Abstract
Background and Aim:
Immunoglobulin G4-associated cholangitis (IAC) shares many similar symptoms with cholangiocarcinoma (CCA). However, the treatment and the prognosis are substantially different. This study aimed to identify the important markers for the differential diagnosis of these 2 diseases.
Methods:
Thirty IAC patients and 275 CCA patients were reviewed retrospectively for their clinical symptoms, serological tests, and imaging characteristics. Posttreatment responses were also studied.
Results:
IgG4 had 100% specificity for IAC at a cutoff of 6 times the upper normal limit. IAC patients had a significantly higher incidence of weight loss (P=0.025) and a higher level of weight loss (P=0.008) than CCA patients. The positive rates of biological markers CA199, CA242, and CEA in CCA and IAC were 81.5% versus 42.9%, 45.5% versus 4.5%, and 29.2% versus 7.1%, respectively. Levels of these tumor markers in CCA were significantly higher than in IAC (P<0.05). The thickened wall [17/18 (94.4%) vs. 3/10 (30%), P=0.001] and the occupying lesion on the bile duct [1/18 (5.6%) vs. 8/10 (80%), P<0.001] were found to be significantly different in IAC and CCA, respectively, by endoscopic ultrasonography. Autoimmune pancreatitis was the most frequently observed comorbidity of IAC (25/30). All IAC patients respond positively to steroid treatment.
Conclusions:
Increased tumor markers, 6-fold higher levels of serum IgG4, and other organs’ involvement could be the reference factors for a differential diagnosis of IAC and CCA. Endoscopic ultrasonography might be an effective imaging tool for diagnosis, although clinical signs and symptoms of IAC and CCA are similar. Experimental steroid treatment can be useful in the diagnosis for certain difficult cases.
Key Words: Immunoglobulin G4-associated cholangitis, cholangiocarcinoma, endoscopic ultrasonography, differential diagnosis
Immunoglobulin G4-associated cholangitis (IAC) was first mentioned by Bjornsson and colleagues in 2007. It is an IgG4-related disease characterized by an elevation of serum IgG4 (sIgG4) and infiltration of IgG4-positive plasma cells in the bile duct, as well as an overproduction of T-helper2 cells.1 The extrahepatic bile duct is often involved and also accompanied with autoimmune pancreatitis (AIP) and other fibrosis. In 2012, Japanese researchers established clinical diagnostic criteria of IAC2 as follows: (1) characteristic biliary imaging findings, (2) an elevation of sIgG4 concentrations (>1350 mg/L), (3) coexistence of other IgG4-related diseases, and (4) characteristic histopathologic features. In addition, the high efficacy of steroid therapy can be a useful indication of IAC.
It is always challenging to distinguish IAC from cholangiocarcinoma (CCA), a relative common malignant disease that manifests similarly, but requires a very different approach of treatment from IAC.3 Radiography features of CCA and IAC are often similar, and CCA can also have elevated sIgG4 levels.4 The correct diagnosis is obviously very important for subsequent treatment and prognosis. Because of the difficulty in obtaining a pathologic biopsy sample in most cases,5 it becomes crucial to differentiate these 2 diseases on the basis of other clinical manifestations. In this study, the primary objective is to identify key factors that may play a role in the differential diagnosis of IAC and CCA. Therefore, we performed a retrospective study with the patients treated in our hospital for either IAC or CCA in the last 3 years.
MATERIALS AND METHODS
Thirty patients were diagnosed with IAC in the Peking Union Medical College Hospital (PUMCH) from January 2011 to September 2014, during which 275 patients were diagnosed with CCA. All patients were retrospectively reviewed and information was collected including their sex, age, symptoms, weight loss (decreased >5% within 6 mo), and serological tests, including biochemical tests, tumor markers, and the sIgG4 level. Imaging characteristics including endoscopic retrograde cholangiopancreatography (ERCP), magnetic resonance cholangiopancreatography (MRCP), computed tomography (CT), B-ultrasound, and endoscopic ultrasonography (EUS) were also collected. The study protocol was approved by the Ethical Committee of PUMCH in May 2013.
