Supplemental Digital Content is available in the text.
Key Words: pain management, randomized controlled trial, systematic review, vaccination, psychological, children, adolescents
Abstract
Background:
This systematic review evaluated the effectiveness of psychological interventions for reducing vaccination pain and related outcomes in children and adolescents.
Design/Methods:
Database searches identified relevant randomized and quasi-randomized controlled trials. Data were extracted and pooled using established methods. Pain, fear, and distress were considered critically important outcomes.
Results:
Twenty-two studies were included; 2 included adolescents. Findings showed no benefit of false suggestion (n=240) for pain (standardized mean difference [SMD] −0.21 [−0.47, 0.05]) or distress (SMD −0.28 [−0.59, 0.11]), or for use of repeated reassurance (n=82) for pain (SMD −0.18 [−0.92, 0.56]), fear (SMD −0.18 [−0.71, 0.36]), or distress (SMD 0.10 [−0.33, 0.54]). Verbal distraction (n=46) showed reduced distress (SMD −1.22 [−1.87, −0.58]), but not reduced pain (SMD −0.27 [−1.02, 0.47]). Similarly, video distraction (n=328) showed reduced distress (SMD −0.58 [−0.82, −0.34]), but not reduced pain (SMD −0.88 [−1.78, 0.02]) or fear (SMD 0.08 [−0.25, 0.41]). Music distraction demonstrated reduced pain when used with children (n=417) (SMD −0.45 [−0.71, −0.18]), but not with adolescents (n=118) (SMD −0.04 [−0.42, 0.34]). Breathing with a toy (n=368) showed benefit for pain (SMD −0.49 [−0.85, −0.13]), but not fear (SMD −0.60 [−1.22, 0.02]); whereas breathing without a toy (n=136) showed no benefit for pain (SMD −0.27 [−0.61, 0.07]) or fear (SMD −0.36 [−0.86, 0.15]). There was no benefit for a breathing intervention (cough) in children and adolescents (n=136) for pain (SMD −0.17 [−0.41, 0.07]).
Conclusions:
Psychological interventions with some evidence of benefit in children include: verbal distraction, video distraction, music distraction, and breathing with a toy.
Vaccine injections are unique in that they are regularly experienced by children who are healthy as well as those who have chronic illness, making them the most common painful medical procedure performed worldwide.1 Multipronged approaches to pain management include pharmacological, psychological, procedural, and physical strategies, all of which have been studied to reduce the pain and distress associated with vaccine injections.2–4 Of these approaches, psychological interventions hold considerable appeal to families given that they capitalize on strategies that children and parents already engage in naturally to some extent (eg, distraction), and, due to their nonpharmacological nature, are generally met with higher acceptance by parents. Many psychological interventions are simple and require minimal or no training, are able to be implemented directly by children, parents, and immunizers, and are applicable across a wide age range. Furthermore, they generally capitalize on available resources, making them easy to implement across different clinical settings.5
In a previous knowledge synthesis on this topic, support was found for several different psychological interventions for vaccination pain, including breathing exercises, child-led or nurse-led distraction, and combined cognitive-behavioral interventions (ie, strategies aimed at modifying emotions, behaviors, and cognitions).3 These interventions were subsequently incorporated into a clinical practice guideline for childhood vaccination pain management.6 Since the original guideline was developed, additional research in the area has been published. Furthermore, the previous systematic review and meta-analysis grouped together infants and children, and omitted adolescents; this led to a gap in knowledge synthesis and recommendations for each pediatric population who present unique developmental considerations.3 Given recent evidence suggesting possible differences in treatment efficacy based on intervention characteristics,5 alternative approaches to examining the literature are warranted, in particular, the type of distracter used. Our previous synthesis examined the literature according to the individual directing the intervention.3 The current systematic review and meta-analysis was therefore undertaken to provide the evidence base for an update and expansion of the original guideline in the specific area of psychological interventions for children and adolescents undergoing vaccine injections and evaluated the data according to the type of distractor used.
This review reports the results for trials that evaluated the effect of any of the following psychological interventions for the management of vaccination pain and related outcomes: (1) false suggestion, (2) repeated reassurance, (3) verbal distraction, (4) video distraction, (5) music distraction, (6) breathing with toy, (7) breathing without toy, and (8) breathing intervention (cough). Separate papers explore the effectiveness of psychological interventions in young children (0 to 3 y)7 and adults,8 as well as pharmacological, physical, procedural, and process approaches for infants, children, adolescents, and adults.
METHODS
This systematic review was conducted as part of the Canadian multidisciplinary Help ELiminate Pain in Kids and Adults (HELPinKids&Adults) team, which was assembled with the goal of developing an evidenced-based clinical practice guideline, and undertaking knowledge translation activities, for reducing vaccination pain. As such, an identical methodological approach was applied across reviewed areas (psychological, pharmacological, physical, procedural, and process) for reducing vaccination injection pain. A separate manuscript describes this methodological approach in greater detail.9
In brief, systematic review and meta-analytic methodologies were informed by GRADE (Grading of Recommendations, Assessment, Development and Evaluation)10 and the Cochrane Collaboration.11 The search was developed in consultation with an experienced librarian and included the following databases: EMBASE, Medline, PsycINFO, and CINAHL. Search results were screened for eligibility.9 Peer-reviewed publications (full or short report) and published academic theses/dissertations were included. Through a voting process, the HELPinKids&Adults team identified clinical questions (ie, psychological interventions) to be examined, as well as critical and important outcomes to be included in each review. Specifically, candidate questions were identified based on prior clinical practice guidelines,3 clinical experience, and knowledge of existing research. Clinical questions were retained if considered important by at least two-thirds of the HELPinKids&Adults team, and were modified as appropriate after preliminary review and discussion of the research evidence by the HELPinKids&Adults team.9 Two of the included clinical questions pertained to individuals across the lifespan (ie, use of false suggestion or repeated reassurance); however, evidence was only available from children.
This review focused on studies of psychological interventions including children (aged above 3 to 12 y) and adolescents (aged above 12 to 17 y) undergoing vaccination in any setting using randomized or quasi-randomized study designs. Only simple psychological interventions were sought for inclusion (ie, those involving distraction, and/or interactions between children and parent/nurse/immunizer). More complex psychological interventions, such as hypnosis, were not included, as they typically require special training to be implemented. Psychological interventions related to treating high needle fear are discussed in another review in this series.12 Pain and fear were typically prioritized as critically important outcomes, respectively, defined as self-report of pain or self-report of fear during vaccination. The overall effectiveness of an intervention was determined according to the effects on critically important outcomes. Distress was also accepted as a critically important outcome if children below 7 years were included in the evidence base, due to the possibility that self-report was unreliable.13–15 Distress was defined as observer ratings of an individual’s behavioral response during vaccination (ie, pain, fear, distress). When available, other important outcomes included procedure outcomes (eg, procedure success, duration), parent fear, use of the intervention, compliance, memory, preference, and satisfaction.
As per the standard approach across reviewed content areas,9 outcomes that were assessed at multiple time-points during the vaccination procedure were analyzed as follows: (1) the preprocedure phase, which occurred postintervention but before vaccine injection(s); (2) the acute phase (within the first minute of needle puncture and vaccine injection); and (3) the recovery phase (1 to 5 min after vaccine injection(s)). Phases were combined when outcomes were not assessed separately for each phase (eg, acute+recovery). Delayed onset of pain (ie, pain occurring hours to days after injection) was not considered. Data from multiple observers assessing the same outcome (eg, parent-rated child distress, clinician-rated child distress, observational behavior coding) was combined into a single point estimate and associated variance before inclusion in the meta-analysis using established methods.16
Attempts were made to contact study authors when data necessary for pooling were not included in published papers (ie, means, SDs). An emphasis was placed on including data from all possible studies. As such, when means and SDs were not available, they were estimated from medians, ranges, SEs, 95% confidence intervals (CIs), or graphs. This was done only as needed, on a very restricted predefined basis, and followed established methods.17
Data was pooled using RevMan (version 5.2, Cochrane Collaboration, Copenhagen, Denmark), and effects of interventions were expressed as a standardized mean difference (SMD) with accompanying 95% CI or relative risk and CI, as appropriate. Separate analyses were conducted for children (above 3 to 12 y old) and adolescents (above 12 to 17 y old) when possible. A random effects model was used for all analyses. Statistical heterogeneity was assessed using I2 and χ2 tests. Additional post hoc analyses were carried out to examine the effects of study methodology and/or heterogeneity. Risk of bias was assessed for critical outcomes for all included studies using the Cochrane risk of bias tool (https://bmg.cochrane.org/assessing-risk-bias-included-studies).
