992) with high levels of PUFAs. The occurrence of high levels of tocopherols and PUFAs in seeds suggests that tocopherols may protect storage lipids from oxidation. However, like the tocopherols in photosynthetic tissues, functions for the high seed tocopherol levels are unclear, though attempts have been made to correlate seed tocopherol content with either seed PUFA levels or seed viability during storage. In some studies, a positive correlation has been reported, whereas other studies have failed to observe such a relationship (Wilson and McDonald, 1986; KamalEldin and Andersson, 1997).
To address the functions of tocopherols in plants, we have isolated a series of tocopherol-deficient mutants in Arabidopsis. Prior studies have shown that disruption of the VTE1 locus, which encodes tocopherol cyclase, results in replacement of tocopherols with the redox-active, lipid-soluble pathway intermediate DMPBQ (Figure 1) (Porfirova et al., 2002; Sattler et al., 2003). In contrast with vte1, disruption of the VTE2 locus, which encodes homogentisate phytyl transferase, eliminates tocopherols without causing accumulation of pathway intermediates (Figure 1). The vte2 mutants thus represent the first land plants in which the primary lipophilic antioxidant of plastids, tocopherols, and all redox-active pathway intermediates have been eliminated. Herein, we describe the use of these vte mutants to elucidate specific tocopherol functions in plants.
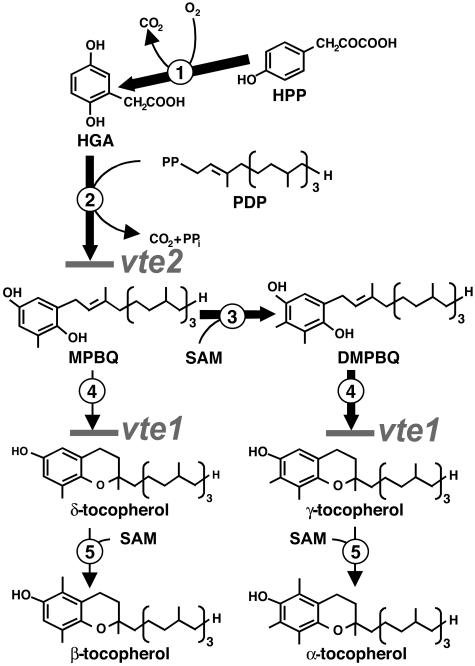
Figure 1.
The Tocopherol Pathway in Arabidopsis.
The enzymes are numbered as follows: 1, hydroxyphenylpyruvate dioxygenase; 2, homogentisate phytyl transferase; 3, 2-methyl-6-phytyl-1,4-benzoquinol methyltransferase; 4, tocopherol cyclase; 5, γ-tocopherol methyltransferase. The gray lines indicate the locations in the pathway blocked by vte1 and vte2. Bold arrows represent the steps leading to γ-tocopherol, the most abundant tocopherol produced in wild-type Arabidopsis seed. HPP, hydroxyphenylpyruvate; HGA, homogentisic acid; MPBQ, 2-methyl-6-phytyl-1,4-benzoquinol; SAM, S-adenosyl l-Met.
RESULTS
Isolation and Characterization of vte1 and vte2
vte1-1 and vte2-1 were isolated in an HPLC-based screen for mutants with altered leaf tocopherol profiles from M3 ethyl methanesulfonate–mutagenized Arabidopsis (Columbia ecotype [Col]) seeds (Sattler et al., 2003). A second vte2 allele, vte2-2 (Wassilewskija ecotype [Ws]) was isolated through a PCR-based reverse genetics screen for T-DNA insertions in At2g18950 (homogentisate phytyl transferase). A complementation test indicated that vte2-1 and vte2-2 were allelic. All three mutants lacked tocopherols in seed (Table 1) and leaf tissues (data not shown). vte1-1 seeds contained 609 ± 54 pmol/mg of the tocopherol pathway intermediate DMPBQ, which is comparable to the 749 ± 7 pmol/mg of tocopherols contained in wild-type (Col) seeds. Unlike vte1, the vte2 mutants and the wild type do not accumulate any tocopherol pathway intermediates (Table 1). Both vte2 alleles are semidominant; plants heterozygous for either allele contained reduced levels of tocopherols in both seed and leaf tissues (∼75 and 50%, respectively, relative to the wild type). These observations support prior work indicating that the homogentisate phytyl transferase is a limiting step in tocopherol synthesis (Collakova and DellaPenna, 2003a). DNA sequencing was used to determine the mutation sites of the three mutants; vte1-1 was previously published (Sattler et al., 2003). vte2-1 contained a nonsense mutation at amino acid 208 of 393 (TGG to TGA; Trp to stop), and vte2-2 contained a T-DNA insertion at amino acid 195. Because tocopherols were not observed under any conditions or in any tissues in all three mutants and because all three mutations cause truncations of the respective native polypeptide near the middle of the protein, each mutant is considered a null allele.
Table 1.
Tocopherol and DMPBQ Content in Wild-Type and vte Seeds
| Tocopherols
|
|||||
|---|---|---|---|---|---|
| Genotype | α | γ | δ | Total | DMPBQ |
| Col | 12.2 ± 0.3 | 683.6 ± 6.2 | 53.2 ± 0.6 | 749.0 ± 6.5 | 0 |
| vte1-1 | 0 | 0 | 0 | 0 | 609 ± 54 |
| vte2-1 | 0 | 0 | 0 | 0 | 0 |
| Ws | 12.6 ± 1.2 | 665.5 ± 8.4 | 68.6 ± 1.0 | 746.7 ± 8.8 | 0 |
| vte2-2 | 0 | 0 | 0 | 0 | 0 |
Tocopherols and DMPQ were analyzed by HPLC (see Methods) and are expressed as pmol/mg seed. The data are the means ± sd (n = 4).
Seed Longevity in vte1 and vte2
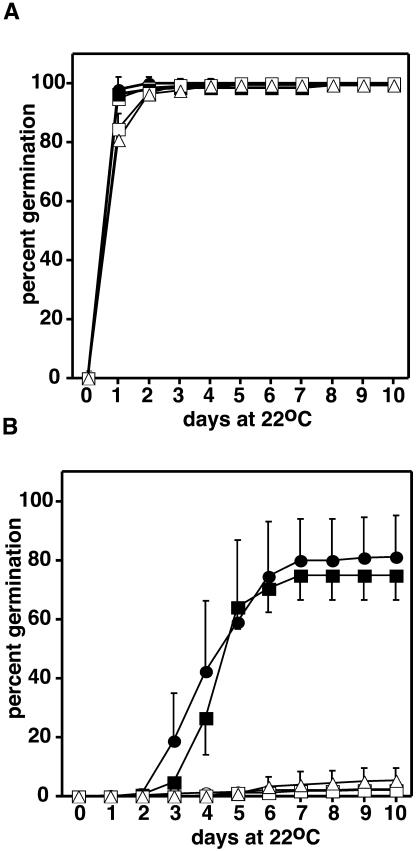
Previous research has shown that extended storage of seeds (i.e., several months to several years) results in a gradual loss of seed viability (Wilson and McDonald, 1986). Initial observations of the vte2 mutants indicated that seed viability was variably compromised during extended storage of different seed lots. Seed producers have developed accelerated aging tests, the short-term exposure of seeds to elevated temperature and relative humidity, as a rapid method to mimic the effects of natural seed aging on viability. To test the role of tocopherols in seed longevity, 2-month-old vte1-1, vte2-1, vte2-2, and corresponding wild-type seed were treated at 40°C, 100% RH for 3 d, imbibed at 4°C for 5 d to break dormancy, and then placed at 22°C and germination monitored by root radical emergence. All untreated genotypes showed >95% germination by 2 d (Figure 2A), indicating that germination per se is not affected by tocopherol deficiency. In response to accelerated aging treatment, wild-type germination did not plateau until 7 d and was reduced 20 to 30% relative to untreated controls (Figure 2B). Subjecting vte1-1, vte2-1, and vte2-2 seed to the same treatment resulted in >90% reduction in germination. These data clearly demonstrate an essential role for tocopherols in seed longevity and suggest that the presence of DMPBQ in vte1-1 seed cannot compensate for tocopherols under these conditions.
Figure 2.
Germination of Wild-Type and Tocopherol-Deficient Arabidopsis Seeds.
(A) Germination of unaged 2-month-old seeds.
(B) Germination of 2-month-old seeds subjected to accelerated aging treatment (40°C for 72 h at 100% RH). Germination was defined as the emergence of the root radical and was scored daily. The zero day time point is seed that have been imbibed for 5 d at 4°C. Closed circle, Col (wild type); open circle, vte2-2 (Col); open triangle, vte1-1 (Col); closed square, Ws (wild type); open square, vte2-2 (Ws).
The data in (A) and (B) are means, and error bars are sd (n = 4).
