Supplemental Digital Content is available in the text.
Key Words: infant, toddler, pain management, randomized controlled trial, quasi-randomized controlled trial, systematic review, vaccination
Abstract
Background:
This systematic review evaluated the effectiveness of distraction for reducing infant distress during vaccinations in young children aged 0 to 3 years.
Design/Methods:
Database searches identified relevant randomized and quasi-randomized controlled trials. Three separate clinical questions related to variants of the psychological strategy of distraction (directed video; directed toy; nondirected toy) were pursued. Distress was identified as the critical outcome to assess the benefits of distraction and extracted from relevant trials. Distress was analyzed by phase of procedure (distress preprocedure; distress acute; distress recovery; idiosyncratic phases based on some or all of the 3 aforementioned phases).
Results:
Ten studies were included in the review. Significant results are presented herein. For directed video distraction, moderate quality evidence suggested that distress was lowered in the treatment group standardized mean difference (SMD −0.68 lower [95% confidence interval (CI), −1.04 to −0.32]) for the acute+recovery phase as well as the preprocedure phase (SMD −0.49 lower [95% CI, −7.6 to −0.22]). For directed toy distraction, the analysis of low-quality evidence for a combined preprocedure+acute+recovery phase of distress (analysis n=81), suggested that distress was lowered in the treatment group (SMD −0.47 lower [95% CI, −0.91 to −0.02]). An effect for nondirected toy distraction was also seen, analyzing very–low-quality evidence, for the acute distress phase (n=290; SMD −0.93 lower [95% CI, −1.86 to 0.00]).
Conclusion:
Generally low-quality to very–low-quality evidence suggests that there may be an effect of directed (toy and video) and nondirected toy distraction for children aged 0 to 3 years, for certain phases of the vaccination.
Early childhood is a period of exponential cognitive development. Although there are similar neural structures involved in infant and older human pain-related responsivity, the actual coordination and modulation of stress responsivity has been posited to differ due to developmental stage.1 One key aspect of the infant stage of development, particularly relevant to psychological strategies such as distraction, is the dependence of the infant on caregiver for regulation of the distress state. Infants move from being completely dependent on a caregiver for regulation of distress at birth and move toward self-regulation by the preschool years.1 This stage of development needs to be taken into account when understanding effective pain management strategies.
In the first version of a clinical practice guideline about vaccination pain management by our team HELPinKids (now Help ELiminate Pain in Kids & Adults), the stage of infancy was not formally built into the psychological strategies’ section.2 This current review (and the updated clinical practice guidelines3) augments previous work by discussing infant psychological strategies, namely distraction, in a stand-alone review. The emerging cognitive ability to self-regulate is a pivotal reason why the decision was made to analyze the impact of distraction interventions for pain management during vaccination in young children (0 to 3 y) separately from other age groups in the series of reviews. This review compliments other work done in this series where distraction, among other psychological strategies, is handled separately for older children, adolescents, and adults.4,5 Moreover, the reader’s attention is also directed to other concurrent reviews in this series in which infant pain management strategies, such as pharmacological (eg, sucrose, topical anesthetics),6 physical, and procedural strategies7 are reviewed.
For the purposes of the current review, infant psychological strategies, that is, strategies seen as having a primarily cognitive mechanism related to modulating infant pain response, has been limited to the practice of distraction. Given the cognitive development of the infant, distraction was the only infant pain management strategy that was seen as having a primarily cognitive mechanism.
In a recent update of an established Cochrane systematic review on nonpharmacological pain management strategies in young children (0 to 3 y), results from 5 randomized controlled trials were analyzed that included distraction interventions.8 Two types of distraction were analyzed—toy and video. Results were analyzed separately for pain reactivity (<30 s after needle) and immediate pain regulation (>30 s after needle). Low-quality to very–low-quality evidence suggested that while toy distraction had no effect on pain scores, video distraction did result in lower scores in the treatment group for both the pain reactivity and immediate regulation phases.
The current review builds on prior work by broadening the literature base in which clinical recommendations can be made to include both quasi-randomized and randomized controlled trials. The inclusion of these albeit lower quality trials increases the international generalizability of the findings as the inclusion of quasi-randomized trials leads to the inclusion of research from middle-income countries in Asia and Europe. Moreover, it allows for the current clinical practice guidelines to draw upon a greater number of studies.
METHODS
This review was conducted as a part of the Help ELiminate Pain in Kids & Adults (HELPinKids&Adults synthesis and dissemination initiative). One overall search strategy was used to provide an umbrella search that would elicit all experimental studies designed to manage vaccination pain. An academic librarian, experienced in systematic reviews, created the search strategy with input from the clinical lead authors. Tailored searches (inception to February 26, 2015) were created for 5 databases: EMBASE, Medline, PsycInfo, CINAHL, and ProQuest Dissertations & Theses Global. Details of the screening strategy and extraction methodology are provided elsewhere in this series.9 The systematic review was registered with PROSPERO and both the Grading of Assessments, Recommendations, Development and Evaluation (GRADE)10 and Cochrane11 methodologies guided the knowledge synthesis.