Statistical Analysis
Data were analyzed using SPSS version 13.0 (SPSS Inc., Chicago, IL). The primary outcome consisted of the clinical parameters that showed significant differences in IAC and CCA. Differences between the groups were evaluated using the independent samples t test, the χ2 test, the Mann-Whitney U, or the Fisher test according to their characteristic. In all tests, P values <0.05 were considered statistically significant. Receiver operating characteristic curves were used to estimate the diagnostic application of sIgG4 levels (Youden Index=sensitivity+specificity−1).
RESULTS
Demographical Data and Symptoms
Thirty patients (21 male and 9 female; median age 59.0±12.7 y; ranging from 28 to 83 y) were diagnosed with IAC, with the criteria described in the introduction section, and 275 CCA patients (170 male and 105 female; median age 61.8±11.3 y; ranging from 30 to 89 y) were diagnosed with histopathology and/or cytology. There was no significant difference in the gender and the age between the 2 groups (Table 1).
TABLE 1.
Demographic Data and Symptoms of IAC and CCA Patients
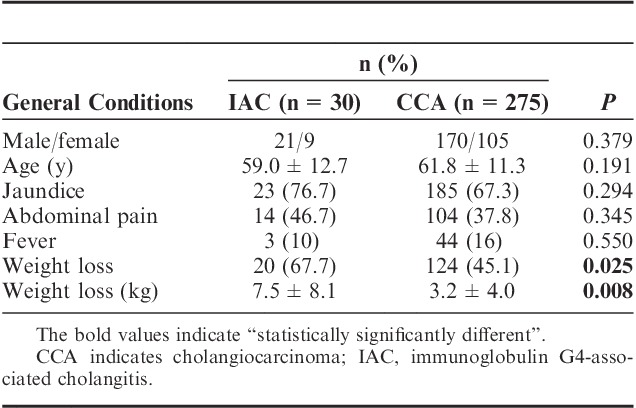
As shown in Table 1, a significantly higher number of IAC patients experienced weight loss than CCA patients (66.7% in IAC vs. 45.1% in CCA, P=0.025). Moreover, IAC patients had a significantly higher level of weight loss than CCA patients (7.5±8.1 vs. 3.2±4.0 kg, P=0.008). No difference was observed in the incidence of jaundice and abdominal pain. On comparing the prognosis of the 2 groups, IAC patients had a significantly longer survival time than CCA patients (P<0.001). The survival times of the 2 groups are shown in Figure 1.
FIGURE 1.
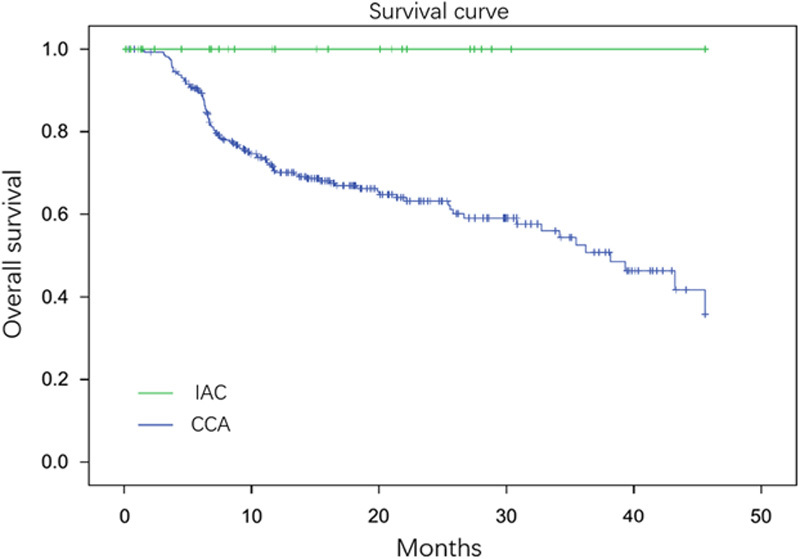
The survival curve of IAC and CCA patients. Thirty IAC patients and 275 CCA patients were reviewed and plotted on the curve. The survival time was significant between these 2 groups (P<0.001). CCA indicates cholangiocarcinoma; IAC, immunoglobulin G4-associated cholangitis.