Evidence profiles and summary of findings tables were created using the GRADE profiler software (version 3.6.1). When analyses demonstrated a consistent benefit of the intervention across critically important outcomes, it was said to have “benefit across all measured outcomes.” Findings were described as “mixed” when results were inconsistent across critically important outcomes, and were described as having “no evidence of a benefit” when any statistical evidence of benefit was lacking.
RESULTS
Database searches returned a total of 114,251 citations, including 32,155 duplicates. An additional 138 citations were identified from manual searches. The remaining 82,234 citations were reviewed for eligibility by 2 members of the HELPinKids&Adults (Help ELiminate Pain in Kids and Adults) Team: Taddio, A., McMurtry C.M., Chambers C.T., Pillai Riddell R., Shah V., Noel M., MacDonald N.E., Rogers J., Bucci L., Mousmanis P., Halperin S.A., Bowles S., Halpert C., Ipp M., Rieder M., Robson K., Asmundson G.J.G., Antony M., Alexander D., Appleton M., Dubey V., Hanrahan A., Lockett D., Scott J., Votta Bleeker E.). Twenty-two studies investigating psychological interventions in children and/or adolescents were identified and included in the review.18–39 In 1 case, there were multiple publications emanating from the same study, including a dissertation and published manuscript of the same data.37 Most studies used a between-subjects (parallel) design (n=20), with 2 studies using a within-subjects (cross-over) design.23,38 Data were provided for 2 or more treatment groups from all trials. Three trials examined multiple psychological interventions, with different treatment groups included in their respective clinical questions.27,29,33 Twenty studies included children only (above 3 to 12 y old), 1 study included adolescents only (above 12 to 17 y old), and 1 study included both children and adolescents. Two studies were excluded due to the: (1) study design not being randomized or quasi-randomized (n=1)40; and (2) intervention was not psychological (n=1).41 See Figure 1 for a flowchart depicting study identification, screening, and inclusion. Table 1 outlines the clinical questions and critically important and important outcomes. Table 2 describes characteristics of included trials for each clinical question examining psychological interventions in children and/or adolescents.
FIGURE 1.
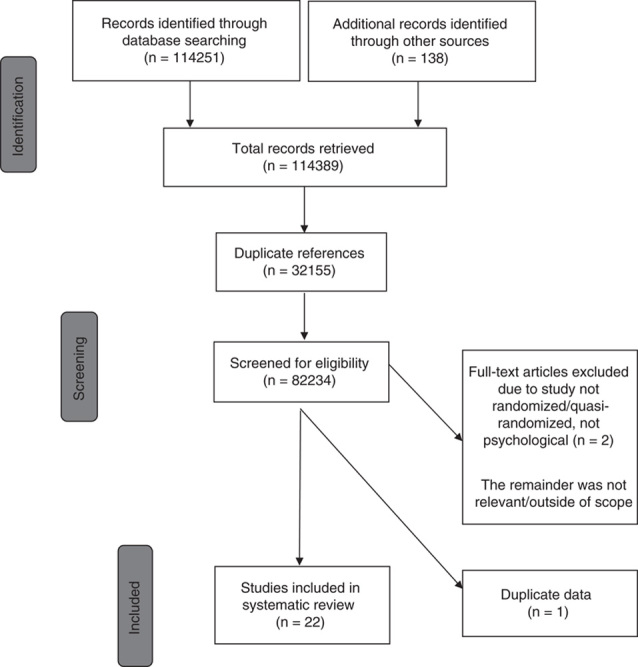
Flowchart of study identification, screening, and inclusion.
TABLE 1.
Clinical Questions and Outcomes
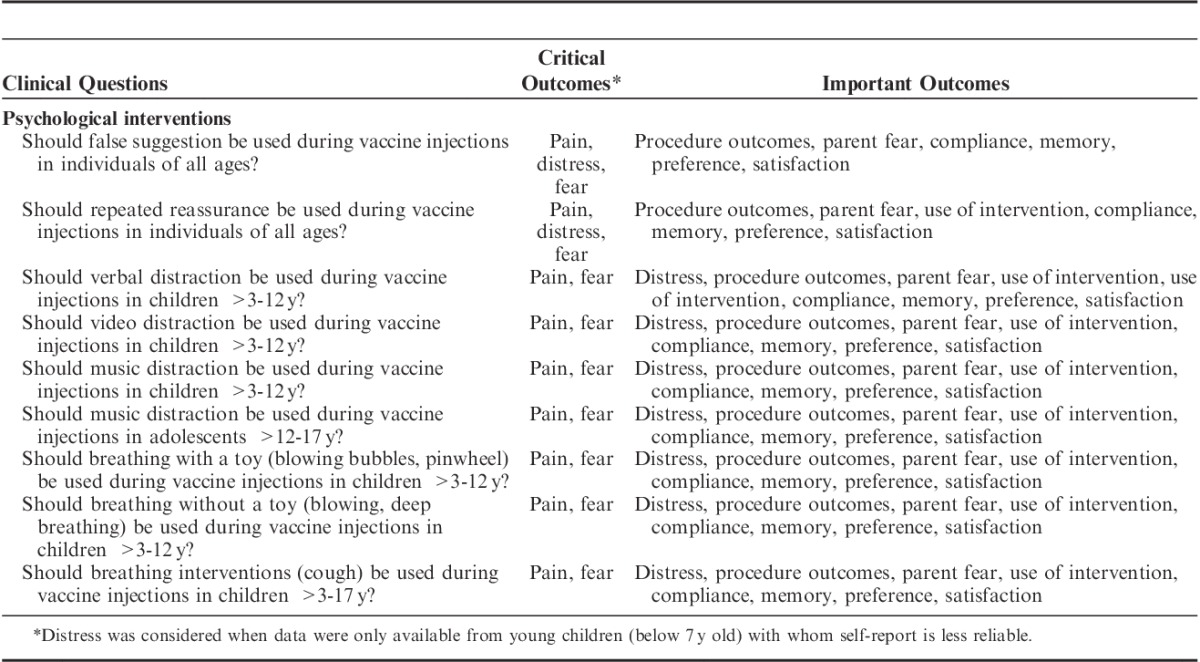
TABLE 2.