Distress was defined as the critically important outcome in this review, as the focus on infants and young children meant that self-report of pain was not possible. The clinical questions on distraction and the prioritization of distress for infants were shaped by team discussions of the larger project with the clinical leads (A.T., C.M.M., V.S., R.P.R., C.C., and M.N.) and rated (in terms of importance) using electronic spreadsheet ballots by the entire HELPinKids&Adults team. Three clinical questions on infant distraction studies were agreed upon for inclusion: directed video distraction, directed toy distraction, and nondirected toy distraction (Table 1). The prefix of “directed” versus “nondirected” was added to delineate studies where an adult actually attempted to engage the young child in the distraction, versus studies that simply exposed an infant to the distractor. When possible, outcomes that were deemed important (rather than critical) by the team were analyzed for completeness and results are presented in the Supplemental Digital Content (see SDC Figures 1 to 3: Supplemental Digital Content 1, http://links.lww.com/CJP/A243, Supplemental Digital Content 2, http://links.lww.com/CJP/A244, Supplemental Digital Content 3, http://links.lww.com/CJP/A245 and SDC Tables 1 to 3, Supplemental Digital Content 4, http://links.lww.com/CJP/A246, Supplemental Digital Content 5, http://links.lww.com/CJP/A247, Supplemental Digital Content 6, http://links.lww.com/CJP/A248) accompanying this paper. However, only the critical outcome of distress will be discussed in this review.
TABLE 1.
Clinical Questions and Outcomes for Infant Psychological Interventions
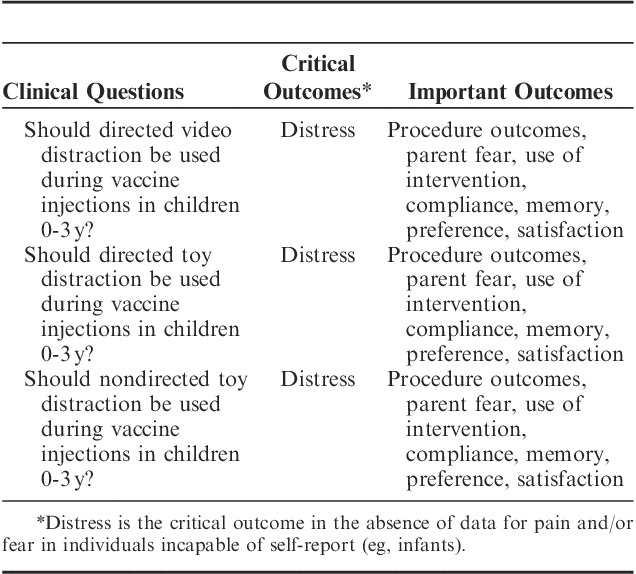
The distinction between the use of distress versus pain in the current review is predicated on the assumption that pain is a subjective experience and, therefore, one must be capable of reliably and validly reporting their pain. In contrast, distress was seen as a less specific yet equally critical outcome for infants’ responses so as not to discriminate against children who cannot self-report. Thus, our use of the term “distress” does not distinguish between fear or pain (contrary with the other reviews of older age spans) because the level of negative impact during the medical procedure was always obtained through a proxy (eg, parent report of pain or observational coding of distress behaviors).
The Cochrane risk of bias tool (https://bmg.cochrane.org/assessing-risk-bias-included-studies) was used to evaluate methodological limitations and the RevMan software program (version 5.2; Cochrane Collaboration, Copenhagen, Denmark) was used to pool the data. The effect of each intervention was expressed as a standardized mean difference (SMD) with accompanying 95% confidence interval (CI) or relative risk and CI, as appropriate. A random-effects model was used for all analyses. Statistical heterogeneity was assessed using I2 and χ2 tests.3
Distress was also subdivided according to the temporal phase of the vaccination. Different potential factors relate to infant pain-related distress before the needle, immediately after the needle, and in the period that follows the peak distress after needle.12 Accordingly, to characterize the impact of the intervention on pain-related distress, distress was analyzed separately for: (1) the preprocedure phase, which occurred postintervention but before vaccine injection(s); (2) the acute procedure phase (within the first minute of needle puncture and vaccine injection); and (3) the recovery procedure phase (1 to 5 min after vaccine injection(s). In addition, some idiosyncratic combinations of before needle, needle, and recovery phases were used by researchers and these were analyzed separately. Pain that did not occur in the immediate minutes postvaccination (eg, parents have reported that infant postvaccination pain lasts beyond the day of injection13) was not analyzed.
