Abstract
Extracorporeal membrane oxygenation (ECMO) for severe acute respiratory failure was proposed more than 40 years ago. Despite the publication of the ARDSNet study and adoption of lung protective ventilation, the mortality for acute respiratory failure due to acute respiratory distress syndrome has continued to remain high. This technology has evolved over the past couple of decades and has been noted to be safe and successful, especially during the worldwide H1N1 influenza pandemic with good survival rates. The primary indications for ECMO in acute respiratory failure include severe refractory hypoxemic and hypercarbic respiratory failure in spite of maximum lung protective ventilatory support. Various triage criteria have been described and published. Contraindications exist when application of ECMO may be futile or technically impossible. Knowledge and appreciation of the circuit, cannulae, and the physiology of gas exchange with ECMO are necessary to ensure lung rest, efficiency of oxygenation, and ventilation as well as troubleshooting problems. Anticoagulation is a major concern with ECMO, and the evidence is evolving with respect to diagnostic testing and use of anticoagulants. Clinical management of the patient includes comprehensive critical care addressing sedation and neurologic issues, ensuring lung recruitment, diuresis, early enteral nutrition, treatment and surveillance of infections, and multisystem organ support. Newer technology that delinks oxygenation and ventilation by extracorporeal carbon dioxide removal may lead to ultra-lung protective ventilation, avoidance of endotracheal intubation in some situations, and ambulatory therapies as a bridge to lung transplantation. Risks, complications, and long-term outcomes and resources need to be considered and weighed in before widespread application. Ethical challenges are a reality and a multidisciplinary approach that should be adopted for every case in consideration.
Keywords: Acute kidney injury, Acute respiratory failure, Anticoagulation, Mechanical ventilator, Postextracorporeal membrane oxygenation syndrome, Sepsis, Triage, Venovenous extracorporeal membrane oxygenation
INTRODUCTION
Despite the publication of the ARDSNet study[1] and adoption of lung protective ventilation, the mortality for acute respiratory failure due to acute lung injury and acute respiratory distress syndrome (ALI/ARDS) has continued to remain high ~47.8% as per the ALIEN study which assessed incidence of ARDS postlung protective ventilation.[2] For arterial oxygen/inspired oxygen ratios (PaO2/FiO2) <100, hospital and intensive care unit mortality has ranged from 50% to 80%.[3] ARDS is often the first step in the common, final pathway to the cascade of multiorgan dysfunction syndrome and death.[4] Beyond lung protective ventilation, other strategies that have shown some positive evidence includes use of neuromuscular blockade[5] and prone positioning;[6] however, these strategies are not always successful, and extracorporeal means of gas exchange may be necessary for refractory hypoxemic/hypercarbic respiratory failure.[7,8] Use of extracorporeal membrane oxygenation (ECMO) for severe acute respiratory failure was proposed more than 40 years ago.[9,10] This technology has evolved over the past couple of decades and has been noted to be safe and successful, especially during the H1N1 influenza epidemic with good survival rates.
VENOVENOUS EXTRACORPOREAL MEMBRANE OXYGENATION - EVIDENCE FROM LANDMARK TRIALS
There have been three major prospective trials in the last 40 years using ECMO in severe acute respiratory failure. The first one sponsored by the National Institutes of Health by Zapol et al. in 1979 using venoarterial (VA) mode showed no improvement in survival, but no lung protective strategy was applied.[11] The patients had prolonged mechanical ventilation before initiation of ECMO, and there was little or no prior ECMO experience in the centers.
The Morris trial in 1994 compared pressure control-inverse ratio ventilation with extracorporeal carbon dioxide (CO2) removal (ECCO2 R) through ECMO.[12] No significant difference in survival was noted in the two groups. There was no substantial lung rest with peak inspiratory pressures reaching 50 cm of H2O. The CESAR trial published in 2009 randomized 180 adult patients with severe reversible respiratory failure to conventional management versus referral to an ECMO center for consideration of ECMO.[13] A 16% absolute reduction in the primary endpoint of death or severe disability in the ECMO-referred group was noted.[13] The lack of a standardized ventilator management protocol in the control group was among its criticisms.[14] The extracorporeal life support organization (ELSO) registry was created at the University of Michigan which recorded data from its member institutions and reported survival to discharge of 55% in a study.[15] As of July 2015, the numbers from the ELSO registry indicate survival to discharge of 58% for acute respiratory failure.[16] In an adult trauma cohort, 64.7% survival was noted in adult trauma patients with acute hypoxemic respiratory failure.[17] The Australian and New Zealand network reported 75% survival to discharge in suspected H1N1-associated ARDS treated with ECMO.[18] The Italian ECMO network had survival of 78% in patients receiving ECMO within 7 days from the onset of mechanical ventilation.[19] Suffice it to say that as technology improves, selection criteria become more specific, the argument for use of ECMO in severe acute respiratory failure increases.
VENOVENOUS EXTRACORPOREAL MEMBRANE OXYGENATION INDICATIONS
The primary indications for venovenous (VV)-ECMO in acute respiratory failure include:
Hypoxemic respiratory failure
Hypercarbic respiratory failure
Respiratory failure in lung transplant
Bronchopleural fistulas and pulmonary air leaks
Complex airway management.
HYPOXIC AND HYPERCARBIC RESPIRATORY FAILURE - TRIAGE CRITERIA
Various triage criteria have been described and published. Overall, there should be potentially reversible cause of acute respiratory failure with significant bilateral lung injury and associated inability to oxygenate and ventilate despite protective, lung ventilation. The P/F ratios (partial pressure of arterial O2/FiO2 = Fraction of inspired O2) and the Murray score[20] have been used. Although not widely prevalent, it consists of the following criteria:
The ELSO criteria[16] for consideration of VV-ECMO in hypoxic respiratory failure include the following:
Consider for PaO2/FiO2 ratios <150 on FiO2 >90% and Murray score 2–3
Indicated for PaO2/FiO2 <100 on FiO2 >90% and Murray score 3–4 despite optimal care for 6 h or more.
Brodie and Bacchetta[7] targeted P/F ratios of <80 with high positive end-expiratory pressure (PEEP) (15–20) as an indication for consideration of ECMO. Other criteria include hypercarbia with high plateau pressures >30 cmH2O and a pH of 7.15, air-leak syndromes, lung transplant patient, and immediate respiratory collapse (massive pulmonary embolism [PE], airway, etc.). The French EOLIA trial (ClinicalTrials.gov; Identifier: NCT0147070) criteria include patients with P/F ratio of <50 with FiO2 >80% for >3 h or P/F <80 for 6 h. pH <7.25 for >6 h despite optimum mechanical ventilation is also included as criteria.
As far as contraindications are concerned, most experienced centers and authors report futility if the patient has been ventilated on high settings for >7 days. Additional concerns include limited vascular access and irreversible conditions such as metastatic cancer, brain injury, contraindication to use of anticoagulant therapy, inability to receive blood products, high body mass index >45, and major immunosuppression. Although not a definitive contraindication, the outcomes and risks have been described to be worse with increasing age. Septic shock used to be a contraindication for VV-ECMO. There is a growing opinion that this may not be an absolute contraindication with ECMO centers reporting successful outcomes in those with septic shock. In addition, patients on vasopressors/inotropes have also been reported to improve with weaning of vasoactive drugs subsequent to ECMO initiation, giving credence to the belief that the need for them is due to right ventricular dysfunction and biotrauma as a result of injurious ventilator settings. The risk-benefit ratio of ECMO in patients who are on vasopressors should be considered on a case-by-case basis. Despite technological advancement, complications do occur with ECMO therapy, notably, bleeding, infection, and mechanical complications. Increased use of ECMO, with its associated needs for training expertise and resources, may also lead to increase in hospital costs. Prognostic scores can provide guidance to allow institutions to appropriately allocate resources and benchmark mortality outcomes. The respiratory extracorporeal membrane oxygenation survival prediction (RESP) score was developed from patients who have received ECMO for severe acute respiratory failure and comprises 2355 patients from multiple countries over a 13-year period.[21] This large population has allowed creation of a well-calibrated and discriminatory survival tool comprising 12 pre-ECMO variables (RESP score; http://www.respscore.com). Several other predictive mortality risk models are currently available. The ECMOnet score, published by the Italian network in 2012, was developed on 60 patients with influenza A (H1N1)-associated ARDS and was secondarily validated on a cohort of 74 influenza A (H1N1) international patients.[22] Recently, the Predicting dEath for SEvere ARDS on VV-ECMO score was constructed from 140 ECMO-treated patients with ARDS admitted to 3 French intensive care units.[23] These tools need further validation and may provide value to the bedside provider when considering risk-benefit ratio of ECMO in severe acute respiratory failure.
