Abstract
Objective:
The incidence of human papillomavirus (HPV)-related oral malignancies is increasing among HIV-infected populations, and the prevalence of oral warts has reportedly increased among HIV patients receiving antiretroviral therapy (ART). We explored whether ART initiation among treatment-naive HIV-positive adults is followed by a change in oral HPV infection or the occurrence of oral warts.
Design:
Prospective, observational study.
Methods:
HIV-1 infected, ART-naive adults initiating ART in a clinical trial were enrolled. End points included detection of HPV DNA in throat-washes, changes in CD4+ T-cell count and HIV RNA, and oral wart diagnosis.
Results:
Among 388 participants, 18% had at least one HPV genotype present before initiating ART, and 24% had at least one genotype present after 12–24 weeks of ART. Among those with undetectable oral HPV DNA before ART, median change in CD4+ count from study entry to 4 weeks after ART initiation was larger for those with detectable HPV DNA during follow-up than those without (P = 0.003). Both prevalence and incidence of oral warts were low (3% of participants having oral warts at study entry; 2.5% acquiring oral warts during 48 weeks of follow-up).
Conclusion:
These results suggest: effective immune control of HPV in the oral cavity of HIV-infected patients is not reconstituted by 24 weeks of ART; whereas ART initiation was not followed by an increase in oral warts, we observed an increase in oral HPV DNA detection after 12–24 weeks. The prevalence of HPV-associated oral malignancies may continue to increase in the modern ART era.
Keywords: HIV, human papillomavirus, immune reconstitution, oral warts
Introduction
Oncogenic human papillomavirus (HPV) genotypes associated with the pathogenesis of oropharyngeal cancer and nononcogenic genotypes associated with development of oral warts are both found in the oral cavity [1]. Studies report up to 56% of HIV-infected adults have detectable oral HPV DNA, a proportion substantially higher than reported in non-HIV-infected populations [2–5], and that oral HPV DNA and oncogenic HPV genotypes are more prevalent and have a higher incidence among HIV-positive than HIV-negative MSM [6]. There are also recent reports of an increasing incidence of HPV-related oropharyngeal malignancies among the US population living with HIV infection, despite the widespread availability of effective combination antiretroviral therapy (ART) for almost two decades [7–9]. However, the impact of ART and associated immune reconstitution on HPV replication in the oropharynx has not previously been prospectively examined.
Retrospective studies have reported that the use and increased duration of ART were associated with the detection of HPV DNA in oral secretions [4], and that oral HPV DNA has been detected more frequently in HIV-infected patients who were virologically suppressed than those not suppressed on ART [5]. Studies have also suggested that the prevalence of HPV-associated oral warts is increased in HIV-infected patients after initiating ART [10,11]. These findings are counterintuitive since the degree of immune reconstitution resulting from virologically effective combination ART controls other AIDS-associated opportunistic infections caused by DNA viruses, such as herpes simplex virus (HSV), cytomegalovirus (CMV), Epstein–Barr virus, and JC virus [12].
These retrospective findings led us to hypothesize that HPV in the oropharynx would persist in HIV-infected patients after ART initiation. If true, the potential for development of HPV-related oral warts and carcinomas might further increase among HIV-infected individuals on ART, who already have an increased risk of solid tumors despite long-term suppression of HIV replication [10]. In particular, the risk of oropharyngeal, anal, and cervical cancer (all of which can be HPV-associated) are 3–60-fold higher among HIV-infected patients in the modern ART era than in the contemporaneous general population, and the incidence of oropharyngeal and anal cancer have significantly increased in the HIV-infected population compared with the premodern ART era when survival among the HIV-infected was much shorter [10]. Therefore, AIDS Clinical Trial Group (ACTG) protocol A5272 sought to explore the changes that occur in oral HPV DNA detection among treatment-naive HIV-positive adults over the first 24 weeks after initiating ART, as well as the prevalence of oral warts prior to and 48 weeks after ART initiation. We further explored oral HPV DNA detection in relation to immune reconstitution, measured by change in the absolute CD4+ cell count change and control of HIV replication. Our analyses accounted for oral sex behavior prior and during the study period.
Methods
Study design and population
A5272 was a prospective, observational study of HIV-1 infected ART-naïve adults (≥18 years old) initiating ART in a controlled clinical trial [13,14] (Table 1). Participants were seen at five visits over 48 weeks. Visits were scheduled at two time points within 1 week prior to ART initiation (study preentry and entry), during a 12–18-week time-interval following ART initiation, and at 24 and 48 weeks after ART initiation. All participants provided written informed consent. The protocol was approved by the institutional review boards of all participating sites.
Table 1.
Characteristics of participants (N = 388)a in AIDS Clinical Trial Group 5272 enrolled at 39 Clinical Research Sites from 2 January 2010 to 4 June 2012 prior to antiretroviral therapy initiation as part of a controlled clinical trial.b
| Characteristics | N (%) |
| Sex | |
| Women | 75 (19) |
| Men | 313 (81) |
| Race/ethnicity | |
| Black, non-Hispanic | 152 (39) |
| White, non-Hispanic | 139 (36) |
| Hispanic | 87 (22) |
| Other | 10 (3) |
| Intravenous injection drug use | |
| Never | 365 (94) |
| Previously | 23 (6) |
| Reported number of oral sex partners during 6 months prior to ART initiation | |
| 0 | 77 (26) |
| 1 | 94 (31) |
| 2 or more | 128 (43) |
| Reported number of oral sex partners between baseline visit and 24-week visit (or following ART initiation) | |
| 0 | 145 (41) |
| 1 | 118 (33) |
| 2 or more | 91 (26) |
| Median (Q1, Q3) | |
| Age at study entry (years) | 38 (29–47) |
| CD4+ T-cell count (cells/μl) | |
| At study entry (N = 385) | 328 (174–455) |
| 4 weeks after initiating ART (N = 359) | 402 (249–559) |
| 12–18 weeks after initiating ART (N = 388) | 447 (290–592) |
| 24 weeks after initiating ART (N = 387) | 483 (311–647) |
| Plasma HIV-1 RNA (log10 copies/ml) | |
| At study entry (N = 387) | 4.68 (4.20–5.22) |
| 4 weeks on ART (N = 379) | 2.40 (1.81–2.89) |
| 12–18 weeks on ART (N = 388) | <1.60 (1.60–1.77) |
| 24 weeks on ART (N = 387) | <1.60 (1.60–1.60) |
ACTG, AIDS Clinical Trial Group; ART, antiretroviral therapy; HPV, human papillomavirus.