The Phenotypes of vte1 and vte2 in Seedlings
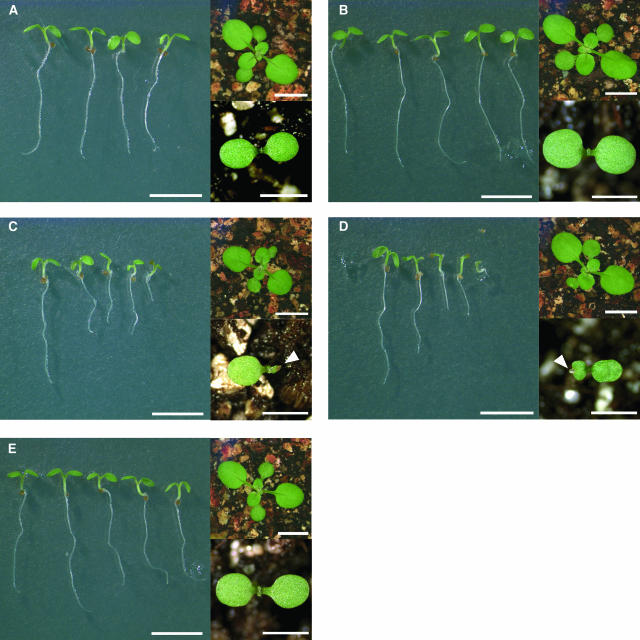
Although untreated vte1, vte2, and wild-type seed did not differ in their germination rates (Figure 3A), early seedling growth and development was severely impacted by the absence of tocopherols in vte2 but not in vte1 mutants (Figures 3A to 3E). Seedlings of both vte2 alleles were generally smaller than those of the wild type and exhibited a wide range of cotyledon defects, including failure to fully expand one or both cotyledons and sectors devoid of chlorophyll at the cotyledon tips (Figures 3C and 3D, bottom insets). Seed size and yield per plant were not affected by the vte2 mutations, and vte2 embryo development was indistinguishable from that of the wild type, indicating that the vte2 cotyledon defects occur during germination and not embryogenesis (data not shown). Leaf development was also normal in vte2; consequently, the plants that survived germination and early seedling development were indistinguishable from the wild type (Figures 3A to 3E, top insets). Based on the phenotype of the vte2 mutant, we can conclude that tocopherols also play a critical role in early seedling development. However, because vte1 was indistinguishable from the wild type, the DMPBQ accumulated by vte1 must functionally compensate to a large degree for the absence of tocopherols during germination and early seedling development.
Figure 3.
Tocopherol-Deficient Mutants in Arabidopsis.
Large panels show representative 6-d-old seedlings grown on media without a carbon source; bars = 5 mm. Top insets are 14-d-old soil-grown plants; bars = 5 mm. Bottom insets are representative cotyledons from 6-d-old soil-grown seedlings; bars = 2 mm. The arrowheads indicate defective cotyledons in the vte2 mutants.
(A) Col (wild type).
(B) Ws (wild type).
(C) vte2-1 (Col).
(D) vte2-2 (Ws).
(E) vte1-1 (Col).
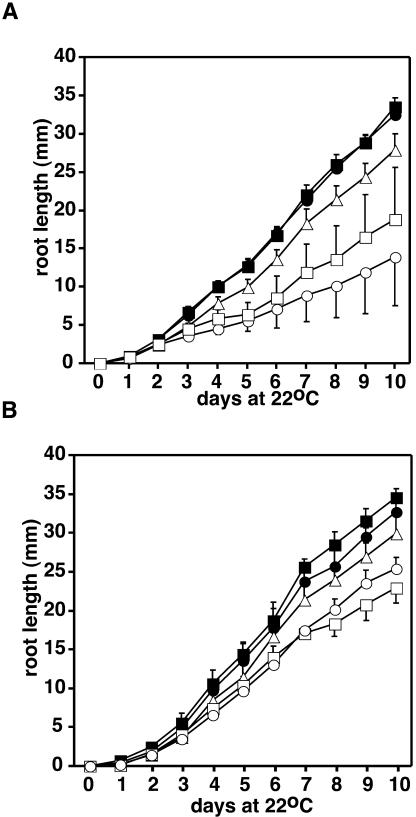
To quantify the growth defects of vte2, root length was measured as a nondestructive assay of seedling growth rates. vte2 root elongation was severely impacted relative to the wild type, whereas vte1 was only slightly affected (Figure 4A). The large error bars for vte2 root growth reflect the wide range of phenotypic severity observed in vte2 mutant seedlings (Figures 4A and 4B). When vte2 was germinated and grown in the presence of an exogenous carbon source (2% sucrose), growth rates were partially rescued and variation was significantly reduced (Figure 4B). vte1 seedling growth was not affected by exogenous sucrose. These data suggest that the phenotype of vte2 seedlings is attributable in part to a limitation in carbon availability during seedling development. The initial source of fixed carbon for seedlings is catabolism of storage lipids through β-oxidation in glyoxysomes and subsequent gluconeogenesis in the mitochondria and cytosol.
Figure 4.
Root Growth of Wild-Type and Tocopherol-Deficient Arabidopsis Seedlings.
(A) Root growth without an exogenous carbon source.
(B) Root growth in the presence of 2% sucrose. Root growth was measured each day in seedlings grown vertically on Petri plates containing 0.5× MS media with or without sucrose. Closed circle, Col (wild type); open circle, vte2-1 (Col); open triangle, vte1-1 (Col); closed square, Ws (wild type); open square vte2-2 (Ws).
The data in (A) and (B) are means, and error bars are sd (n = 15).
Fatty Acid Composition in the vte Mutants
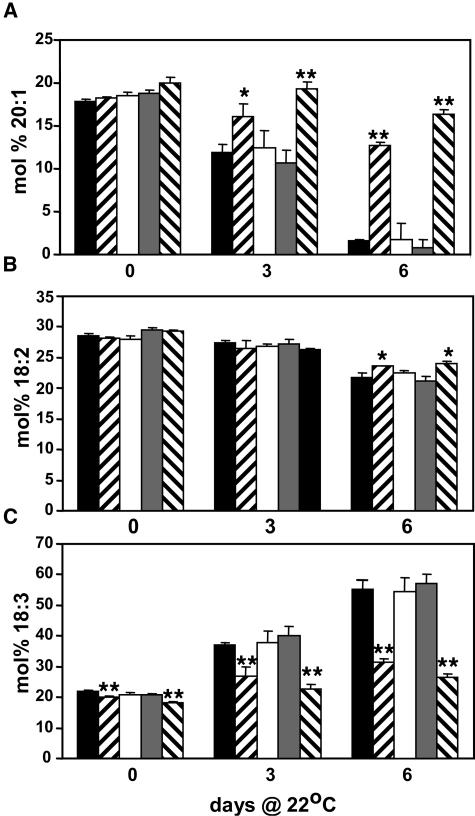
To determine whether metabolism of storage lipids was impacted in vte2, the fatty acid composition of total lipids during seedling development was determined by gas chromatography. The dry seed fatty acid composition of all genotypes was nearly identical (data not shown). The fatty acid composition of seed imbibed for 5 d at 4°C (Figures 5A to 5C, zero days at 22°C) was also nearly identical, with the notable exception of a small but significant decrease in linolenic acid in both vte2 alleles relative to the wild type. In Arabidopsis, eicosenoic acid (20:1) is a marker for the level of storage lipids present because it is found almost entirely in triacylglyerols (Lemieux et al., 1990; Eastmond et al., 2000). The mol % of 20:1 in imbibed seed of all genotypes was similar and progressively decreased during 6 d of seedling growth (Figure 5A). However, at 3 d postgermination, the 20:1 mol % in vte2 mutants was significantly higher than the corresponding wild type and 8- to 20-fold that of the wild type by 6 d postgermination (Figure 5A). 20:1 content in vte1 were indistinguishable from the wild type at all time points. By 6 d postgermination, the mol % linolenic acid of the wild type and vte1 nearly tripled, whereas linoleic acid decreased ∼25% (Figures 5B and 5C). During this same time frame, the linolenic acid composition of vte2 mutants increased only 50%, whereas linoleic acid decreased 10% (Figures 5B and 5C). These data indicate that vte2 mutants are unable to efficiently metabolize seed storage lipids, which may explain in part the growth defect of vte2 seedlings.
Figure 5.
Fatty Acid Composition in Wild-Type and Tocopherol-Deficient Arabidopsis Seed and Seedlings.
(A) mol % of 20:1 (eicosenoic acid).
(B) mol % of 18:2 (linoleic acid).
(C) mol % of 18:3 (linolenic acid).
The zero day time point is seed that have been imbibed for 5 d at 4°C. Col (black bars); vte2-1 (right hatched bars); vte1-1 (white bars); Ws (gray bars); vte2-2 (left hatched bars). The differences between vte2 alleles and their respective wild-type backgrounds were statistically significant (Student's t test, * P < 0.05 and ** P < 0.01). The data in (A) to (C) are means, and error bars are sd (n = 4).