VENOVENOUS EXTRACORPOREAL MEMBRANE OXYGENATION AS BRIDGE TO LUNG TRANSPLANTATION
Patients with chronic respiratory failure in need of lung transplantation used to be ineligible for a lung transplant if they deteriorated to require mechanical ventilation or ECMO,[24,25] but several centers have indicated good outcomes with early ECMO reducing the complications associated with mechanical ventilation in this cohort.[26,27] ECMO can replace mechanical ventilation and reduce the problems of ventilator-associated complications.[7] Ability to transition to ECMO early enables aggressive physical therapy and pulmonary rehabilitation before lung transplant.[28,29] It should be noted that there has to be reasonable functional status prior to acute decompensation for consideration of ECMO. In addition, there is increased risk of primary graft dysfunction and need for ECMO postoperatively in these patients as well. The shorter duration of pretransplant ECMO will lead to better overall outcomes. ECMO has also been used postlung transplant in patients for primary graft dysfunction. Five percent of lung transplant patients need ECMO postoperatively.[30] This is considered for Grade III primary graft dysfunction with a P/F ratio <200 and X-ray signs of diffuse infiltrates, especially when the FiO2 is >60% and peak pressures are >35.[30]
VENOVENOUS EXTRACORPOREAL MEMBRANE OXYGENATION FOR OTHER CAUSES OF SEVERE ACUTE RESPIRATORY FAILURE
The other less common causes of need for VV-ECMO include status asthmaticus and hypercarbic respiratory failure, severe air leak syndrome, and complex airway procedures. Patients with acute pulmonary hypertension and right heart failure decompensate rapidly into cardiogenic shock and multiorgan failure. Supporting the right ventricle by VA-ECMO has been used in these patients as a bridge to recovery or transplant. Similarly, patients with massive PE either due to venous clots or amniotic fluid/air embolism may require VA-ECMO to support their hemodynamics during the acute decompensation phase. VA-ECMO is outside the scope of this review. Severe air-leak syndromes such as bronchopleural fistulas may benefit from VV-ECMO by allowing reduction of peak and mean airway pressure with improved ability of healing or endoscopic/surgical repair.
VV-ECMO has been used to provide gas exchange during tracheal resection in patients.[31,32] Successful use in patients with status asthmaticus has also been demonstrated.[33,34] Use in patients with diffuse alveolar hemorrhage attributed to vasculitis, collagen vascular disease, and other causes has been successful.[35,36] With newer circuits and oxygenators, the need for anticoagulation is low; therefore, ECMO can be considered in this population with high bleeding risk.
CANNULATION TECHNIQUES FOR VENOVENOUS EXTRACORPOREAL MEMBRANE OXYGENATION
With VV-ECMO, blood entering the ECMO circuit is typically drained from the superior vena cava (SVC), inferior vena cava (IVC), and/or a large vein such as the femoral vein. Blood is then typically returned to the patient in or near the right atrium. Blood drainage and return can be accomplished using a single, dual-lumen cannula, or multiple cannulas. It is generally accepted that the largest cannula that can be inserted safely should be used to maximize the degree of ECMO support. Standard VV-ECMO configurations include femoral-femoral, femoro-internal jugular, and bicaval access can be accomplished by a single dual-lumen catheter technique as described below.
A single, dual-lumen cannula such as the Avalon Elite® (Maquet) (MAQUET Holding B.V. & Co. KG Rastatt Germany) is inserted into the right internal jugular, and the tip advanced past the right atrium and into the IVC [Figure 1]. The size of these cannulae in adults range from 16 to 31 Fr and have 3 blood access ports - 2 for drainage and 1 for return. The drainage ports rest in the IVC and SVC, while the return (i.e., outflow) port rests in the right atrium, between the drainage ports. Importantly, the return port is directional (i.e., blood exits only medially) and theoretically points towards the tricuspid valve. This directional outflow was designed to minimize “recirculation,” the phenomenon by which blood from the outflow is inadvertently sent back to the venous ports of the cannula instead of towards the tricuspid and into the right ventricle. It is recommended that this cannula be placed under fluoroscopy and/or transesophageal echocardiography to ensure the proper direction of blood outflow towards the tricuspid valve. A malpositioned dual-lumen cannula that is rotated, so the outflow is pointed away from the tricuspid valve can result in significant recirculation and significantly affect the degree of support offered to the patient by the ECMO circuit.
Figure 1.
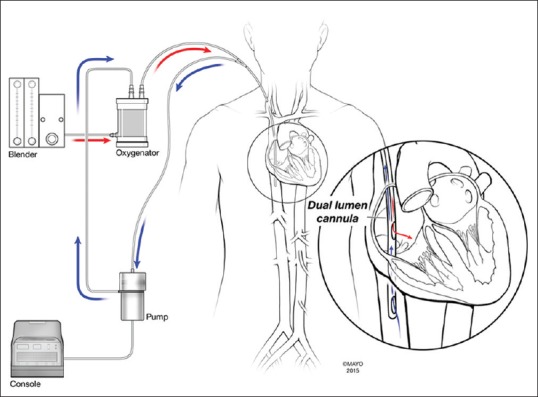
Basic schema of the dual-lumen access and extracorporeal membrane oxygenation circuit
The most common multi-cannula approach uses a long drainage cannula inserted peripherally into the femoral vein and advanced to the retrohepatic IVC. The return (ECMO outflow) cannula may then be placed in the right internal jugular and advanced to the SVC [Figure 2]. The return cannula may also be placed peripherally and run alongside the drainage cannula, but advanced beyond the tip of the drainage cannula to minimize recirculation.
Figure 2.
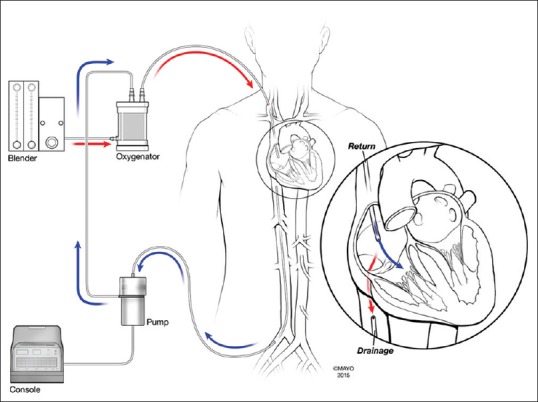
Basic schema of a femorojugular access and extracorporeal membrane oxygenation circuit
EXTRACORPOREAL MEMBRANE OXYGENATION CIRCUITS
ECMO circuits and components have become much simpler in recent years, resulting in safer circuits with less anticoagulation requirements. A typical, modern circuit consists of the following path of blood flow, with only minor accessories: Venous cannula → venous line → blood pump → oxygenator → outflow (i.e., “arterial” or oxygenated) line → outflow (or return) cannula. The most modern circuits, such as the Cardiohelp system (Maquet© (MAQUET Holding B.V. & Co. KG Rastatt Germany)), offer even, simple, highly integrated circuits. Most modern adult ECMO circuits use centrifugal blood pumps as opposed to roller pumps. These pumps consist of polycarbonate housing with a magnetically rotated impeller that uses centrifugal force to propel blood. The disposable centrifugal pump is attached to a motor which is controlled by a small console. The newest generation (and most expensive) centrifugal pump is the CentriMag® (Thoratec) blood pump (St. Jude Medical, California, USA) in which the impeller is also magnetically levitated so that there are no bearings and no mechanical interaction between the housing and the impeller. This design results in no friction between surfaces, and theoretically less heat generation and less hemolysis.
The newest generation is the hollow fiber polymethylpentene (PMP) oxygenator (Quadrox D from Maquet), being the most common in the United States. This oxygenator has a low prime (mL), high flow, high gas exchange, and high rated flow. The Medos Hilite® (Medos Medizintechnik AG Stolberg Germany) is another PMP oxygenator that has recently entered the market.
A typical adult ECMO circuit consists of the aforementioned blood pump and oxygenator, connected to each other and to the cannulas using 3/8" diameter PVC tubing which may be biological (heparin-based) or synthetic. While patients on ECMO do still typically require anticoagulation while on long-term ECMO, these coatings and simplified circuits have led clinicians to safely withhold pharmacological anticoagulation when necessary.