aParticipants who had evaluable throat wash HPV DNA results from both time points before initiating ART and again after 12–18 and 24 weeks of ART.
bACTG Protocol A5257: phase III comparative study of three nonnucleoside reverse-transcriptase inhibitor-sparing antiretroviral regimens for treatment-naive HIV-1-infected volunteers (N = 268) [14], ACTG Protocol A5280: Prospective, randomized, double-blind phase II trial of vitamin D and calcium in HIV-1-infected individuals initiating efavirenz/emtricitabine/tenofovir (N = 87), ACTG Protocol A5303: phase IIb, double-blind, placebo-controlled, randomized trial of a novel, maraviroc-containing antiretroviral regimen in treatment-naïve HIV-1 infected participants (N = 25) and two industry-sponsored ART treatment trials (N = 120), ERTO: Antiretroviral activity and tolerability of etravirine in treatment-naïve adults with HIV-1 infection and Gilead GS-US-264–0110 Study: Evaluation of the safety and efficacy of emtricitabine (FTC)/rilpivirine (RPV)/tenofovir disoproxil fumarate (TDF) compared with tefavirenz/FTC/TDF in ART-naive HIV-1 infected adults [13,14].
Variables and measures
Throat-wash specimens were collected at each of the first four time points (study preentry, study entry, any time between weeks 12 and 18, and week 24). An oral soft tissue examination was performed at entry and weeks 12–18, 24, and 48. CD4+ T-cell count and plasma HIV-1 RNA (using an assay with a lower level of detectability of 40 copies/ml) were measured at entry and weeks 4, 12–18, 24, and 48 (or at discontinuation if a participant exited the study prematurely). Detailed medical history was obtained and a sexual behavior questionnaire administered at study entry. The questionnaire was repeated 24 weeks after ART initiation.
Oral human papillomavirus detection and typing
Real-time PCR reactions were performed using Roche Lightcycler TaqMan master mix. Each reaction consisted of 1X Roche Lightcycler TaqMan master mix (Roche Diagnostics, Indianapolis, Indiana, USA), HPV-specific primer pairs and fluorescently-tagged probes for types 6, 11, 16, and 18 in a total reaction volume of 10 ml as previously described [15]. Real-time PCR reactions that target the E1 region of HPV were performed using Roche Lightcycler Sybr Green master mix (Roche Diagnostics), and sequencing of PCR products was performed as previously described [15].
Statistical analyses
Standard summary statistics were used to describe sample characteristics [proportions for categorical variables and median with first and third (Q1 and Q3) quartiles for continuous variables]. We computed prevalence of oral warts at study entry and incidence over the 48 weeks following ART initiation. Baseline prevalence of oral HPV infection was defined as having HPV DNA of any type detectable in at least one of the two throat wash specimens collected before ART initiation. Incidence was the proportion of participants who had oral HPV detected at one or both follow-up visits (weeks 12–18 and/or week 24 after initiating ART) among those undetectable at study preentry and entry. Our analysis included only those participants who had available specimens for all four time points. To account for oral sex behavior, which may be associated with acquisition of oral HPV during the study period, we compared number of oral sex partners reported during the past 6 months at study entry and 24 weeks following ART initiation using: a nonparametric approach (Wilcoxon signed-rank test); and a contingency table method after categorizing participants as reporting 0, 1, or more than 1 partners (Fisher's exact test).
We estimated prevalence of oral type-specific HPV infection at baseline and 12–24 weeks follow-up. Finally, we determined the proportion of ‘oral HPV persisters’ (individuals who had the same HPV genotype detected at preentry or entry and again at weeks 12–18 or 24); ‘oral HPV clearers’ (individuals who had a specific genotype detected at preentry or entry but not subsequently at weeks 12–18 or 24; and ‘oral HPV acquirers’ (individuals with a specific genotype detected at weeks 12–18 or 24 that was not detected at preentry or entry). We also assessed proportions of persisters, clearers, and acquirers for oncogenic vs. nononcogenic HPV genotypes and by gender.
We computed median change in absolute CD4+ T-cell count and HIV viral load between study entry and three follow-up time points (weeks 4, 12–18, and 24) with a Mann–Whitney rank-sum test to compare distribution of the change in absolute CD4+ T-cell count and HIV RNA load at these three time points among those who did not have oral HPV DNA detected over time, and among those who did. We also compared age, and number of past-6-month oral sex partners reported at the 24-week visit, between these two groups using a Mann–Whitney rank-sum test.
Results
Sample characteristics
A total of 39 sites (see appendix) recruited 500 participants from 2 January 2010 to 4 June 2012. Among the 500 enrolled in this protocol, 388 had evaluable throat-wash HPV DNA results from both time points before initiating ART and again after 12–18 and 24 weeks of ART and are included in this analysis. We found no difference with respect to race, history of injection drug use, entry CD4+ T-cell count or HIV RNA load between the 388 included in the analysis and the 112 excluded because of missing HPV DNA data points.