Serological Tests
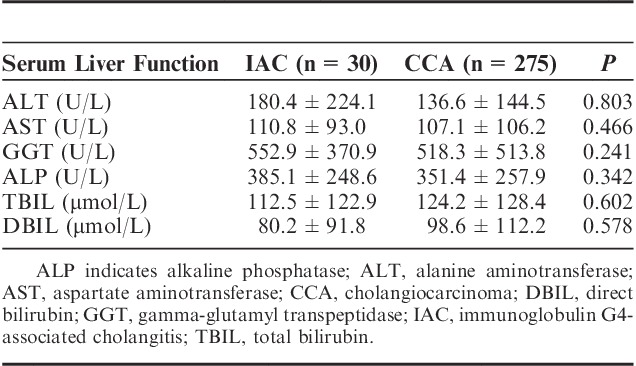
CCA patients demonstrated significantly higher positive rates of tumor markers, including CA199, CA242, and CEA, compared with IAC patients. Positive rates of CA199, CA242, and CEA in CCA patients compared with IAC patients were 81.5% versus 42.9%, 45.5% versus 4.5%, and 29.2% versus 7.1%, respectively. In addition, average serological levels of these tumor markers in positive CCA patients were significantly higher than those in positive IAC patients (P<0.05 in all cases) (Table 2). There were no significant differences in the serum biochemistry tests including ALT, AST, GGT, ALP, TBil, and DBil between IAC and CCA patients (Table 3).
TABLE 2.
Tumor Maker Detection in the IAC and the CCA Groups
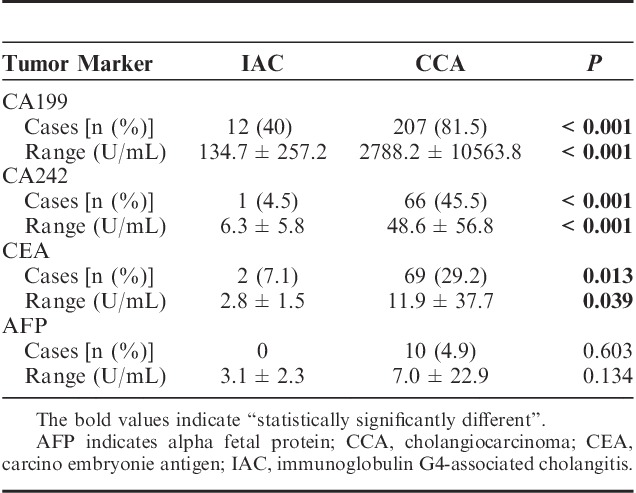
TABLE 3.
Serological Measurement for the Liver Function of IAC and CCA Patients
Thirty-one CCA patients were tested for their sIgG4 level, among whom 16.1% (5/31) were found to have an elevated level with a range between 29 and 8230 mg/L and an average of 896.3 mg/L. Almost 100% of the IAC patients showed elevated sIgG4 ranging between 1650 and 78,590 mg/L, with an average of 16028.6 mg/L. When a cutoff level was set at 6 times the upper normal limit, the area under the curve for sIgG4 was 0.981 in receiver operating characteristic analysis and sIgG4 had 100% specificity for IAC. On the basis of the Youden index calculation, the best cutoff value for sIgG4 in this cohort was 1575 mg/L with 100% sensitivity and 87.1% specificity.
Imaging
Bile duct–occupying lesions were detected with ERCP, MRCP, CT, B-ultrasound, or EUS. An occupying lesion was defined as a thickening of the bile duct wall with a very clear margin. As shown in Table 4, the thickening wall (P=0.001) and the occupying lesion (P<0.001) of the duct were found significantly different in IAC and CCA by EUS. A typical EUS image is shown in Figure 2.
TABLE 4.