PHYSIOLOGY OF LUNG SUPPORT ON VENOVENOUS EXTRACORPOREAL MEMBRANE OXYGENATION AND ESSENTIALS OF PATIENT MANAGEMENT
Despite the evolution in circuit design and enhanced experience over recent years, management of the ECMO patient can remain complex and requires a good understanding of the underlying physiology. The primary rationale for initiating VV extracorporeal life support stems from the need to improve systemic oxygen delivery (DO2). Normally, oxygen content in the blood is determined by hemoglobin concentration, hemoglobin oxygen saturation, and dissolved oxygen, with delivery controlled by cardiac output.[37,38]
During VV-ECMO, when no effective gas exchange occurs within the native lungs, oxygenation of the blood is fully dependent on the ECMO system and its interaction with the patient's native physiology. On VV-ECMO, hemoglobin concentration and native cardiac output continue to play a major role in DO2; however, arterial oxygen saturation (SaO2) is now dependent on extracorporeal blood flow (ECBF) and gas exchange across the membrane oxygenator (MO).[39]
Membrane oxygenator oxygen transfer
Two chambers make up the MO, in which gas flows within the hollow PMP fibers and blood on the outside, allowing for diffusion of oxygen and CO2 along this semi-permeable membrane.[40] Due to the nonmicroporous composition of these fibers, liquid should not diffuse across, allowing for extended durability of these oxygenators compared to older ones.[41] The “sweep” gas circulated through the MO is a combination of oxygen and ambient air that is combined and controlled with a “blender.” Blood oxygenation within the MO is reliant on the hemoglobin concentration, thickness of the blood film, blood flow rate through the MO, oxygen saturation of the inflow blood, MO membrane surface area, and diffusibility through the hollow microfibers.[42,43,44]
Extracorporeal blood flow
Because VV-ECMO is a partial bypass system, the fully saturated ECMO outflow blood mixes with the native venous blood to comprise the patient's SaO2.[40] ECBF and recirculation of oxygenated blood within the ECMO circuit are major determinants of arterial oxygen saturation. To a variable degree, any native pulmonary function, oxygen extraction within the lungs, and coronary venous blood return also contribute.[45,46] The optimal circuit flow in adults is usually 50–100 mL/kg/min, which in a centrifugal pump system is both preload and afterload dependent.[38,40,47]
The main limiting factor to ECBF is frequent drainage through the inflow cannula. Size, positioning, and the number of drainage holes in the inflow cannula have a great impact on resistance to blood flow, which increases directly with length and inversely with the fourth power of the radius.[40,48] Reduced flow related to insufficient preload or drainage is frequently seen with an increase in negative pressure (<−120 mmHg) from the inflow line. This can present in the setting of reduced venous volume or compliance, cannula obstruction, or compression of the inflow circuit. Further or sudden “suctioning” around the cannula can cause “chatter” along the tubing.[40,49,50] While intravascular depletion is the most common reason for this, coughing, cannula malposition, tube kinking, cardiac tamponade, or pneumothoraces may also be a cause.[40] Increased negative pressures, even for a very short time, may produce high shear forces and subsequent cavitation, cellular destruction, and hemolysis.[51]
Reactions to neutralize reduced drainage may include infusion of volume, ensuring circuit patency, and temporarily reducing revolutions per minute on the pump.[49] If sufficient blood flow cannot be obtained despite preload optimization and cannula repositioning, a second drainage cannula may be placed.[40,52]
Centrifugal pumps are also afterload sensitive. In the ECMO circuit, afterload may be related to outflow cannula size and positioning, hydrostatic pressure in the venous system, and the pressure change across the MO.[49] The intrinsic blood flow resistance produced by any MO, especially the PMP oxygenator, is usually less than the critical threshold of 200 mmHg, and therefore infrequently significant. An acute rise in transmembrane pressure however may represent acute thrombosis.[49]
Recirculation
When flow is increased in an attempt to improve SaO2, a phenomenon called recirculation can occur. Like a functional shunt, this process describes recycling of oxygenated blood from the outflow cannula directly back into the inflow cannula. The fraction of blood recirculated is influenced by cannula configuration and positioning, intravascular volume status, pump flow rate, and native Q.[45,53,54] Recirculation reduces the fraction of oxygenated ECMO blood to native Q, thereby reducing SaO2. Manipulation of the Q remains an interesting concept, as the need for higher Q has been suggested to maintain forward flow and less recirculation.[53,54,55] At some threshold, however, the increase in Q will reduce the fraction of ECBF to Q enough to result in worsening hypoxia.
Measurement of recirculation has been studied using various techniques, like comparing PaO2 from the outflow cannula to PvO2 from the inflow cannula, use of dilutional ultrasound,[56,57] and other techniques studied include thermodilution, transthoracic echocardiography, and lithium dilution.[56,58,59,60]
Different cannulation configurations have shown varying degrees of recirculation as it has the distance between the inflow and outflow cannulas. When clinically significant, recirculation may be improved by adjusting cannula position, manipulating other factors to allow for decreased flow, adding a second drainage cannula, or using a well-placed dual-lumen cannula.[52,55,61]
Hemoglobin concentration
The optimal hemoglobin concentration in ECMO patients remains a topic of much controversy. Previous guidelines have suggested maintaining a normal hemoglobin and hematocrit; however, many hospitals practice a restrictive transfusion strategy.[42] The risks and benefits of both strategies must be weighed. Red cell transfusion in the critically ill may contribute to volume overload, an immunologic response, increased infections, ALI, and increased mortality with ARDS.[39,62] When considering a patient's DO2, hemoglobin concentration, however, does still play a considerable role. While adequate DO2 can be obtained in the anemic patient with sufficient ECBF, patients in whom increasing flow is not achievable may require a higher transfusion threshold to allow for adequate DO2 at lower blood flow rates.[39] A recent study, however, showed that a restrictive transfusion protocol did not result in an increased morbidity.[63]
Oxygen consumption
VV-ECMO is implemented and manipulated to improve DO2; however, when this remains insufficient despite best management of ECBF, attempts in reducing oxygen consumption (VO2) may be considered.[41,54] Temporizing measures such as increased sedation, paralysis, hypothermia, and even negative inotropy have been used in these situations.[40,64]
MONITORING
Oxygenation
While it is recommended to maintain a DO2/VO2 ratio of at least 3:1 on VV-ECMO, the complex interaction between patient and circuit physiology makes monitoring of oxygen delivery in these patients challenging.[54] Because there are numerous variables including venous oxygen saturation, ECBF to Q ratio, MO function, degree of recirculation, and hemoglobin which contribute to DO2 and VO2 on VV-ECMO, the SaO2 alone may be a poor indicator of oxygenation.[39] For this reason, a SaO2 >80% may frequently be considered sufficient.[47,54] Normally, the mixed venous hemoglobin saturation is a good reflection of the oxygen delivery and consumption ratio; however, on VV-ECMO, this will be inaccurate due to the mixing of oxygenated and de-oxygenated blood in the right ventricle.[48] The oxygen saturation of ECMO inflow blood from the vena cava should be comparable to a central venous sample; however, may also be inaccurate with any degree of recirculation.[40,48] Given these challenges, other markers of end-organ perfusion and signs of anaerobic metabolisms, such as elevated lactate, may be helpful.
Hemodynamics
Hemodynamic changes are noted pre- and post-ECMO initiation. Before ECMO, patients have high ventilator pressures, decreased venous return, high pulmonary vascular resistance, and poor perfusion with high systemic vascular resistance. Hypoxia may also lead to reduced contractility and need for inotropes. Once ECMO is commenced, pulmonary vascular resistance is reduced with a concomitant fall in peak ventilator pressures, venous return increases with improved perfusion, and systemic vascular resistance is reduced. Inotropes can be weaned with improved cardiac contractility. Hemodynamic monitoring may be challenging in these patients. Transthoracic or transesophageal echocardiography may provide the best assessment of biventricular function. Pulmonary artery catheterization is difficult after ECMO cannulas are placed and may not be of value. Thermodilution techniques for cardiac output may be difficult to interpret. Newer hemodynamic monitoring devices such as FloTrac™ and NICOM™ (Edwards life sciences, USA) are investigational in this population. Central venous pressure can be monitored with a central access/peripherally inserted central catheter line, but the trends are of value rather than an absolute number due to ECMO flows interfering with appropriateness of measurements. Physiologic markers such as blood pressure, urine output, and extremities examination are valuable data for functional hemodynamic monitoring.
Carbon dioxide removal
CO2 transfer through the MO is much more efficient than that of oxygen due to greater solubility and subsequently enhanced diffusion.[47] Elimination of CO2 is dependent on MO function and surface area as well as the PCO2 of the inflow blood and sweep gas flow.[48] A goal PCO2 can be easily manipulated by adjusting the sweep gas flow on the blender. Due to the high capacity for CO2 removal with modern MO's, the necessary blood flow rate to achieve normocarbia alone is only about 10–15 mL/kg/min.[45] This concept has prompted the development of low-flow extracorporeal CO2 removal systems. During initiation of VV-ECMO, the sweep gas flow is usually set at the same rate as the ECBF. This can then be titrated to optimal pH and PCO2 with repeat blood gas analysis.[40]
MECHANICAL VENTILATION CONSIDERATIONS DURING VENOVENOUS EXTRACORPOREAL MEMBRANE OXYGENATION
VV-ECMO requires that the patient has a functional cardiovascular system and can maintain cardiac output to facilitate gas transport to and from the tissues. Gas exchange is dependent on blood flow to and from the oxygenator membrane and oxygen carrying capacity of the hemoglobin. Mixing of the oxygenated infusion blood and the deoxygenated right atrial blood produces a SaO2 of about 80%, and improving lung function can increase the SaO2 even further. Manipulating vent settings are not advised to improve oxygenation. ECMO circuit flow rates and increasing hematocrit are more appropriate interventions.
Very little is known about the optimum ventilator settings while using VV-ECMO. Based on a combination of animal data, observational studies, and previous randomized trials, Schmidt et al.[65] defined four main objectives for mechanical ventilation with the main goal of promoting ultra-protective ventilation:
-
Limit alveolar strain
- Target a tidal volume of < 4 mL/kg predicted body weight or a peak pressure of 20–25 cmH2O
- Limit the respiratory rate (<10).
-
Limit atelectrauma
- Use a high level of PEEP (≥10 cmH2O).
-
Limit reabsorption atelectasis
- Use a low FiO2
- Use adequate PEEP.
-
Avoid overdistention
- Monitor transpulmonary pressure.
