Abstract
Mechanical ventilation remains the cornerstone in the management of severe acute respiratory failure. Acute respiratory distress syndrome (ARDS) is the most common cause of respiratory failure. It is associated with substantial mortality, and unmanageable refractory hypoxemia remains the most feared clinical possibility. If hypoxemia persists despite application of lung protective ventilation, additional therapies including inhaled vasodilators, prone positioning, recruitment maneuvers, high-frequency oscillatory ventilation, neuromuscular blockade (NMB), and extracorporeal membrane oxygenation may be needed. NMB and prone ventilation are modalities that have been clearly linked to reduced mortality in ARDS. Rescue therapies pose a clinical challenge requiring a precarious balance of risks and benefits, as well as, in-depth knowledge of therapeutic limitations.
Keywords: Acute respiratory distress syndrome, Extracorporeal membrane oxygenation, Prone ventilation, Refractory hypoxemia
INTRODUCTION
Refractory hypoxemia is an infrequent but a well-established emergency in Intensive Care Unit (ICU). There is no standard definition of this condition so far. Physiologically, it has been defined as increase in partial pressure of oxygen in arterial blood (PaO2) of <5 mmHg if fraction of inspired oxygen (FiO2) is increased by 0.1. For practical purposes, various criteria have been applied. Most commonly it is considered as either (1) PaO2 ≤60 mmHg or (2) PaO2/FiO2 ≤100 on FiO2 of 0.8-1.0 with a positive end-expiratory pressure (PEEP) of >30 cm H2O with plateau pressure >30 cm H2O.[1,2]
Mortality associated with refractory hypoxemia remains unacceptably high. Clinically, it is encountered in patients with acute respiratory distress syndrome (ARDS). ARDS is a clinical syndrome characterized by severe hypoxemia, bilateral infiltrates on chest radiograph, and reduced pulmonary compliance [Figure 1]. ARDS has been reclassified as per “Berlin definition” according to PaO2/FiO2 ratio for a moderate PEEP as mild, moderate, or severe ARDS [Table 1].[3] Berlin definition has been found to have a better prediction for death as compared to previous American-European Consensus Conference definition of ARDS.
Figure 1.
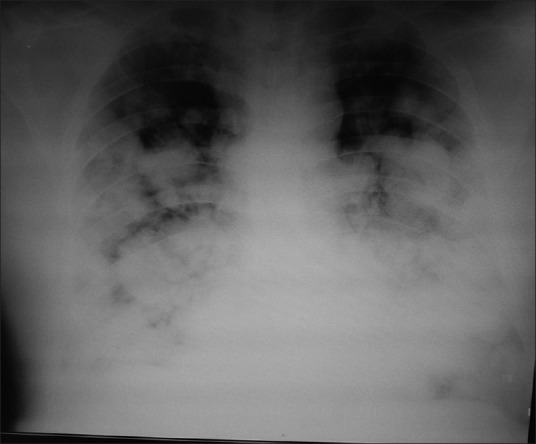
Chest radiograph of acute respiratory distress syndrome
Table 1.
Berlin definition of acute respiratory distress syndrome
| Within 1st week of known clinical insult or new or worsening respiratory symptoms |
| Bilateral opacities on chest imaging not fully explained by effusions, lobar/lung collapse, or nodules |
| Respiratory failure not explained by cardiac failure or fluid overload |
| Need objective assessment such as echocardiography to exclude hydrostatic edema if no risk factor present |
| Impaired oxygenation |
| Mild: 200< PaO2/FiO2 <300 with PEEP or CPAP ≥5 cm H2O |
| Moderate: 100< PaO2/FiO2 <200 with PEEP ≥5 cm H2O |
| Severe: PaO2/FiO2 <100 with PEEP ≥5 cm H2O |
PaO2: Partial pressure of oxygen in arterial blood, FiO2: Fraction of inspired oxygen, PEEP: Positive end-expiratory pressure, CPAP: Continuous positive airway pressure
Other than this, oxygenation index (OI) has been found to be useful in patients with ARDS. OI incorporates both severities of hypoxemia (PaO2/FiO2) and means airway pressure (MAP) into a single variable.