Imaging Comparison of IAC and CCA Patients by Different Radiologic Methods
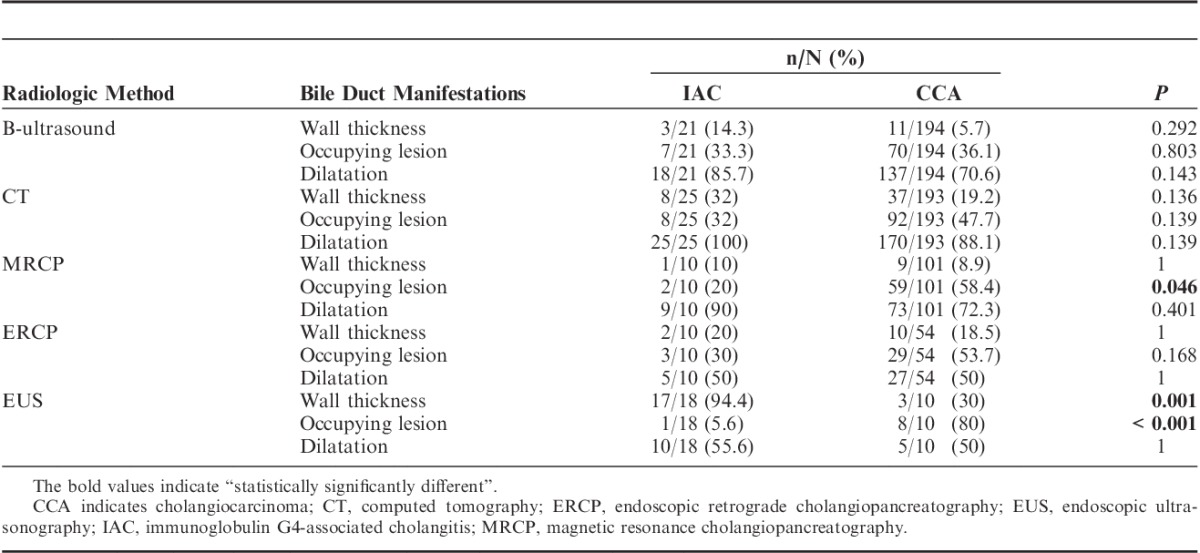
FIGURE 2.
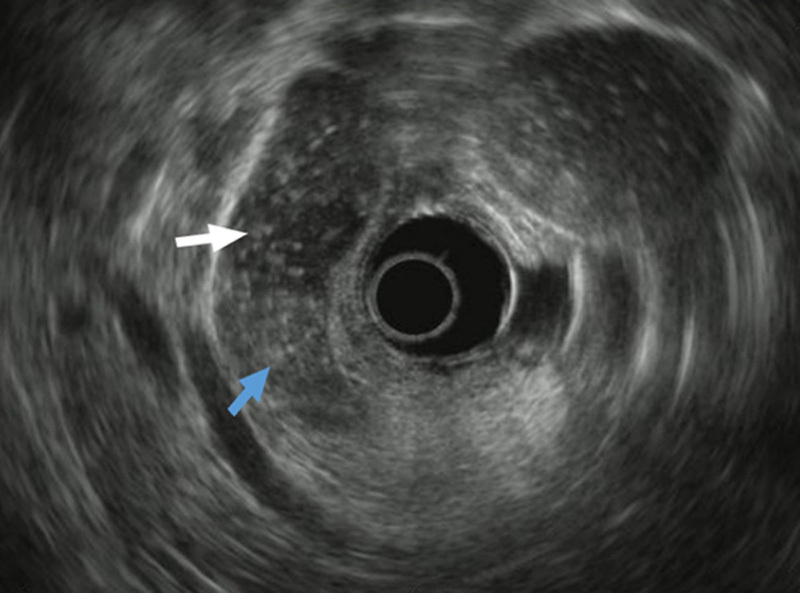
An example of an image taken with endoscopic ultrasonography that exhibited an occupying lesion of the bile duct. The blue arrow indicates the clear margin of the lesion. The white arrow indicates the bile duct.
Other Organ Involvements
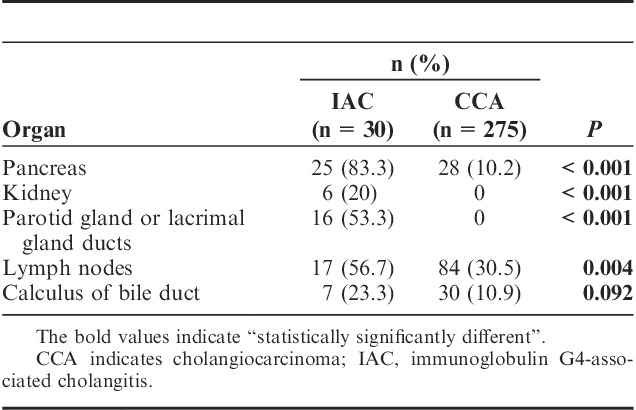
AIP was the most frequent comorbidity of IAC and the incidence reached as high as 83.3% in this study. The imaging diagnosis for AIP included diffused pancreatic enlargement, irregular narrowing of the main pancreatic duct, and bile duct strictures. Among the 30 IAC patients, 12 AIPs were identified with EUS and 13 with other imaging measurements. However, only 10.2% of the CCA patients were found to have pancreas involvement and presented as tumor invasion. Kidney (20%) and parotid gland or lacrimal gland (53.3%) involvement were also present in IAC patients, whereas none was found in CCA. Both groups had hepatic hilar lymph nodule hyperplasia, but the percentage of incidents in IAC patients was significantly higher (56.7% vs. 30.5%, P=0.004). The summary of organ involvement is listed in Table 5.