Among the 388 participants included in this analysis, the majority was men (81%) and had never used illicit intravenous drugs (94%; Table 1). One-third were White non-Hispanic, 39% were Black non-Hispanic, and the median age at enrollment was 38 years. The median CD4+ T-cell count at study entry was 328 cells/μl, and median plasma HIV RNA was 4.68 log10 copies/ml. After 24 weeks of ART, the median CD4+ T-cell count was 483 cells/μl, the plasma HIV RNA was less than 1.60 log10 copies/ml; 81% of participants had an undetectable viral load (<40 copies/ml), and 100% had less than 500 copies/ml.
Detection of human papillomavirus DNA
Seventy study participants [18%, 95% confidence interval (CI): 14%, 22%] had HPV DNA detected in their throat wash prior to initiating ART, and 93 study participants (24%, 95% CI: 20%, 29%) had HPV DNA detected after 12–18 or 24 weeks of ART (Table 2). Among those who did not have HPV DNA present in their throat wash before initiating ART, 15% (95% CI: 11%, 20%) had an oral specimen in which HPV DNA was detected after 12–18 or 24 weeks of ART. In addition, after 12–18 or 24 weeks of ART, 20% (95% CI: 16%, 24%) of the entire cohort had a new HPV genotype detected in throat wash that was not identified in the specimens obtained before initiating ART (Fig. 1). Also, among the 68 study participants who had typeable HPV DNA present before initiating ART, only 46 (75%, 95% CI: 63%, 86%) cleared one or more of these genotypes during follow-up, whereas 15 (28%, 95% CI: 17%, 41%) persisted in having at least one of the genotypes present before ART detected during follow-up.
Table 2.
Prevalence of oral human papillomavirus DNA among participants in AIDS Clinical Trial Group 5272 at baseline (preentry or entry) and at follow-up (weeks 12–18 or week 24).a
| Prevalence | Total (N = 388) n (%) [95% CI]b | Men (N = 313) n (%) [95% CI]b | Women (N = 75) n (%) [95% CI]b | P valuec |
| Any oral HPV genotype detected at baseline | 70 (18) [14, 22] | 62 (20) [16, 25] | 8 (11) [5, 20] | 0.06 |
| Any oral oncogenic HPV genotype detected at baseline | 18 (5) [3, 7] | 14 (4) [2, 7] | 4 (5) [1, 13] | 0.75 |
| Any oral HPV genotype detected during follow-up | 93 (24) [20, 29] | 71 (23) [18, 28] | 22 (29) [19, 41] | 0.23 |
| Any oral oncogenic HPV genotype detected during follow-up | 21 (5) [3, 8] | 14 (4) [2, 7] | 7 (9) [4, 18] | 0.10 |
CI, confidence interval; HPV, human papillomavirus.
aThe mean difference between reported number of oral sex partners in the past 6 months assessed at the 24-week visit and at the baseline visit was −1.59. A Wilcoxon signed-rank test revealed that this difference was statistically significant (P < 0.0001), indicating that participants had significantly fewer oral sex partners during the 6 months following ART initiation than in the 6 months prior to ART initiation.
b95% confidence interval for the percentage.
cχ2 test comparing HPV DNA prevalence between men and women.
Fig. 1.
Oral HPV genotype change over time.
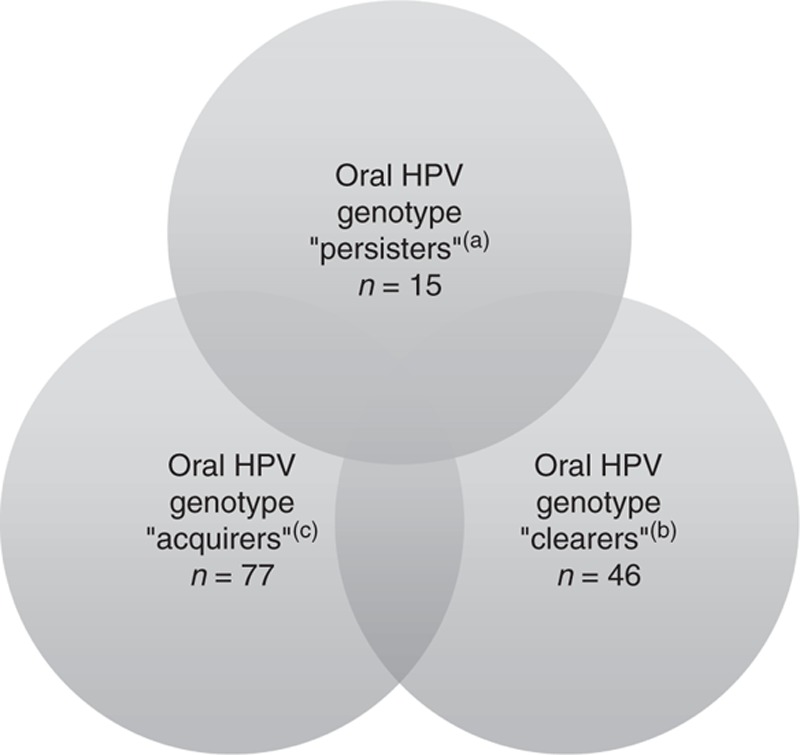
(a) ‘Persisters’ are defined as individuals who had at least one genotype of HPV detected in throat washings at either the screening/preentry or the entry visit and again had that same type detected at either weeks 12–18 or 24. Note: six participants persisted with one HPV genotype and acquired a new one. (b) ‘Clearers’ are defined as individuals who had at least one genotype of HPV detected in throat washings at either the screening/preentry or the entry visit but did not have that genotype detected at week 12–18 or 24. (c) ‘Acquirers’ are defined as individuals who were had at least one genotype of HPV detected in throat washings at either week 12–18 or 24 for which that genotype was not detected at either the screening/preentry or the entry visits. Note: 16 participants had at least one HPV genotype detected at one of the baseline visits but not at either of the follow-up visits and had a new genotype, not detected at baseline that was detected in at least one of the follow-up visits. HPV, human papillomavirus.