Characteristics of the Trials Included in the Systematic Review

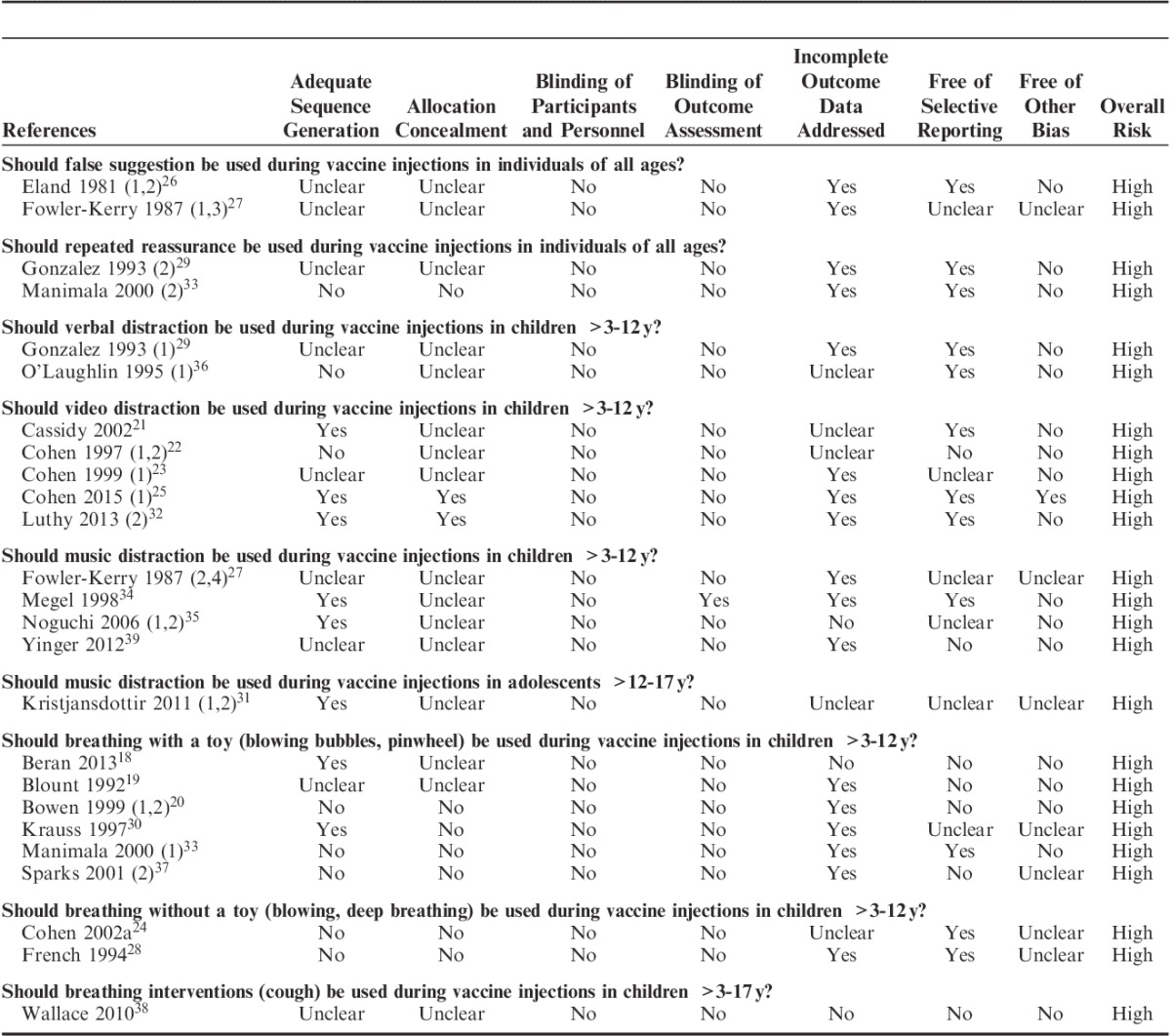
Quality of Studies and Risk of Bias
Assessment of risk of bias for all included trials for critically important outcomes are reported in Table 3. All trials had high overall risk of bias, primarily due to lack of blinding of: participants, clinicians administering the intervention, and/or individual providing the ratings of critically important outcomes of pain, fear, and/or distress.
TABLE 3.
Assessment of Risk of Bias of Included Trials for Critical Outcomes
Overall Quality of Evidence and Treatment Effects
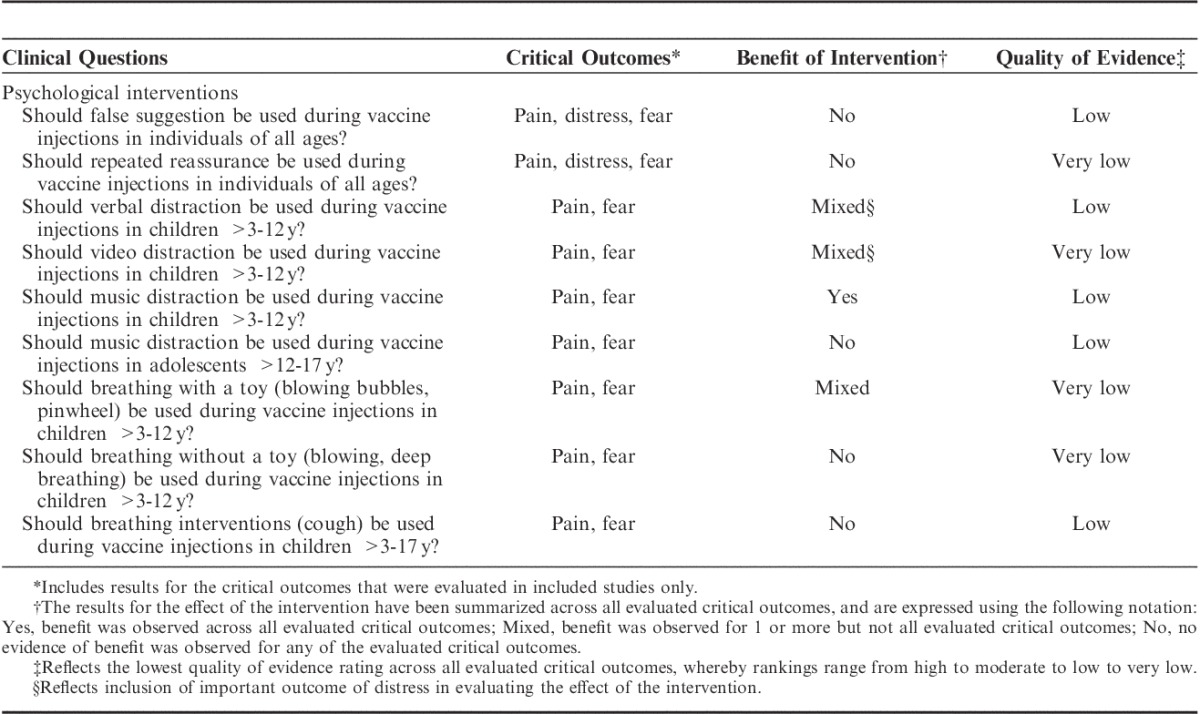
For all clinical questions, results for critically important outcomes only are described below, and are summarized in Table 4. More detailed GRADE Evidence Profiles and Summary of Findings tables (Tables, Supplemental Digital Content 1 to 9, http://links.lww.com/CJP/A183, http://links.lww.com/CJP/A184, http://links.lww.com/CJP/A185, http://links.lww.com/CJP/A186, http://links.lww.com/CJP/A187, http://links.lww.com/CJP/A188, http://links.lww.com/CJP/A189, http://links.lww.com/CJP/A190, http://links.lww.com/CJP/A191,) and accompanying Forest plots (Figures, Supplemental Digital Content 1 to 9, http://links.lww.com/CJP/A192, http://links.lww.com/CJP/A193, http://links.lww.com/CJP/A194, http://links.lww.com/CJP/A195, http://links.lww.com/CJP/A196, http://links.lww.com/CJP/A197, http://links.lww.com/CJP/A198, http://links.lww.com/CJP/A199, http://links.lww.com/CJP/A200), for all critically important and important outcomes are provided as Supplemental Digital Content.
TABLE 4.
Summary of Results for Critically Important Outcomes
Should False Suggestion be Used During Vaccine Injections in Individuals of All Ages?
Two trials including 240 children aged 4 to 7 years investigated the impact of false suggestion.26,27 In both trials, children were told by the immunizer or researcher that something was being done to help make the injection easier or less painful. Depending on the treatment group, this was accompanied by a potentially pain reducing intervention (ie, music distraction or vapocoolant) or a placebo (ie, wearing headphones with no music or aerosol spray). There was low quality of evidence for the critically important outcome of pain, largely due to inconsistent blinding of immunizer and outcome assessor, as well as selective outcome reporting (Table, Supplemental Digital Content 1, http://links.lww.com/CJP/A183). Both trials found no benefit of suggestion for the critically important outcome of pain: SMD −0.21 (−0.47, 0.05). Findings were consistent with and without the data from false suggestion with placebo intervention groups. No trials examined the critically important outcome of fear. Given the young age of participants, distress was also examined for the 1 trial containing these data,26 which showed no benefit of suggestions for preprocedural distress: SMD −0.28 (−0.91, 0.34) (Table, Supplemental Digital Content 1, http://links.lww.com/CJP/A183 and Figure, Supplemental Digital Content 10, http://links.lww.com/CJP/A192). No other important outcomes were assessed.