Lipid Oxidation
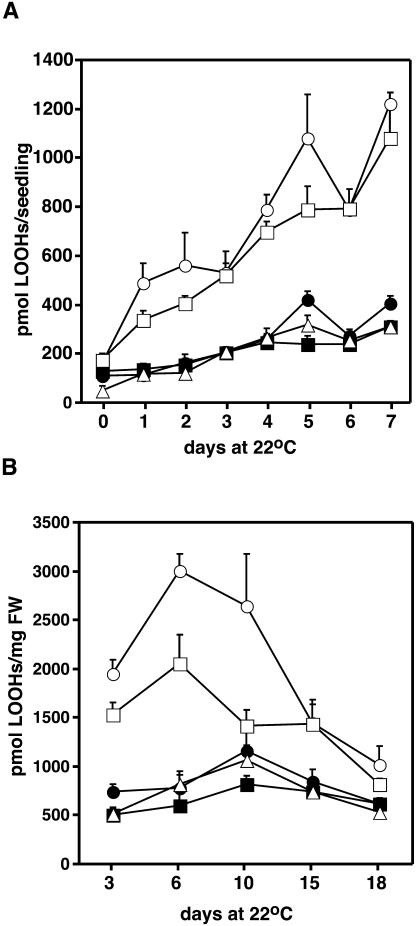
Given the phenotype of vte2 seedlings and the role of tocopherols in protecting PUFAs from lipid peroxidation in animal and in vitro systems (Fukuzawa et al., 1982; Girotti, 1998; Liebler, 1998; Brigelius-Flohe and Traber, 1999; Wang and Quinn, 2000), the levels of lipid hydroperoxides (LOOH) were determined during the first 7 d of seedling growth using the ferrous oxidation xylenol orange (FOX) assay (Griffiths et al., 2000). Imbibed 2-month-old seed of all genotypes contained low and similar levels of LOOHs (Figure 6A). However, the LOOH levels of both vte2 alleles increased significantly the first day of germination, before cotyledons emerge in the wild type, and continued to rise for 7 d. At any time point, vte2 LOOH levels per seedling were twofold to fourfold higher than those of the wild type or vte1 (Figure 6A), which were indistinguishable from one another. In a second experiment, lipid peroxidation in seedlings was measured over a longer duration and normalized to fresh weight (Figure 6B). The results of the second experiment were comparable to first: the vte2 alleles had LOOH levels threefold to fourfold higher than those of the wild type at 3 and 6 d and thereafter gradually declined to near wild-type levels by 18 d (Figure 6B). vte1 LOOH levels were similar to those of the wild type at all time points (Figures 6A and 6B). Together, these results indicate that tocopherols are essential for protecting PUFAs from lipid peroxidation during early seedling growth and that the vte2 cotyledon phenotype is likely a consequence of oxidative damage to lipids.
Figure 6.
Lipid Peroxidation in Wild-Type and Tocopherol-Deficient Arabidopsis Seedlings.
Lipid peroxidation in germinating seedlings as measured by the FOX assay (Griffiths et al., 2000). Hydrogen peroxide was used to construct a standard curve, and LOOH levels were expressed as picomole LOOHs per seedling (A) or picomole LOOHs per milligram of fresh weight (B). Closed circle, Col (wild type); open circle, vte2-1 (Col); open triangle, vte1-1 (Col); closed squre, Ws (wild type); open square, vte2-2 (Ws). The zero day time point is seed that have been imbibed for 5 d at 4°C. The differences between vte2 alleles and their respective wild-type backgrounds were statistically significant (Student's t test, P < 0.01) for all time points except zero days. Data are means, and error bars are sd (n = 4).
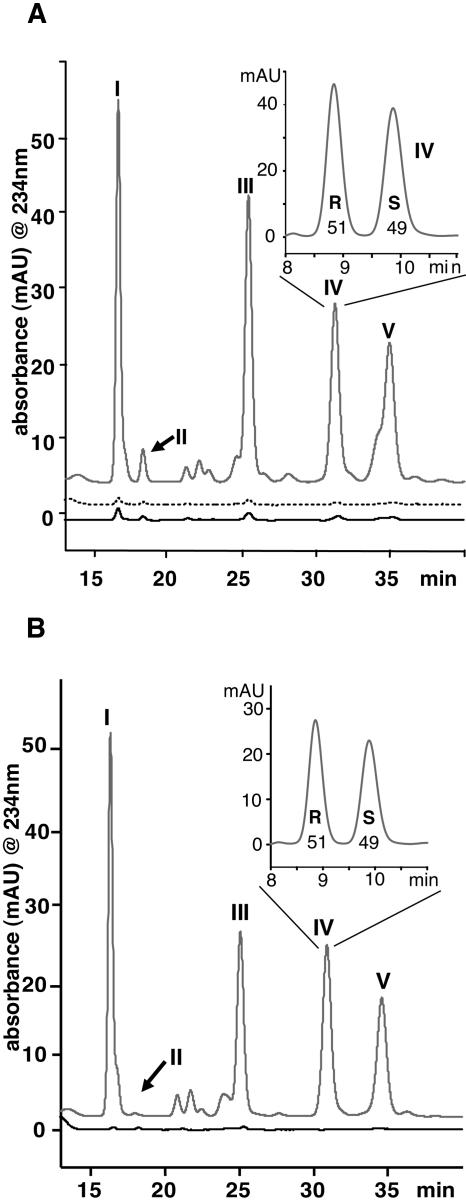
To further assess the consequences of elevated lipid peroxidation in the vte2 mutants, total lipid extracts were saponified, and oxidized fatty acid species and levels were analyzed by HPLC and gas chromatography–mass spectrometry (GC-MS). LOOHs can be reduced to lipid hydroxides (LOHs) in vivo and during saponification (Gardner et al., 1993). Consistent with the low level of lipid peroxidation observed at 3 and 6 d postimbibition in the wild type and vte1 (Figure 6), the levels of individual and total hydroxy fatty acids in these genotypes were also near or below the 10 pmol detection limit of the method (Figure 7, Table 2). By contrast, large increases in hydroxy fatty acids were observed in both vte2 alleles, with the major species being 9- and 13-hydroxy octadecadienoic acid (HODE) as both cis/trans and trans/trans isomers (Figure 7). Other oxidized fatty acids, including those that comigrated with 9- and 13-hydroxy octadecatrienoic acid (HOTE) standards, were also present at lower levels in vte2. The relatively low levels of HOTEs relative to HODEs in vte2 suggest that the former are being further modified or oxidized in vivo. The total hydroxy fatty acid level of vte2 seedlings at 3 and 6 d postgermination was up to 100-fold higher than either the wild type or vte1 (Table 2) and accounted for ∼4% of all PUFAs in vte2. These data suggest that in contrast with the wild type and vte1, lipid oxidation is largely uncontrolled during germination and early seedling growth in vte2.
Figure 7.
LOH Levels in Wild-Type and Tocopherol-Deficient Arabidopsis Seedlings.
Normal phase HPLC analysis (Feussner et al., 1995) of LOHs and LOOHs from saponified lipids of 6-d-old seedlings. The identity of peaks I, III, IV, and V from vte2-1 were confirmed by GC-MS. I, 13-HODE cis/trans; II, 13-HOTE; III, 13-HODE trans/trans; IV, 9-HODE cis/trans; V, 9-HODE trans/trans. mAU, milliabsorbance units.
(A) Columbia, wild type (black line), vte1-1 (dashed line), and vte2-1 (gray line). Inset, chiral phase HPLC analysis (Feussner et al., 1995) to determine the enantiomeric composition of peak IV, 9-HODE cis/trans. The ratio of R and S enantiomers is indicated.
(B) Ws, wild type (black line) and vte2-2 (gray line). Inset, chiral phase HPLC analysis (Feussner et al., 1995) to determine the enantiomeric composition of peak IV, 9-HODE cis/trans. The ratio of R and S enantiomers is indicated.
Table 2.
Fatty Acid Hydroxide Analysis of Germinating Seedlings
| Genotype | 13-HODE cis/trans | 13-HODE trans/trans | 13-HOTE | 9-HODE cis/trans | 9-HODE trans/trans | ΣLOH |
|---|---|---|---|---|---|---|
| 0 d | ||||||
| Col | NDa | ND | ND | ND | ND | ND |
| vte1-1 | ND | ND | ND | ND | ND | ND |
| vte2-1 | 0.4 ± 0.3 | 0.3 ± 0.3 | ND | 0 ± 0.3 | 0 ± 0.2 | 0.3 ± 0.5 |
| Ws | ND | ND | ND | ND | ND | ND |
| vte2-2 | 1.4 ± 0.6 | 1.8 ± 0.9 | ND | 1.0 ± 0.5 | 0.8 ± 0.5 | 2.6 ± 1.3 |
| 3 d | ||||||
| Col | 0.4 ± 0.2 | 0.6 ± 0.3 | ND | 0.3 ± 0.2 | ND | 0.4 ± 0.1 |
| vte1-1 | 0.6 ± 0.1 | 0.3 ± 0.2 | 0 ± 0.1 | 0.3 ± 0.1 | 0 ± 0.1 | 0.4 ± 0.2 |
| vte2-1 | 27.7 ± 2.7 (53:47) | 34.5 ± 5.4 | 2.2 ± 0.8 (56:44) | 23.0 ± 0.1 (50:50) | 20.1 ± 2.4 | 48.3 ± 6.0 |
| Ws | 0.5 ± 0.2 | ND | ND | ND | ND | 0.1 ± 0.1 |
| vte2-2 | 25.6 ± 1.3 (52:48) | 30.0 ± 2.5 | 1.3 ± 0.5 (60:40) | 20.5 ± 1.6 (51:49) | 16.7 ± 1.1 | 46.6 ± 3.8 |
| 6 d | ||||||
| Col | 1.2 ± 0.2 (51:49) | 1.0 ± 0.7 | 0.1 ± 0.1 | 0.6 ± 0.4 (52:48) | 0.6 ± 0.4 | 0.8 ± 0.4 |
| vte1-1 | 0.8 ± 0.1 (54:46) | 0.9 ± 0.2 | ND | 0.6 ± 0.1 (50:50) | 0.3 ± 0.2 | 0.7 ± 0.1 |
| vte2-1 | 31.3 ± 6.3 (52:48) | 30.0 ± 13.9 | 0.5 ± 0.9 (55:45) | 28.0 ± 4.6 (51:49) | 24.9 ± 9.2 | 42.6 ± 13.2 |
| Ws | 0.3 ± 0.1 (52:48) | 0.4 ± 0.2 | 0.0 ± 0.1 | 0.1 ± 0.2 (47:53) | 0.3 ± 0.2 | 0.2 ± 0.1 |
| vte2-2 | 30.8 ± 3.9 (52:48) | 27.1 ± 6.0 | 0.3 ± 0.3 (56:44) | 26.1 ± 3.1 (51:49) | 19.8 ± 1.3 | 42.1 ± 6.2 |
The five prevalent hydroxy fatty acid species were quantified using normal phase HPLC. Each compound is expressed as pmol/nmol of the corresponding PUFA (linoleic or linolenic acid) from which it was derived in the sample. The enantiomeric composition of 9-HODE cis/trans, 13-HOTE, and 13-HODE cis/trans were determined by chiral phase HPLC. The ratio of R to S is given in parentheses for samples that contained sufficient quantities for chiral analysis. Chiral analysis indicated that both 9- and 13-HODE trans/trans peaks contained two enantiomers in relatively equal proportions, though R and S could not be assigned because of the lack of commercially available standards. ΣLOH is sum of all LOHs expressed as pmol/nmol total PUFAs (linoleic and linolenic acid). The zero day time point is seed that have been imbibed for 5 d at 4°C. The data are the means ± sd (n = 4). The LOH values for both vte2-1 and vte2-2 were highly significant (Student's t test, P < 0.01) at all points relative to the wild type.