While the major goals of mechanical ventilation are well-defined, the details such as mode of ventilation, breath type, respiratory, PEEP, and FiO2 are still unknown. Marhong et al. conducted an international survey looking at the ventilator management practices of ELSO-registered centers.[66] Only 27% of centers reported having a mechanical ventilation protocol for ECMO patients. Sixty-two percent of centers reported using controlled ventilation while only 27% reported using a spontaneous mode. Pressure-controlled breathing is the most popular mode in the initial phase of ARDS with ECMO.[65] Advantages of using pressure-controlled ventilation are to limit alveolar strain and trend changes in lung compliance by watching the change in tidal volume as the lung recovers. Spontaneous ventilation is preferred to preserve respiratory muscle function, avoid ventilator-associated diaphragmatic dysfunction, and improving ventilation-perfusion mismatch.[65,67] Use of modes such as airway pressure release ventilation (APRV) and naturally adjusted ventilatory assist or settings to increase the I-time for recruitment to provide more time for spontaneous breathing have been described.[68] Patients in the ELSO registry,[16] CESAR trial,[13] and the REVA trial[69] were treated with a variety of variations on the theme of “lung rest.”[70] All the studies target FiO2 of 30–60%, PEEP ~10, respiratory rate 10–20 and volume control or pressure control mode with plateau pressure ~20–25. The EOLIA trial (ClinicalTrials.gov; Identifier: NCT01470703), currently recruiting patients, plans to use conventional low tidal volume settings to keep plateau pressure <20 and APRV with Phigh/Plow settings of 20/10.
High-frequency percussive ventilation (HFPV) can provide recruitment maneuvers in this patient cohort while maintaining lung protective ventilation and time on ECMO has been noted to have been reduced by the protocolized use of HFPV.[70] HFPV is delivered by the volume diffusive respirator and is different from other modes. It comprises of a time-regulated convective component and a superimposed sub-tidal volume percussive component of about 30 cc.[70] A unique sliding piston called a Phasitron® (Synchronics Electronics Pvt. Ltd. Electronic Engineering Solutions, India) provides percussive and convective components. This enables a combination of diffusive intrapulmonary gas mixing due to the percussive feature with an intermittent scheduled convective tidal exchange of variable length and profile.[70,71,72] Mobilization of secretions and alveolar recruitment ensues without causing over-distension in the injured lung. More research is needed to assess its role compared to pressure/volume control ventilation in patients with VV-ECMO.
Additional lung recruitment strategies including positional therapy, MetaNeb® therapy, diuresis, pleural drainage, and therapeutic bronchoscopy may lead to enhanced lung recovery. Inhaled nitric oxide as an adjunct therapy may be attempted in the severely hypoxemic patient, but only anecdotal data exists. Prone positioning may be considered in selected patients difficult to wean or remaining very hypoxemic despite VV-ECMO supports.[73]
Patients are evaluated daily for lung recovery, the timing of which is variable. Evidence of recovery includes a clearing X-ray, decreasing white blood counts, weaning of vasopressors, net negative fluid balance, and the evidence of intrinsic pulmonary function with improving oxygen saturation as the ECMO FiO2 is weaned. For now, we have good evidence to use an ultra-protective ventilation strategy and minimize lung damage.
TRACHEOSTOMY
Patients on VV-ECMO due to ARDS may benefit from early tracheostomy due to the prolonged ventilation that may be necessary.[74] Tracheostomy may enable less discomfort, improved oral care, and airway security. Reduced agitation and improved physical therapy and rehabilitation may also be possible with a tracheostomy. Use of anticoagulation should not be a contraindication to tracheostomy. Short interruptions are tolerated.[75]
ANTICOAGULATION
Anticoagulation is a major concern in patients due to inadequate (thrombotic) or excessive anticoagulation (hemorrhagic complications).[76] Blood-device interaction leads to widespread inflammatory and prothrombotic response. With ECMO initiation, consumptive coagulopathy and dilution of coagulation factors occur. Platelet activation and aggregation ensues and coagulation factors are consumed continuously, and fibrinolysis becomes a factor by day 5 of ECMO.[76] Thrombin is also generated which leads to clot formation in the circuit and microcirculation.
Heparin is the default anticoagulant for ECMO. Derived from bovine and porcine intestine and lung, heparin binds to antithrombin, and the complex leads to a 1000-time increase in antithrombin activity and free Xa and thrombin (less of VIIa, Xia, and IXa). Antithrombin III decreased with the institution of ECMO,[77] leading to a procoagulant state and decreased in heparin responsiveness.[76] Monitoring of anticoagulation can be done using different assays. Activated clotting time (ACT) is an inexpensive bedside whole blood assay that measures degree of anticoagulation. ACT values, although useful, are also affected by thrombocytopenia, platelet dysfunction or inhibition (e.g. GpIIb/IIIa inhibitors), hypothermia, antithrombin III level, patient age, hemodilution, hypofibrinogenemia, and oral anticoagulants.[76] In contrast to 400–480 as a target for cardiopulmonary bypass, 160–220 is the range acceptable for ECMO. ACT monitoring alone may not be adequate representation of anticoagulation monitoring for patients requiring ECMO. Activated partial thromboplastin time (aPTT) monitoring has been used and a target of 50–80 is often considered acceptable; however, the sensitivity of aPTT to unfractionated heparin is altered in the setting of inflammation, hemodilution, etc. Anti-Xa levels are often considered more reliable and a functional measure of heparin anticoagulation. Levels between 0.3 and 0.7 u/mL are considered adequate. Viscoelastic tests (thromboelastography [TEG®] or rotational thromboelastometry) can be helpful to diagnose an underlying hypo- or hyper-coagulable state that may suggest a bleeding or thrombotic tendency, respectively.[76] It should be noted that novel centrifugal pumps, PMP oxygenator, and heparin-coated circuits ensure less-clotting challenges. Large cannulas with higher flows also reduce risk of thrombosis.
NEUROLOGIC CONSIDERATIONS
Patients that require VV-ECMO for any amount of time are predisposed to not only neurologic injury but neurocognitive changes. The use of anticoagulation, prolonged neuromuscular blockade, and high-dose sedation are all risk factors for brain injury and neurocognitive changes after VV-ECMO. One recent study published in 2014 reported a rate of neurological complications (intracranial hemorrhage, ischemic stroke, seizure, and encephalopathy) as high as 60% in all comers of ECMO patients (n = 212).[78]
In a recent retrospective review of 23,950 patients on ECMO (either VA or VV), an incidence of 7.7% of stroke either hemorrhagic or ischemic was noted.[79] Patients with a hemorrhagic stroke had a 60% mortality rate compared with about 50% mortality rate in patients who suffered an ischemic stroke.[79] Identifying neurologic injuries while a patient is on VV-ECMO can be technically challenging. Other diagnostic modalities such as transcranial Doppler, near-infrared spectrometry, and electroencephalogram to help identify neurologic injuries are investigational at this time. ECMO is not an absolute contraindication for craniotomy or ventricular drain placement. In ischemic stroke, standard stroke treatments apply with careful consideration of intravenous tissue plasminogen activator (TPA) administration. Intraarterial TPA and local thrombectomy procedures may be more appropriate as they carry less bleeding risk.
A survey of intensivists’ sedation practices on VV-ECMO identified that only 44% of practitioners use a daily sedation vacation to assess neurologic status.[80] Confounding factors for sedation vacation involve mechanical complication concerns, hemodynamic instability, and refractory and unsafe hypoxia even on the VV-ECMO circuit. Yet, current guidelines do recommend daily sedation vacations in VV-ECMO patients to assess neurologic injuries. Additional challenges with drug metabolism include the circuit itself as well as the systemic inflammation that results from prolonged use of an extracorporeal circuit. Sequestration of drugs in the circuit, increased volume of distribution, and decreased clearance are the major pharmacokinetic changes associated with ECMO,[81] although further data are needed to characterize and model the kinetics and dynamics of drug effect with extracorporeal circulations. There is a significant increase in dose requirement for morphine and midazolam during ECMO.[82] Ketamine infusion can be used as an adjunctive sedative agent in patients receiving ECMO and may decrease concurrent sedative and/or opioid infusions without altering Richmond agitation-sedation scale. The hemodynamic effects of ketamine may provide the benefit of decreasing vasopressor requirements.[83]
NUTRITION/GASTROINTESTINAL MANAGEMENT
Supporting all organ systems is imperative with ECMO, including maintaining the integrity of the gastrointestinal (GI) tract mucosa. Proper prophylaxis with proton-pump inhibitors or histamine blockers, assuring normal perfusion, assuring adequate bowel care, and assuring nutrition are all measures that should be taken with each VV-ECMO patient. Despite adequate GI prophylaxis and nutrition, GI bleeding has been reported as high as 7% incidence and 8% have liver dysfunction. Mesenteric ischemia incidence is unknown, but yet a feared complication.[84]
Heparin anticoagulation should be reversed for at least 6 h before endoscopy. Furthermore, reversing coagulopathy via TEG appears to be most efficacious. Once bleeding is controlled, restarting anticoagulation within 24 h will decrease thromboembolic injuries that accompany VV-ECMO.[85] In the event of mesenteric ischemia or perforated viscous necessitating major surgical procedure, heparin should be held for 6 h if feasible.