Multiple observers may have provided data on the same outcome (eg, observer-coded child distress, parent-rated child distress), data from multiple time points within the same procedure phase (eg, multiple pain scores in the first minute postvaccination), or both. These multiple data points were pooled before inclusion in the meta-analysis using established methods.9
Evidence profiles and summary of findings tables were created using the GRADE profiler software (version 3.6.1) in which all judgments pertaining to evaluation of quality of evidence were recorded. When findings demonstrated any benefit across critical outcomes, the intervention was recommended but would be qualified by the quality of the evidence.
RESULTS
As denoted in Figure 1, a total of 114,251 references were retrieved from the databases during the umbrella search. Ten studies14–23 that evaluated directed video distraction, directed toy distraction, and nondirected toy distraction were obtained relevant to the current review and it was determined that one of the studies was a duplicate (a thesis and a published manuscript).18
FIGURE 1.
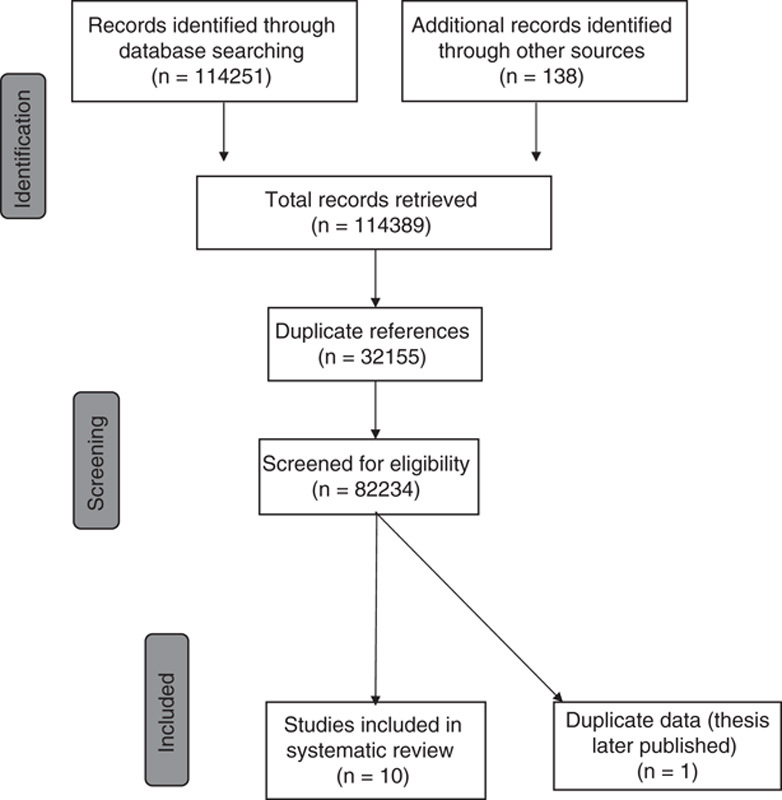
Flow chart of studies for infant distraction trials.
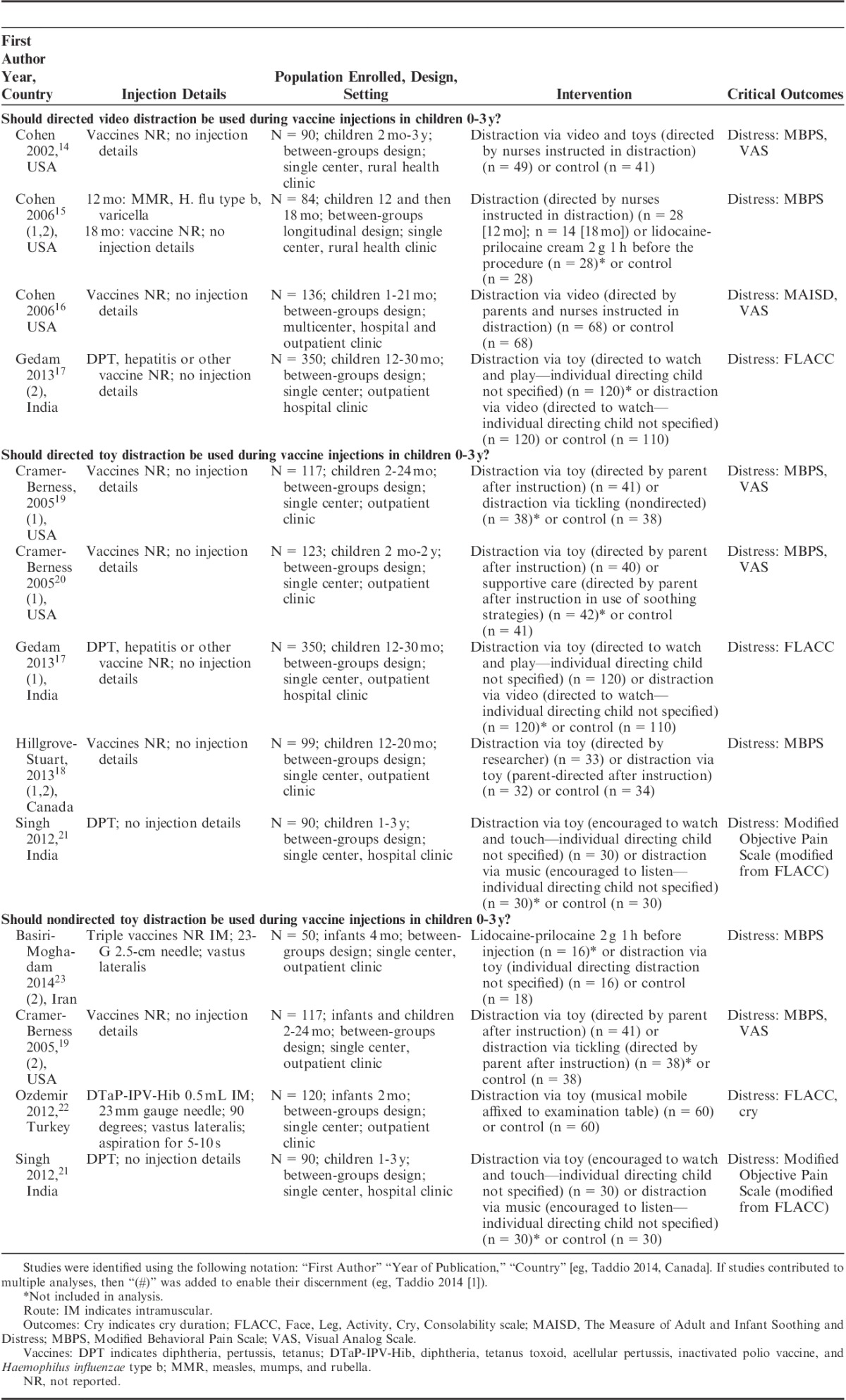
Characteristics of included distraction trials are displayed in Table 2. All included studies provided data for at least one of the 3 analyses. One study provided treatment arms for 2 of the 3 clinical questions.19 Two studies provided multiple treatment arms for analyses within the same clinical question.15,18
TABLE 2.
Characteristics for Included Studies
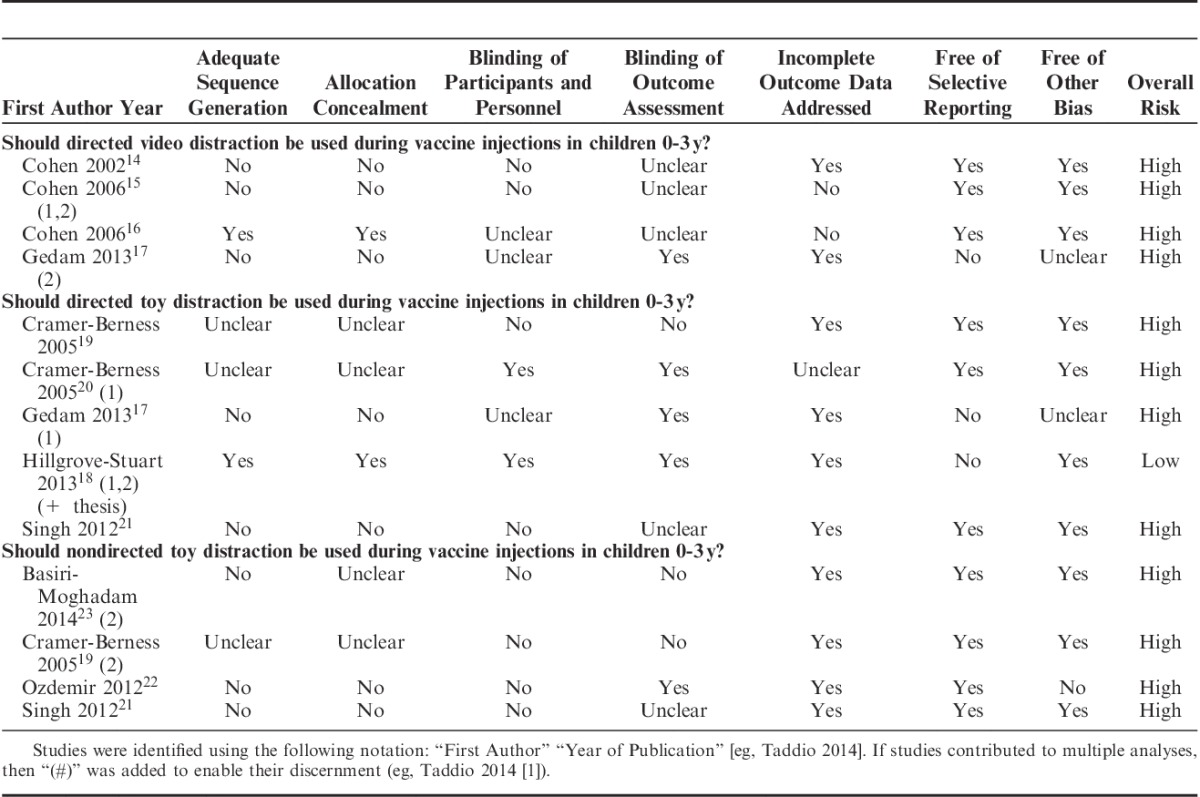
Quality of Studies and Risk of Bias
Table 3 shows the results for the risk of bias assessment for critical outcomes. All trials had a high overall risk of bias primarily because of lack of blinding of important personnel, and methodological issues related to randomization procedures (ie, allocation concealment and adequate sequence generation).