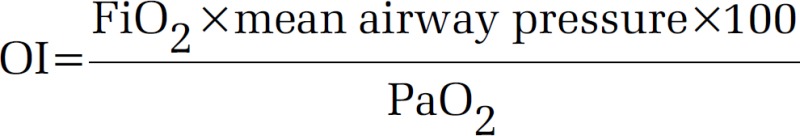
A high OI in ARDS has been found to independently predict mortality. An OI >30 usually indicates refractory hypoxemia.[4]
Protective lung strategy also known as “open lung approach (OLA)” is the standard of care for the management of patients with ARDS. It is a combination of low tidal volume ventilation (LTVV) strategy and application of PEEP above lower inflection point. LTVV is also regarded as lung protective ventilation. It relies on the fact that low tidal volumes result in lesser alveolar over distension. Alveolar over distension has been considered as the principal cause of ventilator-associated lung injury. Collective evidence supports the use of LTVV in ARDS based primarily on the multicenter ARMA trial and a meta-analysis of six randomized controlled trials (RCTs). The LTVV group in both were associated with a low mortality rate (31% vs. 40%, 34.2% vs. 41%, respectively). LTVV frequently results in alveolar hypoventilation leading to permissive hypercapnia. LTVV also induces shear injury due to repetitive opening and closing of alveoli with each breath. To overcome this drawback, PEEP should be applied above lower inflection point to prevent cyclic atelectasis. So far no universally accepted method of applying PEEP has been established. Different RCTs have used different approaches. Applying the highest PEEP limiting the plateau pressure to 28–30 cm of H2O seems like a reasonable approach.[5,6,7] “OLA” strategy is sufficient to oxygenate the majority of patients with ARDS. A select minority of ARDS patients, however, continues to have profound hypoxemia and may require the one of the rescue strategies for optimal oxygenation and ventilation.
Sometimes, in such patients increasing the I: E ratio by prolonging the inspiratory time results in improvement in oxygenation. This is also known as inverse ratio ventilation (IRV) if inspiratory time surpasses the expiratory time.[8] It can be directly achieved during pressure limited ventilation. IRV, however, may result in air trapping, auto-PEEP, hemodynamic compromise, and barotrauma. It also requires significant sedation or neuromuscular blockade (NMB).
Before using the rescue therapies, it is also imperative, however, not to overlook simple measures such as the use of adequate sedation and analgesia to ensure patient-ventilator synchrony and appropriate fluid management. These may prove to be beneficial in some subset of patients.
NEUROMUSCULAR BLOCKADE
NMB has been suggested as an adjunctive therapy for refractory hypoxemia due to three potential benefits. Spontaneously, breathing patients with ARDS usually have a high respiratory drive thereby generating larger than targeted tidal volumes per breath that predispose patients to risk of volutrauma and biotrauma. NMB eliminates muscle activity thereby resulting in decreased oxygen consumption, and improved patient-ventilator synchrony, as was seen in ACURASYS trial. In this RCT, 340 patients of severe ARDS receiving mechanical ventilation were randomized to either cisatracurium infusion or placebo groups within the first 48 h. On regression analysis, an improved 90 days survival rate was found in cisatracurium group after adjustment for severity of ARDS (hazard ratio 0.68; 95% confidence interval [CI], 0.48–0.98, P = 0.04). A recent meta-analysis has also shown the use of NMB in early ARDS to be associated with improved mortality rate (relative risk 0.66, 95% CI, 0.50–0.87). NMBs should, however, be used judiciously as paralysis interferes with neurological assessment and has been found to be associated with critical illness polyneuropathy and posttraumatic stress disorder when compared to patients receiving sedation alone.[9,10] It is necessary to limit their usage to shortest possible time.
RECRUITMENT MANEUVERS
Recruitment maneuver (RM) is the process of transiently increasing transpulmonary pressure with the aim of recruiting the collapsed alveoli. Generally, it is defined as, intermittent or sustained, application of pressure higher than that applied for a normal breath for a short period of time (usually up to 40 s). “Sigh” and “sustained inflation” are the two most commonly used RMs. While using Sigh method tidal volume or PEEP is increased to a prespecified plateau pressure for one or few breaths per minute. In the sustained inflation method, airways are pressurized to a specific level and maintained for a specified duration, for example, application of 40 cm of H2O airway pressure for 40 s. Other RMs are intermittent PEEP increase and pressure control with PEEP. Most of the studies on RMs have studied the effect on physiological outcome such as oxygenation and not on clinical outcomes. Moreover, despite a convincing theoretical advantage, none of the RCTs and meta-analysis have shown any survival benefit or reduced length of ICU stay. Moreover, the improvement in oxygenation has also been observed to be short lived, and efforts have been made to overcome this with decremental PEEP trial. Significant alveolar overdistension does not occur with a single RM but may occur if RMs are done frequently. Various undesirable effects such as hemodynamic instability, barotrauma, arrhythmias, and patient-ventilator asynchrony have been found to occur. Therefore, RMs are best applied when the patient is hemodynamically stable, adequately sedated with peak recruiting pressures <50 cm of water during the maneuver. It is necessary to be vigilant during RM, with immediate termination if hemodynamic instability develops.[11,12,13] At present, there is insufficient evidence to support their use in patients with ARDS. Use of RMs may have to be individualized as patients with ARDS are a heterogeneous population.[14]