Enteral nutrition was the most commonly used nutrition-delivery mode in ECMO patients but is frequently interrupted and associated with caloric and protein deficits.[86,87] Enteral tube feeding is generally better tolerated in VV-ECMO patients compared to VA-ECMO patients, due to the preservation of pulsatile flow. No standard tube feeding regimen has been identified, but other organ system dysfunction, use of propofol, and body habitus should be considered when deciding on the type of tube feed. Consultation with a nutritionist is imperative as part of a multidisciplinary team.
RENAL COMPLICATIONS
Acute kidney injury incidence may be as high as 72% and underestimated by current definitions employed in the ELSO registry.[88] Indication for renal replacement therapy (RRT) varies by center location.[88] Most patients who become dialysis dependent do so within 48 h of ECMO initiation.[89] Fluid overload is often the primary cause for RRT in the VV-ECMO population to hasten recovery from ARDS.
INFECTIONS/ANTIBIOTICS IN VENOVENOUS EXTRACORPOREAL MEMBRANE OXYGENATION
Infections are common with a reported incidence of 11.7% from the ELSO registry for a rate of 30.6/1000 ECMO days in adults.[90] Coagulase-negative staphylococci (15.9%) were the most common organisms cultured followed by species of Candida (12.7%), and Pseudomonas (10.5%). The probability of infections increases with the duration of support and the severity of critical illness before initiation of ECMO. Infections affect length of stay but do not have an impact on mortality.[90]
WEANING STRATEGY
Patients improving on VV-ECMO will have lactate clearance, usually developing a metabolic alkalosis, facilitate weaning of vasoactive drips, develop a negative fluid balance as well as reductions in white count, clearance of chest X-ray, and improvement in mental status. While institutions have different ECMO weaning strategies, the usual approach to weaning VV-ECMO support involves weaning ECMO FiO2 and sweep gas to maintain PaO2 to 50–80 when there is observed clearing of the chest X-ray and noted improvement in oxygen saturation due to contribution from the lung. Flows can be reduced to 2–3 L/min. Ventilator support should be increased simultaneously to lung protective settings (e.g. IP of 20–25/tidal volume of 6 mL/kg, PEEP of 5–10, and rate of ~15). A step often applied includes increasing FiO2 on the ventilator to 100%. If this is associated with O2 saturation's increase to 100%, it is called a positive Cilley study and is predictive of weaning ECMO successfully. Sweep is disconnected and ECMO FiO2 is dropped down to 21% and patient is decannulated. Weaning of ECMO flows may not be necessary in VV-ECMO, although if flows are 5–6 L/min, it may be prudent to drop it to the 2–3 L/min range to avoid hemodynamic perturbations such as right ventricular/left ventricular dysfunction. This can happen when the patient is suddenly switched off ECMO and the intravascular volume of distribution of blood changes acutely. Weaning to less than 2 L/min or so may lead to increased risk of circuit clots.
ETHICS AND FUTILITY
Significant conundrums exist if patients cannot be weaned from ECMO. Most studies have indicated the average duration of ECMO run to be 10–20 days from the H1N1 cohorts. However, longer duration of ECMO support has been reported anecdotally – 3–6 months long.[91] Lung transplantation has also been performed subsequent to severe refractory ARDS.[92] However, the feasibility of lung transplant evaluation and ethics of withdrawal of care is hotly debated. Ethical dilemmas might arise when patients sustained on ECMO are no longer eligible for transplantation (and thus might no longer be eligible for ECMO), especially if they continue to enjoy an acceptable quality of life.[93] Balancing resources, ethics, and addressing patient's goals of care need to be addressed in ways that are considered compassionate and fair.[93]
POSTEXTRACORPOREAL MEMBRANE OXYGENATION SYNDROME
Management of patients after decannulation needs to be carefully coordinated. Risk of fluid overload and right ventricular failure exists due to sudden change in the volume of distribution. PE from deep vein thrombosis (DVT) in the central vessels as a result of the large cannulas is a major concern. Anticoagulation may be necessary postdecannulation if DVT or PE occurs. Serial surveillance is recommended. Some authors recommend inserting an IVC filter before decannulation by guidewire exchange of the femoral venous ECMO cannula. Sepsis can occur postdecannulation and fever may manifest itself after loss of thermoregulation from the ECMO circuit. Long-term neurocognitive outcomes are challenging to deal with and will require physical and psychological rehabilitation.
A BRIEF INTRODUCTION TO EXTRACORPOREAL CARBON DIOXIDE REMOVAL
Failure to implement lung-protective ventilation may be one of the reasons mortality rates have not improved.[94,95,96] Health care providers report that high blood CO2 (hypercapnia), or its effects, were significant barriers to achieving lung protective ventilation.[97] Hypercapnia is a consequence of lung protective ventilation because reduced ventilation volumes prevent adequate pulmonary CO2 removal. Hypercapnia complicates 14–100% of cases depending on the severity.[98,99]
There is also growing literature that reduced ventilator-related lung injury may be achieved by ultra-protective lung ventilation (further lowering tidal volumes to 3–4 mL/kg) by reducing barotrauma, volutrauma, atelectrauma, and biotrauma. Terragni et al.[99] demonstrated with chest computed tomography that approximately one-third of patients with severe ARDS, ventilated with tidal volumes of 6 mL/kg of predicted body weight, had evidence of alveolar overdistension. Hager et al.[100] showed that a plateau pressure of 30 cmH2O in some patients may not be safe and suggested that the lower the plateau pressure, the lower the mortality rate. ECCO2 R is a technology that involves removal of blood from the patient, which is pumped through an artificial lung (oxygenator membrane) where CO2 is removed and subsequently the purified blood is returned to the patient.[101] ECCO2 R may benefit lung protective ventilation and ultra-protective lung ventilation by delinking CO2 removal from the mechanical ventilator strategy. ECCO2 R devices have been developed and literature on ECCO2 R strategies has seen proliferation. Zimmermann et al. used pumpless arteriovenous extracorporeal life support in 51 patients with ARDS characterized by ratios of partial pressure of arterial oxygen to fraction of inspired oxygen of 70-200 mmHg, and showed that tidal volumes and inspiratory pressures could be reduced with efficient CO2 removal and low rate of adverse events.[102] Another study used dual-lumen catheters and noted improved CO2 removal and enhanced lung protection with tidal volumes <6 mL/kg.[99] The Hemolung® is a bicaval cannula-based device that has been reported to benefit low-flow ECCO2 R and remove clinically useful levels of CO2.[103,104] Interventional lung assist devices have also been noted to be associated with high transplantation and survival rates.[105] Delinking oxygenation and ventilation by using ECCO2 R devices may benefit ultra-protective lung ventilation in ARDS and also may lead to avoiding endotracheal intubation in hypercapnic respiratory failure as may occur in cardiopulmonary disease, cystic fibrosis patients awaiting lung transplant, etc., Paracorporeal-assist lung devices are also being developed that would function similar to mechanical circulatory assist devices and perform the role of gas exchange as a rescue therapy, bridge to recovery or lung transplantation.[106]
CONCLUSIONS
Improvements in technology have led to the realization of dreams of incorporating extracorporeal gas exchange bypassing the lung in severe acute respiratory failure. Evidence is growing for the optimum timing, disease characteristics, and indications for ECMO in this cohort. ECMO should be considered for patients with life-threatening hypoxemia or hypercapnia refractory to conventional mechanical ventilation. Risks, complications, and long-term outcomes and resources need to be considered and weighed in before widespread application. Ethical challenges are a reality and a multidisciplinary approach that should be adopted for every case in consideration.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 2.Villar J, Blanco J, Añón JM, Santos-Bouza A, Blanch L, Ambrós A, et al. The ALIEN study: Incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med. 2011;37:1932–41. doi: 10.1007/s00134-011-2380-4. [DOI] [PubMed] [Google Scholar]
- 3.Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: A randomized controlled trial. JAMA. 2008;299:637–45. doi: 10.1001/jama.299.6.637. [DOI] [PubMed] [Google Scholar]
- 4.Koh Y. Update in acute respiratory distress syndrome. J Intensive Care. 2014;2:2. doi: 10.1186/2052-0492-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–16. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 6.Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–68. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 7.Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011;365:1905–14. doi: 10.1056/NEJMct1103720. [DOI] [PubMed] [Google Scholar]
- 8.Terragni P, Faggiano C, Ranieri VM. Extracorporeal membrane oxygenation in adult patients with acute respiratory distress syndrome. Curr Opin Crit Care. 2014;20:86–91. doi: 10.1097/MCC.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 9.Hill JD, O’Brien TG, Murray JJ, Dontigny L, Bramson ML, Osborn JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome).Use of the Bramson membrane lung. N Engl J Med. 1972;286:629–34. doi: 10.1056/NEJM197203232861204. [DOI] [PubMed] [Google Scholar]
- 10.Bartlett RH, Gazzaniga AB, Jefferies MR, Huxtable RF, Haiduc NJ, Fong SW. Extracorporeal membrane oxygenation (ECMO) cardiopulmonary support in infancy. Trans Am Soc Artif Intern Organs. 1976;22:80–93. [PubMed] [Google Scholar]
- 11.Zapol WM, Snider MT, Hill JD, Fallat RJ, Bartlett RH, Edmunds LH, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA. 1979;242:2193–6. doi: 10.1001/jama.242.20.2193. [DOI] [PubMed] [Google Scholar]
- 12.Morris AH, Wallace CJ, Menlove RL, Clemmer TP, Orme JF, Jr, Weaver LK. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO 2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149(2 Pt 1):295–305. doi: 10.1164/ajrccm.149.2.8306022. [DOI] [PubMed] [Google Scholar]
- 13.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet. 2009;374:1351–63. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 14.Wallace DJ, Milbrandt EB, Boujoukos A. Ave, CESAR, morituri te salutant! (Hail, CESAR, those who are about to die salute you!) Crit Care. 2010;14:308. doi: 10.1186/cc8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paden ML, Conrad SA, Rycus PT, Thiagarajan RR; ELSO Registry. Extracorporeal life support organization registry report 2012. ASAIO J. 2013;59:202–10. doi: 10.1097/MAT.0b013e3182904a52. [DOI] [PubMed] [Google Scholar]
- 16.Extracorporeal Life Support Organization. ECLS Registry Report. 2015. [Last accessed on 2015 Oct 15]. Available from: https://www.elso.org/Registry/Statistics/InternationalSummary.aspx .