TABLE 3.
Assessment of Risk of Bias of Included Trials for Critical Outcomes
Overall Quality of Evidence and Treatment Effects
A quantitative summary of the treatment effects for available critical outcomes is provided below. Table 4 displays a qualitative summary of these results. Supporting GRADE Evidence Profiles and Summary of Findings tables for critically important and important outcomes can be found in the Supplemental Digital Content to the paper. A full summary of findings Table with GRADE criteria is provided for each clinical question.
TABLE 4.
Summary of Results for Critically Important Outcomes for Infant Psychological Interventions
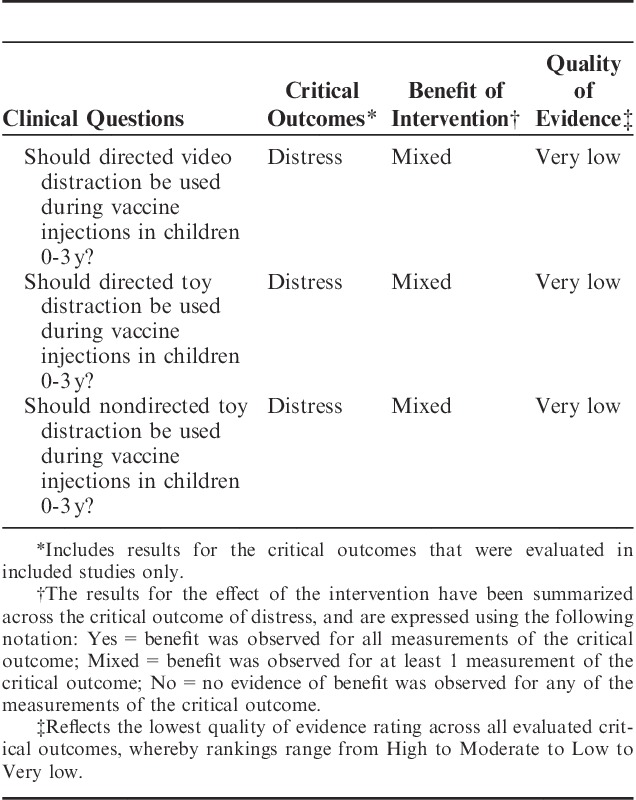
Should Directed Video Distraction be Used During Vaccine Injections With Children Between 0 and 3 Years of Age?
Four trials14–17 were included in this analysis with 5 distress outcomes based on temporal phases of the vaccination (distress acute,14,16,17 distress recovery,14 distress acute+recovery,16 distress preprocedure+acute,15 distress preprocedure14,16). The risk of bias was high in all 4 studies and the overall quality of evidence across studies ranged from moderate to very low for the 5 distress outcomes evaluated. Quality issues related mainly to randomization and blinding. Results were mixed across different distress indicators. One moderate quality analysis (n=126) revealed a benefit of directed video distraction on the combined phase of distress acute+recovery: (SMD −0.68 [95% CI, −1.04 to −0.32]). In another analysis of preprocedure distress (n=216), there was a positive impact of directed video distraction: (SMD −0.49 [95% CI, −0.76 to −0.22]). No other distress phase analyses were significant.
Should Directed Toy Distraction be Used During Vaccine Injections With Children Between 0 and 3 Years of Age?