TABLE 5.
Other Organ Involvement in IAC and CCA Patients
Response to Treatment
When diagnosed or highly suspected, IAC patients were treated with steroid therapy (initial prednisolone dose as 30 mg/d for 2 wk). Almost 100% IAC patients showed complete response. The average sIgG4 and TBil levels of IAC patients decreased to 6278.37 mg/L and 26.14 μmol/L, respectively. Prednisolone application resulted in a decrease in the sIgG4 level in all IAC patients, and a decrease in the bilirubin level was noticed in 80.77% of the IAC patients. Differences between pretreatment and posttreatment were statistically significant (P<0.001 in both).
DISCUSSION
IAC was recently recognized as an independent disease from other IgG4-related diseases, and there are no epidemiology data for IAC based on a large population.6 Differential diagnosis between IAC and CCA can be challenging as both diseases share several symptoms and signs.7 Obstructive jaundice accompanied with skin pruritus, abdominal discomfort, and/or weight loss have been the most common symptoms in both IAC and CCA patients.8–10 IAC patients may be positive for tumor markers, whereas CCA patient can also exhibit elevated sIgG4. Imaging studies can also demonstrate many similarities including obstruction, dilatation, and a thickening wall of the bile duct.
In this study, we examined the clinical data collected from patients who were diagnosed with either IAC or CAA. Weight loss in IAC patients was one of the symptoms that was significantly different from that of CCA patients. A prolonged duration of inflammatory response may also play a role. We observed similar incidences of IAC in both male and female patients, which agrees with other studies reported earlier.11 No significant demographic differences were found between IAC and CCA patients.
The sIgG4 level is a major characteristic of IgG4-related diseases. The production of IgG4 is related to the expression of several immune genetic factors, such as MHCII, polymorphism of nuclear factor-κB, and Fc-receptor-like (FCRL) 3.10 Other scholars proposed the “induction and progression” biphasic mechanism,12 in which decreased naive Tregs may induce a Th1 immune response with the release of proinflammatory cytokines to antigens such as self-antigen or microorganisms. Subsequently, Th2-type immune responses may be involved in the disease progression, resulting in the production of IgG4. In the IAC diagnosis criteria proposed by Japanese scholars, the minimum level of IgG4 was set as 1350 mg/L.2 However, the specificity at this cutoff is not sufficient to distinguish IAC and CCA. Oseini et al13 found that out of the 126 CCA patients, 17 (13.5%) had elevated sIgG4 (>1400 mg/L) and 4 (3.2%) had a >2-fold (>2800 mg/L) increase. In our study, 16.1% of the CCA patients had an elevated sIgG4 level (range, 29 to 8230 mg/L), which could mislead to an IAC diagnosis, although the level was significantly lower than that of the IAC group. On the basis of this study, we concluded that the best cutoff value for sIgG4 level was 1575 mg/L, with 100% sensitivity and 87.1% specificity. Our study suggested a cutoff level that was 6-fold higher than the upper normal limit of IgG4, which was different from the 4-fold criteria proposed previously by Oseini et al.13
CA199 presents in the fetal gastrointestinal and pancreatic epithelium, whereas its serum level in adults is very low. Its expression is elevated in adenocarcinoma cells, and is released into the blood through the thoracic duct. Therefore, it can be a useful marker for the diagnosis of pancreatic carcinoma, gastric carcinoma, CCA, and intrahepatic CCA.14 The serum level of CA199 can also be elevated in pancreatitis, obstructive jaundice, and sclerosing diseases, which may be produced by abnormal epithelial cells.15–17 In our study, the serum CA199 level was found to be increased in most of the CCA patients. In IAC patients, the serum CA199 level also increased, but at a significantly lower incidence and a significantly lower level. Similarly, CEA and CA242 were also found in the normal tissue.18 The incidence and elevated levels of CEA and CA242 in CCA patients were significantly higher than those in IAC patients. These findings were in line with the studies published earlier.14,19,20
Space-occupying lesions of the biliary tract are often diagnosed by B-ultrasound, CT, MRI, or ERCP. Endoscopic nasobiliary drainage is used to decrease obstructive jaundice. Both IAC and CCA patients exhibit similar imaging characteristics, such as dilatation, thickening wall, or occupying lesion, which makes it difficult to distinguish one from the other. On reviewing the cases in our study, we found that these 3 manifestations exhibited significantly differently under EUS between IAC and CCA, which could make EUS a valuable tool to distinguish these 2 diseases. This finding was consistent with a previous study.21
Multiple organ involvement, including most commonly the pancreas,19 the kidney, and the salivary and the lacrimal glands, is characteristic of IAC.22,23 In this study, we had similar observations: 83.3% of the IAC patients had obvious involvements of the pancreas, 20% had involvement of the kidney, and 53.3% had involvement of the salivary or the lacrimal glands. In contrast, in CCA patients, the biliary tract was always the only involved organ at an early stage and few had other organs involved even though neighboring tissue/organ invasion and distant metastasis could occur at middle and advanced stages.