ND, not detected.
The 13-HOTE and 9- and 13-HODE cis/trans peaks in vte2 were collected and subjected to chiral phase HPLC analysis (Feussner et al., 1995) to determine their enantiomeric compositions. Hydroxy fatty acids derived from the action of lipoxygenases are predominantly S enantiomers, whereas those derived from the nonenzymatic oxidation of PUFAs yield racemic mixtures of R and S enantiomers (Feussner et al., 1995). All cis/trans vte2 LOH peaks at all time points contained R and S enantiomers in nearly equal proportions (insets of Figure 7, Table 2). The trans/trans isomers of 9-HODE and 13-HODE, which are not products of known lipoxygenases, also contained both enantiomers at near equal proportions. The presence of trans/trans isomers of 9-HODE and 13-HODE at approximately equimolar levels to their corresponding cis/trans isomers in vte2 mutants is also consistent with autoxidation (Chan and Levett, 1977b). Together, these results indicate that the elevated lipid oxidation in vte2 seedlings is attributable primarily to nonenzymatic oxidation of PUFAs.
DISCUSSION
Tocopherols are synthesized by all plants and have long been assumed to play important but undefined roles in various aspects of plant biology. Studies in animals and artificial membrane systems have shown that tocopherols scavenge and quench various ROS and lipid oxidation products, stabilize membranes, and modulate signal transduction (Brigelius-Flohe and Traber, 1999; Wang and Quinn, 2000; Yamauchi et al., 2001; Clement et al., 2002). Whether any of these functions also occurs in plants, or whether additional novel plant-specific tocopherol functions exist, has yet to be determined. As a first step toward elucidating tocopherol function in plants, we have used two classes of Arabidopsis tocopherol-deficient mutants, vte1 and vte2, to define several specific roles for tocopherols in seed viability and seedling growth and development.
Tocopherols Are Required for Seed Longevity
Seed longevity is an important trait from both ecological and agricultural perspectives and has been studied in considerable detail (for review, see McDonald, 1999). Lipid oxidation has been proposed to be a significant factor in seed longevity, with some studies reporting an inverse correlation of various lipid oxidation products and seed longevity during natural and accelerated aging (Harman and Mattick, 1976; Wilson and McDonald, 1986; Bailly et al., 1998). However, other studies have failed to observe such a relationship (Priestley and Leopold, 1979; Bailly et al., 2002) and instead suggest that other processes, such as membrane integrity, the levels of other antioxidants, damage to nucleic acids and proteins, and the activity of ROS detoxifying enzymes (catalase, superoxide dismutase, glutathione reductase, and ascorbate peroxidase) (McDonald, 1999) are key determinants of seed longevity. It has also been impossible to establish a defined role for tocopherols in seed longevity because some studies have shown a correlation of tocopherol content or degradation with longevity, whereas others have not (Priestley et al., 1980; Gorecki and Harman, 1987; Senaratna et al., 1988; Simontacchi et al., 1993; Basra et al., 1994). The two classes of vte mutants have conclusively demonstrated that tocopherols are essential components for seed longevity in Arabidopsis. Although nearly identical phenotypes were observed for vte1 and vte2 in accelerated aging tests (Figure 2), the assay is an extreme test of seed viability because even wild-type seed had delayed germination and reduced viability after treatment. More moderate treatments may show differential effects of the two vte mutations on seed longevity as when different seed lots were stored under ambient conditions for up to 1 year, vte2 germination and viability was consistently lower than comparably aged vte1 (data not shown). Detailed biochemical analyses are required to determine the precise mechanism(s) responsible for loss of seed longevity during natural aging or accelerated aging treatments in the absence of tocopherols in the vte mutants.
Primary Metabolism in Seedlings in the Absence of Tocopherols
Germination and the first days of seedling development are critical periods in a plant's lifecycle during which seedlings are heterotrophic and rely entirely on β-oxidation of storage lipids (triacylglycerols [TAGs]) for fixed carbon and energy. In the vte2 mutants, catabolism of seed storage lipids is severely impacted, and unexpanded sectors devoid of chlorophyll at the cotyledon tips bear a striking resemblance to β-oxidation mutants of Arabidopsis (Hayashi et al., 1998; Germain et al., 2001). The partial rescue of vte2 seedling root growth by supplementation of media with exogenous sucrose is consistent with a shortage of fixed carbon contributing to the growth defects of vte2 seedlings (Figures 3 and 4).
Fatty acid analysis demonstrated that vte2 seedlings do not efficiently breakdown storage lipids, as indicated by both the elevated level of total fatty acids and 20:1, a biochemical marker for storage lipids, during early vte2 seedling development in comparison with the wild type. During the first 6 d of growth in wild-type and vte1 seedlings, storage lipids are efficiently metabolized, total fatty acid content decreases 70 to 75%, and 20:1 mol % and absolute levels decrease 95 and 98%, respectively. During the same time frame in vte2, total fatty acid levels only decrease 60 to 65%, whereas 20:1 levels decrease 20 to 30% as mol % and 75% in absolute level (nmol per seedling). Thus, whereas β-oxidation of storage lipids still occurs in vte2, it is not as efficient as in the wild type or vte1-1 and in turn severely restricts the energy and biochemical building blocks available to developing vte2 seedlings.
In addition to 20:1, the metabolism of linoleic (18:2) and linolenic acid (18:3) was also negatively impacted in vte2. The 18:2 content of vte2 seedlings did not decrease as rapidly as developing wild-type seedlings. The mol % of 18:3, a major component of chloroplast membranes (Miquel and Browse, 1992), nearly tripled during the first 6 d of wild-type and vte1-1 seedling growth. By contrast, the 18:3 content of vte2 only increased 50% during this time frame likely because of delayed chloroplast development and enhanced 18:3 oxidation in vte2 cotyledons. If one uses 18:3 levels as an indicator of chloroplast development and function, photoautotrophy is also likely delayed and impaired in vte2, which would further limit energy and fixed carbon availability during vte2 seedling development. The diminished catabolism of storage lipids and decreased de novo lipid synthesis are likely the root cause of the many pleiotropic effects in vte2 seedlings and are in turn brought about by elevated oxidative damage to PUFAs and other cellular components in the absence of tocopherols.
Lipid Oxidation in the Absence of Tocopherols
The best-described and most oft attributed function of tocopherols is their involvement by various mechanisms in protecting PUFAs from oxidation. Tocopherols quench singlet oxygen and can scavenge various ROS and ROS by-products, including lipid peroxyl radicals (Girotti, 1998). During scavenging, tocopherols can form tocopheroxyl radicals that can be recycled back to the corresponding tocopherol by reacting with ascorbate or other antioxidants or covalently react with free radicals and form tocopherol derivatives that are not as readily recycled (Liebler, 1993). In vte2 seedlings, the absence of tocopherols results in apparently uncontrolled PUFA oxidation, and levels of LOOHs and LOHs are threefold to fourfold and >100 fold higher, respectively, than either the wild type or vte1. Oxidized lipids account for >4% of total PUFAs in vte2. The elevated level of LOH relative to LOOH in vte2 is likely because LOOHs are highly reactive and therefore less stable than LOHs both in vivo and in vitro. In wild-type Arabidopsis and most other plants, LOOH levels are kept relatively low during germination and, based on the vte2 phenotype, are detrimental at elevated levels. A notable exception is germinating cucumber (Cucumis sativus) seedlings where a single lipoxygenase-derived LOOH product (13S-hydroperoxy octadecatrienoic acid [HPODE]) accumulates to high levels in TAGs and presumably targets the acyl chain for catabolism. However, unlike in vte2, the LOOH produced in cucumber seedlings is part of the normal germination process (Feussner et al., 1995) and is apparently not detrimental.