PRONE VENTILATION
Delivering mechanical ventilation with patient in prone position is defined as prone ventilation. Prone ventilation has gained popularity in recent times following the latest multicenter Proning Severe ARDS patients (PROSEVA) study.[15] PROSEVA was an RCT where 466 patients with severe ARDS (PaO2/FiO2 ratio <150 mmHg with FiO2 ≥0.6 and PEEP ≥5 cm of water) were subjected to either prone ventilation for at least 16 h or were made to continue in the supine position after 12-24 h of initiation of conventional mechanical ventilation. The study was carried out at centers which were routinely performing prone ventilation for several years. Prone ventilation was associated with a major decrease in 28 days and 90 days mortality (16% vs. 33%; P < 0.001 and 29% vs. 41%; P < 0.001, respectively), increased ventilator-free days (14 vs. 10 days) and reduced time to extubation. Subsequent meta-analysis has also made similar observations. Improvement in oxygenation with prone ventilation is multifactorial. The proposed mechanisms are change in regional diaphragm motions, increased functional and residual capacity, better ventilation-perfusion matching, better secretion clearance, reduced ventilator-induced lung injury (VILI), and anterior shift of the mediastinal structures in prone position. During prone ventilation, response is difficult to predict, and may be related to the cause of ARDS (pulmonary vs. extra pulmonary), stage of ARDS (early vs. late), severity of hypoxemia, and the patient's body habitus.[16] Responders usually continue to have improved oxygenation for hours once they are shifted back to supine position. An increase in PaO2 by 10 mmHg over the first 30 min of prone ventilation usually predicts a sustained increase in PaO2 with prone ventilation. Few investigators have observed that prone ventilation is helpful in the presence of elevated intra-abdominal pressures, and in those patients, whose chest compliance reduces in the prone position.[17,18,19] These findings have not been validated yet. Indications, contraindications, and complications of prone ventilation are ellicited in Table 2.
Table 2.
Prone ventilation-contraindications and complications
| Absolute Contraindications |
| Spinal instability |
| Shock (mean arterial pressure <65 mmHg) |
| Severe traumatic injuries (unstable fractures) |
| Pregnancy |
| Tracheal surgery within 2 weeks |
| Hemorrhagic shock |
| Massive hemoptysis |
| Elevated intracranial pressure |
| Relative Contraindications |
| Anterior chest tubes with air leaks |
| Recent pacemaker |
| Severe burns |
| Major abdominal surgery |
| Deep venous thrombosis treated for <2 days |
| Lung transplant recipients |
| Complications |
| Pressure ulcers |
| Unplanned extubation |
| Loss of vascular access |
| Nerve compression |
| Retinal damage |
| Crush injury |
Various authors have recommended early (up to 36 h after intubation), high dose prone ventilation (for 12–18 consecutive h/day) as a rescue strategy in patients with severe hypoxemia. Safety considerations have to be taken into account during the process of prone ventilation. It is a labor-intensive procedure requiring a coordinated effort and minimum of three staffs. Special attention has to be paid to keep all the lines and tubes secured during the pruning process. “Log Roll” method should be used. Some commercial devices are available to assist the movement, including a bed, which can help both in initiating and maintaining the prone position. “Rotoprone” is a commercially available bed that facilitates prone positioning and also provides some continuous rotation if desired. Head of the bed should be elevated to 30–45° once the patient settles in the prone position. This helps in minimizing facial and ocular edema and improves gastric emptying. Cautious enteral feeding with proper positioning, adequate tracheal cuff inflation, close monitoring, and judicious use of prokinetic agents is recommended these days.
No additional monitoring is required in prone ventilation, although the need for endotracheal suctioning should be regularly assessed. Paralytic agents have not been found to be essentially required and may be actually harmful in exacerbating the supradiaphragmatic alveolar collapse.
Recently, investigators have also reported successful prone ventilation in nonintubated patients with hypoxemic respiratory failure.[20]
HIGH-FREQUENCY OSCILLATORY VENTILATION
High-frequency oscillatory ventilation (HFOV) is a type of ventilation combining high respiratory rate (3–15 Hz, >900 breaths/min) and tidal volumes smaller than even the anatomical dead space volume, through the endotracheal tube [Figure 2]. An oscillatory pump is required for this purpose.[21,22,23] It basically maintains a constant distending MAP around which the oscillations take place. This strategy results in homogenous distribution of ventilation by maintaining a high MAP, reduces the risk of VILI and hyperinflation. Tracheal gas insufflation may also be tried during HFOV as it may improve gas exchange. HFOV should be avoided in obstructive airways disease. HFOV can result in hemodynamic compromise, and frequent need for sedation and NMB to abolish patient-ventilator asynchrony.[24,25]
Figure 2.