Should Repeated Reassurance be Used During Vaccine Injections in Individuals of All Ages?
Two trials including 82 children aged 3 to 7 years investigated repeated reassurance by parents during vaccination.29,33 Parents were trained before the procedure through oral instruction, modeling, and practice; during the vaccination, parents were repeatedly prompted to engage in reassurance. For example, saying reassuring statements such as “You’re ok” or “It’s almost over.” There was low quality of evidence for the critically important outcome of pain, and very low quality of evidence for the critically important outcome of fear, largely due to inconsistent blinding of participants, immunizers, and outcome assessors, and contamination of treatment effects in the control group (ie, parents engaging in reassurance) (Table, Supplemental Digital Content 2, http://links.lww.com/CJP/A184). One trial29 found no benefit for the critically important outcome of pain (SMD −0.18 [−0.92, 0.56]), whereas the other trial33 found no benefit for the critically important outcome of preprocedural fear (SMD −0.18 [−0.71, 0.36]). Given the young age of participants, distress was also examined (preprocedure, acute, and recovery distress combined) and showed no benefit of repeated reassurance in both trials: SMD 0.10 (−0.33, 0.54) (Table, Supplemental Digital Content 2, http://links.lww.com/CJP/A184 and Figure, Supplemental Digital Content 11, http://links.lww.com/CJP/A193). Other assessed important outcomes included parent fear and parent use of intervention.
Should Verbal Distraction be Used During Vaccine Injections in Children >3 to 12 Years?
Two trials including 46 children aged 3 to 7 years investigated the impact of verbal distraction.29,36 Verbal distraction involved an adult attracting the child’s attention away from the needle by using their voice only; no additional physical, visual, or auditory distracter is used. In both trials, verbal distraction was provided by mothers who received instruction (written or oral) about how to engage in distraction with their child during the vaccine injection (eg, talking, counting, singing, reciting a poem/rhyme). There was low quality of evidence for outcome data pertaining to all assessed outcomes, largely due to lack of blinding of immunizers and/or outcome assessors (Table, Supplemental Digital Content 3, http://links.lww.com/CJP/A185). Only 1 trial29 examined the critically important outcome of pain and found no benefit of verbal distraction: SMD −0.27 (−1.02, 0.47). No trials examined the critically important outcome of fear. Given the young age of participants, distress was also examined, which contained data from both trials (preprocedure, acute, and recovery distress combined) and showed a significant benefit of verbal distraction: SMD −1.22 (−1.87, −0.58) (Table, Supplemental Digital Content 3, http://links.lww.com/CJP/A185 and Figure, Supplemental Digital Content 12, http://links.lww.com/CJP/A194). Other assessed important outcomes included parents’ use of the intervention.
Should Video Distraction be Used During Vaccine Injections in Children >3 to 12 Years?
Five trials including 328 children aged 2 to 12 years investigated the impact of video distraction.21–23,25,32 In these studies, interventions generally involved having the child watch an age-appropriate movie on a television screen or portable DVD player. In 3 trials, children were able to choose from a selection of movies and received additional distraction coaching while watching the movie from a nurse and/or parent.22,23,25 There was very low quality of evidence for outcome data pertaining to critically important outcomes of pain and fear, largely due to lack of blinding of immunizers and/or outcome assessors, inclusion of cross-over and quasi-randomized trials, and possible contamination of treatment effects in control groups (eg, engaging in distraction) (Table, Supplemental Digital Content 4, http://links.lww.com/CJP/A186). Four trials21–23,25 found no benefit for the critically important outcome of pain: SMD −0.88 (−1.78, 0.02). Only 1 trial23 examined the critically important outcome of fear and also found no benefit of video distraction: SMD 0.08 (−0.25, 0.41). The important outcome of distress was also considered given the younger age of children (below 7 y old) providing self-report in 3 of 4 trials, the reliance on data from a single cross-over study for self-reported fear, as well as the inclusion of 1 trial that did not examine critically important outcomes of pain or fear.32 All 5 trials examined distress during at least 1 phase of treatment, with evidence of benefit of video distraction during the preprocedure (SMD −0.65 [−1.18, −0.12]), acute (SMD −0.96 [−1.85, −0.08]), and preprocedure+acute+recovery (SMD −0.58 [−0.82, −0.34]) phases (Table, Supplemental Digital Content 4, http://links.lww.com/CJP/A186 and Figure, Supplemental Digital Content 13, http://links.lww.com/CJP/A195). Other assessed important outcomes included parent fear, immunizer fear, parent and child preferences, and use of intervention by children, parents, and/or immunizers.
Should Music Distraction be Used During Vaccine Injections in Children >3 to 12 Years?
Four trials including 417 children aged 3 to 7 years investigated the impact of music distraction in children.27,34,35,39 In 3 of these studies, children listened to music using headphones27,34,35; in 1 study, children engaged in live music with a music therapist.39 There was low quality of evidence for outcome data pertaining to the critically important outcome of pain and important outcome of distress, largely due to inconsistent blinding of participants, immunizers, and outcome assessors (Table, Supplemental Digital Content 5, http://links.lww.com/CJP/A187). Three trials that could be pooled for the critically important outcome of pain27,34,35 found a benefit of music distraction: SMD −0.45 (−0.71, −0.18) (Table, Supplemental Digital Content 5, http://links.lww.com/CJP/A187 and Figure, Supplemental Digital Content 14, http://links.lww.com/CJP/A196). No trials examined the critically important outcome of fear.
Given the young age of participants, the important outcome of distress was also considered and was assessed in 2 trials at various phases of the procedure. There was a beneficial effect on preprocedure distress (SMD −0.48 [−0.86, −0.10]) and acute distress (SMD −0.49 [−0.87, −0.11]). Whereas, there was no benefit on distress during the acute plus recovery phases combined (SMD −0.27 [−0.65, 0.10]), or during the recovery phase only (SMD −0.09 [−0.46, 0.29]) (Table, Supplemental Digital Content 5, http://links.lww.com/CJP/A187 and Figure, Supplemental Digital Content 14, http://links.lww.com/CJP/A196). Other assessed important outcomes included procedure duration, parent preferences, and child use of intervention.
Should Music Distraction be Used During Vaccine Injections in Adolescents >12 to 17 Years?
One trial including 118 adolescents aged 13 to 15 years investigated the impact of music distraction in adolescents.31 In this trial, adolescents listened to music of their choice from an available selection. Half of adolescents who received the intervention wore headphones, whereas the other half did not. There was low quality of evidence due to lack of blinding of participants, who reported no benefit of the intervention for the critical outcome of pain: SMD −0.04 (−0.42, 0.34) (Table, Supplemental Digital Content 6, http://links.lww.com/CJP/A188 and Figure, Supplemental Digital Content 15, http://links.lww.com/CJP/A197). No data were available for the critically important outcome of fear or other important outcomes.
Should Breathing With a Toy (Blowing Bubbles, Pinwheel) be Used During Vaccine Injections in Children >3 to 12 Years?
Six trials including 368 children aged 3 to 9 years investigated the impact of breathing with a toy in children.18,19,20,30,33,37 In all studies, children were directed to blow on a toy (ie, party blower, pinwheel, bubbles, small toy). One study provided instruction from a robot to blow on a dusty toy,18 and in 3 trials, children were supported with additional rehearsal or coaching from parents, researchers, or immunizers.19,30,33 There was very low quality of evidence for outcome data pertaining to critically important outcomes of pain and fear, largely due to lack of blinding of immunizers, participants, and/or outcome assessors, inclusion of quasi-randomized trials, and possible contamination of treatment effects in control groups (Table, Supplemental Digital Content 7, http://links.lww.com/CJP/A189).
Two trials18,37 found a benefit of breathing with a toy for the critically important outcome of pain: SMD −0.49 (−0.85, −0.13), whereas 2 different trials20,33 found no benefit of breathing with a toy for the critically important outcome of fear preprocedure (SMD −0.53 [−1.07, 0.01]) or acute fear (SMD −0.60 [−1.22, 0.02]).