The results of normal and chiral phase HPLC analysis are consistent with increased lipid oxidation in vte2 being attributable to a large increase in the nonenzymatic, peroxyradical-mediated production of LOOHs. These data indicate that reduction of LOOHs to LOHs is a major route whereby vte2 cells can reduce the levels of highly reactive LOOHs generated in the absence of tocopherols. Several enzymes that catalyze such reactions have been characterized in plants (Herbette et al., 2002; Dietz, 2003; Konig et al., 2003). The major LOHs accumulated by vte2 are near equal mixtures of cis/trans and trans/trans structural isomers of HODEs (oxidized 18:2 derivatives) composed of near equimolar R and S stereoisomers, the chemical hallmarks of nonenzymatic lipid oxidation (Chan and Levett, 1977a, 1997b). That HODEs (oxidized 18:2 derivatives) are much more abundant than HOTEs (oxidized 18:3 derivatives) is surprising because the fatty acid precursors for each are present at similar levels in developing vte2 seedlings. Furthermore, 18:3 is at least as susceptible to oxidation as 18:2 both in vivo and in vitro (Berger et al., 2001), making it unlikely that 18:3 is specifically not oxidized in vte2.
The most likely explanation for low HOTE levels in vte2 is that hydroperoxyoctadecatrienoic acids (HPOTEs) are being converted into products other than HOTEs and/or that other hydroperoxides (i.e., cyclic peroxides) are being generated. There is substantial evidence that nonenzymatic oxidation and modification of HPOTEs can occur both in vitro and in vivo (Porter et al., 1995; Kohlmann et al., 1999; Imbusch and Mueller, 2000). In addition, numerous enzymes that use HPOTEs as substrates to generate products other than HOTEs have also been documented in plants (e.g., allene oxide synthase, hydroperoxide lyase, epoxy alcohol synthase, and divinyl ether synthase) (Creelman and Mulpuri, 2002; Feussner and Wasternack, 2002; Howe and Schilmiller, 2002). Such enzymes may play a role in the low HOTE levels observed in vte2, but most are capable of using both HPODE and HPOTE as substrates (Creelman and Mulpuri, 2002; Feussner and Wasternack, 2002; Howe and Schilmiller, 2002). An enzymatic explanation for the differing HOTE and HODE levels in vte2 would require enzymes that preferentially act upon HPOTEs. Hydroperoxide lyase is one such example and is 10-fold more active toward 13-HPOTE than 13-HPODE (Bate et al., 1998; Matsui et al., 1999). Because the HPLC method used in this study only resolves a limited range of lipid oxidation products, mainly LOHs, none of the alternative enzymatic or nonenzymatic possibilities for low HOTE levels in vte2 can be eliminated.
Interestingly, both enzymatic and nonenzymatic products of lipid peroxidation (oxylipins) have been shown to activate signal transduction and gene expression in response to pathogens and herbivores (Stintzi et al., 2001; Almeras et al., 2003; Thoma et al., 2003). Some oxylipins and oxylipin-derived products may also have cytotoxic properties (Weber et al., 1999; Vollenweider et al., 2000; Taylor et al., 2002; Almeras et al., 2003). Therefore, it seems likely that in addition to limitations in energy and fixed carbon brought about by the diminished lipid catabolism in vte2, the extremely elevated production of lipid oxidation products (oxylipins) may also contribute to the pleiotropic defects during vte2 seedling growth and development.
Model for Tocopherol Function in Limiting Lipid Oxidation in Developing Seedlings
The phenotypic effects of vte2 are limited to cotyledons and early seedling development (Figure 3), suggesting that the impact of tocopherol deficiency is less severe in leaves or, more likely, that other processes can compensate for the absence of tocopherols in mature leaves. Our results suggest that germination and early seedling development are an oxidative bottleneck for plants in which tocopherols play an essential role. The mobilization of seed storage lipids by β-oxidation and gluconeogenesis are two extremely active processes during germination and likely sources of elevated ROS in vte2 seedlings. β-Oxidation occurs in the glyoxysome and produces one molecule of hydrogen peroxide per cycle, whereas the mitochondrial electron transport chain can produce superoxide and hydrogen peroxide as by-products of gluconeogenesis (Perl-Treves and Perl, 2002). As cotyledons mature and become photosynthetically competent, photosynthesis may provide an additional source of ROS (Perl-Treves and Perl, 2002), which could further exacerbate vte2 seedling growth and development. Regardless of the ROS source, our results indicate that tocopherols are essential for protecting PUFAs from the damaging effects of ROS during germination and early seedling growth.
In addition to an increased level of ROS during and after germination, the lipid concentrations of seeds far exceeds that of leaf tissue and is present primarily as TAGs. In such a situation, other components of the plant antioxidant network, such as ascorbate and glutathione, which are hydrophillic molecules, may be ill adapted to compensate for the absence of tocopherols in vte2 seedlings. The massive levels of lipid oxidation observed in vte2 seedlings suggest that tocopherols protect PUFAs in storage lipids from oxidation, which would require association of tocopherols with oil bodies in seed. Tocopherol synthesis has been convincingly shown to be localized in plastids in vegetative tissues and fruit (Soll et al., 1980, 1985; Lichtenthaler et al., 1981; Fryer, 1992; Kruk and Strzalka, 1995; Arango and Heise, 1998). However, in soybean (Glycine max) seeds and seedlings, the major tocopherol present, γ-tocopherol, was associated with the oil bodies (Yamauchi and Matsushita, 1976). Perhaps in oil seed, which are specialized for the synthesis, accumulation, and storage of oil, tocopherols are transported or incorporated into oil bodies by an as yet unidentified mechanism.
The Difference between vte1 and vte2 Phenotypes
The vte2 mutants demonstrate that tocopherols are essential for seed longevity and for protecting PUFAs from oxidation during germination and early seedling growth. It is therefore somewhat surprising that vte1, which is also deficient in tocopherols, exhibits root growth, 20:1 degradation, and LOOH and LOH levels during germination and early seedling growth that are nearly identical to those of the wild type. These results indicate that, with the exception of seed longevity (Figure 2B), the DMPBQ that accumulates in vte1 (Table 1; Sattler et al., 2003) can functionally compensate for the absence of tocopherols. This is likely because, like tocopherols, DMPBQ is also hydrophobic and in its quinol form can donate two electrons and thereby act as a lipophilic antioxidant. An obvious question then becomes, why is it that tocopherols (the product of the VTE1 locus) and not DMPBQ are evolutionarily conserved in the plant kingdom?
The answer appears to reside in the essential role tocopherols play in seed longevity, where vte1 and vte2 show similarly severe phenotypes and DMPBQ cannot functionally replace tocopherols. There are several possible reasons tocopherols are specifically required for seed longevity. First, unlike DMPBQ, tocopherols can donate a single electron (Liebler, 1993; KamalEldin and Appelqvist, 1996), which may influence how efficiently or effectively specific ROS or free radicals (especially lipid peroxyradicals) are scavenged in seed. Second, tocopheroxyl radicals can be chemically recycled (reduced) (Liebler, 1993), whereas quinones, like DMPBQ, generally require enzymatic reduction coupled to a cofactor (NADH/NADPH) or electron transport chain (Liebler, 1998). Finally, the chromanol ring, which is unique to tocopherols, allows quenching of singlet oxygen (Fukuzawa et al., 1982), a function that DMPBQ lacks. Thus, whereas a naturally occurring vte1 mutation might not be as strongly selected against during germination and early seedling development as vte2 mutants, a vte1 mutant would likely be as strongly selected against as vte2 during seed dormancy in a natural environment.
Conclusions
The evolution of seeds was a key step in the successful radiation of land plants into a variety of ecological niches. Seeds permitted the production of quiescent progeny with high-energy lipid stores that could be rapidly mobilized as a fixed carbon source upon germination. The ability of seeds to remain quiescent but viable during unfavorable environmental conditions and then rapidly germinate and become established as photoautotrophs when environmental conditions become favorable was clearly a key evolutionary advantage in the conquering of land by plants. Tocopherol synthesis was apparently a critical biochemical pathway for the evolution of seeds as evidenced by the conservation of tocopherol synthesis in all extant seed-bearing plants, the presence of high levels of tocopherols in all seeds, and the phenotype of vte mutants.
METHODS
Mutant Isolation and Characterization
vte1-1 and vte2-1 were isolated in an HPLC-based screen for mutants with altered leaf tocopherol profiles from M3 ethyl methanesulfonate–mutagenized Arabidopsis thaliana (Col; Lehle Seed, Round Rock, TX) (Sattler et al., 2003). vte2-2 was isolated through a PCR-based reverse genetics screen for T-DNA insertions in At2g18950 (homogentisate phytyl transferase) from the Wisconsin Knockout Facility (www.biotech.wisc.edu/Arabidopsis/). The vte2-2 mutant is in the Ws ecotype. All three mutants were backcrossed to the wild type three times. vte2-2 segregated 3:1 for the selectable marker BastaR. Tocopherol and prenyl quinone analyses were described previously (Sattler et al., 2003).