Histopathologic examination has been the gold standard and definitive method for IAC and CCA diagnosis. However, obtaining pathologic samples by puncture or ERCP brush before surgery is invasive and may not be suitable for all patients, such as patients who are old, patients with coagulopathy, or those with high bilirubin.24 In addition, the positive rate of brush check is low.25 Therefore, clinical examination and experimental treatment are very important for a differential diagnosis. We observed complete response of IAC patients to the steroid treatment, although in some cases the stent placement also played a role in the symptom alleviation.
There are certain limitations to this study. The retrospective nature of this study made it difficult to obtain data of a single variable from all patients. The case number of the IAC was low, which may affect the significance of the study. The use of a stent in some patients could be another factor in alleviating symptoms in IAC patient, which was not taken into account for the response to steroid treatment because of the limited numbers.
CONCLUSIONS
Our study suggested that 6-fold higher levels of sIgG4, tumor markers (CA199, CEA, and CA242), and other organ involvement could be used as reference criteria for the differential diagnosis of IAC and CCA. EUS might be the best imaging tool. For difficult cases, experimental steroid treatment can be used for further diagnosis under appropriate conditions.
Footnotes
Supported by the National Natural Science Foundation of China (30901453), the National Key Technology Research and Development Program of China (2012BAI06B01), and the Student Innovation Training Program of Peking Union Medical College (2015zlgc0612).
Disclosure: The authors declare no conflict of interest.
REFERENCES
- 1.Bjornsson E, Chari ST, Smyrk TC, et al. Immunoglobulin G4 associated cholangitis: description of an emerging clinical entity based on review of the literature. Hepatology. 2007;45:1547–1554. [DOI] [PubMed] [Google Scholar]
- 2.Ohara H, Okazaki K, Tsubouchi H, et al. Clinical diagnostic criteria of IgG4-related sclerosing cholangitis 2012. J Hepatobiliary Pancreat Sci. 2012;19:536–542. [DOI] [PubMed] [Google Scholar]
- 3.Fisher SB, Fisher KE, Maithel SK. Molecular targeted therapy for biliary tract malignancy: defining the target. Hepatobiliary Surg Nutr. 2012;1:53–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Resheq YJ, Quaas A, von Renteln D, et al. Infiltration of peritumoural but tumour-free parenchyma with IgG4-positive plasma cells in hilar cholangiocarcinoma and pancreatic adenocarcinoma. Dig Liver Dis. 2013;45:859–865. [DOI] [PubMed] [Google Scholar]
- 5.Tabata T, Kamisawa T, Hara S, et al. Differentiating immunoglobulin g4-related sclerosing cholangitis from hilar cholangiocarcinoma. Gut Liver. 2013;7:234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khosroshahi A, Stone JH. A clinical overview of IgG4-related systemic disease. Curr Opin Rheumatol. 2011;23:57–66. [DOI] [PubMed] [Google Scholar]
- 7.Cardinale V, Bragazzi MC, Carpino G, et al. Cholangiocarcinoma: increasing burden of classifications. Hepatobiliary Surg Nutr. 2013;2:272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chi T, Xu Y, Lu X. Obstructive jaundice suspected hilar cholangiocarcinoma. Hepatobiliary Surg Nutr. 2014;3:102–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Zhao C, Shen Y. Autoimmune cholangitis and cholangiocarcinoma. J Gastroenterol Hepatol. 2012;27:1783–1789. [DOI] [PubMed] [Google Scholar]