Five trials17–21 evaluating of the effect directed toy distraction were included, evaluating 5 distress outcomes (distress acute,17–21 distress acute+recovery,19,20 distress recovery,18 distress preprocedure,19,20 distress preprocedure+acute+recovery20). The risk of bias was high in 417,19,20,21 of the 5 studies. Overall quality across studies ranged from low to very low for the distress outcomes evaluated and results were mixed. Challenges in quality related mainly to randomization detailing. Using data from 1 low-quality trial (n=81), the (SMD −0.47 [95% CI, −0.91 to −0.02]) for a combined phase of distress preprocedure+acute+recovery showed a positive impact of directed toy distraction on infant distress. No other distress phase analyses were significant.
Should Nondirected Toy Distraction be Used During Vaccine Injections With Children Between 0 and 3 Years of Age?
Four trials19,21–23 were included in this analysis for 3 distress outcomes (distress acute,19,21–23 distress acute+recovery,20,22 distress preprocedure20). The risk of bias was high in all 4 studies. Across the different analyses on the 3 distress outcomes, overall quality of the studies meta-analyzed ranged from very low to low and results were mixed. Quality ratings were impacted due to both issues with randomization and blinding. Using data from 4 trials (n=290), only the results for acute distress showed a favorable impact of nondirected toy distraction: (SMD −0.93 [95% CI, −1.86 to 0.00]).
DISCUSSION
Building on a broader international research base, the current systematic review set out to review randomized and quasi-randomized controlled trials on distraction as a pain management strategy for distress in young children aged 0 to 3 years. There was some evidence of benefit for directed video and toy distraction and nondirected toy distraction; however, benefit was not consistently observed across phases of the vaccination procedure. The evidence for all the interventions was generally either of low quality (due to issues with randomization or blinding) or only based on evidence from 1 experimental study.
There was little pattern to the significance of findings due to the use of idiosyncratic time phases. However, it is perhaps noteworthy that in 2 of the significant findings, a longer time epoch was used. The reliability of the pain-related distress measurement may have been increased due to the longer sampling of time on which the distress measurement was based. By reducing the noise of the measurement this may have increased the probability of showing a significant effect. Conversely, adding to the noise of measurement within our analyses was the age differences across studies.
As mentioned, infancy is a period encompassing the steepest trajectory of development across the lifespan. Both individual studies and our analyses of studies often synthesized findings based on infants and young children from 2 months up to 3 years. Developmentally, this type of averaging obscures our understanding of pain management. A recent age-sensitive analysis of developmental differences in infant pain responsivity over the first year of life clearly demonstrated that researchers who conduct infant pain management randomized controlled trials must pay greater attention to age differences within infancy.24 Moreover, the receptivity of infants to distraction is hypothesized to vary due to developing motor and cognitive capacities. On the basis of developmental milestones, a 2-month old would seem less oriented to benefit from distraction. However, a child older than 12 months would seem to have greater ability to benefit from distraction, owing in part to the emerging ability to enjoy joint attention with a caregiver and the motor control to manage self-orientation to an external stimulus.
Furthermore, because of the central importance of the primary caregiver to understanding pain responses and management in early childhood,25 another important factor to incorporate into trials is the agent of distraction is (ie, who is doing the distracting), whether it is a primary caregiver, nonprimary caregiver, nurse, or physician. Although 1 trial did address this question with null results (hypothesized because of the short amount of time in which the distractor was used across treatment groups), this implementation factor should be more systematically investigated18 in trials discerning the impact of distraction for early childhood pain management during vaccination.
The promising findings on video distraction for both the preprocedure phase and the acute+recovery phase mirrored the updated Cochrane Review8 recommendations, a review based solely on randomized controlled trials. This is despite the inclusion in the current review of a large nonrandomized controlled trial that was not a part of the Cochrane analysis.17 However, the findings on the toy distraction disagreed with the updated Cochrane Review on the topic, which did not find an effect of toy distraction, regardless of the phase of the vaccination. The results of the current review may have differed due to the inclusion of 3 additional studies.15,19,21 Thus, based on analyses within the current review, weak recommendations are being made for both toy and video distractors. However, further work should explore other types of distraction that may be especially helpful during the infant and early childhood stage of development (eg, face-to-face engagement with a caregiver while cradled for young infants).
Another possible reason for the equivocal results of distraction across distress outcomes within this review may relate to the timing of distraction—specifically, there may be specific times during vaccination when distraction may be optimally engaged to help young children. Observational and experimental research has shown that infants who are distressed before the needle will have higher pain scores after the needle.18,26 Thus, there would be sufficient reason to hypothesize that distraction should begin early enough to sufficiently engage a child before the needle, thus mitigating the acute and recovery pain responses after needle. Conducting a randomized controlled trial where distraction is initiated at a number of different intervals (eg, 1 min before needle [no distress], right after needle [high distress], 2 min after needle [moderate distress]) with strict controls to equalize proximity to caregiver among treatment groups would help clarify this issue.