High-frequency ventilator
Two recent RCTs “Oscillation in ARDS (OSCAR),” “OSCILLATE” and a recent meta-analysis failed to show any mortality benefit with HFOV. The OSCILLATE trial was conducted in 39 ICUs in five countries.[26] Patients were randomized to either HFOV or an ARDSnet ventilation strategy. This study was terminated early because of higher in-hospital mortality in HFOV group versus the control group (47% vs. 35% P = 0.005). HFOV group was also observed to have more need for sedatives, paralytics, and vasopressors. The OSCAR trial was conducted in the United Kingdom. Eight hundred patients were enrolled for the study.[27] This study also failed to demonstrate a survival benefit though no harm was observed with HFOV. Currently, it is not recommended as an initial strategy for ARDS. Many have put this failure secondary to delay in resorting to this technique once ARDSnet strategy has failed.
Efforts have been made to study the effect of HFOV in combination with other interventions such as inhaled nitric oxide (INO) or RM. It has also been found to have a beneficial role in maintaining oxygenation when the patient is returned to supine position after a session of prone ventilation. All these combinations are yet to show any clinical benefit. Another potential use of HFOV can be in severe ARDS with bronchopleural fistula.[28]
More research is required for optimal utilization of HFOV as this modality definitely has a sound theoretical basis.
AIRWAY PRESSURE RELEASE VENTILATION
Airway pressure release ventilation (APRV) is primarily a combination of pressure controlled ventilation and IRV. It is a time triggered, pressure targeted, and time cycled mode of ventilation. It requires setting up of two pressures. Higher pressure is maintained during inspiration which accounts for about 80–95% of the respiratory cycle time. Lower pressure is maintained during the shortened expiratory time. The patient is allowed to breathe spontaneously at both pressures, i.e. full breathing cycle. It has also been found to have a lung protective effect as it ventilates the patient on the steep or advantageous portion of the pressure-volume curve, if properly adjusted.[29,30,31] Spontaneous ventilation precludes the need for sedatives and vasopressors, ensures homogenous distribution of ventilation, and augments cardiac filling. In APRV, MAP increases without much increase in peak pressures. In addition, the lower pressure during expiration helps hemodynamic stability. It mimics IRV in a paralyzed patient. This mode has been found to be associated with shorter ICU stay and duration of ventilation in various studies in ARDS patients. However, a recent RCT of APRV was abandoned for futility. So far there is no evidence to suggest mortality benefit with APRV in ARDS. Hence, current evidence does not support the use of APRV, and further studies are required to establish its role in ARDS.[32,33,34]
INHALED PULMONARY VASODILATORS
Inhaled pulmonary vasodilators cause selective pulmonary vasodilatation in well-ventilated lung units resulting in better ventilation-perfusion matching and improved oxygenation in ARDS patients [Figures 3 and 4]. INO and prostacyclins are commonly used for this purpose. Inhaled vasodilators have short half-lives and therefore are associated with lesser systemic side effects such as hypotension. INO is costly, gets rapidly inactivated by hemoglobin, requires specialized equipment, and can result in methemoglobinemia (usually above 40 ppm, dose range - 2–80 ppm). INO increases the risk of renal failure and usually results in rebound pulmonary hypertension if withdrawn too quickly.[35,36,37,38] INO has been observed to result in transient improvement in oxygenation without any effect on survival or ventilator-free days. Response has been observed to be better among patients without sepsis or septic shock than in patients with septic shock. It has been suggested that significant improvement in oxygenation within the 1st h of initiation of INO should be present to justify its continued use, particularly keeping in mind its cost.
Figure 3.
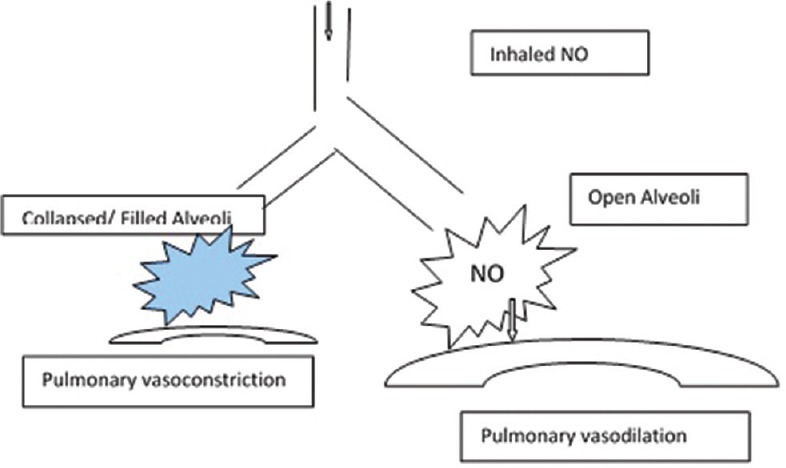
Inhaled nitric oxide is able to reach the well-ventilated alveoli resulting in vasodilation
Figure 4.