- 17.Guirand DM, Okoye OT, Schmidt BS, Mansfield NJ, Aden JK, Martin RS, et al. Venovenous extracorporeal life support improves survival in adult trauma patients with acute hypoxemic respiratory failure: A multicenter retrospective cohort study. J Trauma Acute Care Surg. 2014;76:1275–81. doi: 10.1097/TA.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 18.Davies A, Jones D, Bailey M, Beca J, Bellomo R, et al. Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Extracorporeal membrane oxygenation for 2009 influenza A (H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–95. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 19.Patroniti N, Zangrillo A, Pappalardo F, Peris A, Cianchi G, Braschi A, et al. The Italian ECMO network experience during the 2009 influenza A (H1N1) pandemic: Preparation for severe respiratory emergency outbreaks. Intensive Care Med. 2011;37:1447–57. doi: 10.1007/s00134-011-2301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–3. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus PT, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med. 2014;189:1374–82. doi: 10.1164/rccm.201311-2023OC. [DOI] [PubMed] [Google Scholar]
- 22.Pappalardo F, Pieri M, Greco T, Patroniti N, Pesenti A, Arcadipane A, et al. Predicting mortality risk in patients undergoing venovenous ECMO for ARDS due to influenza A (H1N1) pneumonia: The ECMOnet score. Intensive Care Med. 2013;39:275–81. doi: 10.1007/s00134-012-2747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt M, Zogheib E, Rozé H, Repesse X, Lebreton G, Luyt CE, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med. 2013;39:1704–13. doi: 10.1007/s00134-013-3037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maurer JR, Frost AE, Estenne M, Higenbottam T, Glanville AR. International guidelines for the selection of lung transplant candidates. The International Society for Heart and Lung Transplantation, the American Thoracic Society, the American Society of Transplant Physicians, the European Respiratory Society. Transplantation. 1998;66:951–6. doi: 10.1097/00007890-199810150-00033. [DOI] [PubMed] [Google Scholar]
- 25.Mason DP, Thuita L, Nowicki ER, Murthy SC, Pettersson GB, Blackstone EH. Should lung transplantation be performed for patients on mechanical respiratory support? The US experience. J Thorac Cardiovasc Surg. 2010;139:765–73. doi: 10.1016/j.jtcvs.2009.09.031. e1. [DOI] [PubMed] [Google Scholar]
- 26.Toyoda Y, Bhama JK, Shigemura N, Zaldonis D, Pilewski J, Crespo M, et al. Efficacy of extracorporeal membrane oxygenation as a bridge to lung transplantation. J Thorac Cardiovasc Surg. 2013;145:1065–70. doi: 10.1016/j.jtcvs.2012.12.067. [DOI] [PubMed] [Google Scholar]
- 27.Javidfar J, Brodie D, Iribarne A, Jurado J, Lavelle M, Brenner K, et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation and recovery. J Thorac Cardiovasc Surg. 2012;144:716–21. doi: 10.1016/j.jtcvs.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 28.Nosotti M, Rosso L, Tosi D, Palleschi A, Mendogni P, Nataloni IF, et al. Extracorporeal membrane oxygenation with spontaneous breathing as a bridge to lung transplantation. Interact Cardiovasc Thorac Surg. 2013;16:55–9. doi: 10.1093/icvts/ivs433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuehner T, Kuehn C, Hadem J, Wiesner O, Gottlieb J, Tudorache I, et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med. 2012;185:763–8. doi: 10.1164/rccm.201109-1599OC. [DOI] [PubMed] [Google Scholar]
- 30.Gulack BC, Hirji SA, Hartwig MG. Bridge to lung transplantation and rescue post-transplant: The expanding role of extracorporeal membrane oxygenation. J Thorac Dis. 2014;6:1070–9. doi: 10.3978/j.issn.2072-1439.2014.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keeyapaj W, Alfirevic A. Carinal resection using an airway exchange catheter-assisted venovenous ECMO technique. Can J Anaesth. 2012;59:1075–6. doi: 10.1007/s12630-012-9773-x. [DOI] [PubMed] [Google Scholar]
- 32.Smith IJ, Sidebotham DA, McGeorge AD, Dorman EB, Wilsher ML, Kolbe J. Use of extracorporeal membrane oxygenation during resection of tracheal papillomatosis. Anesthesiology. 2009;110:427–9. doi: 10.1097/ALN.0b013e3181943288. [DOI] [PubMed] [Google Scholar]
- 33.Coleman NE, Dalton HJ. Extracorporeal life support for status asthmaticus: The breath of life that's often forgotten. Crit Care. 2009;13:136. doi: 10.1186/cc7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iwamoto T, Ikeda K, Nakajima H, Suga M, Kumano K, Hiraguri M, et al. Extracorporeal membrane oxygenation is indicated for status asthmaticus refractory to maximal conventional therapy. Ann Allergy Asthma Immunol. 2013;110:300–1. doi: 10.1016/j.anai.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 35.Abrams D, Agerstrand CL, Biscotti M, Burkart KM, Bacchetta M, Brodie D. Extracorporeal membrane oxygenation in the management of diffuse alveolar hemorrhage. ASAIO J. 2015;61:216–8. doi: 10.1097/MAT.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 36.Pacheco Claudio C, Charbonney E, Durand M, Kolan C, Laskine M. Extracorporeal membrane oxygenation in diffuse alveolar hemorrhage secondary to systemic lupus erythematosus. J Clin Med Res. 2014;6:145–8. doi: 10.14740/jocmr1685w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vincent JL, De Backer D. Oxygen transport-the oxygen delivery controversy. Intensive Care Med. 2004;30:1990–6. doi: 10.1007/s00134-004-2384-4. [DOI] [PubMed] [Google Scholar]
- 38.Bartlett RH, Gattinoni L. Current status of extracorporeal life support (ECMO) for cardiopulmonary failure. Minerva Anestesiol. 2010;76:534–40. [PubMed] [Google Scholar]
- 39.Spinelli E, Bartlett RH. Relationship between hemoglobin concentration and extracorporeal blood flow as determinants of oxygen delivery during venovenous extracorporeal membrane oxygenation: A mathematical model. ASAIO J. 2014;60:688–93. doi: 10.1097/MAT.0000000000000125. [DOI] [PubMed] [Google Scholar]
- 40.Sidebotham D, McGeorge A, McGuinness S, Edwards M, Willcox T, Beca J. Extracorporeal membrane oxygenation for treating severe cardiac and respiratory disease in adults: Part 1 – Overview of extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth. 2009;23:886–92. doi: 10.1053/j.jvca.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Agerstrand CL, Bacchetta MD, Brodie D. ECMO for adult respiratory failure: Current use and evolving applications. ASAIO J. 2014;60:255–62. doi: 10.1097/MAT.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt M, Tachon G, Devilliers C, Muller G, Hekimian G, Bréchot N, et al. Blood oxygenation and decarboxylation determinants during venovenous ECMO for respiratory failure in adults. Intensive Care Med. 2013;39:838–46. doi: 10.1007/s00134-012-2785-8. [DOI] [PubMed] [Google Scholar]
- 43.Combes A, Bréchot N, Luyt CE, Schmidt M. What is the niche for extracorporeal membrane oxygenation in severe acute respiratory distress syndrome? Curr Opin Crit Care. 2012;18:527–32. doi: 10.1097/MCC.0b013e328357f090. [DOI] [PubMed] [Google Scholar]
- 44.Drinker PA, Lehr JL. Engineering aspects of ECMO technology. Artif Organs. 1978;2:6–11. doi: 10.1111/j.1525-1594.1978.tb00997.x. [DOI] [PubMed] [Google Scholar]
- 45.Messaï E, Bouguerra A, Harmelin G, Di Lascio G, Cianchi G, Bonacchi M. A new formula for determining arterial oxygen saturation during venovenous extracorporeal oxygenation. Intensive Care Med. 2013;39:327–34. doi: 10.1007/s00134-012-2756-0. [DOI] [PubMed] [Google Scholar]
- 46.Finney SJ. Extracorporeal support for patients with acute respiratory distress syndrome. Eur Respir Rev. 2014;23:379–89. doi: 10.1183/09059180.00005514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacLaren G, Combes A, Bartlett RH. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: Life support in the new era. Intensive Care Med. 2012;38:210–20. doi: 10.1007/s00134-011-2439-2. [DOI] [PubMed] [Google Scholar]