Growth Conditions
Arabidopsis plants were grown at 22°C under a 12-h photoperiod (120 μE) in a vermiculite and potting soil mixture and fertilized with Hoagland solution. Alternatively, seedlings were grown on Petri plates containing 0.5× Murashige and Skoog basal medium (MS salts; Sigma, St. Louis, MO) and 1.0% phytoagar under the same growth parameters as above. For root growth assays, 15 seedlings for each genotype were grown vertically on Petri plates.
Accelerated Aging
Two-month-old seeds were subjected to accelerated aging using the wire-mesh method (Elias and Copeland, 2001). Seeds were placed above 100 mL of water on a wire-mesh tray in accelerated aging boxes (11 cm × 11 cm × 3.5 cm) and the lid sealed. Four sets of 100 seed were used for each genotype. Each seed set came from an individual plant, and all plants were grown simultaneously under the same conditions. Seed were aged for 72 h at 40°C and 100% RH, placed on filter paper moistened with water, and incubated at 4°C for 5 d to imbibe and break dormancy. Unaged seed (controls) were also incubated on filter paper moistened with water at 4°C for 5 d. After cold treatment, seed were placed at 22°C under a 12-h photoperiod (140 μE). Germination was scored each day by root radical emergence for 10 d.
FOX Assay
The FOX assay for determining lipid peroxides was performed as previously described (Griffiths et al., 2000). Total lipids were extracted in a 96-well format as previously described (Tian et al., 2003). Forty seedlings (four samples per genotype) were extracted using 400 μL of methanol:dichloromethane (1:1 [v/v]) containing 0.05% butylated hydroxytoluene and 50 μL of 150 mM acetic acid using two 4-mm glass beads and a commercial paint shaker, shaking for 5 min. Lipids were partitioned into the organic phase by adding 300 μL of water, vortexing and centrifugation at 3750g. Half of the organic phase was incubated for 30 min with an equal volume of 10 mM triphenyl phosphine to reduce lipid peroxides, and half was left untreated. The triphenyl phosphine–treated samples were used to determine assay background not attributed to lipid peroxides. The lipid extracts were incubated at room temperature with 0.5 mL of FOX solution for 30 min. Immediately after incubation the absorbance was measured at 560 nm. A standard curve was constructed using hydrogen peroxide as previously described (DeLong et al., 2002). The reactivity of 18:2-derived LOOHs with the FOX reagent is nearly identical to hydrogen peroxide (DeLong et al., 2002).
Lipid Analysis
Fatty acid methyl esters were extracted from 20 seedlings (six samples per genotype) and subjected to GC analysis as previously described (Focks and Benning, 1998). For hydroxy fatty acid analysis, the extraction protocol and reverse, normal, and chiral phase HPLC analyses were as previously described (Feussner et al., 1995; Berger et al., 2001). Total lipids from 200 mg of fresh weight seedlings (four samples per genotype) were extracted, saponified with 40% KOH, and neutralized with an equal volume of glacial acetic acid. 18:2, 18:3, and hydroxy fatty acids were separated using reverse phase HPLC. The peaks containing fatty acid hydroxides were collected, dried under nitrogen gas, and subjected to normal phase HPLC analysis. Individual fatty acid peaks were collected and analyzed by chiral phase HPLC to determine enantiomeric composition. HPLC analysis was performed using an Agilent 1100 series HPLC (Sigma) on a Spherisorb ODS-2 5-μm, 250 × 4.6-mm reverse phase column (Column Engineering, Ontario, CA), a ReliaSil Silica 5-μm, 250 × 4.6-mm normal phase column (Column Engineering), or an ODH 5-μm, 250 × 4.6-mm chiral phase column (Daicel Industries, Chiral Technologies, Exton, PA) at 30°C with a flow rate of 1 mL min−1 and methanol:water:acetic acid (85:15:0.1 [v/v/v]) (reverse phase), hexane:isopropanol:acetic acid (100:1.5:0.1 [v/v/v]) (normal phase), or hexane:isopropanol:trifluoroacetic acid (100:5:0.1 [v/v/v]) (chiral phase). 18:2 and 18:3 were detected at 210 nm and hydroxy fatty acids at 234 nm using a photodiode array detector. Commercially available hydroxy fatty acids standards (Cayman Chemicals, Ann Arbor, MI) were used to identify compounds based on HPLC and GC retention time and mass spectra and to construct standard curves. For quantification of 13-HODE trans/trans and 9-HODE trans/trans, the standard curves from their corresponding cis/trans isomers were used because trans/trans standards were not commercially available.
GC-MS was used to confirm the identity of 9-HODE cis/trans, 9-HODE trans/trans, 13-HODE cis/trans, and 13-HODE trans/trans. For GC-MS, the hydroxy fatty acids were methylated using ethereal diazomethane for 30 s and dried under nitrogen gas. The hydroxyl group was trimethylsilyl derivatized by treating the sample with 300 μL of N,O-bis(trimethylsilyl)trifluoroacetamide (Sigma):pyridine 1:1 (v/v) for 30 min at 60°C, dried with nitrogen gas, and resuspended in 100 μL of heptane. The derivatized samples were analyzed on electron ionization GC-MS using a Hewlett-Packard 5890 quadrupole instrument with a HP-5 column (30 m × 0.32 mm), with helium as the carrier gas. The column temperature program was 90°C for 3 min, then to 250°C at 5°/min, then at 250°C for 10 min. The mass spectra were obtained at 70 eV at 250°C. The mass spectra of 13-HODE cis/trans and trans/trans isomers had an initial mass ion of 382 mass-to-charge ratio and identical fragmentation patterns. Likewise, the mass spectra of 9-HODE cis/trans and trans/trans isomers had an initial mass ion of 382 mass-to-charge ratio and identical fragmentation patterns. The 9-HODE and 13-HODE spectra matched previously published mass spectra (Abian et al., 1991; Wu et al., 1995).
Acknowledgments
We thank C. Benning, A. Cernac, and J. Ohlrogge for their critical advice and assistance with the lipid analysis and the members of the DellaPenna lab for reviewing the manuscript. We also acknowledge Chris Cook for his technical assistance. This work was support by National Science Foundation grant MCB-023529 to D.D.P.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Dean DellaPenna (dellapen@msu.edu).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.021360.
References
- Abian, J., Gelpi, E., and Pages, M. (1991). Effect of abscisic-acid on the linoleic-acid metabolism in developing maize embryos. Plant Physiol. 95, 1277–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeras, E., Stolz, S., Vollenweider, S., Reymond, P., Mene-Saffrane, L., and Farmer, E.E. (2003). Reactive electrophile species activate defense gene expression in Arabidopsis. Plant J. 34, 202–216. [DOI] [PubMed] [Google Scholar]
- Arango, Y., and Heise, K.P. (1998). Localization of alpha-tocopherol synthesis in chromoplast envelope membranes of Capsicum annuum L. fruits. J. Exp. Bot. 49, 1259–1262. [Google Scholar]
- Azzi, A., Breyer, I., Feher, M., Pastori, M., Ricciarelli, R., Spycher, S., Staffieri, M., Stocker, A., Zimmer, S., and Zingg, J.M. (2000). Specific cellular responses to alpha-tocopherol. J. Nutr. 130, 1649–1652. [DOI] [PubMed] [Google Scholar]
- Bailly, C., Benamar, A., Corbineau, F., and Come, D. (1998). Free radical scavenging as affected by accelerated ageing and subsequent priming in sunflower seeds. Physiol. Plant 104, 646–652. [Google Scholar]
- Bailly, C., Bogatek-Leszczynska, R., Come, D., and Corbineau, F. (2002). Changes in activities of antioxidant enzymes and lipoxygenase during growth of sunflower seedlings from seeds of different vigour. Seed Sci. Res. 12, 47–55. [Google Scholar]
- Basra, A.S., Singh, B., and Malik, C.P. (1994). Amelioration of the effects of aging in onion seeds by osmotic priming and associated changes in oxidative metabolism. Biol. Plant. 36, 365–371. [Google Scholar]
- Bate, N.J., Sivasankar, S., Moxon, C., Riley, J.M.C., Thompson, J.E., and Rothstein, S.J. (1998). Molecular characterization of an Arabidopsis gene encoding hydroperoxide lyase, a cytochrome P-450 that is wound inducible. Plant Physiol. 117, 1393–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, S., Weichert, H., Porzel, A., Wasternack, C., Kuhn, H., and Feussner, I. (2001). Enzymatic and non-enzymatic lipid peroxidation in leaf development. Biochim. Biophys. Acta 1533, 266–276. [DOI] [PubMed] [Google Scholar]
- Brigelius-Flohe, R., and Traber, M.G. (1999). Vitamin E: Function and metabolism. FASEB J. 13, 1145–1155. [PubMed] [Google Scholar]
- Cahoon, E.B., Hall, S.E., Ripp, K.G., Ganzke, T.S., Hitz, W.D., and Coughlan, S.J. (2003). Metabolic redesign of vitamin E biosynthesis in plants for tocotrienol production and increased antioxidant content. Nat. Biotechnol. 21, 1082–1087. [DOI] [PubMed] [Google Scholar]
- Chan, H.W.S., and Levett, G. (1977. a). Autoxidation of methyl linolenate: Analysis of methyl hydroxylinolenate isomers by high-performance liquid- chromatography. Lipids 12, 837–840. [DOI] [PubMed] [Google Scholar]
- Chan, H.W.S., and Levett, G. (1977. b). Autoxidation of methyl linoleate: Separation and analysis of isomeric mixtures of methyl linoleate hydroperoxides and methyl hydroxylinoleates. Lipids 12, 99–104. [DOI] [PubMed] [Google Scholar]