The proportion of individuals with detectable HPV DNA at baseline or follow-up was not different between men and women (Table 2). Among those who had evidence of HPV oral infection before initiation of ART, 63% had discordant HPV DNA test results between the preentry and entry visits (one was positive and one negative). Similar analysis after ART initiation revealed 69% had discordant test results at 12–18 weeks and 24 weeks.
The mean difference in the number of oral sex partners reported in the past 6 months assessed at the 24-week visit vs. the baseline visit was −1.59 (P < 0.0001, Wilcoxon signed-rank test), Furthermore, contingency table analysis showed 44% reported more than one oral sex partners in the past 6 months at study entry vs. 29% when assessed at 24-week follow-up (P < 0.0001; note: proportions are slightly different from those in Table 1 because of some missing observations in the cross-tabulation).
Detection of genotype-specific oral human papillomavirus infection
The most common genotypes of HPV detected in throat wash specimens at baseline was HPV 44 (18%), followed by HPV 32 (16%), which is commonly found in focal epithelial hyperplasia, followed by HPV 16 (14%), an oncogenic genotype (Fig. 2). Six of the 20 genotypes detected were high-risk oncogenic genotypes (16, 35, 39, 56, 73, and 82). The prevalence of any oncogenic genotype was 5% (95% CI: 3%, 7%) before and 5% (95% CI: 3%, 8%) after 12–24 weeks of ART (Table 2). Among participants with an oral oncogenic HPV genotype detected at baseline, 58% cleared it after 12–24 weeks of ART, whereas 42% either persisted with the same type, or acquired another oncogenic type while clearing the genotype present at baseline. Four percentage of participants acquired a new oncogenic genotype that was not present at baseline.
Fig. 2.
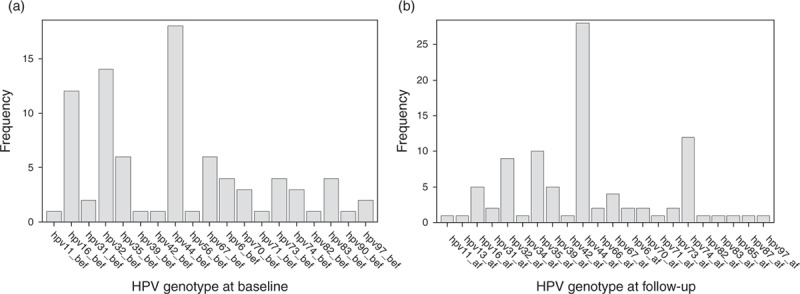
(a) Frequency of each HPV genotype at baseline (preentry and entry); (b) frequency of each HPV genotype at follow-up (12–18 weeks and 24 weeks). HPV, human papillomavirus.
New oral human papillomavirus DNA infection by change in CD4+ T-cell count and HIV RNA load over time
Among participants who had undetectable oral HPV DNA before ART, the median change in CD4+ T-cell count from study entry to 4 weeks after ART initiation was significantly larger in the subset who had detectable HPV DNA during follow-up than in the subset who did not (P = 0.003; Table 3). A difference was also observed with larger CD4+ T-cell increases among the acquirers at 12–18 weeks after ART initiation (P = 0.04), but this was not statistically significant at the 0.05 level at 24 weeks after ART initiation (P = 0.08). With respect to change in plasma HIV RNA from baseline to 4 weeks, or 12–18, or 24 weeks after ART initiation, no difference was observed when comparing those who had detectable oral HPV DNA after ART initiation with those who did not.
Table 3.
Comparison, among those who did not have detectable oral human papillomavirus DNA at baseline, of absolute CD4+ T cell and HIV RNA changes during follow-up based on whether oral human papillomavirus DNA was detected within 24 weeks after antiretroviral therapy initiation.
| CD4+ T-cell count change after ART initiation | Participants with no detectable oral HPV DNA over 24 weeks after ART initiationa | Participants who had detectable oral HPV DNA within 24 weeks after ART initiationa | P valueb | |
| N = 245 | N = 47 | |||
| At week 4 | Median [Q1; Q3] | 79 [20–132] | 125 [54–213] | 0.003 |
| N = 267 | N = 48 | |||
| At weeks 12–18 | Median [Q1; Q3] | 116 [53–195] | 163 [77–274] | 0.04 |
| N = 267 | N = 48 | |||
| At week 24 | Median [Q1; Q3] | 144 [71–245] | 190 [94–292] | 0.08 |
| Log10 HIV RNA load change after ART initiation | ||||
| N = 264 | N = 48 | |||
| At week 4 | Median [Q1; Q3] | 2 [2–3] | 2 [2–3] | 0.34 |
| N = 270 | N = 48 | |||
| At weeks 12–18 | Median [Q1; Q3] | 2 [2–2] | 2 [2–2] | 0.73 |
| N = 270 | N = 48 | |||
| At week 24 | Median [Q1; Q3] | 2 [2–2] | 2 [2–2] | 0 79 |
ART, antiretroviral therapy; HPV, human papillomavirus.
aA Mann–Whitney rank-sum test comparing age and number of past 6-month oral sex partners reported at the 24-week visit among participants with and without detectable oral HPV DNA over 24 weeks after ART initiation revealed no statistically significant difference between the two groups for either variable (P = 0.202 and P = 0.342, respectively).
bMann–Whitney rank-sum test.