Although distraction may offer some benefits in reducing child distress during vaccination, observational research suggests that it is not a commonly occurring strategy during routine infant vaccine injections. In the largest longitudinal observational study conducted during infant immunization, including over 760 parent-infant dyads,27 the natural occurrence and effects of parental use of distraction were examined over the first year of life. Systematic analyses suggested a clear developmental trend in the naturalistic use of distraction (ie, distraction techniques that parents employed with no coaching). Parents used distraction increasingly as the infant aged from 2 to 12 months. However, even at 12 months, during any given phase of the vaccination (ie, preprocedure, acute after needle, recovery before needle) the maximum average amount of time that parents used distraction hovered around 10%. Current parental practices during routine immunizations, namely the natural occurrence yet mixed effectiveness, further strengthens the justification for more research on the role of distraction as a pain management strategy in early childhood.
Although strict protocols for systematic reviews were followed in the current review, there are a number of limitations that warrant caution. First, the quantity and quality of the studies are not adequate to base strong recommendations in either direction. Moreover, as noted earlier, the age of children in most of these studies encompassed large developmental spans during infancy. Despite this knowledge, the paucity of literature did not permit more finely grained age analyses in this review. Another limitation that is pertinent to understanding distraction on the infant is the role of holding. The position of the child is a crucial element to the execution of distraction; therefore, future researchers on this topic are strongly encouraged to provide this methodological detail. On the basis of evidence presented elsewhere in this series,7 it is posited that holding an infant in the caregivers’ arms is the optimal position for distraction in young infants (ie, less than 1 y of age), whereas the exact positioning of older infants (eg, toddlers over 1 y of age) in relation to the caregiver should depend on child preference. Finally, given the lack of any correction applied to the entire set of analyses, there is a chance that one of the positive results reflects type II error (the existence of a significant effect when in fact no such effect exists).
Despite these limitations, the current review adds to the literature base for pain management for vaccination due to the use of a stringent methodology, the attention paid to temporal phases of the vaccination, and a developmental attunement to early childhood throughout analysis and interpretation. There is sufficient evidence from these studies to weakly suggest that there may be some benefit for distraction via toy and video to infants and young children (0 to 3 y), whether directed or nondirected; albeit the effect is not robust. The use of distraction during early childhood should not interfere with a young child’s core developmental need for proximity to the caregiver during times of pain-related distress.25
Although the feasibility of video distraction in low-resource environments is challenging, other types of distraction (eg, toys, car keys, objects in clinic setting) can be low cost, easily transferable interventions with minimal impact to clinical flow. Moreover, researchers should focus their attention on rigorous trial execution that considers developmental stage and timing of the distraction more closely and the interaction between distraction and other measures of infant soothing (eg, holding).
Supplementary Material
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.clinicalpain.com.
HELPinKIDS (Help ELiminate Pain in KIDS) Team: Ipp M., MacDonald N. E., Rogers J., Bucci L., Mousmanis P., Lang E., Halperin S. A., Bowles S., Halpert C., Rieder M., Robson K., Asmundson G. J. G., Uleryk E., Antony M. M., Dubey V., Hanrahan A., Lockett D., Scott J., Votta Bleeker E.
Supported by the Canadian Institutes of Health Research (CIHR), Ottawa, ON, Canada (KRS 132031). Open access funding was provided by the Mayday Fund in the United States. A. T., declares a grant from Pfizer, and study supplies from Natus and Ferndale. C.T. C., declares consultation fees from Abbvie. E. L. is a member of the GRADE working group and declares consultation fees from the International Liaison Committee on Resuscitation (ILCOR).L. B., declares a relationship with government agencies and grants from Merck, GSK, Novartis, Sanofi, and Pfizer. S.A. H., declares grants from GSK, Sanofi, Novartis, Pfizer, Merck, PREVENT, ImmunoVaccine, NovaVax, Janssen, and Folia.