- Chan, S.S., Monteiro, H.P., Schindler, F., Stern, A., and Junqueira, V.B.C. (2001). Alpha-tocopherol modulates tyrosine phosphorylation in human neutrophils by inhibition of protein kinase C activity and activation of tyrosine phosphatases. Free Radic. Res. 35, 843–856. [DOI] [PubMed] [Google Scholar]
- Cheng, Z., Sattler, S., Maeda, H., Sakuragi, Y., Bryant, D.A., and DellaPenna, D. (2003). Highly divergent methyltransferases catalyze a conserved reaction in tocopherol and plastoquinone synthesis in cyanobacteria and photosynthetic eukaryotes. Plant Cell 15, 2343–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement, S.A., Tan, C.C., Guo, J.L., Kitta, K., and Suzuki, Y.J. (2002). Roles of protein kinase C and alpha-tocopherol in regulation of signal transduction for GATA-4 phosphorylation in HL-1 cardiac muscle cells. Free Radic. Biol. Med. 32, 341–349. [DOI] [PubMed] [Google Scholar]
- Collakova, E., and DellaPenna, D. (2001). Isolation and functional analysis of homogentisate phytyltransferase from Synechocystis sp. PCC 6803 and Arabidopsis. Plant Physiol. 127, 1113–1124. [PMC free article] [PubMed] [Google Scholar]
- Collakova, E., and DellaPenna, D. (2003. a). Homogentisate phytyltransferase activity is limiting for tocopherol biosynthesis in Arabidopsis. Plant Physiol. 131, 632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collakova, E., and DellaPenna, D. (2003. b). The role of homogentisate phytyltransferase and other tocopherol pathway enzymes in the regulation of tocopherol synthesis during abiotic stress. Plant Physiol. 133, 930–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman, R.A., and Mulpuri, R. (2002). The oxylipin pathway in Arabidopsis. In The Arabidopsis Book, C.R. Somerville and E.M. Meyerowitz, eds (Rockville, MD: American Society of Plant Biologists), doi/10.1199/tab.0012, http://www.aspb.org/publications/arabidopsis/. [DOI] [PMC free article] [PubMed]
- DeLong, J.M., Prange, R.K., Hodges, D.M., Forney, C.F., Bishop, M.C., and Quilliam, M. (2002). Using a modified ferrous oxidation-xylenol orange (FOX) assay for detection of lipid hydroperoxides in plant tissue. J. Agric. Food Chem. 50, 248–254. [DOI] [PubMed] [Google Scholar]
- Dietz, K.J. (2003). Plant peroxiredoxins. Annu. Rev. Plant Biol. 54, 93–107. [DOI] [PubMed] [Google Scholar]
- Eastmond, P.J., Germain, V., Lange, P.R., Bryce, J.H., Smith, S.M., and Graham, I.A. (2000). Postgerminative growth and lipid catabolism in oilseeds lacking the glyoxylate cycle. Proc. Natl. Acad. Sci. USA 97, 5669–5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias, S.G., and Copeland, L.O. (2001). Physiological and harvest maturity of canola in relation to seed quality. Agron. J. 93, 1054–1058. [Google Scholar]
- Feussner, I., and Wasternack, C. (2002). The lipoxygenase pathway. Annu. Rev. Plant Biol. 53, 275–297. [DOI] [PubMed] [Google Scholar]
- Feussner, I., Wasternack, C., Kindl, H., and Kuhn, H. (1995). Lipoxygenase-catalyzed oxygenation of storage lipids is implicated in lipid mobilization during germination. Proc. Natl. Acad. Sci. USA 92, 11849–11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focks, N., and Benning, C. (1998). wrinkled1: A novel, low-seed-oil mutant of Arabidopsis with a deficiency in the seed-specific regulation of carbohydrate metabolism. Plant Physiol. 118, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer, M.J. (1992). The antioxidant effects of thylakoid vitamin-E (alpha-tocopherol). Plant Cell Environ. 15, 381–392. [Google Scholar]
- Fukuzawa, K., Tokumura, A., Ouchi, S., and Tsukatani, H. (1982). Antioxidant activities of tocopherols on Fe2+-ascorbate-induced lipid peroxidation in lecithin liposomes. Lipids 17, 511–513. [DOI] [PubMed] [Google Scholar]
- Gardner, H.W., Simpson, T.D., and Hamberg, M. (1993). Transformation of fatty-acid hydroperoxides by alkali and characterization of products. Lipids 28, 487–495. [Google Scholar]
- Germain, V., Rylott, E.L., Larson, T.R., Sherson, S.M., Bechtold, N., Carde, J.P., Bryce, J.H., Graham, I.A., and Smith, S.M. (2001). Requirement for 3-ketoacyl-CoA thiolase-2 in peroxisome development, fatty acid beta-oxidation and breakdown of triacylglycerol in lipid bodies of Arabidopsis seedlings. Plant J. 28, 1–12. [DOI] [PubMed] [Google Scholar]
- Girotti, A.W. (1998). Lipid hydroperoxide generation, turnover, and effector action in biological systems. J. Lipid Res. 39, 1529. [PubMed] [Google Scholar]
- Gorecki, R.J., and Harman, G.E. (1987). Effects of antioxidants on viability and vigor of aging pea-seeds. Seed Sci. Technol. 15, 109–117. [Google Scholar]
- Griffiths, G., Leverentz, M., Silkowski, H., Gill, N., and Sanchez-Serrano, J.J. (2000). Lipid hydroperoxide levels in plant tissues. J. Exp. Bot. 51, 1363–1370. [PubMed] [Google Scholar]
- Harman, G.E., and Mattick, L.R. (1976). Association of lipid oxidation with seed aging and death. Nature 260, 323–324. [Google Scholar]
- Hayashi, M., Toriyama, K., Kondo, M., and Nishimura, M. (1998). 2,4-Dichlorophenoxybutyric acid-resistant mutants of Arabidopsis have defects in glyoxysomal fatty acid beta-oxidation. Plant Cell 10, 183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbette, S., Lenne, C., Leblanc, N., Julien, J.L., Drevet, J.R., and Roeckel-Drevet, P. (2002). Two GPX-like proteins from Lycopersicon esculentum and Helianthus annuus are antioxidant enzymes with phospholipid hydroperoxide glutathione peroxidase and thioredoxin peroxidase activities. Eur. J. Biochem. 269, 2414–2420. [DOI] [PubMed] [Google Scholar]
- Howe, G.A., and Schilmiller, A.L. (2002). Oxylipin metabolism in response to stress. Curr. Opin. Plant Biol. 5, 230–236. [DOI] [PubMed] [Google Scholar]
- Imbusch, R., and Mueller, M.J. (2000). Analysis of oxidative stress and wound-inducible dinor isoprostanes F-1 (phytoprostanes F-1) in plants. Plant Physiol. 124, 1293–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal-Eldin, A., and Andersson, R. (1997). A multivariate study of the correlation between tocopherol content and fatty acid composition in vegetable oils. J. Am. Oil Chem. Soc. 74, 375–380. [Google Scholar]
- Kamal-Eldin, A., and Appelqvist, L.A. (1996). The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 31, 671–701. [DOI] [PubMed] [Google Scholar]
- Kohlmann, M., Bachmann, A., Weichert, H., Kolbe, A., Balkenhohl, T., Wasternack, C., and Feussner, I. (1999). Formation of lipoxygenase-pathway-derived aldehydes in barley leaves upon methyl jasmonate treatment. Eur. J. Biochem. 260, 885–895. [DOI] [PubMed] [Google Scholar]
- Konig, J., Lotte, K., Plessow, R., Brockhinke, A., Baier, M., and Dietz, K.J. (2003). Reaction mechanism of plant 2-Cys peroxiredoxin. Role of the C terminus and the quaternary structure. J. Biol. Chem. 278, 24409–24420. [DOI] [PubMed] [Google Scholar]
- Kruk, J., and Strzalka, K. (1995). Occurrence and function of alpha-tocopherol quinone in plants. J. Plant Physiol. 145, 405–409. [Google Scholar]
- Lemieux, B., Miquel, M., Somerville, C., and Browse, J. (1990). Mutants of Arabidopsis with alterations in seed lipid fatty-acid composition. Theor. Appl. Genet. 80, 234–240. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler, H.K., Prenzel, U., Douce, R., and Joyard, J. (1981). Localization of prenylquinones in the envelope of spinach chloroplasts. Biochim. Biophys. Acta 641, 99–105. [DOI] [PubMed] [Google Scholar]
- Liebler, D.C. (1993). The role of metabolism in the antioxidant function of vitamin E. Crit. Rev. Toxicol. 23, 147–169. [DOI] [PubMed] [Google Scholar]
- Liebler, D.C. (1998). Antioxidant chemistry of alpha-tocopherol in biological systems. Roles of redox cycles and metabolism. Subcell. Biochem. 30, 301–317. [DOI] [PubMed] [Google Scholar]
- Liebler, D.C., and Burr, J.A. (2000). Antioxidant reactions of alpha-tocopherolhydroquinone. Lipids 35, 1045–1047. [DOI] [PubMed] [Google Scholar]
- Mansfield, S.G., and Briarty, L.G. (1992). Cotyledon cell-development in Arabidopsis thaliana during reserve deposition. Can. J. Bot. 70, 151–164. [Google Scholar]
- Matsui, K., Wilkinson, J., Hiatt, B., Knauf, V., and Kajiwara, T. (1999). Molecular cloning and expression of Arabidopsis fatty acid hydroperoxide lyase. Plant Cell Physiol. 40, 477–481. [DOI] [PubMed] [Google Scholar]
- McDonald, M.B. (1999). Seed deterioration: Physiology, repair and assessment. Seed Sci. Technol. 27, 177–237. [Google Scholar]
- Miquel, M., and Browse, J. (1992). Arabidopsis mutants deficient in polyunsaturated fatty acid synthesis. Biochemical and genetic characterization of a plant oleoyl-phosphatidylcholine desaturase. J. Biol. Chem. 267, 1502–1509. [PubMed] [Google Scholar]
- Munne-Bosch, S., and Alegre, L. (2002). The function of tocopherols and tocotrienols in plants. Crit. Rev. Plant Sci. 21, 31–57. [Google Scholar]
- Nobata, Y., Urakaze, M., Temaru, R., Sato, A., Nakamura, N., Yamazaki, K., Kishida, M., Takata, M., and Kobayashi, M. (2002). Alpha-tocopherol inhibits IL-8 synthesis induced by thrombin and high glucose in endothelial cells. Horm. Metab. Res. 34, 49–54. [DOI] [PubMed] [Google Scholar]
- Perl-Treves, R., and Perl, A. (2002). Oxaditive stress: An introduction. In Oxidative Stress in Plants, D. Inzé and M. Van Montagu, eds (London, New York: Taylor & Francis), pp. 1–32.