Given the young age of participants, the important outcome of distress was also considered. Two trials18,20 found a benefit of breathing with a toy for acute distress: SMD −0.80 (−1.17, −0.42); and 4 trials18,19,30,33 found a benefit for preprocedure+acute+recovery phases combined: SMD −0.55 (−0.82, −0.28) (Table, Supplemental Digital Content 7, http://links.lww.com/CJP/A189 and Figure, Supplemental Digital Content 16, http://links.lww.com/CJP/A198). Other assessed important outcomes included parent fear, child and parent use of intervention, and child and parent preferences.
Should Breathing Without a Toy (Blowing, Deep Breathing) be Used During Vaccine Injections in Children >3 to 12 Years?
Two trials including 136 children aged 3 to 7 years investigated the impact of breathing without a toy in children.24,28 In 1 study, children were taught deep breathing, in addition to coping skills24; in the other study, were instructed to blow out air during the injection.28 In both trials, children were given time to practice the skills before the injection. There was very low quality of evidence for outcome data pertaining to critically important outcomes of pain and fear, largely due to lack of blinding of participants and outcome assessors, inclusion of quasi-randomized trials, and selective outcome reporting (Table, Supplemental Digital Content 8, http://links.lww.com/CJP/A190). Both trials found no benefit of breathing without a toy for the critically important outcome of pain: SMD −0.27 (−0.61, 0.07). One trial24 examined the critically important outcome of fear and found no benefit of the intervention: SMD −0.36 (−0.86, 0.15). Given the young age of participants, the important outcome of distress was also considered. Distress was examined in both trials, although the phase of procedure was unclear. No benefit of breathing without a toy was observed: SMD −0.27 (−0.61, 0.07) (Table, Supplemental Digital Content 8, http://links.lww.com/CJP/A190 and Figure, Supplemental Digital Content 17, http://links.lww.com/CJP/A199). No other important outcomes were assessed.
Should a Breathing Intervention (Cough) be Used During Vaccine Injections in Children >3 to 17 Years?
One trial including 136 children (aged 4 to 5 y) and adolescents (aged 11 to 13 y) investigated the impact of a breathing intervention (cough).38 Children and adolescents were asked to cough once before and once at the time of the injection. There was low quality of evidence for outcome data pertaining to the critically important outcome of pain, largely due to lack of blinding of participants and inclusion of a cross-over trial (Table, Supplemental Digital Content 9, http://links.lww.com/CJP/A191). No benefit was found for the critically important outcome of pain: SMD −0.17 (−0.41, 0.07). No data were available for the critically important outcome of fear (Table, Supplemental Digital Content 9, http://links.lww.com/CJP/A191 and Figure, Supplemental Digital Content 18, http://links.lww.com/CJP/A200). Other assessed important outcomes included distress and child satisfaction.
DISCUSSION
This systematic review was conducted to investigate the effectiveness of various psychological interventions used by children, adolescents, their parents, and/or immunizers to reduce adverse effects from vaccine injections including pain and pain-related outcomes. Only simple psychological interventions were considered, such as those including distraction and/or interactions between children and parents, nurses, and/or immunizers. There was some evidence to support the following interventions in children: verbal distraction, video distraction, music distraction, and breathing with a toy. Available evidence was insufficient to support the following interventions with children: false suggestion, repeated reassurance, and breathing without a toy. There was insufficient evidence to support use of breathing intervention (cough) with children or adolescents, or use of music distraction with adolescents.
The only psychological intervention with consistent evidence supporting its use across pain and pain-related outcomes was music distraction in children younger than 12 years old. Benefit was shown in studies that used age-appropriate recorded music delivered to children using headphones, as well as more involved live music distraction interventions provided by a music therapist. Behavioral distraction (ie, requiring children to do something distracting) is a generally effective coping strategy in young children,42 and in most of the included trials, children received additional support to engage fully with the music. The positive benefit of music in children is promising, as it can rely on minimal resources and no training to be implemented effectively by parents or immunizers. In general, music seems to be an effective pain management strategy for children, with supportive evidence from other types of medical procedures.43
The results were mixed regarding the benefit of verbal distraction in children. Child ratings of pain indicated no benefit from the intervention, whereas observer ratings of the child’s distress were reduced. In both trials, mothers received instruction on how to verbally distract their child by counting, singing, or talking about topics other than the vaccine injection. This pattern of findings, including benefit for reducing observed child distress but not self-reported pain, has been noted in studies examining parent-led distraction for other types of needle procedures.44 Although providing instruction to parents was shown to increase their use of distraction with their child during vaccine injections, equivocal findings with regards to self-reported pain may be explained due to the mix of parent behaviors observed in both the distraction intervention and the control group.29 More specifically, some mothers in the distraction intervention group also engaged in behaviors that have been shown to increase children’s pain (ie, reassurance),45 and some mothers in the control group naturally engaged in distraction.29 Although not examined in the included trial, increased doses of verbal distraction from parents have been associated with greater reduction of pain and distress in children undergoing other needle procedures regardless of training in verbal distraction.46 Furthermore, not all parents are effective distraction coaches. In particular, highly distressed parents seem less able to successfully distract their child.47,48 None of the included studies examined nurse-led verbal distraction; however, other nurse-led psychological interventions have previously been shown to be effective for vaccine injections,3 and may pose a reasonable alternative when parents are highly distressed. Relatively minor resources are needed to instruct parents in use of verbal distraction (eg, providing a pamphlet).36 Furthermore, parents are typically present at vaccine injections with young children, making this a very feasible intervention to implement.
The results were also mixed for video distraction with demonstrated benefit of reduced distress across all procedure phases (pre, acute, and recovery), but not reduced pain or fear. Given that distraction is most effective when it is interesting, enjoyable, and engaging, the child’s ability to choose and interact with the video distracter may be critical.49 The reviewed video distraction interventions generally relied on older technology (ie, DVD players and televisions). This may pose some impediment to clinical settings when required resources are limited or unavailable for families to use. Readily available smartphones and smart devices offer a feasible and promising alternative, and are already being used by some in clinical practice to manage pediatric procedural pain.50 In support of this hypothesis, a recent nonrandomized study reported reduced distress in children aged 2 to 5 when iPads were used to distract them during immunizations; however, it should be noted that lack of randomization makes this study at high risk of bias.40 Interactive distraction interventions show some evidence for increased efficacy over more passive distraction for reducing distress during pediatric needle procedures.5 Given the many highly interactive videos and games available on smart devices, their use for vaccine injections is worthy of future research.
Findings showed mixed benefit for the use of breathing with a toy, but no support for breathing without a toy or for a breathing intervention (cough). The type of breathing children and/or adolescents were instructed to do as part of these interventions (ie, blowing out air, coughing) may have been insufficient to induce any sort of relaxation response and/or distract children on their own. Research has shown that relaxation during breathing is an important mechanism for modulating physiological responses to stress and influencing pain perception, as compared with simply attending to the breath in the absence of efforts to relax.51 It is likely that the small toys that assisted children during the “breathing with a toy” interventions also served as distracters (eg, bubbles, pinwheel, party blower), thereby potentially bolstering the effectiveness of the intervention on pain. As is noted in this review and in others, distraction is a generally effective strategy for reducing pain and pain-related outcomes during pediatric medical procedures.5,49,52 Thus, the availability of a toy may have enhanced the efficacy of breathing alone by enhanced distraction. However, no trials provided a head-to-head comparison of breathing with and without a toy, making it difficult to conclude what components of the intervention were the most effective.