Inhaled nitric oxide being given to a patient on mechanical ventilation
Another alternative therapy is inhaled prostacyclin. Prostaglandin I-2 (epoprostenol) is the most commonly used prostacyclin. It is less costly as compared to INO and does not require specialized equipment. Head to head comparisons of these two agents have shown equivocal results.[39] Currently, these can be used only as temporizing measures to a more definitive intervention such as extracorporeal membrane oxygenation (ECMO) in ARDS patients.
EXTRACORPOREAL MEMBRANE OXYGENATION
ECMO is a technique in which blood is removed from the patient and made to pass through an artificial lung and returned to the patient again. This technology has been used successfully in neonatal or pediatric respiratory failure. There were, however, conflicting results observed in adult population until the conventional ventilatory support versus ECMO for severe adult respiratory failure (CESAR) trial. In this largest RCT on ECMO, patients with severe hypoxemia were randomized to either standard ventilatory strategy and other rescue modalities at their original hospital or transferred to a center highly skilled in performing ECMO. This trial reported survival at 6 months or the absence of severe disability in 63% of ECMO patients versus 47% of the standard therapy patients (P = 0.003).This study has been criticized for various reasons. Patients in the control group were ventilated at different centers with a nonstandardized protocols, conventional ventilation, or high-frequency ventilation, and about 30% of patients did not receive lung protective ventilatory strategy. All ECMO patients were treated in the same center, and many of the patients assigned to ECMO group actually did not receive ECMO. No data about ventilatory parameters during the study have been presented. These observations may indicate toward a mortality benefit related more to a specialized center versus a regional center, and not convincingly to a clear benefit of ECMO.
Noah et al. published data on the use of ECMO in patients with severe H1N1 influenza-related ARDS in 2011. They reported that patients referral and management to an ECMO center had a lower mortality rate when compared to those managed without ECMO (23.7% vs. 52.5%, respectively). On the other hand, Pham et al. also published their data of severe H1N1 ARDS patients treated with ECMO in France from 2009 to 2011. There was no difference in ICU mortality upon propensity method (1:1) cohort analysis between the ECMO and the non-ECMO group (50% vs. 40%).[40,41,42,43,44]
Despite these contradictory findings, ECMO remains an important tool for managing life-threatening hypoxemia. It is usually used as a last resort in severe ARDS, and, therefore, its all the more essential to initiate ECMO timely in the course of the disease [Figure 5]. It should be used in refractory hypoxemia due to a potential reversible cause, <7 days of present stay on mechanical ventilation, age <65 years, no significant comorbidities, no contraindication to anticoagulation, and no significant neurological dysfunction.
Figure 5.
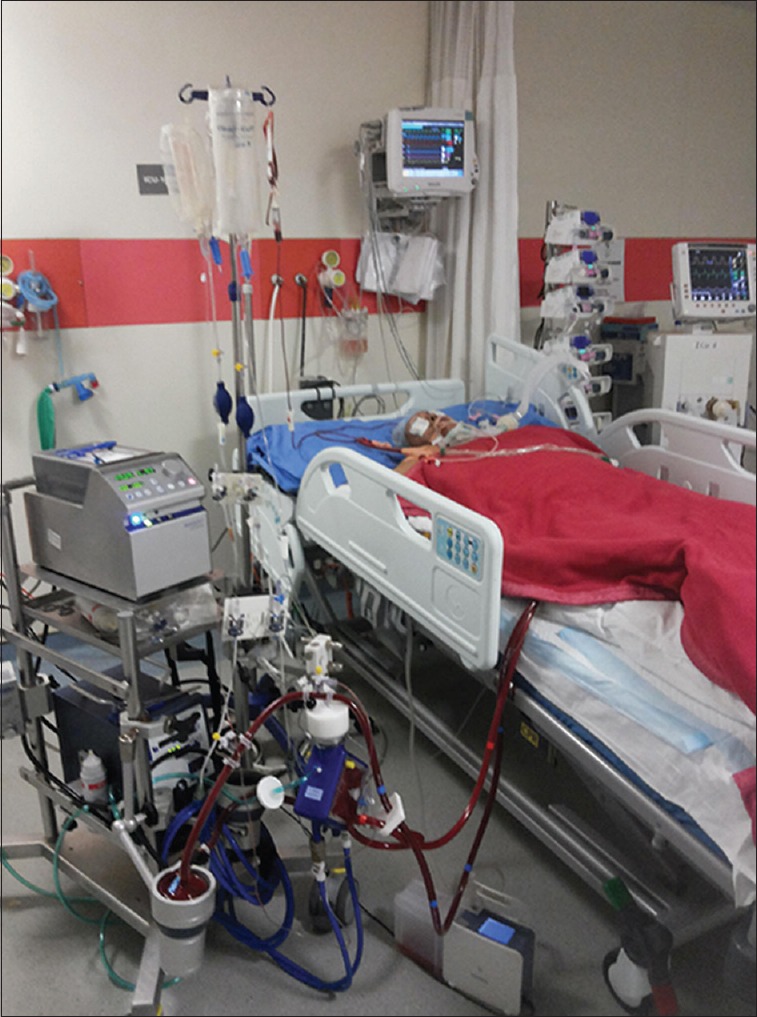
Patient on extracorporeal membrane oxygenation
Resource availability, infrastructure, expertise, cost, and serious complications [Table 3] remain the major limitations to ECMO use in ARDS patients.