- Porfirova, S., Bergmuller, E., Tropf, S., Lemke, R., and Dormann, P. (2002). Isolation of an Arabidopsis mutant lacking vitamin E and identification of a cyclase essential for all tocopherol biosynthesis. Proc. Natl. Acad. Sci. USA 99, 12495–12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter, N.A., Caldwell, S.E., and Mills, K.A. (1995). Mechanisms of free-radical oxidation of unsaturated lipids. Lipids 30, 277–290. [DOI] [PubMed] [Google Scholar]
- Priestley, D.A., and Leopold, A.C. (1979). Absence of lipid oxidation during accelerated aging of soybean seeds. Plant Physiol. 63, 726–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestley, D.A., McBride, M.B., and Leopold, C. (1980). Tocopherol and organic free-radical levels in soybean seeds during natural and accelerated aging. Plant Physiol. 66, 715–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher, L.M., Miao, L., Sinha, N., and Lucas, W.J. (2001). Sucrose export defective1 encodes a novel protein implicated in chloroplast-to-nucleus signaling. Plant Cell 13, 1127–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciarelli, R., Zingg, J.M., and Azzi, A. (2001). Vitamin E 80th anniversary: A double life, not only fighting radicals. IUBMB Life 52, 71–76. [DOI] [PubMed] [Google Scholar]
- Sattler, S.E., Cahoon, E.B., Coughlan, S.J., and DellaPenna, D. (2003). Characterization of tocopherol cyclases from higher plants and cyanobacteria. Evolutionary implications for tocopherol synthesis and function. Plant Physiol. 132, 2184–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savidge, B., Weiss, J.D., Wong, Y.H., Lassner, M.W., Mitsky, T.A., Shewmaker, C.K., Post-Beittenmiller, D., and Valentin, H.E. (2002). Isolation and characterization of homogentisate phytyltransferase genes from Synechocystis sp. PCC 6803 and Arabidopsis. Plant Physiol. 129, 321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen, C.K., Khanna, S., Roy, S., and Packer, L. (2000). Molecular basis of vitamin E action. Tocotrienol potently inhibits glutamate-induced pp60(c-Src) kinase activation and death of HT4 neuronal cells. J. Biol. Chem. 275, 13049–13055. [DOI] [PubMed] [Google Scholar]
- Senaratna, T., Gusse, J.F., and McKersie, B.D. (1988). Age-induced changes in cellular membranes of imbibed soybean seed axes. Physiol. Plant 73, 85–91. [Google Scholar]
- Sheppard, A.J., Pennington, J.A.T., and Weihrauch, J.L. (1993). Analysis and distribution of vitamin E in vegetable oils and foods. In Vitamin E in Health and Disease, L. Packer and J. Fuchs, eds (New York: Marcel Dekker), pp. 9–31.
- Shintani, D., and DellaPenna, D. (1998). Elevating the vitamin E content of plants through metabolic engineering. Science 282, 2098–2100. [DOI] [PubMed] [Google Scholar]
- Shintani, D.K., Cheng, Z., and DellaPenna, D. (2002). The role of 2-methyl-6-phytylbenzoquinone methyltransferase in determining tocopherol composition in Synechocystis sp. PCC6803. FEBS Lett. 511, 1–5. [DOI] [PubMed] [Google Scholar]
- Simontacchi, M., Caro, A., Fraga, C.G., and Puntarulo, S. (1993). Oxidative stress affects alpha-tocopherol content in soybean embryonic axes upon imbibition and following germination. Plant Physiol. 103, 949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll, J., Douce, R., and Schultz, G. (1980). Site of biosynthesis of alpha-tocopherol in spinach chloroplasts. FEBS Lett. 112, 243–246. [Google Scholar]
- Soll, J., Schultz, G., Joyard, J., Douce, R., and Block, M.A. (1985). Localization and synthesis of prenylquinones in isolated outer and inner envelope membranes from spinach chloroplasts. Arch. Biochem. Biophys. 238, 290–299. [DOI] [PubMed] [Google Scholar]
- Stintzi, A., Weber, H., Reymond, P., Browse, J., and Farmer, E.E. (2001). Plant defense in the absence of jasmonic acid: The role of cyclopentenones. Proc. Natl. Acad. Sci. USA 98, 12837–12842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, N.L., Day, D.A., and Millar, A.H. (2002). Environmental stress causes oxidative damage to plant mitochondria leading to inhibition of glycine decarboxylase. J. Biol. Chem. 277, 42663–42668. [DOI] [PubMed] [Google Scholar]
- Thoma, I., Loeffler, C., Sinha, A.K., Gupta, M., Krischke, M., Steffan, B., Roitsch, T., and Mueller, M.J. (2003). Cyclopentenone isoprostanes induced by reactive oxygen species trigger defense gene activation and phytoalexin accumulation in plants. Plant J. 34, 363–375. [DOI] [PubMed] [Google Scholar]
- Tian, L., Magallanes-Lundback, M., Musetti, V., and DellaPenna, D. (2003). Functional analysis of beta- and epsilon-ring carotenoid hydroxylases in Arabidopsis. Plant Cell 15, 1320–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsegaye, Y., Shintani, D.K., and DellaPenna, D. (2002). Overexpression of the enzyme p-hydroxyphenolpyruvate dioxygenase in Arabidopsis and its relation to tocopherol biosynthesis. Plant Physiol. Biochem. 40, 913–920. [Google Scholar]
- Vollenweider, S., Weber, H., Stolz, S., Chetelat, A., and Farmer, E.E. (2000). Fatty acid ketodienes and fatty acid ketotrienes: Michael addition acceptors that accumulate in wounded and diseased Arabidopsis leaves. Plant J. 24, 467–476. [DOI] [PubMed] [Google Scholar]
- Wang, X., and Quinn, P.J. (2000). The location and function of vitamin E in membranes (review). Mol. Membr. Biol. 17, 143–156. [DOI] [PubMed] [Google Scholar]
- Weber, H., Chetelat, A., Caldelari, D., and Farmer, E.E. (1999). Divinyl ether fatty acid synthesis in late blight-diseased potato leaves. Plant Cell 11, 485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, D.O., and McDonald, M.B. (1986). The lipid peroxidation model of seed aging. Seed Sci. Technol. 14, 269–300. [Google Scholar]
- Wu, Z.C., Robinson, D.S., Domoney, C., and Casey, R. (1995). High-performance liquid-chromatographic analysis of the products of linoleic-acid oxidation catalyzed by pea (Pisum sativum) seed lipoxygenases. J. Agric. Food Chem. 43, 337–342. [Google Scholar]
- Yamauchi, J., Iwamoto, T., Kida, S., Masushige, S., Yamada, K., and Esashi, T. (2001). Tocopherol-associated protein is a ligand-dependent transcriptional activator. Biochem. Biophys. Res. Commun. 285, 295–299. [DOI] [PubMed] [Google Scholar]
- Yamauchi, R., and Matsushita, S. (1976). Quantitative changes in tocopherols and their intracellular distribution in cotyledons accompanying with soybean germination. J. Agric. Chem. Soc. Jpn. 50, 525–529. [Google Scholar]