- 48.Bartlett RH. Extracorporeal life support for cardiopulmonary failure. Curr Probl Surg. 1990;27:621–705. doi: 10.1016/0011-3840(90)90015-w. [DOI] [PubMed] [Google Scholar]
- 49.Lehle K, Philipp A, Müller T, Schettler F, Bein T, Schmid C, et al. Flow dynamics of different adult ECMO systems: A clinical evaluation. Artif Organs. 2014;38:391–8. doi: 10.1111/aor.12180. [DOI] [PubMed] [Google Scholar]
- 50.Combes A, Bacchetta M, Brodie D, Müller T, Pellegrino V. Extracorporeal membrane oxygenation for respiratory failure in adults. Curr Opin Crit Care. 2012;18:99–104. doi: 10.1097/MCC.0b013e32834ef412. [DOI] [PubMed] [Google Scholar]
- 51.Spurlock DJ, Ranney DN, Fracz EM, Mazur DE, Bartlet RH, Haft JW. In vitro testing of a novel blood pump designed for temporary extracorporeal support. ASAIO J. 2012;58:109–14. doi: 10.1097/MAT.0b013e318245d356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ichiba S, Peek GJ, Sosnowski AW, Brennan KJ, Firmin RK. Modifying a venovenous extracorporeal membrane oxygenation circuit to reduce recirculation. Ann Thorac Surg. 2000;69:298–9. doi: 10.1016/s0003-4975(99)01227-8. [DOI] [PubMed] [Google Scholar]
- 53.Locker GJ, Losert H, Schellongowski P, Thalhammer F, Knapp S, Laczika KF, et al. Bedside exclusion of clinically significant recirculation volume during venovenous ECMO using conventional blood gas analyses. J Clin Anesth. 2003;15:441–5. doi: 10.1016/s0952-8180(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 54.Annich GA, Lynch W, McLaren G, Wilson J, Bartlett R. 4th ed. Ann Arbor, MI: Extracorporeal Life Support Organization; 2012. ECMO: Extracorporeal Cardiopulmonary Support in Critical Care. [Google Scholar]
- 55.Broman M, Frenckner B, Bjällmark A, Broomé M. Recirculation during veno-venous extra-corporeal membrane oxygenation – A simulation study. Int J Artif Organs. 2015;38:23–30. doi: 10.5301/ijao.5000373. [DOI] [PubMed] [Google Scholar]
- 56.Darling EM, Crowell T, Searles BE. Use of dilutional ultrasound monitoring to detect changes in recirculation during venovenous extracorporeal membrane oxygenation in swine. ASAIO J. 2006;52:522–4. doi: 10.1097/01.mat.0000237589.20935.a4. [DOI] [PubMed] [Google Scholar]
- 57.van Heijst AF, van der Staak FH, de Haan AF, Liem KD, Festen C, Geven WB, et al. Recirculation in double lumen catheter veno-venous extracorporeal membrane oxygenation measured by an ultrasound dilution technique. ASAIO J. 2001;47:372–6. doi: 10.1097/00002480-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 58.Sreenan C, Osiovich H, Cheung PY, Lemke RP. Quantification of recirculation by thermodilution during venovenous extracorporeal membrane oxygenation. J Pediatr Surg. 2000;35:1411–4. doi: 10.1053/jpsu.2000.16402. [DOI] [PubMed] [Google Scholar]
- 59.Körver EP, Ganushchak YM, Simons AP, Donker DW, Maessen JG, Weerwind PW. Quantification of recirculation as an adjuvant to transthoracic echocardiography for optimization of dual-lumen extracorporeal life support. Intensive Care Med. 2012;38:906–9. doi: 10.1007/s00134-012-2534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Linton R, Turtle M, Band D, O’Brien T, Jonas M. In vitro evaluation of a new lithium dilution method of measuring cardiac output and shunt fraction in patients undergoing venovenous extracorporeal membrane oxygenation. Crit Care Med. 1998;26:174–7. doi: 10.1097/00003246-199801000-00035. [DOI] [PubMed] [Google Scholar]
- 61.Abrams D, Bacchetta M, Brodie D. Recirculation in venovenous extracorporeal membrane oxygenation. ASAIO J. 2015;61:115–21. doi: 10.1097/MAT.0000000000000179. [DOI] [PubMed] [Google Scholar]
- 62.Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: Potential role of red cell transfusion. Crit Care Med. 2005;33:1191–8. doi: 10.1097/01.ccm.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- 63.Voelker MT, Busch T, Bercker S, Fichtner F, Kaisers UX, Laudi S. Restrictive transfusion practice during extracorporeal membrane oxygenation therapy for severe acute respiratory distress syndrome. Artif Organs. 2015;39:374–8. doi: 10.1111/aor.12385. [DOI] [PubMed] [Google Scholar]
- 64.Kimmoun A, Vanhuyse F, Levy B. Improving blood oxygenation during venovenous ECMO for ARDS. Intensive Care Med. 2013;39:1161–2. doi: 10.1007/s00134-013-2903-2. [DOI] [PubMed] [Google Scholar]
- 65.Schmidt M, Stewart C, Bailey M, Nieszkowska A, Kelly J, Murphy L, et al. Mechanical ventilation management during extracorporeal membrane oxygenation for acute respiratory distress syndrome: A retrospective international multicenter study. Crit Care Med. 2015;43:654–64. doi: 10.1097/CCM.0000000000000753. [DOI] [PubMed] [Google Scholar]
- 66.Marhong JD, Telesnicki T, Munshi L, Del Sorbo L, Detsky M, Fan E. Mechanical ventilation during extracorporeal membrane oxygenation. An international survey. Ann Am Thorac Soc. 2014;11:956–61. doi: 10.1513/AnnalsATS.201403-100BC. [DOI] [PubMed] [Google Scholar]
- 67.Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358:1327–35. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
- 68.Karagiannidis C, Lubnow M, Philipp A, Riegger GA, Schmid C, Pfeifer M, et al. Autoregulation of ventilation with neurally adjusted ventilatory assist on extracorporeal lung support. Intensive Care Med. 2010;36:2038–44. doi: 10.1007/s00134-010-1982-6. [DOI] [PubMed] [Google Scholar]
- 69.Pham T, Combes A, Rozé H, Chevret S, Mercat A, Roch A, et al. Extracorporeal membrane oxygenation for pandemic influenza A (H1N1)-induced acute respiratory distress syndrome: A cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2013;187:276–85. doi: 10.1164/rccm.201205-0815OC. [DOI] [PubMed] [Google Scholar]
- 70.Michaels AJ, Hill JG, Sperley BP, Young BP, Ogston TL, Wiles CL, et al. Use of HFPV for adults with ARDS: The protocolized use of high-frequency percussive ventilation for adults with acute respiratory failure treated with extracorporeal membrane oxygenation. ASAIO J. 2015;61:345–9. doi: 10.1097/MAT.0000000000000196. [DOI] [PubMed] [Google Scholar]
- 71.Eastman A, Holland D, Higgins J, Smith B, Delagarza J, Olson C, et al. High-frequency percussive ventilation improves oxygenation in trauma patients with acute respiratory distress syndrome: A retrospective review. Am J Surg. 2006;192:191–5. doi: 10.1016/j.amjsurg.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 72.Salim A, Martin M. High-frequency percussive ventilation. Crit Care Med. 2005;33(3 Suppl):S241–5. doi: 10.1097/01.ccm.0000155921.32083.ce. [DOI] [PubMed] [Google Scholar]
- 73.Guervilly C, Dizier S, Thomas G, Jaussaud N, Morera P, Hraiech S, et al. Comparison of femorofemoral and femorojugular configurations during venovenous extracorporeal membrane oxygenation for severe ARDS. Intensive Care Med. 2014;40:1598–9. doi: 10.1007/s00134-014-3427-0. [DOI] [PubMed] [Google Scholar]
- 74.Del Sorbo L, Cypel M, Fan E. Extracorporeal life support for adults with severe acute respiratory failure. Lancet Respir Med. 2014;2:154–64. doi: 10.1016/S2213-2600(13)70197-8. [DOI] [PubMed] [Google Scholar]
- 75.Braune S, Kienast S, Hadem J, Wiesner O, Wichmann D, Nierhaus A, et al. Safety of percutaneous dilatational tracheostomy in patients on extracorporeal lung support. Intensive Care Med. 2013;39:1792–9. doi: 10.1007/s00134-013-3023-8. [DOI] [PubMed] [Google Scholar]
- 76.Esper SA, Levy JH, Waters JH, Welsby IJ. Extracorporeal membrane oxygenation in the adult: A review of anticoagulation monitoring and transfusion. Anesth Analg. 2014;118:731–43. doi: 10.1213/ANE.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 77.Niebler RA, Christensen M, Berens R, Wellner H, Mikhailov T, Tweddell JS. Antithrombin replacement during extracorporeal membrane oxygenation. Artif Organs. 2011;35:1024–8. doi: 10.1111/j.1525-1594.2011.01384.x. [DOI] [PubMed] [Google Scholar]