We also compared age, and number of past 6-month oral sex partners reported at the 24-week visit among participants with and without detectable oral HPV DNA over 24 weeks after ART initiation, which revealed no statistically significant difference between the two groups for either variable (Mann–Whitney rank-sum test P = 0.202 and P = 0.342, respectively).
Detection of oral warts
At entry, 3% (95% CI: 2%, 5%) of participants were found to have oral warts. After 48 weeks of follow-up, 2.5% (95% CI: 1%, 4%) of study participants who did not have oral warts at entry and had developed oral warts during follow-up.
Discussion
In this prospective study of 388 previously untreated HIV-infected patients, we observed no reduction in overall oral HPV DNA prevalence or in the prevalence of oncogenic oral HPV genotypes after 12–24 weeks of ART despite a high rate of HIV virologic suppression and robust immune reconstitution as indicated by absolute CD4+ T-cell count increase. Among those with oral HPV DNA that could be genotyped before initiating ART, 28% persisted in having at least one of the genotypes still detectable during follow-up; and among participants with an oral oncogenic HPV genotype detected at baseline, 42% either persisted with the same type or acquired another oncogenic type during follow-up. This lack of reduction of overall HPV DNA prevalence was observed despite a significantly lower number of oral sex partners reported by participants during the 6 months following ART initiation than the 6 months prior to initiation.
We also observed that both oral wart prevalence at baseline and incidence during 48 weeks of follow-up were low. Most remarkably, among those who had undetectable oral HPV DNA before ART, the median increase in CD4+ T-cell count from study entry to 4 weeks after ART initiation was paradoxically larger in the subset of study participants with new detectable HPV DNA during follow-up than in the subset that continued to have undetectable HPV DNA in their throat wash specimens.
The prevalence of oral HPV infection and incidence of new oral infection have been previously examined in HIV-infected and uninfected cohorts of men, though not in the context of ART initiation. Kreimer et al. tested oral specimens for HPV DNA at 6-month intervals in 1626 HIV-uninfected men enrolled in Brazil, Mexico, and the United States; MSM made up only 11% of this cohort [16]. The point prevalence of HPV DNA in baseline throat washings was 4%, and the incidence of new HPV infection during 12 months of follow-up among those who were HPV DNA negative at baseline was 4.4% (95% CI 3.5%, 5.6%). The prevalence of oncogenic HPV infection was 2.4% and the incidence of oncogenic HPV infection was 1.7% during 12 months of follow-up. King et al. reported a point prevalence of 13.7% for any oral HPV genotype and 5.9% for oral oncogenic HPV genotypes in a cohort of 151 HIV-uninfected MSM [17]. Mooij et al.[6] reported on a cohort of 453 HIV-uninfected and 314 HIV-infected MSM enrolled in Amsterdam that had considerably higher prevalence rates – 27.6% for any oral HPV DNA in HIV-uninfected MSM and 56.7% in HIV-infected MSM; 8.8 and 24.8% for oncogenic HPV infection, respectively. Among those evaluable at 6-month follow-up, 4.1% of HIV-uninfected MSM and 14.1% of HIV-infected MSM had acquired a new oncogenic oral HPV infection [6]. Among the 388 HIV-infected men enrolled in our cohort, oral HPV prevalence values were between those of these previous studies, perhaps reflecting the increased risk that MSMs have for oral HPV acquisition independent of HIV status [16,17].
The intriguing finding that evidence of HPV DNA was detected more frequently among those who had the largest increases in circulating absolute CD4+ T-cell counts does suggest that activation of latent HPV infection might be an epiphenomenon of immune reconstitution in HIV patients. Replication of the chronic human herpesvirus infections, CMV, and HSV has been prospectively examined among HIV-infected patients initiating ART. Several studies have reported a significant, progressive decline in CMV viremia in the absence of specific anti-CMV therapy after HIV-infected cohorts have initiated ART [18,19]. There is one report that the frequency of vaginal HSV-2 DNA detection among Ugandan HIV-infected women transiently increased immediately after ART initiation, remained elevated in the absence of concomitant acyclovir therapy for 12 weeks, and then returned to pre-ART levels [20]. However, unlike oral HPV DNA detection in HIV-infected patients, the frequency of HSV-2 vaginal detection before and after 6 months of ART in this study was similar to that previously reported in sexually active, HIV-seronegative African women [21]. Nevertheless, this study did find a trend toward those with greater increases in CD4+ T-cell counts after initiating ART having more frequent HSV-2 shedding, as we observed to be statistically significant for oral HPV shedding in our study. This same group more recently reported a transient increase in proportion of Ugandan HIV-infected women from whom CMV DNA could be detected in the vagina between 8 and 16 weeks after ART initiation – perhaps another possible mucosal epiphenomenon of immune reconstitution [22].
Given the increased incidence of HPV-associated oropharyngeal squamous cell carcinoma (SCC) that has been reported in the last 2 decades in the HIV-infected population [9], our findings are concerning. One-third of oral HPV detected in our study were oncogenic genotypes. The greatest risk factor for development of malignancy is HPV persistence. Although this association is best established for cervical cancer where cancer arises from a small, well defined anatomic area (the transformation zone), it may apply to oropharyngeal SCC as well. Although previously published studies have suggested that HPV may persist in oral sites among HIV patients receiving combination ART, this study is the first to prospectively examine the association of initiating ART (and the degree of therapy-related immune reconstitution as measured by absolute CD4+ T-cell count increase) with clearance of oral HPV DNA. Not only did we find no evidence of a reduction in oral HPV resulting from ART, we observed that study participants with the largest increases in circulating absolute CD4+ T-cell count after 12–24 weeks of ART were paradoxically the most likely to have detectable oral HPV DNA. Why the immune benefit induced by ART in controlling other pathogens does not generalize to HPV is unknown. Perhaps, damage to HPV-specific T or B cells occurs early in the course of HIV disease and is irreversible, or ART-induced immune reconstitution might preferentially expand the pool of HPV-specific regulatory T cells, or reconstitution of immune responses targeting other pathogens might adversely affect HPV-specific immune reconstitution.