Contributor Information
Collaborators: HELPinKIDS Team
REFERENCES
- 1.Rifkin-Graboboi A, Borelli JL, Bosquet Enlow M.Zeanah CH. Neurobiology of stress in infancy. Handbook of Infant Mental Health, 3rd ed New York: Guilford Press; 2009:59–79. [Google Scholar]
- 2.Taddio A, Appleton M, Bortolussi R, et al. Reducing the pain of childhood immunization–an evidence-based clinical practice guideline. Can Med Assoc J. 2010;182:E843–E855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taddio A, Appleton M, Bortolussi B, et al. Reducing the pain of childhood vaccination—an evidence-based clinical practice guideline. CMAJ. 2010;182:E843–E855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birnie K, Chambers C, Taddio A, et al. Psychological interventions for vaccine injections in children and adolescents: systematic review of randomized controlled trials and quasi-randomized controlled trials. Clin J Pain. 2015;31(10S):S72–S89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boerner KE, Birnie KA, Chambers CT, et al. HELPinKIDS&Adults Team. Simple psychological interventions for reducing pain from common needle procedures in adults: systematic review of randomized and quasi-randomized controlled trials. Clin J Pain. 2015;31(10S):S90–S98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah VS, Taddio A, McMurtry CM, et al. Pharmacological and combined interventions to reduce vaccine injection iain in children and adults; systematic review and meta-analysis. Clin J Pain. 2015;31(10S):S38–S68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taddio A, Shah VS, McMurtry CM, et al. Procedural and physical interventions for vaccine injections: systematic review of randomized controlled trials and quasi-randomized controlled trials. Clin J Pain. 2015;31(10S):S20–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pillai Riddell RR, Racine NM, Turcotte K, et al. Non-pharmacological management of infant and young child procedural pain. Cochrane Database Systc Rev. 2011;10:CD006275. [DOI] [PubMed] [Google Scholar]
- 9.Taddio A, McMurtry CM, Shah V, et al. Methodology for knowledge synthesis of the management of vaccination pain and needle fear. Clin J Pain. 2015;31(10S):S12–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guyatt GH, Oxman AD, Schunemann HJ, et al. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64:380–382. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JPT, Green S, (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from http://www.cochrane-handbook.org.
- 12.Pillai Riddell RR, Racine N, Craig K, et al. McGrath P, Stevens B, Walker S, Zempsky W. Psychological theories and biopsychosocial models in pediatric pain. The Oxford Textbook of Pediatric Pain. United Kingdom: Oxford; 2013. [Google Scholar]
- 13.Pillai Riddell RR, Stevens BJ, Cohen LL, et al. Predicting maternal and behavioral measures of infant pain: the relative contribution of maternal factors. Pain. 2007;133:138–149. [DOI] [PubMed] [Google Scholar]
- 14.Cohen LL. Reducing infant immunization distress through distraction. Health Psychol. 2002;21:207–211. [PubMed] [Google Scholar]
- 15.Cohen LL, Bernard RS, McClellan CB, et al. Topical anesthesia versus distraction for infants’ immunization distress: evaluation with 6-month follow up. Child Health Care. 2006;35:103–121. [Google Scholar]
- 16.Cohen LL, MacLaren JE, Fortson BL, et al. Randomized clinical trial of distraction for infant immunization pain. Pain. 2006;125:165–171. [DOI] [PubMed] [Google Scholar]
- 17.Gedam DS, Verma M, Patil U, et al. Effect of distraction technique during immunization to reduce behaviour response score (FLACC) to pain in toddlers. J Nepal Paediatr Soc. 2013;33:25–30. [Google Scholar]
- 18.Hillgrove-Stuart J, Pillai Riddell R, Horton R, et al. Toy-mediated distraction: clarifying the role of agent of distraction and preneedle distress in toddlers. Pain Res Manag. 2013;18:197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cramer-Berness LJ. A Comparison of Behavioral Interventions for Infant Immunizations [dissertation]. UMI Dissertations Publishing; 2005:3203883.
- 20.Cramer-Bernss LJ, Friedman AG. Behavioral interventions for infant immunizations. Child Health Care. 2005;34:95–111. [Google Scholar]
- 21.Singh P. Effect of distraction techniques in behaviour responses to pain among toddlers receiving immunisation. Nurs J India. 2012;103:176–179. [PubMed] [Google Scholar]
- 22.Ozdemir FK, Tufekci FG. The effect of using musical mobiles on reducing pain in infants during vaccination. J Res Med Sci. 2012;17:662–667. [PMC free article] [PubMed] [Google Scholar]
- 23.Basiri-Moghadam M, Kianmehr M, Pasban-Noghabi S, et al. Comparison of EMLA cream with rattles on reducing immunization pain in four months infants. JPMA. 2014;64:874–878. [PubMed] [Google Scholar]
- 24.Pillai Riddell RR, Flora DB, Stevens SA, et al. Variability in infant acute pain responding meaningfully obscured by averaging pain responses. Pain. 2013;154:714–721. [DOI] [PubMed] [Google Scholar]
- 25.Pillai Riddell RR, Chambers CT.Anand KJ, Stevens BJ, McGrath P. Parenting and pain during infancy. Pain in Neonates and Infants, 3rd ed Edinburgh: Elsevier Limited; 2007:289–298. [Google Scholar]
- 26.Ahola S, Pillai Riddell RR. Does the Neonatal Facial Coding System differentiate between infants experiencing pain-related and non-pain-related distress? J Pain. 2009;10:214–220. [DOI] [PubMed] [Google Scholar]
- 27.Lisi D, Cambell L, Pillai Riddell R, et al. Naturalis parental pain management during immunizations during the first year of life: observational norms from the OUCH cohort. Pain. 2013;54:1245–1253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