Behaviors of parents and other adults (eg, nurses) have received extensive study in the context of pediatric medical procedures, and have been shown repeatedly to exert helpful and unhelpful influences on children’s pain and distress.53 Although seemingly counterintuitive, a generally consistent finding is that reassurance seems to be unhelpful for children when they are in pain.54,55 One reason may be because children perceive adults as being worried when they reassure, which may in turn increase child distress.45 Although there may be forms of reassurance that are more helpful than others,45 the lack of benefit for repeated reassurance found for vaccine injections in this review is consistent with extant research, and is thus, not recommended when other adult behaviors, such as distraction, are helpful. Although the evidence base consisted of children only, the counterintuitive relationship between reassurance and increased distress has been found in infants54,56,57; furthermore, although in a different context, medically focused reassurance has also been shown to be ineffective for adults with high levels of health anxiety.58
Included trials also assessed the impact of an adult (nurse or researcher) suggesting to the child that something was being done to help make the injection easier. As with repeated reassurance, false suggestion showed no benefit for reducing children’s pain or distress. Suggestion showed no benefit in and of itself, indicating that there was no observed placebo effect induced by a simple statement that some sort of pain management was being used in the absence of a real intervention. There was also no benefit for suggestion used as a means of enhancing the efficacy of another intervention (ie, distraction or vapocoolant). Use of false suggestion as a placebo or to overstate the efficacy of an intervention may be perceived by the person being immunized as deceitful and may lead to distrust of immunizers and health care professionals more broadly, potentially leading to noncompliance with medical care. Thus, use of false or simplistic suggestion may be problematic for individuals of any age being immunized across the lifespan.
Other than the breathing intervention (cough), the only other psychological intervention studied in adolescents was music distraction. In contrast to the clear benefits of music distraction for children below 12 years old, no support was found for use of music distraction with adolescents above 12 years old. This is consistent with a recent systematic review and meta-analysis that found no evidence supporting use of distraction to reduce pain and distress in adolescents across all types of needle procedures.5 Three of the 4 distraction interventions studied, in samples of predominantly adolescents, included music.59-61 However, evidence for music distraction in adolescents based on a single trial in the current review, and the ability to detect differences between treatment and control groups may have been impeded by the very low levels of self-reported pain following the injection in both groups (ie, average pain <1/10) in this study, potentially introducing floor effects.31 Developmental differences are noted in coping strategies, preferences, and self-efficacy across childhood and adolescence.62 In particular, adolescents seem to have different preferences in how they want to cope depending on the stressor, and they increasingly draw on cognitive strategies.62 Although 70% to 80% of the adolescents in the music distraction trial identified using music to cope with emotional stress, only about half indicated they typically use music to cope with pain.31 It may be that the requirement for adolescents to use music during the vaccine injection detracted from use of a more preferred (and effective) coping strategy (eg, positive self-talk). More research is needed to understand whether individual treatment preference impacts the efficacy of a psychological intervention for managing pain and related outcomes from vaccinations.
All of the psychological interventions showing some benefit for reducing pain and/or pain-related outcomes included some form of distraction (ie, verbal, music, video, breathing with a toy). Distraction is the most commonly studied psychological intervention for all procedural pain in children,49 and has been shown to be efficacious for reducing pain across all types of pediatric needle procedures, including vaccine injections.5,52 Although it remains somewhat unclear what the effective components of distraction interventions are specifically, they generally require minimal instruction, and can be easily tailored given the cultural or resource considerations. Thus, while video distraction may have specific technological demands, verbal and music distraction, as well as breathing with a toy, do not. Moreover, the support for the efficacy of these interventions suggests that low-tech modalities can also be engaging and effective in children. This holds broad appeal for global implementation of psychological interventions, as part of a multifaceted approach to pain management. Furthermore, they may be accessible even in areas of the world where pharmacological interventions, such as topical anesthetics (lidocaine-prilocaine), are not. That being said, educating parents through knowledge translation initiatives before the vaccine injection itself, may be helpful given the need for parents to bring necessary distracters or toys (eg, bubbles).
A major limitation of this area of research is that there are several psychological interventions for which the evidence base is relatively limited and is outdated. Six of 9 clinical questions relied on data from only 1 or 2 trials, and half (n=11) of the included studies were published before the year 2000. That being said, the psychological interventions showing benefit in the current review, generally relied on a larger number of trials, and more recent trials, overall. Of particular mention, is the paucity of research available for adolescents given that approximately up to 4 vaccinations are offered in Canada in grades 7 or later.63 The typical setting in which adolescents (and slightly younger school-aged children) undergo vaccine injections is school-based immunization clinics. This setting brings very different circumstances as compared with primary care clinics where younger children are often immunized, perhaps most notably, the absence of parents and presence of peers.64 Fear may be particularly high among children and adolescents receiving vaccine injections at school, and has the potential to spread quickly among peers, a phenomenon referred to as “fear contagion.”65 As a result, adolescents, in particular, are required to become much more autonomous in their use of pain management strategies. Although their confidence, self-reliance, and effective use of coping strategies is more developed than younger children,42 additional research is needed to ensure implementation of effective psychological strategies that match the coping skills and preferences in this age group and for this setting.
Also of note is the very low to low quality of evidence available across all assessed outcomes.10 In large part, this is due to high risk of bias arising from the lack of blinding of participants and outcome assessors, including children, parents, and immunizers. The generally poor quality of evidence has been previously noted, as it is a consistent finding for trials investigating psychological interventions across all types of needle procedures in children and adolescents.5,52,66 This is concerning, as high risk of bias has been associated with exaggerated treatment effects.67 While blinding of children, parents, and immunizers to study group can sometimes be difficult given the nature of psychological interventions (ie, it is difficult to hide the presence of a television or headphones), efforts can be made to blind individuals to study hypotheses, and researcher-ratings of the child’s distress can readily be achieved. Several trials also noted contamination of treatment effects in control groups. Although this may be unavoidable due to the natural engagement of parents or other adults’ in specific behaviors (ie, distraction), it does support the value of assessing the natural occurrence of the intervention in control groups. Future trials need to improve the quality of evidence by considering necessary design considerations a priori. A more detailed discussion of limitations of the available evidence and discussions for future research in all areas of pain management for vaccine injections is also available.68
Despite the unavoidable limitations posed by the available evidence in this area, the current systematic review and meta-analysis was very rigorous in its approach.9 A thorough database search for all relevant studies was undertaken, with consistent a priori decisions for identifying relevant clinical questions and critically important outcomes as derived by a multidisciplinary national panel of experts in vaccination pain management (HELPinKids&Adults team). The application of high quality established methods for pooling data and evaluating the quality of evidence ensures confidence in the review’s findings (GRADE10; Cochrane11). Furthermore, a unique strength arises from the inclusion of this review within a series of similar reviews examining psychological interventions for vaccine injections across the lifespan,7,8 reviews examining physical, procedural, and pharmacological approaches to vaccine pain management, as well as a review on the management of high levels of needle fear.12 The compilation of these reviews in clinical practice guidelines ensures the utility, feasibility, and practicality of a multipronged approach to vaccine pain management and long-term sequelae, and encourages uptake of these findings in clinical practice (also McMurtry CM, Taddio A, Noel M, et al., unpublished data, 2015).69
In summary, a number of psychological interventions show benefit for reducing pain and pain-related outcomes during vaccine injections in children. Effective interventions largely seem to include some degree of distraction, with music distraction being the most consistently beneficial across assessed outcomes for children. In general, effective psychological interventions require minimal training to be implemented by children, parents, and/or immunizers, and can draw from varied available resources, ensuring their clinical utility and appropriateness for children of different ages and at a global level.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to acknowledge Dr Jennifer A. Parker, PhD, Research Associate, Centre for Pediatric Pain Research, IWK Health Centre; Adjunct Professor, Department of Pediatrics, Dalhousie University, Halifax, Canada, Katelynn E. Boerner, BSc (Hons), Department of Psychology and Neuroscience, Dalhousie University, Halifax, Canada, Meghan Schinkel, BSc (Hons), Department of Psychology and Neuroscience, Dalhousie University, Halifax, Canada, and Kristen Higgins, BSc (Hons), Department of Psychology and Neuroscience, Dalhousie University, Halifax, Canada for their research assistance. K.A. Birnie is a Killam Scholar. K.A. Birnie and M. Noel are trainee members of Pain in Child Health (PICH): a strategic training initiative funded by CIHR. C. Chambers was funded by a Canada Research Chair during completion of this work and also by CIHR.
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.clinicalpain.com.
HELPinKids&Adults (Help ELiminate Pain in Kids and Adults) Team: N.E. MacDonald, J. Rogers, L. Bucci, E. Lang, P. Mousmanis, S.A. Halperin, S. Bowles, C. Halpert, M. Ipp, M. Rieder, K. Robson, G.J.G. Asmundson, E. Uleryk, M. Antony, D. Alexander, M. Appleton, V. Dubey, A. Hanrahan, D. Lockett, J. Scott, E. Votta Bleeker.