Table 3.
Extracorporeal membrane oxygenation related complications
| Bleeding - in 30-40% of patients |
| Thromboembolism |
| Cannulation related: Vessel perforation, arterial dissection, distal ischemia |
| Heparin-induced thrombocytopenia |
| VA ECMO-related |
| Pulmonary hemorrhage |
| Cardiac thrombosis |
| Coronary or cerebral hypoxia |
ECMO: Extracorporeal membrane oxygenation, VA: Venoarterial
It is a useful strategy as it provides oxygenation and ventilation, minimizes barotrauma and allows complete bed rest. In case of isolated respiratory failure, it can be accomplished by venovenous approach. It can be continued until adequate lung function recovery. In case of associated hemodynamic instability venoarterial, ECMO should be used. It definitely has a role in young adult patients with single organ failure due to a reversible lung pathology. However, more evidence is needed before it can be widely adopted as a routine standard of care.[45]
Extracorporeal CO2 removal is a variant of conventional ECMO, where the circuit pump is eliminated. Patient's native cardiac output serves the purpose of the pump in this pumpless arteriovenous extracorporeal lung assist. It enables a more protective ventilatory strategy where the gas exchange occurs across the circuit membrane by diffusion. It, however, cannot provide full extracorporeal oxygenation. It can only be used for severe hypercarbia in the presence of adequate oxygenation and relative hemodynamic stability.[46,47]
CONCLUSIONS
Refractory hypoxemia is encountered in clinical practice in ARDS patients. “Open lung strategy” of low tidal volume with high PEEP and permissive hypercapnia is successful in the majority of ARDS patients. Life-threatening hypoxemia usually accounts for <15% of mortality in ARDS. Aggressive interventions are needed to manage critical hypoxia and minimize organ failure. First line therapies are inhaled vasodilators, prone positioning, RM, and HFOV. ECMO is usually considered as second-line rescue therapy once the first line rescue therapies fail. Although these interventions look appealing, convincing evidence is lacking to warrant their routine use except for prone ventilation. There are sound physiological reasons for each of the therapies but well-designed, adequately powered RCTs demonstrating their efficacy are lacking. Benefits and risks of each modality, clinician familiarity, local expertise, resource availability, and cost considerations have to be taken into account before resorting to these therapies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Villar J, Kacmarek RM. Rescue strategies for refractory hypoxemia: A critical appraisal. Medicine Reports. 2009;1:91–4. doi: 10.3410/M1-91. doi:10.3410/M1-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esan A, Hess DR, Raoof S, George L, Sessler CN. HYPERLINK “/article.aspx%3FarticleID=1086443” Severe HypoxemicRespiratory Failure: Part - 1- Ventilatory Strategies. Chest. 2010;137:1203–16. doi: 10.1378/chest.09-2415. doi:10.1378/chest.09-2415. [DOI] [PubMed] [Google Scholar]
- 3.Modrykamien AM, Gupta P. The acute respiratory distress syndrome. Proc (Bayl Univ Med Cent) 2015;28:163–71. doi: 10.1080/08998280.2015.11929219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seeley E, McAuley DF, Eisner M, Miletin M, Matthay MA, Kallet RH. Predictors of mortality in acute lung injury during the era of lung protective ventilation. Thorax. 2008;63:994–8. doi: 10.1136/thx.2007.093658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: A randomized controlled trial. JAMA. 2008;299:646–55. doi: 10.1001/jama.299.6.646. [DOI] [PubMed] [Google Scholar]
- 6.Brower RG, Matthay MA, Morris A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The acute respiratory distress syndrome network. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 7.Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–36. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 8.Esan A, Hess DR, Raoof S, George L, Sessler CN. Severe hypoxemic respiratory failure: Part 1 – Ventilatory strategies. Chest. 2010;137:1203–16. doi: 10.1378/chest.09-2415. [DOI] [PubMed] [Google Scholar]
- 9.Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–16. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 10.Artigas A, Bernard GR, Carlet J, Dreyfuss D, Gattinoni L, Hudson L, et al. The American-European consensus conference on ARDS, part 2.Ventilatory, pharmacologic, supportive therapy, study design strategies and issues related to recovery and remodeling. Intensive Care Med. 1998;24:378–98. doi: 10.1007/s001340050585. [DOI] [PubMed] [Google Scholar]