These findings underscore the importance of advancing knowledge of immune system control of HPV and determinants of HPV-specific immune deficits that are associated with loss of that control in HIV-infected people. Peripheral blood mononuclear cells and saliva specimens were obtained and stored from time points both before and after 24 weeks of ART in our study. The next step will be to measure HPV-specific immune responses in our cohort. Though we are not aware of any T-cell functional assays that can reliably detect HPV-specific responses in stored peripheral blood mononuclear cells from HIV-infected patients (e.g. cytokine production or T-cell proliferation), there are ELISA assays that have been used in animal and human vaccination studies to measure HPV genotype-specific neutralizing antibody titers in mucosal secretions, including saliva [23–25].
One limitation of this study was incomplete follow-up. Of the 500 study participants enrolled, evaluable oral specimens for HPV DNA were not obtained from 6% of study participants at week 16 and 13% at week 24. However, we were able to obtain an evaluable oral specimen at both of the pre-ART visits and both follow-up time points in 388 (78%) study participants. Although oral HPV infection can be chronic, the presence of HPV DNA in the oral cavity may be intermittent [26]. Thus, our collection of two throat wash specimens from before ART initiation and two specimens during the period 12–24 weeks afterward improved the accuracy of the results reported here, which was also demonstrated by the poor concordance we observed between the two specimens that were collected before ART as well as the two collected during 12–24 weeks of follow-up. All other large observational studies of oral HPV have obtained only a single sample at baseline and at follow-up intervals.
In conclusion, a causal link between HPV infection in the oral cavity and oropharyngeal SCC, the higher prevalence and incidence of oral HPV infection in men with HIV than among men who are HIV-negative, and our observation in an HIV-infected cohort of the failure of ART to decrease oral HPV infection all lead us to be concerned that oropharyngeal SCC may continue to increase among those who are HIV-infected. Perhaps, wide adoption of HPV vaccination for adolescent males might ultimately reduce oropharyngeal SCC incidence, even among those with concomitant HIV infection. In the meantime, research studies that can define how HIV, despite ART, permits unrestricted HPV infection in the oral cavity are needed to provide the scientific underpinning for other preventive strategies.
Acknowledgements
We would like to express our sincere appreciation to A5272 study participants, to Dr Isaac Rodriguez-Chavez for reviewing the manuscript, and to the investigative teams of the ACTG clinical research sites that enrolled participants in protocol A5272. The following Clinical Research Site (CRS) teams (names of CRS team members who contributed to the study are listed before the CRS name for each site), which enrolled participants for Protocol A5272, are listed in the order of highest to lowest accrual number.
Dee Dee Pacheo and Leticia Muttera – UC San Diego (Site 701) Grant AI069432.
Pablo Tebas MD and Aleshia Thomas BSN RN – University of Pennsylvania (Site 6201) ACTG CTU Grant UMIA068636.
Elizabeth Lindsey RN and Truus Delfos-Broner CNM – Alabama CRS (Site 31788) ACTG CTU Grant 2UM1AI069452-08.
Hannah Edmondson RN MPH and Lorraine Sanchez-Keeland PA-C – University of Southern California (Site 1201) Grant AI069428.
Mary Adams RN and Christine Hurley RN – University of Rochester (Site 31787) ACTG CTU Grant 2UM1AI069511 and UL1 TR000042.
Traci Davis and Kim Whitely – MetroHealth Medical Center (Site 2503) Grant AI-69501.
Annie Luetkemeyer MD and Jay Dwyer RN – UCSF AIDS (Site 801) Grant UM1 AI069496.
Beverly Sha MD and Joan Swiatek, MS APRN – Rush University Medical Center (Site 2702) Grant U01 AI069471.
Dr John Davis MD and Kathy Watson RN – Ohio State University (Site 2301) ACTG CTU Grant UM1AI069494.
Vicki Bailey RN and Husamettin Erdem – Vanderbilt Therapeutics CRS (Site 3652) ACTG CTU Grant 2UM1AI069439–08 and supported in part by the Vanderbilt CTSA grant UL1 TR000445 from NCATS/NIH.
Dr David Reznik and Ericka Patrick – Ponce de Leon (Site 5802) Grants 5U01 AI069418, ACTG CTU Grant 2UM1AI069418–08, UL1TR0000454 and 2P30 AI 50409.
Michael Klebert RN PhD ANP-BC and Lisa Kessels RN BS – Washington University in St. Louis (Site 2101) Grant AI49439.
Michael Yin MD and Jolene Noel-Connor RN – Columbia University P&S CRS (Site 30329) Grant 5UM1AI069470-09.
Christina Megill PA-C and Todd Stroberg RN – Weill Cornell-Chelsea CRS (Site 7803) ACTG CTU Grant UM1AI069419.
Mehri McKellar MD and Vicky Pena RN – Duke University Medical Center (Site 1601) Grant 5U01 AI069484.
Jonathan Oakes BA – UNC Chapel Hill CRS (Site 3201) Grants UM1 AI069423, 1UL1TR001111, and P30 AI50410.
Margaret A. Fischl MD and Hector Bolivar MD – University of Miami AIDS CRS (Site 901) Grant UM1 AI069477-08.