- 10.Okazaki K, Uchida K, Miyoshi H, et al. Recent concepts of autoimmune pancreatitis and IgG4-related disease. Clin Rev Allergy Immunol. 2011;41:126–138. [DOI] [PubMed] [Google Scholar]
- 11.Ghazale A, Chari ST, Zhang L, et al. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology. 2008;134:706–715. [DOI] [PubMed] [Google Scholar]
- 12.Okazaki K, Uchida K, Koyabu M, et al. IgG4 cholangiopathy—current concept, diagnosis, and pathogenesis. J Hepatol. 2014;61:690–695. [DOI] [PubMed] [Google Scholar]
- 13.Oseini AM, Chaiteerakij R, Shire AM, et al. Utility of serum immunoglobulin G4 in distinguishing immunoglobulin G4-associated cholangitis from cholangiocarcinoma. Hepatology. 2011;54:940–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Gao SG, Chen JM, et al. Serum CA242, CA199, CA125, CEA, and TSGF are biomarkers for the efficacy and prognosis of cryoablation in pancreatic cancer patients. Cell Biochem Biophys. 2015;71:1287–1291. [DOI] [PubMed] [Google Scholar]
- 15.Robertson AG, Davidson BR. Mirizzi syndrome complicating an anomalous biliary tract: a novel cause of a hugely elevated CA19-9. Eur J Gastroenterol Hepatol. 2007;19:167–169. [DOI] [PubMed] [Google Scholar]
- 16.Murray MD, Burton FR, Di Bisceglie AM. Markedly elevated serum CA 19-9 levels in association with a benign biliary stricture due to primary sclerosing cholangitis. J Clin Gastroenterol. 2007;41:115–117. [DOI] [PubMed] [Google Scholar]
- 17.Mann DV, Edwards R, Ho S, et al. Elevated tumour marker CA19-9: clinical interpretation and influence of obstructive jaundice. Eur J Surg Oncol. 2000;26:474–479. [DOI] [PubMed] [Google Scholar]
- 18.Hammarstrom S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67–81. [DOI] [PubMed] [Google Scholar]
- 19.Nakazawa T, Naitoh I, Hayashi K, et al. Diagnostic criteria for IgG4-related sclerosing cholangitis based on cholangiographic classification. J Gastroenterol. 2012;47:79–87. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson O, Johansson C, Glimelius B, et al. Sensitivity and specificity of CA242 in gastro-intestinal cancer. A comparison with CEA, CA50 and CA 19-9. Br J Cancer. 1992;65:215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naitoh I, Nakazawa T, Ohara H, et al. Endoscopic transpapillary intraductal ultrasonography and biopsy in the diagnosis of IgG4-related sclerosing cholangitis. J Gastroenterol. 2009;44:1147–1155. [DOI] [PubMed] [Google Scholar]
- 22.Itoh S, Nagasaka T, Suzuki K, et al. Lymphoplasmacytic sclerosing cholangitis: assessment of clinical, CT, and pathological findings. Clin Radiol. 2009;64:1104–1114. [DOI] [PubMed] [Google Scholar]
- 23.Oh HC, Kim MH, Lee KT, et al. Clinical clues to suspicion of IgG4-associated sclerosing cholangitis disguised as primary sclerosing cholangitis or hilar cholangiocarcinoma. J Gastroenterol Hepatol. 2010;25:1831–1837. [DOI] [PubMed] [Google Scholar]
- 24.Fogel EL, Sherman S, Park SH, et al. Therapeutic biliary endoscopy. Endoscopy. 2003;35:156–163. [DOI] [PubMed] [Google Scholar]
- 25.Lee YT, Chan FK, Leung WK, et al. Comparison of EUS and ERCP in the investigation with suspected biliary obstruction caused by choledocholithiasis: a randomized study. Gastrointest Endosc. 2008;67:660–668. [DOI] [PubMed] [Google Scholar]