- 11.Lapinsky SE, Aubin M, Mehta S, Boiteau P, Slutsky AS. Safety and efficacy of a sustained inflation for alveolar recruitment in adults with respiratory failure. Intensive Care Med. 1999;25:1297–301. doi: 10.1007/s001340051061. [DOI] [PubMed] [Google Scholar]
- 12.Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: A randomized controlled trial. JAMA. 2008;299:637–45. doi: 10.1001/jama.299.6.637. [DOI] [PubMed] [Google Scholar]
- 13.Fan E, Wilcox ME, Brower RG, Stewart TE, Mehta S, Lapinsky SE, et al. Recruitment maneuvers for acute lung injury: A systematic review. Am J Respir Crit Care Med. 2008;178:1156–63. doi: 10.1164/rccm.200802-335OC. [DOI] [PubMed] [Google Scholar]
- 14.Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354:1775–86. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- 15.Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–68. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 16.Messerole E, Peine P, Wittkopp S, Marini JJ, Albert RK. The pragmatics of prone positioning. Am J Respir Crit Care Med. 2002;165:1359–63. doi: 10.1164/rccm.2107005. [DOI] [PubMed] [Google Scholar]
- 17.Mure M, Glenny RW, Domino KB, Hlastala MP. Pulmonary gas exchange improves in the prone position with abdominal distension. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1785–90. doi: 10.1164/ajrccm.157.6.9711104. [DOI] [PubMed] [Google Scholar]
- 18.Pelosi P, Tubiolo D, Mascheroni D, Vicardi P, Crotti S, Valenza F, et al. Effects of the prone position on respiratory mechanics and gas exchange during acute lung injury. Am J Respir Crit Care Med. 1998;157:387–93. doi: 10.1164/ajrccm.157.2.97-04023. [DOI] [PubMed] [Google Scholar]
- 19.Firodiya M, Mehta Y, Juneja R, Trehan N. Mechanical ventilation in the prone position: A strategy for acute respiratory failure after cardiac surgery. Indian Heart J. 2001;53:83–6. [PubMed] [Google Scholar]
- 20.Scaravilli V, Grasselli G, Castagna L, Zanella A, Isgrò S, Lucchini A, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: A retrospective study. J Crit Care. 2015;30:1390–4. doi: 10.1016/j.jcrc.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Chan K, Stewart T. Clinical use of high-frequency oscillatory ventilation in adult patients with acute respiratory distress syndrome. Crit Care Med. 2005;33(3 Suppl):S170–4. doi: 10.1097/01.ccm.0000155915.97462.80. [DOI] [PubMed] [Google Scholar]
- 22.Fessler H, Derdak S, Ferguson N, Hager D, Kacmarek R, Thompson T, et al. A protocol for high-frequency oscillatory ventilation inadults: Results from a roundtable discussion. Crit Care Med. 2007;35:1649–54. doi: 10.1097/01.CCM.0000269026.40739.2E. [DOI] [PubMed] [Google Scholar]
- 23.van Heerde M, Roubik K, Kopelent V, Plötz FB, Markhorst DG. Unloading work of breathing during high-frequency oscillatory ventilation: A bench study. Crit Care. 2006;10:R103. doi: 10.1186/cc4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pillow JJ. Tidal volume, recruitment and compliance in HFOV: Same principles, different frequency. Eur Respir J. 2012;40:291–3. doi: 10.1183/09031936.00020012. [DOI] [PubMed] [Google Scholar]
- 25.Pillow JJ. High-frequency oscillatory ventilation: Mechanisms of gas exchange and lung mechanics. Crit Care Med. 2005;33(3 Suppl):S135–41. doi: 10.1097/01.ccm.0000155789.52984.b7. [DOI] [PubMed] [Google Scholar]
- 26.Ferguson ND, Cook DJ, Guyatt GH, Mehta S, Hand L, Austin P, et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368:795–805. doi: 10.1056/NEJMoa1215554. [DOI] [PubMed] [Google Scholar]
- 27.Young D, Lamb SE, Shah S, MacKenzie I, Tunnicliffe W, Lall R, et al. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med. 2013;368:806–13. doi: 10.1056/NEJMoa1215716. [DOI] [PubMed] [Google Scholar]
- 28.Demory D, Michelet P, Arnal JM, Donati S, Forel JM, Gainnier M, et al. High-frequency oscillatory ventilation following prone positioning prevents a further impairment in oxygenation. Crit Care Med. 2007;35:106–11. doi: 10.1097/01.CCM.0000251128.60336.FE. [DOI] [PubMed] [Google Scholar]
- 29.Liu LL, Aldrich JM, Shimabukuro DW, Sullivan KR, Taylor JM, Thornton KC, et al. Special article: Rescue therapies for acute hypoxemic respiratory failure. Anesth Analg. 2010;111:693–702. doi: 10.1213/ANE.0b013e3181e9c356. [DOI] [PubMed] [Google Scholar]