Dr Jorge L. Santana Bagur MD FIDSA and Dr Olga Mendez MD AAHIVS PR-ACTU CRS (Site 5401) ACTG CTU Grant 5UM1AI069415-10.
Rose Kim MD and Yolanda Smith BA - Cooper University Hospital (Site 31476) Grant UM1 AI069503.
Patricia Walton RN and Kristen Allen RN – Case CRS (Site 2501) Grant AI069501.
Roberto C. Arduino MD and Aristoteles Villamil MD – HART (Site 31473) Grant 2MU1 AI069503 and 2UM1 AI068636.
Teri Flynn ANP-BC and Amy Sbrolla RN BSN – Massachusetts General Hospital (Site 101) ACTG CTU Grant 2UM1AI069412-08.
Baiba Berzins MPH and Nina Lambert BSN – Northwestern University CRS (Site 2701) Grant AI069471.
Aadia Rana MD and Deborah Perez RN – The Miriam Hospital (Site 2951) ACTG CTU Grant 2UM1A1069412-08.
Graham Ray and Cathi Basler – University of Colorado (Site 6102) ACTG CTU Grant 2UM1AI069432 and UL1 TR001082.
Charles E. Davis Jr MD and Leonard A. Sowah MBChB MPH – IHV Baltimore Treatment CRS (Site 4651) Grant AI069447.
Dr Margrit Carlson MD and Maria Palmer PA – UCLA Care Center CRS (Site 601) Grant AI069424.
Shelia Dunaway MD and Sheryl S. Storey PA-C – University of Washington ACTU CRS (Site 1401) Grant UM AI069481.
Dr Carl Fichtenbaum and Eva Whitehead RN BSN – University of Cincinnati (Site 2401) Grant UM1 AI069501.
Linda Makohon RN BSN and Leslie Faber RN BSN – Henry Ford Hospital (Site 31472) Grant B40465.
Carol Clark RN and Vicky Watson RN – Virginia Commonwealth University (Site 31475) Grant UL1TR000058.
Mary Albrecht MD and Andrea Kershaw ANP – Beth Israel Deaconess/Harvard (Site 103) Grant AI069472.
David Cohn MD and Diane States RN – Denver Public Health CRS (Site 31470) Grant UM1 AI069503.
Cornelius Van Dam MD and Tim Lane MD – Greensboro CRS (Site 3203) 2UMIAI069423-08.
Rodger D. MacArthur MD and Marti Farrough BSN RN – Wayne State University (Site 31478) Grant 1UM1 AI069503.
Shobha Swaminathan MD and Christie Lyn Costanza MPH – Rutgers New Jersey Medical School CRC (Site 31786) ACTG CTU Grant 2UM1AI069419.
Paul Sax MD and Cheryl Keenan RN BC – Brigham and Women's Hospital (Site 107) Grant UM1AI069472.
Judith A. Aberg MD and Karen Cavanagh RN – New York University/Bellevue ACTU (Site 0401) Grant UM1 AI069532.
Co-authors roles:
Study design and/or protocol development: C.H.S., H.C., J.W.C., R.J.L., M.J., N.R., L.N., J.P., M.A.J.
Data acquisition: R.J.L., N.R.
Laboratory assays: J.W.C., T.S.
Data analysis: A.L., H.C.
Data interpretation: C.H.S., J.W.C., R.J.L., and M.A.J.
Initial drafting of the manuscript: C.H.S. and M.A.J.
Manuscript revision and review of final version: all authors.
Funding: This work was supported by National Institutes of Health Cooperative Agreement U01AI068636 from the National Institute of Allergy and Infectious Diseases and the National Institute of Dental and Craniofacial Research; and U01 AI068634 for the Statistical and Data Management Center for the AIDS Clinical Trials Group. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Regezi JA, Dekker NP, Ramos DM, Li X, Macabeo-Ong M, Jordan RC. Proliferation and invasion factors in HIV-associated dysplastic and nondysplastic oral warts and in oral squamous cell carcinoma: an immunohistochemical and RT-PCR evaluation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002; 94:724–731. [DOI] [PubMed] [Google Scholar]
- 2.Coutlee F, Trottier AM, Ghattas G, Leduc R, Toma E, Sanche G, et al. Risk factors for oral human papillomavirus in adults infected and not infected with human immunodeficiency virus. Sex Transm Dis 1997; 24:23–31. [DOI] [PubMed] [Google Scholar]
- 3.Kreimer AR, Alberg AJ, Daniel R, Gravitt PE, Viscidi R, Garrett ES, et al. Oral human papillomavirus infection in adults is associated with sexual behavior and HIV serostatus. J Infect Dis 2004; 189:686–698. [DOI] [PubMed] [Google Scholar]
- 4.D'Souza G, Fakhry C, Sugar EA, Seaberg EC, Weber K, Minkoff HL, et al. Six-month natural history of oral versus cervical human papillomavirus infection. Int J Cancer 2007; 121:143–150. [DOI] [PubMed] [Google Scholar]
- 5.Cameron JE, Mercante D, O’Brien M, Gaffga AM, Leigh JE, Fidel PL, Jr, et al. The impact of highly active antiretroviral therapy and immunodeficiency on human papillomavirus infection of the oral cavity of human immunodeficiency virus-seropositive adults. Sex Transm Dis 2005; 32:703–709. [DOI] [PubMed] [Google Scholar]
- 6.Mooij SH, Boot HJ, Speksnijder AG, Stolte IG, Meijer CJ, Snijders PJ, et al. Oral human papillomavirus infection in HIV-negative and HIV-infected MSM. AIDS 2013; 27:2117–2128. [DOI] [PubMed] [Google Scholar]
- 7.Clifford GM, Polesel J, Rickenbach M, Dal Maso L, Keiser O, Kofler A, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst 2005; 97:425–432. [DOI] [PubMed] [Google Scholar]
- 8.Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer 2008; 123:187–194. [DOI] [PubMed] [Google Scholar]