- 78.Guttendorf J, Boujoukos AJ, Ren D, Rosenzweig MQ, Hravnak M. Discharge outcome in adults treated with extracorporeal membrane oxygenation. Am J Crit Care. 2014;23:365–77. doi: 10.4037/ajcc2014115. [DOI] [PubMed] [Google Scholar]
- 79.Nasr DM, Rabinstein AA. Neurologic Complications of Extracorporeal Membrane Oxygenation. J Clin Neurol. 2015;11:383–9. doi: 10.3988/jcn.2015.11.4.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buscher H, Vaidiyanathan S, Al-Soufi S, Nguyen DN, Breeding J, Rycus P, et al. Sedation practice in veno-venous extracorporeal membrane oxygenation: An international survey. ASAIO J. 2013;59:636–41. doi: 10.1097/MAT.0b013e3182a84558. [DOI] [PubMed] [Google Scholar]
- 81.Shekar K, Roberts JA, Welch S, Buscher H, Rudham S, Burrows F, et al. ASAP ECMO: Antibiotic, Sedative and Analgesic Pharmacokinetics during Extracorporeal Membrane Oxygenation: A multi-centre study to optimise drug therapy during ECMO. BMC Anesthesiol. 2012;12:29. doi: 10.1186/1471-2253-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shekar K, Roberts JA, Mullany DV, Corley A, Fisquet S, Bull TN, et al. Increased sedation requirements in patients receiving extracorporeal membrane oxygenation for respiratory and cardiorespiratory failure. Anaesth Intensive Care. 2012;40:648–55. doi: 10.1177/0310057X1204000411. [DOI] [PubMed] [Google Scholar]
- 83.Tellor B, Shin N, Graetz TJ, Avidan MS. Ketamine infusion for patients receiving extracorporeal membrane oxygenation support: A case series. F1000Res. 2015;4:16. doi: 10.12688/f1000research.6006.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zangrillo A, Landoni G, Biondi-Zoccai G, Greco M, Greco T, Frati G, et al. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc. 2013;15:172–8. [PubMed] [Google Scholar]
- 85.Oliver WC. Anticoagulation and coagulation management for ECMO. Semin Cardiothorac Vasc Anesth. 2009;13:154–75. doi: 10.1177/1089253209347384. [DOI] [PubMed] [Google Scholar]
- 86.Ferrie S, Herkes R, Forrest P. Nutrition support during extracorporeal membrane oxygenation (ECMO) in adults: A retrospective audit of 86 patients. Intensive Care Med. 2013;39:1989–94. doi: 10.1007/s00134-013-3053-2. [DOI] [PubMed] [Google Scholar]
- 87.Ridley EJ, Davies AR, Robins EJ, Lukas G, Bailey MJ, Fraser JF. Nutrition therapy in adult patients receiving extracorporeal membrane oxygenation: A prospective, multicentre, observational study. Crit Care Resusc. 2015;17:183–9. [PubMed] [Google Scholar]
- 88.Askenazi DJ, Selewski DT, Paden ML, Cooper DS, Bridges BC, Zappitelli M, et al. Renal replacement therapy in critically ill patients receiving extracorporeal membrane oxygenation. Clin J Am Soc Nephrol. 2012;7:1328–36. doi: 10.2215/CJN.12731211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kielstein JT, Heiden AM, Beutel G, Gottlieb J, Wiesner O, Hafer C, et al. Renal function and survival in 200 patients undergoing ECMO therapy. Nephrol Dial Transplant. 2013;28:86–90. doi: 10.1093/ndt/gfs398. [DOI] [PubMed] [Google Scholar]
- 90.Bizzarro MJ, Conrad SA, Kaufman DA, Rycus P. Extracorporeal Life Support Organization Task Force on Infections, Extracorporeal Membrane Oxygenation. Infections acquired during extracorporeal membrane oxygenation in neonates, children, and adults. Pediatr Crit Care Med. 2011;12:277–81. doi: 10.1097/PCC.0b013e3181e28894. [DOI] [PubMed] [Google Scholar]
- 91.Strecker T, Münch F, Weyand M. One hundred ten days of extracorporeal membrane oxygenation in a young woman with postpartum cerebral venous thrombosis and acute respiratory distress syndrome. Heart Surg Forum. 2012;15:180–E181. doi: 10.1532/HSF98.20111068. [DOI] [PubMed] [Google Scholar]
- 92.Tang X, Sun B, He H, Li H, Hu B, Qiu Z, et al. Successful extracorporeal membrane oxygenation therapy as a bridge to sequential bilateral lung transplantation for a patient after severe paraquat poisoning. Clin Toxicol (Phila) 2015;53:908–13. doi: 10.3109/15563650.2015.1082183. [DOI] [PubMed] [Google Scholar]
- 93.Truog RD, Thiagarajan RR, Harrison CH. Ethical dilemmas with the use of ECMO as a bridge to transplantation. Lancet Respir Med. 2015;3:597–8. doi: 10.1016/S2213-2600(15)00233-7. [DOI] [PubMed] [Google Scholar]
- 94.Weinert CR, Gross CR, Marinelli WA. Impact of randomized trial results on acute lung injury ventilator therapy in teaching hospitals. Am J Respir Crit Care Med. 2003;167:1304–9. doi: 10.1164/rccm.200205-478OC. [DOI] [PubMed] [Google Scholar]
- 95.Young MP, Manning HL, Wilson DL, Mette SA, Riker RR, Leiter JC, et al. Ventilation of patients with acute lung injury and acute respiratory distress syndrome: Has new evidence changed clinical practice? Crit Care Med. 2004;32:1260–5. doi: 10.1097/01.ccm.0000127784.54727.56. [DOI] [PubMed] [Google Scholar]
- 96.Kalhan R, Mikkelsen M, Dedhiya P, Christie J, Gaughan C, Lanken PN, et al. Underuse of lung protective ventilation: Analysis of potential factors to explain physician behavior. Crit Care Med. 2006;34:300–6. doi: 10.1097/01.ccm.0000198328.83571.4a. [DOI] [PubMed] [Google Scholar]
- 97.Rubenfeld GD, Cooper C, Carter G, Thompson BT, Hudson LD. Barriers to providing lung-protective ventilation to patients with acute lung injury. Crit Care Med. 2004;32:1289–93. doi: 10.1097/01.ccm.0000127266.39560.96. [DOI] [PubMed] [Google Scholar]
- 98.Kregenow DA, Rubenfeld GD, Hudson LD, Swenson ER. Hypercapnic acidosis and mortality in acute lung injury. Crit Care Med. 2006;34:1–7. doi: 10.1097/01.ccm.0000194533.75481.03. [DOI] [PubMed] [Google Scholar]
- 99.Terragni PP, Del Sorbo L, Mascia L, Urbino R, Martin EL, Birocco A, et al. Tidal volume lower than 6 ml/kg enhances lung protection: Role of extracorporeal carbon dioxide removal. Anesthesiology. 2009;111:826–35. doi: 10.1097/ALN.0b013e3181b764d2. [DOI] [PubMed] [Google Scholar]
- 100.Hager DN, Krishnan JA, Hayden DL, Brower RG. ARDS Clinical Trials Network. Tidal volume reduction in patients with acute lung injury when plateau pressures are not high. Am J Respir Crit Care Med. 2005;172:1241–5. doi: 10.1164/rccm.200501-048CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cove ME, MacLaren G, Federspiel WJ, Kellum JA. Bench to bedside review: Extracorporeal carbon dioxide removal, past present and future. Crit Care. 2012;16:232. doi: 10.1186/cc11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zimmermann M, Bein T, Arlt M, Philipp A, Rupprecht L, Mueller T, et al. Pumpless extracorporeal interventional lung assist in patients with acute respiratory distress syndrome: A prospective pilot study. Crit Care. 2009;13:R10. doi: 10.1186/cc7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Burki NK, Mani RK, Herth FJ, Schmidt W, Teschler H, Bonin F, et al. A novel extracorporeal CO(2) removal system: Results of a pilot study of hypercapnic respiratory failure in patients with COPD. Chest. 2013;143:678–86. doi: 10.1378/chest.12-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lund LW, Federspiel WJ. Removing extra CO 2 in COPD patients. Curr Respir Care Rep. 2013;2:131–8. doi: 10.1007/s13665-013-0057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schellongowski P, Riss K, Staudinger T, Ullrich R, Krenn CG, Sitzwohl C, et al. Extracorporeal CO 2 removal as bridge to lung transplantation in life-threatening hypercapnia. Transpl Int. 2015;28:297–304. doi: 10.1111/tri.12486. [DOI] [PubMed] [Google Scholar]
- 106.Hoganson DM, Gazit AZ, Boston US, Sweet SC, Grady RM, Huddleston CB, et al. Paracorporeal lung assist devices as a bridge to recovery or lung transplantation in neonates and young children. J Thorac Cardiovasc Surg. 2014;147:420–6. doi: 10.1016/j.jtcvs.2013.08.078. [DOI] [PubMed] [Google Scholar]