- 30.Neumann P, Golisch W, Strohmeyer A, Buscher H, Burchardi H, Sydow M. Influence of different release times on spontaneous breathing pattern during airway pressure release ventilation. Intensive Care Med. 2002;28:1742–9. doi: 10.1007/s00134-002-1522-0. [DOI] [PubMed] [Google Scholar]
- 31.Putensen C, Wrigge H. Clinical review: Biphasic positive airway pressure and airway pressure release ventilation. Crit Care. 2004;8:492–7. doi: 10.1186/cc2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sydow M, Burchardi H, Ephraim E, Zielmann S, Crozier TA. Long-term effects of two different ventilatory modes on oxygenation in acute lung injury. Comparison of airway pressure release ventilation and volume-controlled inverse ratio ventilation. Am J Respir Crit Care Med. 1994;149:1550–6. doi: 10.1164/ajrccm.149.6.8004312. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan LJ, Bailey H, Formosa V. Airway pressure release ventilation increases cardiac performance in patients with acute lung injury/adult respiratory distress syndrome. Crit Care. 2001;5:221–6. doi: 10.1186/cc1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Räsänen J, Cane RD, Downs JB, Hurst JM, Jousela IT, Kirby RR, et al. Airway pressure release ventilation during acute lung injury: A prospective multicenter trial. Crit Care Med. 1991;19:1234–41. doi: 10.1097/00003246-199110000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Griffiths MJ, Evans TW. Inhaled nitric oxide therapy in adults. N Engl J Med. 2005;353:2683–95. doi: 10.1056/NEJMra051884. [DOI] [PubMed] [Google Scholar]
- 36.Taylor RW, Zimmerman JL, Dellinger RP, Straube RC, Criner GJ, Davis K, Jr, et al. Low-dose inhaled nitric oxide in patients with acute lung injury: A randomized controlled trial. JAMA. 2004;291:1603–9. doi: 10.1001/jama.291.13.1603. [DOI] [PubMed] [Google Scholar]
- 37.Adhikari NK, Burns KE, Friedrich JO, Granton JT, Cook DJ, Meade MO. Effect of nitric oxide on oxygenation and mortality in acute lung injury: Systematic review and meta-analysis. BMJ. 2007;334:779. doi: 10.1136/bmj.39139.716794.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Makker R, Mehta Y, Trehan N, Bapna R. Therapeutic application of inhaled nitric oxide in adult cardiac surgical patients. Indian Heart J. 2006;58:432–6. [PubMed] [Google Scholar]
- 39.Walmrath D, Schneider T, Schermuly R, Olschewski H, Grimminger F, Seeger W. Direct comparison of inhaled nitric oxide and aerosolized prostacyclin in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;153:991–6. doi: 10.1164/ajrccm.153.3.8630585. [DOI] [PubMed] [Google Scholar]
- 40.Pham T, Combes A, Roze H, Chevret S, Mercat A, Roch A, et al. For the REVA Research Network. Extracorporeal membrane oxygenation for pandemic influenza A (H1N1) – Induced acute respiratory distress syndrome. A cohort study and propensity matched analysis. Am J Respir Crit Care Med. 2013;187:276–85. doi: 10.1164/rccm.201205-0815OC. [DOI] [PubMed] [Google Scholar]
- 41.Patroniti N, Zangrillo A, Pappalardo F, Peris A, Cianchi G, Braschi A, et al. The Italian ECMO network experience during the 2009 influenza A (H1N1) pandemic: Preparation for severe respiratory emergency outbreaks. Intensive Care Med. 2011;37:1447–57. doi: 10.1007/s00134-011-2301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet. 2009;374:1351–63. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 43.Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A (H1N1) JAMA. 2011;306:1659–68. doi: 10.1001/jama.2011.1471. [DOI] [PubMed] [Google Scholar]
- 44.Howard C, Ong P, Thachuthara J, Charlie L, Guntupalli KK. Acute respiratory syndrome. In: Mehta Y, Sharma J, Gupta MK, editors. Text Book of Critical Care. Delhi: Jaypee Brothers; 2015. pp. 334–43. [Google Scholar]
- 45.Betit P. Extracorporeal membrane oxygenation: Quo vadis? Respir Care. 2009;54:948–57. doi: 10.4187/002013209793800402. [DOI] [PubMed] [Google Scholar]
- 46.Meyer A, Strüber M, Fischer S. Advances in extracorporeal ventilation. Anesthesiol Clin. 2008;26:381–91. doi: 10.1016/j.anclin.2008.01.006. viii. [DOI] [PubMed] [Google Scholar]
- 47.Lobaz S, Carey M. Rescue of acute refractory hypercapnia and acidosis secondary to life-threatening asthma with extracorporeal carbon dioxide removal (ECCO2R) JICS. 2011;12:140–2. [Google Scholar]