Supported by the Canadian Institutes of Health Research (CIHR), Ottawa, ON, Canada (KRS 132031). Open access funding was provided by the Mayday Fund in the United States. A. Taddio declares a grant from Pfizer, and study supplies from Natus and Ferndale. C.T. Chambers declares consultation fees from Abbvie. E. Lang is a member of the GRADE working group and declares consultation fees from the International Liaison Committee on Resuscitation (ILCOR). L. Bucci declares a relationship with government agencies and grants from Merck, GSK, Novartis, Sanofi, and Pfizer. S.A. Halperin declares grants from GSK, Sanofi, Novartis, Pfizer, Merck, PREVENT, ImmunoVaccine, NovaVax, Janssen, and Folia. The remaining authors declare no conflict of interest.
Contributor Information
Collaborators: HELPinKids&Adults Team
REFERENCES
- 1.Schechter NL, Zempsky WT, Cohen LL, et al. Pain reduction during pediatric immunizations: evidence-based review and recommendations. Pediatrics. 2007;119:e1184–e1198. [DOI] [PubMed] [Google Scholar]
- 2.Shah V, Taddio A, Rieder MJ, et al. Effectiveness and tolerability of pharmacological and combined interventions for reducing injection pain during routine childhood immunizations: systematic review and meta-analyses. Clin Ther. 2009;31(suppl 2):S104–S151. [DOI] [PubMed] [Google Scholar]
- 3.Chambers CT, Taddio A, Uman LS, et al. Psychological interventions for reducing pain and distress during routine childhood immunizations: a systematic review. Clin Ther. 2009;31:S77–S103. [DOI] [PubMed] [Google Scholar]
- 4.Taddio A, Ilersich AL, Ipp M, et al. Physical interventions and injection techniques for reducing injection pain during routine childhood immunizations: systematic review of randomized controlled trials and quasi-randomized controlled trials. Clin Ther. 2009;31(suppl 2):S48–S76. [DOI] [PubMed] [Google Scholar]
- 5.Birnie KA, Noel M, Parker JA, et al. Systematic review and meta-analysis of distraction and hypnosis for needle-related pain and distress in children and adolescents. J Pediatr Psych. 2014;39:783–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taddio A, Appleton M, Bortolussi B, et al. Reducing the pain of childhood vaccination—an evidence-based clinical practice guideline. CMAJ. 2010;182:E843–E855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pillai Riddell R, Taddio A, McMurtry CM, et al. Psychological interventions for vaccine injections in young children 0 to 3 years: systematic review of randomized controlled trials and quasi-randomized controlled trials. Clin J Pain. 2015;31(10S):S64–S71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boerner KE, Birnie KA, Chambers CT, et al. Simple psychological interventions for reducing pain from common needle procedures in adults: systematic review of randomized and quasi-randomized controlled trials. Clin J Pain. 2015;31(10S):S90–S98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taddio A, McMurtry CM, Shah V, et al. Methodology for knowledge synthesis of the management of vaccination pain and needle fear. Clin J Pain. 2015;31(10S):S12–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guyatt GH, Oxman AD, Schunemann HJ, et al. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64:380–382. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JPT, Green S. (eds) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. (updated September 2011). The Cochrane Collaboration, 2011. Available at: http://www.cochrane-handbook.org. Accessed April 9, 2015.
- 12.McMurtry CM, Taddio A, Noel M, et al. Interventions for individuals with high levels of needle fear: systematic review of randomized controlled trials and quasi randomized controlled trials. Clin J Pain. 2015;31(10S):S109–S123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Baeyer CL, Chambers CT, Forsyth S, et al. Developmental data supporting simplification of self-report pain scales for preschool-age children. J Pain. 2013;14:1116–1121. [DOI] [PubMed] [Google Scholar]
- 14.von Baeyer CL, Uman LS, Chambers CT, et al. Can we screen young children for their ability to provide accurate self-reports of pain? Pain. 2011;152:1327–1333. [DOI] [PubMed] [Google Scholar]
- 15.von Baeyer CL, Forsyth S, Stanford EA, et al. Response biases in preschool children’s ratings of pain in hypothetical situations. Eur J Pain. 2009;13:209–213. [DOI] [PubMed] [Google Scholar]
- 16.Borenstein M, Hedges LV, Higgins JPT, et al. Introduction to Meta-analysis. West Sussex, UK: John Wiley & Sons; 2009. [Google Scholar]
- 17.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beran TN, Ramirez-Serrano A, Vanderkooi OG, et al. Reducing children’s pain and distress towards flu vaccinations: a novel and effective application of humanoid robotics. Vaccine. 2013;31:2772–2777. [DOI] [PubMed] [Google Scholar]
- 19.Blount RL, Bachanas PJ, Powers SW, et al. Training children to cope and parents to coach them during routine immunizations: effects of child, parent and staff behaviors. Behav Ther. 1992;23:689–705. [Google Scholar]
- 20.Bowen AM, Dammeyer MM. Reducing children’s immunization distress in a primary care setting. J Pediatr Nurs. 1999;14:296–303. [DOI] [PubMed] [Google Scholar]
- 21.Cassidy K, Reid GJ, McGrath PJ, et al. Watch needle, watch TV: audiovisual distraction in preschool immunization. Pain Med. 2002;3:108–118. [DOI] [PubMed] [Google Scholar]
- 22.Cohen LL, Blount RL, Panopoulos G. Nurse coaching and cartoon distraction: an effective and practical intervention to reduce child, parent, and nurse distress during immunizations. J Pediatr Psychol. 1997;22:355–370. [DOI] [PubMed] [Google Scholar]
- 23.Cohen LL, Blount RL, Cohen RJ, et al. Comparative study of distraction versus topical anesthesia for pediatric pain management during immunizations. Health Psychol. 1999;18:591–598. [DOI] [PubMed] [Google Scholar]
- 24.Cohen LL, Bernard RS, Greco LA, et al. A child-focused intervention for coping with procedural pain: are parent and nurse coaches necessary? J Pediatr Psychol. 2002;27:749–757. [DOI] [PubMed] [Google Scholar]
- 25.Cohen LL, Rodrigues NP, Lim CS, et al. Automated parent-training for preschooler immunization pain relief: a randomized controlled trial. J Pediatr Psych. 2015;40:526–534. Advance online access. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eland JM. Minimizing pain associated with prekindergarten intramuscular Injections. Issues Compr Pediatr Nurs. 1981;5:361–372. [DOI] [PubMed] [Google Scholar]
- 27.Fowler-Kerry S, Lander JR. Management of injection pain in children. Pain. 1987;30:169–175. [DOI] [PubMed] [Google Scholar]
- 28.French GM, Painter EC, Coury DL. Blowing away shot pain: a technique for pain management during immunization. Pediatrics. 1994;93:384–388. [PubMed] [Google Scholar]
- 29.Gonzalez JC, Routh DK, Armstrong FD. Effects of maternal distraction versus reassurance on children’s reactions to injections. J Pediatr Psych. 1993;18:593–604. [DOI] [PubMed] [Google Scholar]
- 30.Krauss WJ. Videotape Modeling and Parent Participants: Effects on Reducing Distress Behaviour in Children Undergoing Immunization Procedures (dissertation). Fresno, CA: California School of Professional Psychology; 1997. [Google Scholar]
- 31.Kristjansdottir O, Kristjansdottir G. Randomized clinical trial of musical distraction with and without headphones for adolescents’ immunization pain. Scand J Caring Sci. 2011;25:19–26. [DOI] [PubMed] [Google Scholar]
- 32.Luthy KE, Beckstrand RL, Pulsipher A. Evaluation of methods to relieve parental perceptions of vaccine-associated pain and anxiety in children: a pilot study. J Pediatr Health Care. 2013;27:351–358. [DOI] [PubMed] [Google Scholar]