- 9.Patel P, Hanson DL, Sullivan PS, Novak RM, Moorman AC, Tong TC, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Ann Intern Med 2008; 148:728–736. [DOI] [PubMed] [Google Scholar]
- 10.Greenspan D, Canchola AJ, MacPhail LA, Cheikh B, Greenspan JS. Effect of highly active antiretroviral therapy on frequency of oral warts. Lancet 2001; 357:1411–1412. [DOI] [PubMed] [Google Scholar]
- 11.King MD, Reznik DA, O’Daniels CM, Larsen NM, Osterholt D, Blumberg HM. Human papillomavirus-associated oral warts among human immunodeficiency virus-seropositive patients in the era of highly active antiretroviral therapy: an emerging infection. Clin Infect Dis 2002; 34:641–648. [DOI] [PubMed] [Google Scholar]
- 12.Buchacz K, Baker RK, Palella FJ, Jr, Chmiel JS, Lichtenstein KA, Novak RM, et al. AIDS-defining opportunistic illnesses in US patients, 1994-2007: a cohort study. AIDS 2010; 24:1549–1559. [DOI] [PubMed] [Google Scholar]
- 13.Lennox JL, Landovitz RJ, Ribaudo HJ, Ofotokun I, Na LH, Godfrey C, et al. Efficacy and tolerability of 3 nonnucleoside reverse transcriptase inhibitor-sparing antiretroviral regimens for treatment-naive volunteers infected with HIV-1: a randomized, controlled equivalence trial. Ann Intern Med 2014; 161:461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porter DP, Kulkarni R, Fralich T, Miller MD, White KL. Characterization of HIV-1 drug resistance development through week 48 in antiretroviral naive subjects on rilpivirine/emtricitabine/tenofovir DF or efavirenz/emtricitabine/tenofovir DF in the STaR study (GS-US-264-0110). J Acquir Immune Defic Syndr 2014; 65:318–326. [DOI] [PubMed] [Google Scholar]
- 15.Seaman WT, Andrews E, Couch M, Kojic EM, Cu-Uvin S, Palefsky J, et al. Detection and quantitation of HPV in genital and oral tissues and fluids by real time PCR. Virol J 2010; 7:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreimer AR, Pierce Campbell CM, Lin HY, Fulp W, Papenfuss MR, Abrahamsen M, et al. Incidence and clearance of oral human papillomavirus infection in men: the HIM cohort study. Lancet 2013; 382:877–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King EM, Gilson R, Beddows S, Soldan K, Panwar K, Young C, et al. Oral human papillomavirus (HPV) infection in men who have sex with men: prevalence and lack of anogenital concordance. Sex Transm Infect 2015; 91:284–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deayton JR, Sabin CA, Britt WB, Jones IM, Wilson P, Johnson MA, et al. Rapid reconstitution of humoral immunity against cytomegalovirus but not HIV following highly active antiretroviral therapy. AIDS 2002; 16:2129–2135. [DOI] [PubMed] [Google Scholar]
- 19.O'Sullivan CE, Drew WL, McMullen DJ, Miner R, Lee JY, Kaslow RA, et al. Decrease of cytomegalovirus replication in human immunodeficiency virus infected-patients after treatment with highly active antiretroviral therapy. J Infect Dis 1999; 180:847–849. [DOI] [PubMed] [Google Scholar]
- 20.Tobian AA, Grabowski MK, Serwadda D, Newell K, Ssebbowa P, Franco V, et al. Reactivation of herpes simplex virus type 2 after initiation of antiretroviral therapy. J Infect Dis 2013; 208:839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayaud P, Nagot N, Konate I, Ouedraogo A, Weiss HA, Foulongne V, et al. Effect of HIV-1 and antiretroviral therapy on herpes simplex virus type 2: a prospective study in African women. Sex Transm Infect 2008; 84:332–337. [DOI] [PubMed] [Google Scholar]
- 22.Gianella S, Redd AD, Grabowski MK, Tobian AA, Serwadda D, Newell K, et al. Vaginal cytomegalovirus shedding before and after initiation of antiretroviral therapy in Rakai, Uganda. J Infect Dis 2015; 212:899–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kemp TJ, Garcia-Pineres A, Falk RT, Poncelet S, Dessy F, Giannini SL, et al. Evaluation of systemic and mucosal anti-HPV16 and anti-HPV18 antibody responses from vaccinated women. Vaccine 2008; 26:3608–3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balmelli C, Roden R, Potts A, Schiller J, De Grandi P, Nardelli-Haefliger D. Nasal immunization of mice with human papillomavirus type 16 virus-like particles elicits neutralizing antibodies in mucosal secretions. J Virol 1998; 72:8220–8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowe RS, Brown DR, Bryan JT, Cook JC, George HA, Hofmann KJ, et al. Human papillomavirus type 11 (HPV-11) neutralizing antibodies in the serum and genital mucosal secretions of African green monkeys immunized with HPV-11 virus-like particles expressed in yeast. J Infect Dis 1997; 176:1141–1145. [DOI] [PubMed] [Google Scholar]
- 26.Beachler DC, Weber KM, Margolick JB, Strickler HD, Cranston RD, Burk RD, et al. Risk factors for oral HPV infection among a high prevalence population of HIV-positive and at-risk HIV-negative adults. Cancer Epidemiol Biomarkers Prev 2012; 21:122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]