- 33.Manimala R, Blount RL, Cohen LL. The effects of parental reassurance versus distraction on child distress and coping during immunizations. Child Health Care. 2000;29:161–177. [Google Scholar]
- 34.Megel ME, Houser CW, Gleaves LS. Children’s responses to immunizations: lullabies as a distraction. Issues Compr Pediatr Nurs. 1998;21:129–145. [DOI] [PubMed] [Google Scholar]
- 35.Noguchi LK. The effect of music versus nonmusic on behavioral signs of distress and self- report of pain in pediatric injection patients. J Music Ther. 2006;43:16–38. [DOI] [PubMed] [Google Scholar]
- 36.O’Laughlin E, Ridley-Johnson R. Maternal presence during children’s routine immunizations: the effect of mother as observer in reducing child distress. Child Health Care. 1995;24:175–191. [DOI] [PubMed] [Google Scholar]
- 37.Sparks L. Taking the “ouch” out of injections for children: using distraction to decrease pain. MCN Am J Matern Child Nurs. 2001;26:72–78. [DOI] [PubMed] [Google Scholar]
- 38.Wallace DP, Allen KD, Lacroix AE, et al. The “cough trick:” a brief strategy to manage pediatric pain from immunization injections. Pediatrics. 2010;125:e367–e373. [DOI] [PubMed] [Google Scholar]
- 39.Yinger OS. Music Therapy as Procedural Support for Young Children Undergoing Immunizations: A Randomized Controlled Study (Dissertation). Tallahassee, FL: The Florida State University; 2012. [DOI] [PubMed] [Google Scholar]
- 40.Shahid R, Benedict C, Mishra S, et al. Using iPads for distraction to reduce pain during immunizations. Clin Pediatr. 2015;54:145–148. [DOI] [PubMed] [Google Scholar]
- 41.Hedberg AG, Schlong A. Eliminating fainting by school children during mass inoculation clinics. Nurs Res. 1973;22:352–353. [DOI] [PubMed] [Google Scholar]
- 42.Zimmer-Gembeck MJ, Skinner EA. The development of coping across childhood and adolescence: a integrative review and critique of research. Int J Behav Dev. 2011;35:1–17. [Google Scholar]
- 43.Klassen JA, Liang Y, Tjosvold L, et al. Music for pain and anxiety in children undergoing medical procedures: a systematic review of randomized controlled trials. Ambul Pediatr. 2008;8:117–128. [DOI] [PubMed] [Google Scholar]
- 44.McCarthy AM, Kleiber C, Hanrahan K, et al. Impact of parent-provided distraction on child responses to an IV insertion. Child Health Care. 2010;39:125–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McMurtry CM, Chambers CT, McGrath PJ, et al. When “don’t worry” communicates fear: children’s perceptions of parental reassurance and distraction during a painful medical procedure. Pain. 2010;150:52–58. [DOI] [PubMed] [Google Scholar]
- 46.McCarthy AM, Kleiber C, Hanrahan K, et al. Factors explaining children’s responses to intravenous needle insertions. Nurs Res. 2010;59:407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCarthy AM, Kleiber C, Hanrahan K. Matching doses of distraction with child risk for distress during a medical procedure: a randomized clinical trial. Nurs Res. 2014;63:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dahlquist LM, Pendley JS. When distraction fails: parental anxiety and children’s responses to distraction during cancer procedures. J Ped Psych. 2005;30:623–628. [DOI] [PubMed] [Google Scholar]
- 49.Cohen LL, Cousins L, Martin S.McGrath PJ, Stevens BJ, Walker SM, Zempsky W. Procedural pain distraction. Oxford Textbook of Paediatric Pain. Oxford, UK: Oxford University Press; 2014:553–559. [Google Scholar]
- 50.McQueen A, Cress C, Tothy A. Using a tablet computer during pediatric procedures: a case series and review of the “apps”. Pediatr Emer Care. 2012;28:712–714. [DOI] [PubMed] [Google Scholar]
- 51.Busch V, Magerl W, Kern U, et al. The effect of deep and slow breathing on pain perception, autonomic activity, and mood processing: an experimental study. Pain Med. 2012;13:215–228. [DOI] [PubMed] [Google Scholar]
- 52.Uman LS, Birnie KA, Noel M, et al. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev. 2013;10:1–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Birnie KA, Boerner KE, Chambers CT.McGrath PJ, Stevens BJ, Walker SM, Zempsky W. Families and pain. Oxford Textbook of Paediatric Pain. Oxford, UK: Oxford University Press; 2014:111–118. [Google Scholar]
- 54.McMurtry CM, McGrath PJ, Chambers CT. Reassurance can hurt: parental behavior and painful medical procedures. J Pediatr. 2006;148:560–561. [DOI] [PubMed] [Google Scholar]
- 55.McMurtry CM. A Multi-method Examination of Adult Reassurance During Children’s Medical Procedures. Halifax, NS: Dalhousie University; 2009. [Google Scholar]
- 56.Cohen LL, Bernard RS, McClellan CB, et al. Assessing medical room behavior during infants’ painful procedures: The Measure of Adult and Infant Soothing and Distress (MAISD). Child Health Care. 2005;34:81–94. [Google Scholar]
- 57.Racine NM, Pillai Riddell RR, Flora D, et al. A longitudinal examination of verbal reassurance during infant immunization: occurrence and examination of emotional availability as a potential moderator. J Ped Psych. 2012;37:935–944. [DOI] [PubMed] [Google Scholar]
- 58.Lucock MP, Morley S, White C, et al. Responses of consecutive patients to reassurance after gastroscopy: results of self administered questionnaire survey. BMJ. 1997;315:572–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fanurik D, Koh JL, Schmitz ML. Distraction techniques combined with EMLA: effects on IV insertion pain and distress in children. Child Health Care. 2000;29:87–101. [Google Scholar]
- 60.Jeffs DA. A pilot study of distraction for adolescents during allergy testing. J Spec Pediatr Nurs. 2007;12:170–185. [DOI] [PubMed] [Google Scholar]
- 61.Windich-Biermeier A, Sjoberg I, Dale JC, et al. Effects of distraction on pain, fear, and distress during venous port access and venipuncture in children and adolescents with cancer. J Ped Onc Nurs. 2007;24:8–19. [DOI] [PubMed] [Google Scholar]
- 62.Skinner EA, Zimmer-Gembeck MJ. The development of coping. Ann Rev Psychol. 2007;58:119–144. [DOI] [PubMed] [Google Scholar]
- 63.National Advisory Committee on Immunization. Provincial and territorial immunization information. January 16, 2015. Available at: http://healthycanadians.gc.ca/healthy-living-vie-saine/immunization-immunisation/children-enfants/schedule-calendrier-eng.php#info-ns. Accessed April 9, 2015.
- 64.Boerner KE, Gillespie JM, McLaughlin EN, et al. Implementation of evidence-based psychological interventions for pediatric needle pain. Clin Pract Pediatr Psychol. 2014;2:224–235. [Google Scholar]
- 65.Bernard DM, Cooper Robbins SC, McCaffery KJ, et al. The domino effect: adolescent girls’ response to human papillomavirus vaccination. Med J Aust. 2011;194:297–300. [DOI] [PubMed] [Google Scholar]
- 66.Uman LS, Chambers CT, McGrath PJ, et al. Assessing the quality of randomized controlled trials examining psychological interventions for pediatric procedural pain: recommendations for quality improvement. J Pediatr Psych. 2010;35:693–703. [DOI] [PubMed] [Google Scholar]
- 67.Savovic J, Jones H, Altman D, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta-epidemiological studies. Health Technol Assess. 2012;16:1–82. [DOI] [PubMed] [Google Scholar]
- 68.Noel M, Taddio A, McMurtry CM, et al. HELPinKids&Adults knowledge synthesis of vaccination pain management and fear management in individuals with high needle fear: limitations of the evidence and recommendations for future research. Clin J Pain. 2015;31(10S):S124–S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taddio A, McMurtry CM, Shah V, et al. Reducing pain during vaccine injections: clinical practice guideline. CMAJ. 2015; Aug 24. [Epub ahead of print]. DOI:10.1503?/cmaj.150391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


